Significance
Food availability in nature changes continually over an organism’s lifetime. As such, animals must diligently assess resource availability and appropriately allocate reserves that have been stored during times of feast for reproduction, to abate evolutionary pressures during times of famine. Our findings functionally link the availability of somatic (survival-promoting) and germline (reproduction-promoting) lipids to SKN-1 responses to oxidative and nutrient stress. We have defined this physiological response at the molecular, genetic, and organismal levels and identified a specific signaling system for regulating this process within intact animals. These findings will inform not only laboratory-based studies, but also ecological studies that have long sought to functionally integrate oxidative stress responses (like the SKN-1 pathway) into life-history traits.
Keywords: soma, germline, trade-off, lipids, survival
Abstract
Animals in nature are continually challenged by periods of feast and famine as resources inevitably fluctuate, and must allocate somatic reserves for reproduction to abate evolutionary pressures. We identify an age-dependent lipid homeostasis pathway in Caenorhabditis elegans that regulates the mobilization of lipids from the soma to the germline, which supports fecundity but at the cost of survival in nutrient-poor and oxidative stress environments. This trade-off is responsive to the levels of dietary carbohydrates and organismal oleic acid and is coupled to activation of the cytoprotective transcription factor SKN-1 in both laboratory-derived and natural isolates of C. elegans. The homeostatic balance of lipid stores between the somatic and germ cells is mediated by arachidonic acid (omega-6) and eicosapentaenoic acid (omega-3) precursors of eicosanoid signaling molecules. Our results describe a mechanism for resource reallocation within intact animals that influences reproductive fitness at the cost of somatic resilience.
Trade-offs between fecundity and viability fitness components are thought to drive life-history traits when resources are limited (1). In Caenorhabditis elegans, previous studies that removed proliferating germ cells led to an increase in somatic fat (2) and a ∼60% increase in lifespan (3), which is hypothesized to result from the reallocation of germline resources to the soma, promoting survival through enhanced proteostasis (4) and attuned metabolism (5).
Although these previous studies are compelling, the use of reproduction-deficient animals confounds the interpretation of their results with regard to trade-off models, and raises the question of how altered reallocation may affect intact animals. During reproduction, somatic resources are deposited to the germline by the actions of vitellogenins (6), which assemble and transport lipids in the form of yolk from the intestine to developing oocytes. The increased survival of germline-defective animals and their accumulation of somatic lipids suggest that the levels of somatic and germline lipids may influence the age-related decline of somatic cell function in postreproductive life (5). The mechanisms that regulate the distribution of energy resources remain elusive, however.
SKN-1 is the worm homolog of mammalian Nrf2, a cytoprotective transcription factor that impacts multiple aspects of animal physiology (7). Early work on SKN-1 defined its essential roles in development (8) and oxidative stress responses (9), whereas more recent work has identified a role mediating changes in diet availability and composition (10, 11). In the present study, we examined the SKN-1–mediated dietary adaptation pathways (10–12) of C. elegans and uncovered a sophisticated mechanism for mobilizing somatic lipids to the germline when animals sense stressful environments. This altruistic act by the soma impacts organismal viability to promote fecundity during oxidative and nutrient stress conditions. The universality of oxidative stress responses among aerobic organisms is a tantalizing source of energetic “cost” to maintain homeostasis that can compete with resources for reproduction. As such, an understanding of how oxidative stress responses impact reproduction, and vice versa, will likely yield insights into how the complex regulation of survival and reproduction trade-offs depend on resource reallocation (13). Here we report a SKN-1–dependent axis of regulating the distribution of somatic and germ cell resources.
Results
Age-Dependent Somatic Depletion of Fat Is Induced by Activated SKN-1.
Over the course of an individual’s lifespan, lipids are continually mobilized to afford organismal energy demands for growth, cellular maintenance and repair, and reproduction (14). We first examined total fat stores by Oil-red-O (15) (SI Appendix, Fig. S1 A–E) and fixed Nile red (SI Appendix, Fig. S2 A–D) in the standard wild type (WT) laboratory C. elegans strain N2-Bristol throughout reproduction, from early adulthood (72 h postfeeding) through reproductive senescence (144 h postfeeding). (Herein, hours postfeeding refers to the amount of time that animals have been provided with food following synchronization at larval stage 1 via starvation from hatching.) In these animals, similar to most metazoans, somatic lipid stores increased throughout this time period (Fig. 1 A and B and SI Appendix, Figs. S1 A–E and S2 A–D).
Fig. 1.
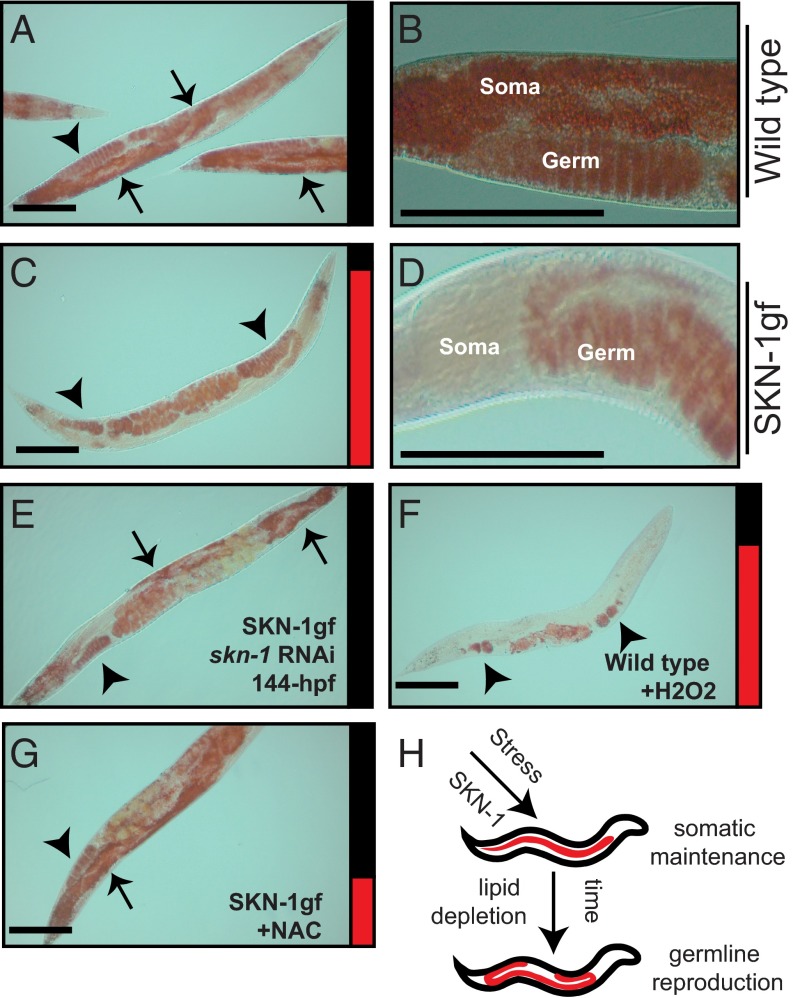
SKN-1 activation mobilizes somatic fat to the germline. (A–D) Oil-red-O staining of somatic and germline lipids in WT animals, but only germline lipids in SKN-1gf mutants, at 144 h postfeeding. (E) skn-1 RNAi suppresses Asdf in SKN-1gf animals. (F) Asdf is induced in WT animals by acute exposure to H2O2. (G) NAC treatment suppresses Asdf in SKN-1gf animals. (H) Cartoon of the Asdf phenotype. Arrows indicate soma, and arrowheads indicate germ. Bar graphs accompanying each panel indicate the percent of population scored with the Asdf phenotype (red) vs. normal lipid distribution (black) from a minimum of two biological replicates for each genotype and condition (SI Appendix, Fig. S3). (Scale bars: 100 μm.)
Based on the recent discovery that SKN-1 can potently influence the ability of organisms to metabolically adapt to changes in the environment (10, 11), we next looked at total fat stores during reproduction in SKN-1 gain-of-function (gf) mutant animals (Fig. 1 C and D and SI Appendix, Figs. S1 A and F–M and S2 E–L) and observed the skn-1–dependent rapid depletion of somatic, but not germline, lipid stores near the end of the reproductive period (Fig. 1 C and D and SI Appendix, Fig. S1 I and M, Fig. S2 H and L, and Table S1), a phenotype that, based on its characteristics, we call the age-dependent somatic depletion of fat (Asdf) phenotype. We assessed the Asdf phenotype in each cohort by quantifying the number of animals that displayed Asdf with those that did not. SI Appendix, Fig. S3 provides all % Asdf measurements. The Asdf phenotype was similar in all SKN-1–activating mutants tested, which includes strains harboring mutations in alh-6 (10, 11) (SI Appendix, Fig. S4 A and B) or wdr-23 (16) (SI Appendix, Fig. S4 C and D), whereas skn-1 RNAi suppressed Asdf in SKN-1gf mutant animals (Fig. 1E). These data indicate that activated SKN-1 is sufficient to induce Asdf.
SKN-1 activation is correlated with increased levels of reactive oxygen species from endogenous sources or environmental exposure to oxidizing agents (17). Following acute exposure to H2O2, which can activate SKN-1, WT animals rapidly (within 12 h) deplete most somatic lipids (Fig. 1F). The Asdf response is not a generalized stress response and is specific to oxidative stress; WT animals exposed to heat (SI Appendix, Fig. S4G) or osmotic (SI Appendix, Fig. S4H) stress environments did not induce the lipid depletion phenotype. Further supporting the need for skn-1 in the Asdf response, skn-1(−/−) null mutants did not deplete somatic fat following H2O2 exposure, and heterozygous skn-1(+/−) animals showed an intermediate response (SI Appendix, Fig. S4 I and J). Asdf was suppressed when animals with activated SKN-1 were treated with the antioxidant N-acetylcysteine (NAC) (Fig. 1G and SI Appendix, Fig. 4 K–N). Intriguingly, treatment of WT animals with NAC or skn-1 RNAi led to excessive accumulation of somatic lipids (SI Appendix, Fig. S4 O–R), similar to the increased fat observed in skn-1(−/−) animals (SI Appendix, Fig. S4 S and T) and consistent with previous reports of lipid phenotypes in animals with reduced skn-1 (18). This finding supports previous predictions in the life-history theory proposing that the energetic costs to maintain organismal oxidative stress capacity over the animal’s lifetime represent a major trade-off variable (19). Taken together, our data indicate that the somatic depletion phenotype is sensitive to oxidative stress and requires SKN-1 (Fig. 1H).
Asdf Mobilizes Somatic Lipids During Nutrient Stress.
Our observation that animals with Asdf retained lipids in the germline suggests that Asdf might result from mobilization of stored somatic lipids to the reproductive system. Members of the vitellogenin family of proteins facilitate transport of stored lipids from the intestine to developing oocytes (20) (Fig. 2A). RNAi of all vit genes tested resulted in suppression of Asdf (i.e., restoration of somatic lipids), indicating that vitellogenesis is required for Asdf in the SKN-1gf mutants (Fig. 2B and SI Appendix, Fig. S5 A–D). The presence of somatic lipids in SKN-1gf animals was restored when vit-2, -3, or -5 was targeted by RNAi, or was even increased with reduced expression of vit-4. As such, the age-dependent loss of lipids in the soma in SKN-1gf animals is not simply the result of somatic utilization, but rather is a consequence of the unidirectional mobilization of stored lipids by the vitellogenins.
Fig. 2.
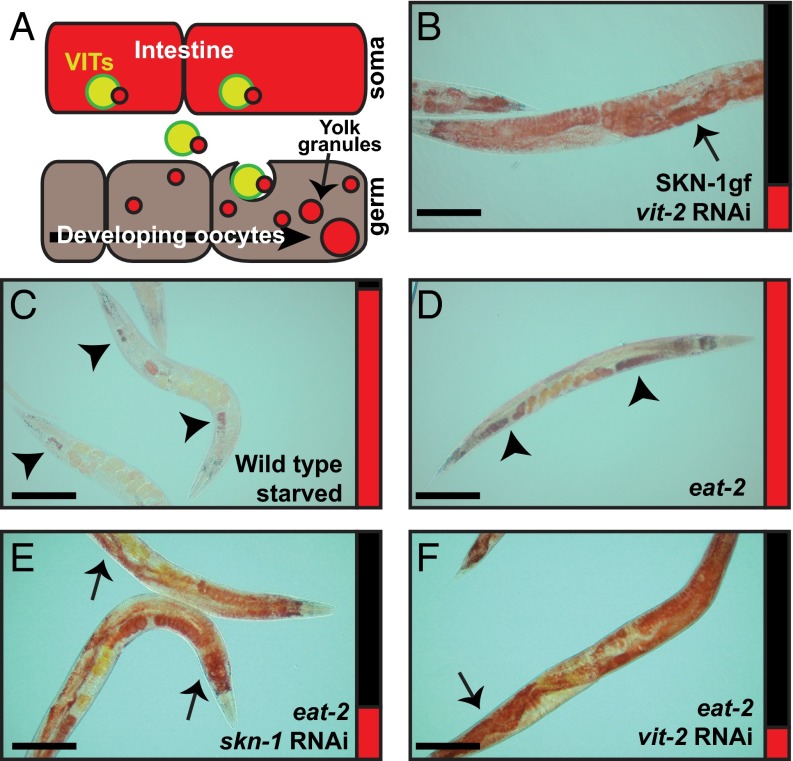
Asdf is a starvation response dependent on vitellogenesis. (A) Cartoon representation of the vitellogenin lipid transport system from the intestine to the germline. (B) vit-2 RNAi suppresses Asdf in SKN-1gf animals. (C) WT animals starved for 24 h deplete somatic lipids but retain a lipid pool in the germline. (D) eat-2(ad456) mutants display Asdf at 144 h postfeeding. (E and F) skn-1 (E) and vit-2 (F) RNAi suppresses Asdf in eat-2 mutant animals. Arrows indicate soma, and arrowheads indicate germ. Bar graphs accompanying each panel indicate the percent of population scored with the Asdf phenotype (red) vs. normal lipid distribution (black) from a minimum of two biological replicates for each genotype and condition (SI Appendix, Fig. S3). (Scale bars: 100 μm.)
SKN-1 activity is essential for the longevity response to dietary deficiencies (21), and starvation itself can induce oxidative stress (22). Indeed, the depletion of stored lipids in WT animals after 24 h of starvation, albeit more extreme, resembled the Asdf observed in well-fed animals with activated SKN-1 (Fig. 2C). Consistent with the idea that the Asdf phenotype in SKN-1gf is a response to a perceived nutritional deficiency, eat-2 mutants, which eat significantly less food than WT animals (23), also displayed Asdf at the same time point in their reproductive span, whereas WT animals failed to display Asdf (Fig. 2D and SI Appendix, Fig. S6 A–D). Asdf was not observed in daf-2/insulin-IGF1 receptor (SI Appendix, Fig. S6 E and F) and isp-1/mitochondrial iron sulfur protein (SI Appendix, Fig. S6 G–J) mutants, and thus is not universal to all longevity-promoting mutations. The Asdf phenotype observed in eat-2 mutants was suppressed by skn-1 (Fig. 2E) and vit-2 (Fig. 2F) RNAi-treated animals. Note that Asdf is suppressed by the HT115 diet and glucose; thus, all RNAi experiments reported herein were performed in an OP50-background RNAi strain (SI Appendix, Fig. S7 and Tables S2 and S3). Our findings support an intriguing model of resource reallocation between the C. elegans soma and germline, where activation of the cytoprotective transcription factor SKN-1 under limited food and oxidative stress leads to the mobilization of stored lipid pools to the germline, presumably to ensure fitness.
Oleic Acid Deficiency Is Sufficient to Induce Asdf.
To understand the mechanisms underlying Asdf, we identified the specific lipid molecules altered in the SKN-1gf mutants by HPLC/GCMS (SI Appendix, Fig. S8 A and B). We noted a significant reduction in C17-branched fatty acids and the monounsaturated fatty acid (MUFA) oleic acid (C18:1 n-9) in the triglyceride fraction of the SKN-1gf mutants compared with WT animals. Oleic acid was the sole lipid species restored to WT levels in SKN-1gf animals when the Asdf phenotype was suppressed by dietary glucose (SI Appendix, Figs. S7 R–U and S8C). C. elegans can synthesize oleic acid and all polyunsaturated fatty acid (PUFA) species from dietary or de novo synthesized C16:0 (24) (SI Appendix, Figs. S8D and S9A).
fat-6 and fat-7 encode the major isoforms of the Δ9 desaturases that convert stearic acid to oleic acid (25). We subsequently tested for a direct relationship between oleic acid and Asdf. First, we decreased fat-6/-7 by RNAi in WT animals, which phenocopied the Asdf phenotype at the same 144-h postfeeding time point observed in the SKN-1gf mutants (Fig. 3A). We measured fat-6 and fat-7 mRNA in SKN-1gf and WT animals and found similar levels of expression. Thus, the Asdf phenotype in SKN-1gf mutant animals is not due simply to reduced expression of the transcripts (SI Appendix, Table S4). Next, we supplemented the OP50 diet fed to SKN-1gf mutants with 160 μM and 320 μM oleic acid and observed a concentration-dependent reversal of Asdf, with 60.7% and 81.6% suppression of the Asdf phenotype, respectively, in this population (Fig. 3B and SI Appendix, Fig. S9 B–G).
Fig. 3.
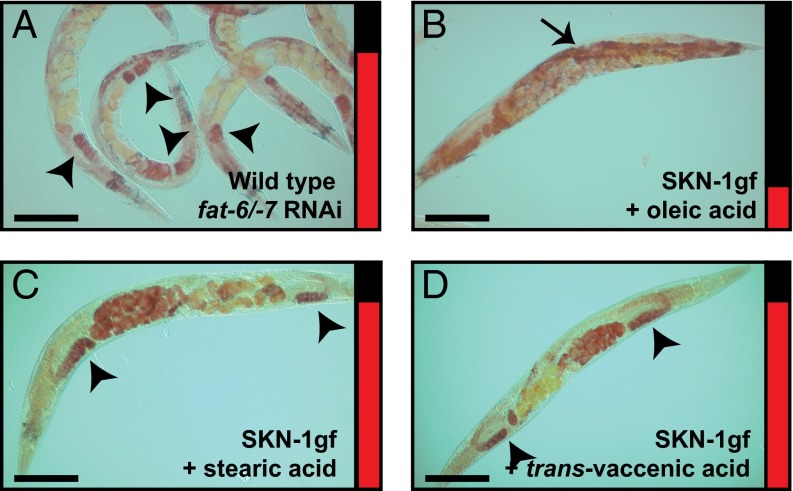
Oleic acid deficiency is causal for Asdf. (A) RNAi inactivation of fat-6/-7 in WT animals is sufficient to induce Asdf. (B) Dietary supplementation of oleic acid suppresses Asdf in SKN-1gf mutant animals. (C and D) Dietary supplementation of stearic acid (C) and trans-vaccenic acid (D) do not suppress Asdf in SKN-1gf mutant animals. Arrows indicate soma, and arrowheads indicate germ. Bar graphs accompanying each panel indicate the percent of population scored with the Asdf phenotype (red) vs. normal lipid distribution (black) from a minimum of two biological replicates for each genotype and condition (SI Appendix, Fig. S3). (Scale bars: 100 μm.)
To test the hypothesis that the suppression of Asdf by oleic acid is related to a general increase in total lipids, we assessed the ability of additional lipid supplements to suppress Asdf. We tested lipid species that are biosynthetic precursors to oleic acid, including C18:0 stearic acid (Fig. 3C and SI Appendix, Fig. S9H) and C12:0 lauric acid (SI Appendix, Fig. S9 I and J), as well lipids that are further desaturated products of oleic acid, including C18:2 n-6 linoleic acid, C18:3 n-3 α-linolenic acid, and C18:3 n-6 γ-linolenic acid. Similar to supplementation with stearic and lauric acid, each of these supplements dramatically increased total fat in WT animals; however, they could not suppress Asdf in SKN-1gf mutants (SI Appendix, Fig. S9 K–P). We also tested trans-vaccenic acid (C18:1 trans-11), a MUFA that can be desaturated by FAT-6 and FAT-7 (26), but found that, unlike oleic acid, it was incapable of any observable suppression of Asdf in the SKN-1gf mutants (Fig. 3D and SI Appendix, Fig. S9Q). Taken together, these findings suggest that a lipid deficiency, specifically in oleic acid (C18:1), is causal for the Asdf phenotype in SKN-1gf animals as animal reproduction declines.
Omega-3 and -6 C20 PUFAs Oppose Asdf.
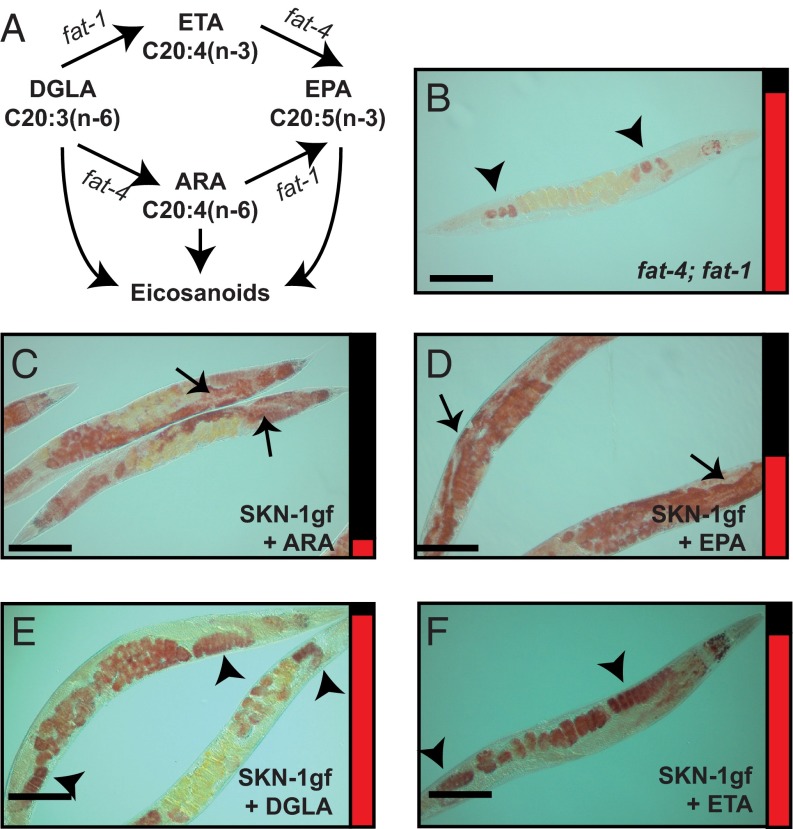
We were surprised to find that lipid defects in the SKN-1gf mutant animals were specific to a single MUFA, oleic acid, and that this defect did not propagate to longer and more unsaturated species (SI Appendix, Fig. S8A). However, in our assessment of the lipid biosynthesis pathways, we uncovered a role for specific C20 omega-3 and omega-6 PUFAs in the regulation of Asdf. Like mammals, C. elegans synthesize a variety of lipid signaling molecules that are epoxy and hydroxyl derivatives of dihomo-γ-linolenic acid (DGLA), arachidonic acid (ARA), and eicosapentaenoic acid (EPA) PUFAs, which influence complex physiological processes that maintain homeostasis (27, 28) (Fig. 4A). DGLA and eicosatetraenoic acid (ETA) are biosynthetic precursors for ARA and EPA, respectively; however, ARA can be further desaturated to make EPA, and thus DGLA is a precursor for both ARA and EPA. fat-4(wa14); fat-1(wa9) double-mutant animals, which cannot generate ARA or EPA (29), prominently displayed Asdf at the same 144-h postfeeding time point, but not early in reproduction at 72 h postfeeding, as was observed in SKN-1gf mutant animals (Fig. 4B and SI Appendix, Fig. S10A). The levels of fat-1 and fat-4 were similar in SKN-1gf and WT animals, indicating that the Asdf phenotype is not due to a reduction in gene expression in SKN-1gf mutant animals (SI Appendix, Table S4).
Fig. 4.
ARA (omega-6) and EPA (omega-3) fatty acids regulate Asdf. (A) Schematic of eicosanoid biosynthesis pathways in C. elegans. (B) ARA and EPA deficient fat-4(wa14); fat-1(wa9) animals induce Asdf at 144 h postfeeding. (C–F) Dietary supplementation of ARA (C) or EPA (D), but not of DGLA (E) or ETA (F), can suppress Asdf in SKN-1gf mutant animals. Arrows indicate soma, and arrowheads indicate germ. Bar graphs accompanying each panel indicate the percent of population scored with the Asdf phenotype (red) vs. normal lipid distribution (black) from a minimum of two biological replicates for each genotype and condition (SI Appendix, Fig. S3). (Scale bars: 100 μm.)
The foregoing data suggest that one function of C20 omega-3 and omega-6 PUFAs is to help maintain the distribution of somatic and germline lipids, and that reduced levels of these lipid species promote Asdf. Treatment of SKN-1gf mutants with 160 μM or 320 μM ARA resulted in potent suppression of Asdf, by 82% and 91%, respectively (Fig. 4C and SI Appendix, Fig. S10 B–E). Similarly, EPA supplementation suppressed Asdf to 40% and 54% of animals at the same concentrations (Fig. 4D and SI Appendix, Fig. S10 F and G). The suppression of Asdf was specific to ARA and EPA; SKN-1gf mutants fed OP50 supplemented with DGLA or ETA, even at high concentrations, still displayed Asdf (Fig. 4 E and F and SI Appendix, Fig. S10 H–J). Taken together, these findings further support a dose-dependent role for specific omega-6 and omega-3 PUFAs in the homeostatic balance of somatic and germline lipid reserves.
Asdf Occurs in Natural Isolates of C. elegans.
C. elegans represent a species of particularly low genetic diversity at the molecular level (30), and recent work to isolate and document the phenotypes of the ever-expanding library of wild C. elegans strains has revealed interesting phenotypic variation among them when cultured under laboratory conditions (31). A dearth of ecological data has hindered a better understanding of the relevance of this variation in the natural context, however (32). We analyzed a small collection of wild isolates of C. elegans and examined the abundance of somatic and germline lipids and their propensity for Asdf (SI Appendix, Fig. S11 A–H and Table S5). None of the wild isolates displayed Asdf at early time points in their reproductive span; however, four of the wild isolate strains tested displayed Asdf at the same 144-h postfeeding time point as animals with activated SKN-1, albeit with varying penetrance. NL7000 and ED3040 had the strongest Asdf phenotype, ED3021 displayed an intermediary phenotype, and ED3049 had a weak Asdf response in this population. RW7000, TR403, CB4856, and CB4869 were most similar to N2-Bristol in that they did not display Asdf at any time point.
Strains NL7000 and RW7000 are isolates of the same strain of Bergerac that recently diverged in the laboratory setting. Although derived from the same parental isolate, NL7000 displays Asdf at 144 h postfeeding, whereas RW7000 does not. Moreover, and consistent with the idea that Asdf promotes reproductive fitness, NL7000 animals have more progeny and remain reproductive longer than RW7000 animals (SI Appendix, Fig. S11I). Taken together, our data suggest that the Asdf phenotype is present in some, but not all, wild C. elegans strains, and that the propensity for Asdf may be correlated with reproductive success.
Asdf Promotes Reproduction at the Cost of Survival.
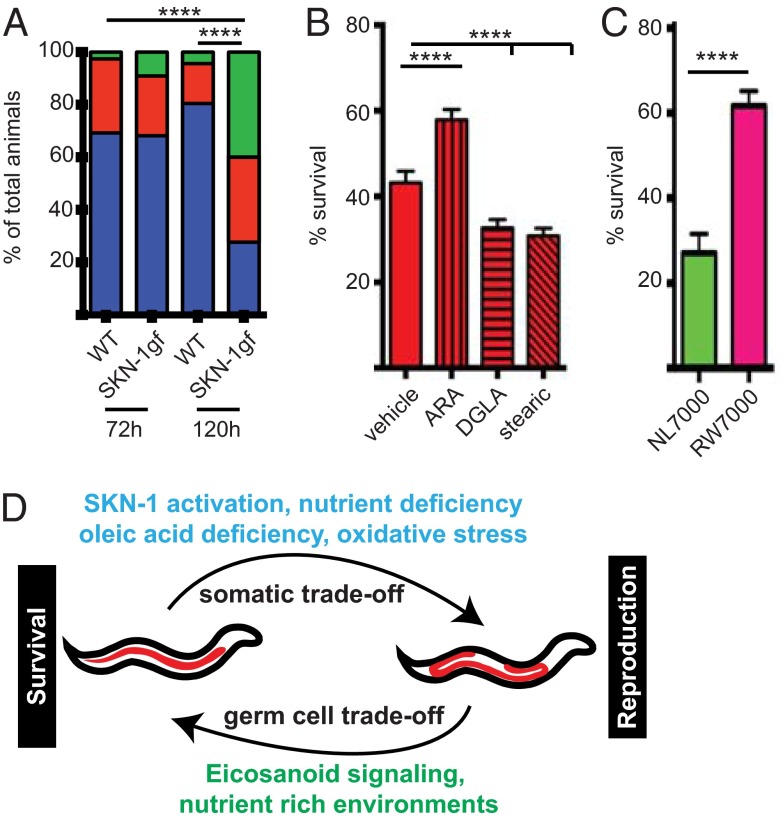
We next assessed the role of Asdf in animal physiology and the resulting impact of deregulating Asdf capacity. During periods of scarce resources, fertile C. elegans hermaphrodites exhibit matricide, an altruistic behavior in which fertilized eggs are held in the uterus and hatch internally, and the resulting larvae feed on the hermaphrodite mother as a nutrient source (33). We observed an intriguing matricide phenomenon that correlated with Asdf in SKN-1gf mutants. When day 3 (120 h postfeeding) adult SKN-1gf mutants with early signs of Asdf were starved for 24 h, they became filled with newly hatched larvae, phenotypically defined as bags of worms (Bag) (Fig. 5A and SI Appendix, Fig. S12 A–D). This is in contrast to day 1 adult (72 h postfeeding) SKN-1gf mutant animals and day 1 or 3 adult WT animals, which have only one, if any, internally hatched larvae after 24 h of starvation. During the 48 h separating these two periods in reproduction, WT C. elegans accumulate lipids in their somatic tissues (SI Appendix, Figs. S1 B–E and S2 A–D), whereas SKN-1gf mutants mobilize somatic fat to the germline (SI Appendix, Figs. S1 F–M and S2 E–L). The Bag phenotype observed in day 3 adult SKN-1gf mutants with Asdf could be a consequence of the Asdf-mediated increase in germline lipids.
Fig. 5.
Asdf fuels germ cell maturation to ensure fitness. (A) Following 24 h of starvation, SKN-1gf mutants display an age-dependent increase in the incidence of matricide (Bag phenotype) that coincides with Asdf and is not induced in WT animals when starved for 24 h. Blue indicates zero to one internal progeny; red, two to four internal progeny; green, five or more internal progeny. ****P < 0.0001, ANOVA. (B) OP50 diet supplemented with ARA, but not with DGLA or stearic acid, can increase somatic resistance to acute H2O2 exposure in SKN-1gf mutant animals at 144 h postfeeding. ****P < 0.0001, ANOVA. (C) Somatic resistance to H2O2 in NL7000 and RW7000 Bergerac strains correlates with Asdf competency. Data are mean ± SEM for at least 40 animals, with a minimum of two biological replicates for each genotype and condition. ****P < 0.0001, two-tailed t test. (D) Model for the mechanisms underlying somatic survival and germline reproduction trade-offs of lipid reallocation within intact animals.
A primary function of somatic cells is to protect the germline, but this comes at the cost of depleting somatic resources. ARA supplementation has been linked to the survival of somatic tissues during starvation and can increase the lifespan of ad libitum-fed WT animals (34). SKN-1gf mutants display significant resilience to H2O2 exposure in early reproductive life compared with WT animals (SI Appendix, Fig. S13 A and B); however, the afforded resistance to exogenous oxidative stress in SKN-1gf mutants declines at 144 h postfeeding (SI Appendix, Fig. S13B). We hypothesized that the reduction in somatic energy reserves as lipids are mobilized to the germline during Asdf is causal for the diminished oxidative stress resistance capacity. To test this, we inhibited Asdf by ARA supplementation to the OP50 diet, which resulted in a marked increase in resilience to acute H2O2 exposure in 144 h postfeeding, but not 80 h postfeeding, SKN-1gf animals (Fig. 5B and SI Appendix, Fig. S13 B and C). The restoration of somatic resistance to oxidative stress was specific, because supplementation with DGLA and stearic acid did not increase survival at either time point (Fig. 5B and SI Appendix, Fig. S13C). Intriguingly, postreproductive WT animals, which no longer need to devote as many resources to reproduction, exhibited a significantly increased survival response to acute H2O2 exposure (SI Appendix, Fig. S13A).
Finally, we examined somatic stress resistance to H2O2 in the NL7000 (Asdf+) and RW7000 (Asdf−) Bergerac strains. Although both strains had enhanced resistance at 72 h postfeeding (SI Appendix, Fig. S13D), NL7000 displayed a significant loss of resilience at 144 h postfeeding, whereas RW7000 was more apt to survive acute exposure to H2O2 (Fig. 5C). These findings are consistent with an increased capacity for stress resistance that is fueled by additional somatic resources, and regulated by specific omega PUFAs.
Taken together, our results describe a pathway for the reallocation of resources between the soma and germ cells of an intact organism (Fig. 5D). Our findings link the availability of somatic and germline lipids to SKN-1 responses to oxidative stress and nutrient limitation. This reallocation impacts somatic survival during stress and reproductive output, which may have universal implications for organisms with specialized soma and germ cells.
Discussion
In the present study, we examined organismal age-related levels of lipids during the C. elegans reproductive span and found a remarkable lipid reallocation phenotype between somatic and germ cells that impacts survival and reproduction trade-offs. We used Oil-red-O and Nile red staining of fixed animals, because the former allows for qualitative assessment of tissue distribution and the latter affords more quantitative measurements, albeit with reduced spatial resolution. We observed similar patterns of lipid distribution with either dye, but each could have unique specificity for different lipid species (35, 36), and differences in the intensity and size of the lipid droplets might reflect a change in the composition of lipid molecules affected.
Our discovery was facilitated by a collection of SKN-1gf mutants that we previously characterized as having reduced lipid levels on fat-inducing diets (10, 11), perhaps owing in part to their starvation-like behaviors, despite being fed ad libitum (12). Although resistant to acute exposure to oxidative stress, none of the constitutively activated SKN-1 mutants have proven to be long-lived. This finding is surprising, given that SKN-1 is a cytoprotective transcription factor essential for mounting an appropriate stress response. The near-complete depletion of somatic lipid reserves from the soma in the animals could explain this lack of longevity in the SKN-1gf mutants. The eventual depletion of somatic lipids was apparent at 144 h postfeeding, but clear differences in lipid abundance between the somatic and germline cells were obvious by 120 h postfeeding. Our data suggest that following the peak of reproduction, somatic resources are mobilized to the germline, but these resources are effectively “wasted” as animals enter reproductive senescence, because postreproductive animals no longer need to devote as many resources to reproduction. Intriguingly, SKN-1gf mutants do indeed have an extended self-reproductive period that does require Asdf, and thus an intriguing model for the function of Asdf is to promote late reproductive output. Although recent reports have shown that mated C. elegans hermaphrodites lose fat after mating, future assessment of the impact of Asdf on the fertility of mated animals will be of great interest, considering that maximal reproductive capacity is limited by sperm production in hermaphrodites (37, 38).
Collectively, our data support a genetic role for skn-1 in the Asdf phenotype. Further refinement of the role SKN-1 plays in the distribution of somatic and germline lipids will be of particular interest. This work expands the known impact of SKN-1 on organismal physiology beyond its role as a mediator of cellular and organismal stress responses (7). One interpretation of this study is that SKN-1 activity is restricted to the soma, which leads to loss of lipids in this compartment; however, the fact that both SKN-1gf and eat-2 mutant animals no longer deplete somatic lipids when vitellogenesis is impaired suggests that mobilization of lipids to the germline is at least partially causal for the loss of somatic lipids. In addition, the supplementation of all lipid species resulted in an increase of somatic fat in WT animals and in SKN-1gf mutants early in reproduction, but only oleic acid, ARA, and EPA could suppress Asdf. The fact that most fatty acid supplements did not impact Asdf but also did not increase somatic stress resistance in the SKN-1gf mutants suggests that the depletion of somatic lipids is not simply a result of increased utilization in the soma.
This lipid reallocation has consequences for both somatic and germline tissues. The enhanced resistance to oxidative stress afforded in the SKN-1gf mutant animals is progressively impaired as animals proceed through reproduction, which correlates with the temporal progression of Asdf. Furthermore, if Asdf is suppressed, then the decline in stress resistance is attenuated. Thus, the reallocation of lipids between the soma and the germline is physiologically relevant, because the ultimate location where the lipids reside impacts the function of that compartment. Although body mass index (BMI) has proven to be an imperfect predictor of human metabolic disease risk (39), recent work has suggested that moderate increases in BMI above “normal” can be protective (40). Perhaps the reduction in mortality resulting from increased somatic reserves is the result of enhanced utilization of those stores for adaptation.
We have identified a role for C20 PUFAs in the mobilization of somatic resources to the germline in the SKN-1gf mutant animals. Dietary supplementation with the omega-6 PUFA ARA and the omega-3 PUFA EPA effectively suppressed Asdf, whereas that with the omega-6 PUFA DGLA did not. ARA, EPA, and DGLA are precursors of specific classes of eicosanoid signaling molecules (41), which play multiple and complex roles in animal physiology. Our finding that only ARA and EPA can suppress Asdf suggests that specific species of eicosanoids could be responsible for the physiological responses that we observed. C20 PUFAs also play a critical role in maintaining membrane fluidity (42), and thus the addition of these C20 PUFAs could alter membrane function and signaling capacity; however, the opposing responses to DGLA compared with ARA and EPA suggest that this is not simply a general disorganization of the lipid bilayer (43). Nevertheless, future assessment of the phospholipid composition of membranes, the signaling pathways that influence Asdf, and the functional consequences of perturbing these components on Asdf capacity and resulting phenotypes will be of great interest.
Although we examined reproductive-stage adults, previous studies of germline starvation responses in developing larvae have documented the scavenging of material from the germline to fuel reproduction (44) and even reproductive diapause (45). The increased germline lipid stores in Asdf+ animals could promote two non-mutually exclusive outcomes: (i) provide additional fuel for the rapid maturation of progeny and (ii) provide adequate nutrients to escape diapause initiation and/or maintenance. Alternatively, because SKN-1gf mutants Bag only when starved at the end of the reproductive period, this phenotype could represent a time-dependent failure to decrease ovulation in response to nutrient limitation when SKN-1 is constitutively activated. Nevertheless, because progeny’s success is subject to the deposition of maternal factors, and their life-history parameters are sensitive to the experiences of the parental and grandparental generations (46–48), future studies to assess the cumulative effects of Asdf capacity on fitness in successive generations are needed.
We analyzed a collection of natural C. elegans isolates from diverse climates that revealed that Asdf capacity is variable in the wild (SI Appendix, Table S5). The RW7000 Bergerac isolate does not display Asdf and has a diminished reproductive period and brood compared with the recently diverged NL7000 strain, which displays Asdf at 144 h postfeeding and has a much larger brood size and a longer self-reproductive period. The number of SNPs between these strains is unknown, and these strains quite possibly could be significantly divergent from each other because they are classical mutator lines, originally used for the active transposons in their genomes. Nonetheless, in light of our finding that single gene mutations are sufficient to induce Asdf, future assessment of the genomic differences between these two strains and all of the wild isolates tested will be of particular interest.
Our results identify a SKN-1 and eicosanoid signaling pathway that balances somatic lipid mobilization to developing germ cells at the cost of survival. Our study provides insight into the trade-offs resulting from the reallocation of lipid stores within intact animals, which are critically important during nutrient and oxidative stress (Fig. 5D). The fundamental similarities of the C. elegans and mammalian lipid metabolism and eicosanoid biosynthesis and signaling pathways (41) suggests that the resource reallocation pathways and resulting trade-offs may be conserved.
Methods
C. elegans was cultured by standard techniques at 20 °C unless noted otherwise. Statistical analyses were performed with GraphPad Prism 6 software. Data are presented as mean ± SEM. Data were analyzed with the unpaired Student t test and two-way ANOVA. All of the methods used in this study are described in detail in SI Appendix.
Supplementary Material
Acknowledgments
We thank L. Thomas and J. Dietrich for technical support and A. Pradhan and J. Lo for a critical reading of the manuscript. We also thank the Caenorhabditis Genetics Center, funded by the National Institutes of Health’s Office of Research Infrastructure Programs (P40 OD010440), for providing some strains. Support for this work was provided by the National Institutes of Health (Grant T32AG000037, to D.A.L.; R00AG032308, to S.P.C.; and R01 GM109028, to S.P.C.), the American Heart Association (S.P.C.), an Ellison New Scholar Award (S.P.C.), and the American Federation for Aging Research (S.P.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1514012112/-/DCSupplemental.
References
- 1.Stearns SC. The Evolution of Life Histories. Oxford Univ Press; Oxford, UK: 1992. [Google Scholar]
- 2.O’Rourke EJ, Soukas AA, Carr CE, Ruvkun G. C. elegans major fats are stored in vesicles distinct from lysosome-related organelles. Cell Metab. 2009;10(5):430–435. doi: 10.1016/j.cmet.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsin H, Kenyon C. Signals from the reproductive system regulate the lifespan of C. elegans. Nature. 1999;399(6734):362–366. doi: 10.1038/20694. [DOI] [PubMed] [Google Scholar]
- 4.Vilchez D, et al. RPN-6 determines C. elegans longevity under proteotoxic stress conditions. Nature. 2012;489(7415):263–268. doi: 10.1038/nature11315. [DOI] [PubMed] [Google Scholar]
- 5.Hansen M, Flatt T, Aguilaniu H. Reproduction, fat metabolism, and life span: What is the connection? Cell Metab. 2013;17(1):10–19. doi: 10.1016/j.cmet.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schneider WJ. Vitellogenin receptors: Oocyte-specific members of the low-density lipoprotein receptor supergene family. Int Rev Cytol. 1996;166:103–137. doi: 10.1016/s0074-7696(08)62507-3. [DOI] [PubMed] [Google Scholar]
- 7.Sykiotis GP, Bohmann D. Stress-activated cap’n’collar transcription factors in aging and human disease. Sci Signal. 2010;3(112):re3. doi: 10.1126/scisignal.3112re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowerman B, Eaton BA, Priess JR. skn-1, a maternally expressed gene required to specify the fate of ventral blastomeres in the early C. elegans embryo. Cell. 1992;68(6):1061–1075. doi: 10.1016/0092-8674(92)90078-q. [DOI] [PubMed] [Google Scholar]
- 9.Sykiotis GP, Bohmann D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev Cell. 2008;14(1):76–85. doi: 10.1016/j.devcel.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pang S, Lynn DA, Lo JY, Paek J, Curran SP. SKN-1 and Nrf2 couple proline catabolism with lipid metabolism during nutrient deprivation. Nat Commun. 2014;5:5048. doi: 10.1038/ncomms6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pang S, Curran SP. Adaptive capacity to bacterial diet modulates aging in C. elegans. Cell Metab. 2014;19(2):221–231. doi: 10.1016/j.cmet.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paek J, et al. Mitochondrial SKN-1/Nrf mediates a conserved starvation response. Cell Metab. 2012;16(4):526–537. doi: 10.1016/j.cmet.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isaksson CS, Sheldon BC, Uller T. The challenges of integrating oxidative stress into life-history biology. Bioscience. 2011;61:194–202. [Google Scholar]
- 14.Efeyan A, Comb WC, Sabatini DM. Nutrient-sensing mechanisms and pathways. Nature. 2015;517(7534):302–310. doi: 10.1038/nature14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pino EC, Webster CM, Carr CE, Soukas AA. Biochemical and high- throughput microscopic assessment of fat mass in Caenorhabditis elegans. J Vis Exp. 2013;73:50180. doi: 10.3791/50180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choe KP, Przybysz AJ, Strange K. The WD40 repeat protein WDR-23 functions with the CUL4/DDB1 ubiquitin ligase to regulate nuclear abundance and activity of SKN-1 in Caenorhabditis elegans. Mol Cell Biol. 2009;29(10):2704–2715. doi: 10.1128/MCB.01811-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.An JH, Blackwell TK. SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes Dev. 2003;17(15):1882–1893. doi: 10.1101/gad.1107803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinbaugh MJ, et al. Lipid-mediated regulation of SKN-1/Nrf in response to germ cell absence. eLife. 2015;4:PMC4541496. doi: 10.7554/eLife.07836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Selman C, Blount JD, Nussey DH, Speakman JR. Oxidative damage, ageing, and life-history evolution: Where now? Trends Ecol Evol. 2012;27(10):570–577. doi: 10.1016/j.tree.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Kimble J, Sharrock WJ. Tissue-specific synthesis of yolk proteins in Caenorhabditis elegans. Dev Biol. 1983;96(1):189–196. doi: 10.1016/0012-1606(83)90322-6. [DOI] [PubMed] [Google Scholar]
- 21.Bishop NA, Guarente L. Two neurons mediate diet restriction-induced longevity in C. elegans. Nature. 2007;447(7144):545–549. doi: 10.1038/nature05904. [DOI] [PubMed] [Google Scholar]
- 22.Scherz-Shouval R, et al. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26(7):1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raizen DM, Lee RY, Avery L. Interacting genes required for pharyngeal excitation by motor neuron MC in Caenorhabditis elegans. Genetics. 1995;141(4):1365–1382. doi: 10.1093/genetics/141.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watts JL. Fat synthesis and adiposity regulation in Caenorhabditis elegans. Trends Endocrinol Metab. 2009;20(2):58–65. doi: 10.1016/j.tem.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brock TJ, Browse J, Watts JL. Genetic regulation of unsaturated fatty acid composition in C. elegans. PLoS Genet. 2006;2(7):e108. doi: 10.1371/journal.pgen.0020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watts JL, Browse J. A palmitoyl-CoA-specific delta9 fatty acid desaturase from Caenorhabditis elegans. Biochem Biophys Res Commun. 2000;272(1):263–269. doi: 10.1006/bbrc.2000.2772. [DOI] [PubMed] [Google Scholar]
- 27.Delmastro-Greenwood M, Freeman BA, Wendell SG. Redox-dependent anti-inflammatory signaling actions of unsaturated fatty acids. Annu Rev Physiol. 2014;76:79–105. doi: 10.1146/annurev-physiol-021113-170341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kosel M, et al. Eicosanoid formation by a cytochrome P450 isoform expressed in the pharynx of Caenorhabditis elegans. Biochem J. 2011;435(3):689–700. doi: 10.1042/BJ20101942. [DOI] [PubMed] [Google Scholar]
- 29.Vásquez V, Krieg M, Lockhead D, Goodman MB. Phospholipids that contain polyunsaturated fatty acids enhance neuronal cell mechanics and touch sensation. Cell Reports. 2014;6(1):70–80. doi: 10.1016/j.celrep.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sivasundar A, Hey J. Population genetics of Caenorhabditis elegans: The paradox of low polymorphism in a widespread species. Genetics. 2003;163(1):147–157. doi: 10.1093/genetics/163.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Bono M, Bargmann CI. Natural variation in a neuropeptide Y receptor homolog modifies social behavior and food response in C. elegans. Cell. 1998;94(5):679–689. doi: 10.1016/s0092-8674(00)81609-8. [DOI] [PubMed] [Google Scholar]
- 32.Barrière A, Félix MA. Isolation of C. elegans and related nematodes. WormBook. 2014 doi: 10.1895/wormbook.1.115.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCulloch D, Gems D. Evolution of male longevity bias in nematodes. Aging Cell. 2003;2(3):165–173. doi: 10.1046/j.1474-9728.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 34.O’Rourke EJ, Kuballa P, Xavier R, Ruvkun G. Omega-6 polyunsaturated fatty acids extend life span through the activation of autophagy. Genes Dev. 2013;27(4):429–440. doi: 10.1101/gad.205294.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greenspan P, Mayer EP, Fowler SD. Nile red: A selective fluorescent stain for intracellular lipid droplets. J Cell Biol. 1985;100(3):965–973. doi: 10.1083/jcb.100.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rudolf M, Curcio CA. Esterified cholesterol is highly localized to Bruch’s membrane, as revealed by lipid histochemistry in whole mounts of human choroid. J Histochem Cytochem. 2009;57(8):731–739. doi: 10.1369/jhc.2009.953448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi C, Murphy CT. Mating induces shrinking and death in Caenorhabditis mothers. Science. 2014;343(6170):536–540. doi: 10.1126/science.1242958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hodgkin J, Barnes TM. More is not better: Brood size and population growth in a self-fertilizing nematode. Proc Biol Sci. 1991;246(1315):19–24. doi: 10.1098/rspb.1991.0119. [DOI] [PubMed] [Google Scholar]
- 39.Conus F, Rabasa-Lhoret R, Peronnet F. 2007. Characteristics of metabolically obese normal-weight (MONW) subjects. Appl Physiol Nutr Metab 32(1):4–12. [DOI] [PubMed]
- 40.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: A systematic review and meta-analysis. JAMA. 2013;309(1):71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vrablik TL, Watts JL. Polyunsaturated fatty acid derived signaling in reproduction and development: Insights from Caenorhabditis elegans and Drosophila melanogaster. Mol Reprod Dev. 2013;80(4):244–259. doi: 10.1002/mrd.22167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watts JL, Browse J. Genetic dissection of polyunsaturated fatty acid synthesis in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2002;99(9):5854–5859. doi: 10.1073/pnas.092064799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Webster CM, Deline ML, Watts JL. Stress response pathways protect germ cells from omega-6 polyunsaturated fatty acid-mediated toxicity in Caenorhabditis elegans. Dev Biol. 2013;373(1):14–25. doi: 10.1016/j.ydbio.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seidel HS, Kimble J. The oogenic germline starvation response in C. elegans. PLoS One. 2011;6(12):e28074. doi: 10.1371/journal.pone.0028074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Angelo G, Van Gilst MR. Starvation protects germline stem cells and extends reproductive longevity in C. elegans. Science. 2009;326(5955):954–958. doi: 10.1126/science.1178343. [DOI] [PubMed] [Google Scholar]
- 46.Greer EL, Brunet A. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell. 2009;8(2):113–127. doi: 10.1111/j.1474-9726.2009.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rechavi O, et al. Starvation-induced transgenerational inheritance of small RNAs in C. elegans. Cell. 2014;158(2):277–287. doi: 10.1016/j.cell.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pang S, Curran SP. Longevity and the long arm of epigenetics: Acquired parental marks influence lifespan across several generations. BioEssays. 2012;34(8):652–65. doi: 10.1002/bies.201200046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.