Significance
High-intensity interval training (HIIT) has become popular because it is a time-efficient way to increase endurance. An intriguing and so-far-unanswered question is how a few minutes of HIIT can be that effective. We exposed recreationally active men to one session of three to six sets of 30-s high-intensity cycling exercise. Muscle biopsies taken 24 h later showed an extensive fragmentation of the sarcoplasmic reticulum (SR) Ca2+ channels, the ryanodine receptor 1 (RyR1). In isolated mouse muscle fibers, this fragmentation was accompanied by increased SR Ca2+ leak, which can trigger mitochondrial biogenesis. The HIIT-induced RyR1 fragmentation did not occur in muscles exposed to antioxidant, which offers an explanation for why antioxidants blunt effects of endurance training.
Keywords: ryanodine receptor 1, high-intensity exercise, skeletal muscle, Ca2+, reactive oxygen species
Abstract
High-intensity interval training (HIIT) is a time-efficient way of improving physical performance in healthy subjects and in patients with common chronic diseases, but less so in elite endurance athletes. The mechanisms underlying the effectiveness of HIIT are uncertain. Here, recreationally active human subjects performed highly demanding HIIT consisting of 30-s bouts of all-out cycling with 4-min rest in between bouts (≤3 min total exercise time). Skeletal muscle biopsies taken 24 h after the HIIT exercise showed an extensive fragmentation of the sarcoplasmic reticulum (SR) Ca2+ release channel, the ryanodine receptor type 1 (RyR1). The HIIT exercise also caused a prolonged force depression and triggered major changes in the expression of genes related to endurance exercise. Subsequent experiments on elite endurance athletes performing the same HIIT exercise showed no RyR1 fragmentation or prolonged changes in the expression of endurance-related genes. Finally, mechanistic experiments performed on isolated mouse muscles exposed to HIIT-mimicking stimulation showed reactive oxygen/nitrogen species (ROS)-dependent RyR1 fragmentation, calpain activation, increased SR Ca2+ leak at rest, and depressed force production due to impaired SR Ca2+ release upon stimulation. In conclusion, HIIT exercise induces a ROS-dependent RyR1 fragmentation in muscles of recreationally active subjects, and the resulting changes in muscle fiber Ca2+-handling trigger muscular adaptations. However, the same HIIT exercise does not cause RyR1 fragmentation in muscles of elite endurance athletes, which may explain why HIIT is less effective in this group.
It is increasingly clear that regular physical exercise plays a key role in the general well-being, disease prevention, and longevity of humans. Impaired muscle function manifesting as muscle weakness and premature fatigue development are major health problems associated with the normal aging process as well as with numerous common diseases (1). Physical exercise has a fundamental role in preventing and/or reversing these muscle problems, and training also improves the general health status in numerous diseases (2–4). On the other side of the spectrum, excessive muscle use can induce prolonged force depressions, which may set the limit on training tolerance and performance of top athletes (5, 6).
Recent studies imply a key role of the sarcoplasmic reticulum (SR) Ca2+ release channel, the ryanodine receptor 1 (RyR1), in the reduced muscle strength observed in numerous physiological conditions, such as after strenuous endurance training (6), in situations with prolonged stress (7), and in normal aging (8, 9). Defective RyR1 function is also implied in several pathological states, including generalized inflammatory disorders (10), heart failure (11), and inherited conditions such as malignant hyperthermia (12) and Duchenne muscular dystrophy (13). In many of the above conditions, there is a link between the impaired RyR1 function and modifications induced by reactive oxygen/nitrogen species (ROS) (6, 8, 10, 12, 13). Conversely, altered RyR1 function may also be beneficial by increasing the cytosolic free [Ca2+] ([Ca2+]i) at rest, which can stimulate mitochondrial biogenesis and thereby increase fatigue resistance (14–16). Intriguingly, effective antioxidant treatment hampers beneficial adaptations triggered by endurance training (17–19), and this effect might be due to antioxidants preventing ROS-induced modifications of RyR1 (20).
A high-intensity interval training (HIIT) session typically consists of a series of brief bursts of vigorous physical exercise separated by periods of rest or low-intensity exercise. A major asset of HIIT is that beneficial adaptations can be obtained with much shorter exercise duration than with traditional endurance training (21–25). HIIT has been shown to effectively stimulate mitochondrial biogenesis in skeletal muscle and increase endurance in untrained and recreationally active healthy subjects (22, 26), whereas positive effects in elite endurance athletes are less clear (21, 27, 28). Moreover, HIIT improves health and physical performance in various pathological conditions, including cardiovascular disease, obesity, and type 2 diabetes (29, 30). Thus, short bouts of vigorous physical exercise trigger intracellular signaling of large enough magnitude and duration to induce extensive beneficial adaptations in skeletal muscle. The initial signaling that triggers these adaptations is not known.
In this study, we tested the hypothesis that a single session of HIIT induces ROS-dependent RyR1 modifications. These modifications might cause prolonged force depression due to impaired SR Ca2+ release during contractions. Conversely, they may also initiate beneficial muscular adaptations due to increased SR Ca2+ leak at rest.
Results
HIIT Causes Fragmentation of RyR1 in Recreationally Active Men.
In an initial experiment to test whether a brief period of HIIT exercise can induce long-lasting changes in muscle function, recreationally active males (SI Appendix, Table S1) performed three 30-s all-out bouts of cycling (i.e., only 90 s of total exercise time) with 4-min rest between bouts. Subsequent contractions produced by electrical stimulation of knee extensors revealed a marked length-independent force decrease, especially at low (10 Hz) stimulation frequency, which was not fully recovered even 24 h after the brief HIIT exercise (SI Appendix, Fig. S1A). Thus, these initial experiments show that as little as three 30-s intervals of HIIT exercise can induce long-lasting impairments in contractile function.
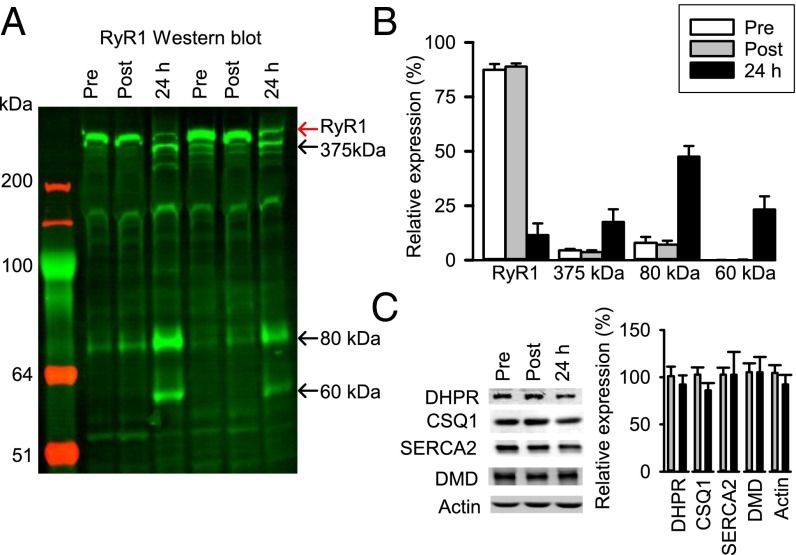
In the next series of experiments, recreationally active males performed six 30-s all-out cycling bouts, and biopsies were taken from the vastus lateralis muscle before and at ∼10 min and 24 h after the cycling bouts (SI Appendix, Fig. S1B). To assess changes in RyR1 induced by this HIIT exercise, Western blot experiments were performed with a polyclonal antibody targeted against the last nine amino acids on the C-terminal end of human RyR (no. 5029; gift from Andrew Marks, Columbia University, New York). These experiments showed no obvious change in RyR1 directly after the HIIT exercise, but 24 h later, only ∼15% remained as the full-sized RyR1 monomer, and instead major fragments emerged at ∼375, 80, and 60 kDa (Fig. 1 A and B). Similarly, a commercially available mouse monoclonal anti-RyR1 antibody (ab2868; Abcam) showed a shift from the full-length RyR1 monomer to a ∼375-kDa fragment 24 h after exercise (SI Appendix, Fig. S2); note that the ab2868 antibody did not detect the smaller ∼80- and 60-kDa fragments, possibly because the cleavage sites then interfered with the binding site of this antibody. Conversely, neither the t-tubular voltage sensor (the dihydropyridine receptor; DHPR), the SR Ca2+ pump (SERCA2), the SR Ca2+ buffer (calsequestrin 1; CSQ1), nor the structural proteins dystrophin (DMD) and actin showed any change in expression or signs of fragmentation after the HIIT exercise (Fig. 1C). Moreover, Western blotting to assess the amount of ubiquitin-conjugated proteins showed no general difference between before and 10 min and 24 h after HIIT exercise (SI Appendix, Fig. S3).
Fig. 1.
HIIT exercise induces extensive RyR1 fragmentation in recreationally active subjects. (A) Representative Western blot reveals decreased expression of full-sized RyR1 (red arrow) 24 h after exercise, which was accompanied by the appearance of fragments of ∼375, 80, and 60 kDa (indicated by black arrows). (B) Mean relative distribution of native RyR1 and its fragments from four subjects; the total intensity of all four analyzed bands was set to 100% at each time point in each subject. (C) Representative Western blots and mean data of DHPR, SERCA2, CSQ1, DMD, and actin expression ∼10 min (n = 11) and 24 h (n = 5) after exercise; relative expression before exercise was set to 100% in each subject. Data are expressed as mean ± SEM.
To investigate whether other types of exhaustive exercise also result in RyR1 fragmentation, we studied RyR1 modifications induced by a marathon foot race performed by male subjects regularly doing endurance training at a recreational level. Western blotting showed neither decreased RyR1 expression nor fragmentation at 1 and 24 h after the marathon race (SI Appendix, Fig. S4A). However, RyR1 immunoprecipitation experiments revealed a marked dissociation of the channel-stabilizing subunit calstabin1 (also known as FKBP12; SI Appendix, Fig. S4B), which is consistent with previous results obtained after strenuous endurance exercise and in muscle pathologies and which has been linked to increased RyR1 Ca2+ leakage (6–13). Thus, the extensive challenge to muscle integrity caused by marathon running resulted in destabilizing changes to RyR1, but no fragmentation.
HIIT Causes Force Depression Due to Defective SR Ca2+ Release in Muscle Fibers.
Tentative mechanisms underlying the decrease in contractile performance during and after the HIIT exercise were assessed both at the neuronal and muscular levels (SI Appendix, Fig. S5). Mean power output decreased as the series of cycling bouts progressed, being decreased by ∼25% in the sixth bout, and this decrease occurred despite constant neuronal activation (SI Appendix, Fig. S6). Maximum voluntary contraction (MVC) force was decreased by ∼40% immediately and 5 min after the repeated cycling bouts, and again this decrease was not accompanied by any reduction in neuronal activation (SI Appendix, Fig. S7).
We used supramaximal electrical stimulation of the femoral nerve to assess knee extensor muscle function without influence from neuronal activation. The force induced by 10- and 100-Hz doublet stimulation as well as the rate of twitch force development were substantially decreased immediately and 5 min after the six cycling bouts (SI Appendix, Fig. S8 A–C). Conversely, the membrane excitability seemed unaffected by the HIIT exercise, as judged from measurements of the muscle compound action potential (M wave) in response to a single electrical impulse (SI Appendix, Fig. S8D). Intriguingly, no statistically significant differences from prefatigue values were observed when the above MVC contractions and experiments with electrical femoral nerve stimulation were performed 24 h after exercise—i.e., at the time when RyR1 Western blots show extensive fragmentation.
Thus far, our results show a force depression induced by a single session of HIIT that is due to defective function within the muscle fibers. The close to normal action potential characteristics (i.e., virtually unaltered M-wave properties) after exercise indicate that the force depression is due to factor(s) intrinsic to the muscle fibers—i.e., decreased SR Ca2+ release and/or impaired myofibrillar contractile function. To distinguish between these two possibilities, we measured the force produced during direct stimulation of the contractile proteins in skinned fibers obtained from vastus lateralis muscle biopsies taken before and ∼10 min after the repeated cycling bouts. The results showed no HIIT exercise-induced change in maximum Ca2+-activated force or myofibrillar Ca2+ sensitivity (SI Appendix, Fig. S9). Note that the cycling performed during the HIIT exercise involved mainly concentric contractions. A long-lasting force depression was observed after unaccustomed eccentric contractions, but such contractions resulted in severe impairments in myofibrillar contractility and a shift of the active force–length relationship toward longer lengths (31); neither of these defects were observed after the present HIIT exercise (see also SI Appendix, Fig. S1A). Thus, contractile function of the myofibrillar proteins was not impaired after the HIIT exercise, and the mechanism behind the force depression can be narrowed down to defective SR Ca2+ release.
Elite Endurance Athletes Develop a Prolonged HIIT-Induced Force Depression, but No RyR1 Fragmentation.
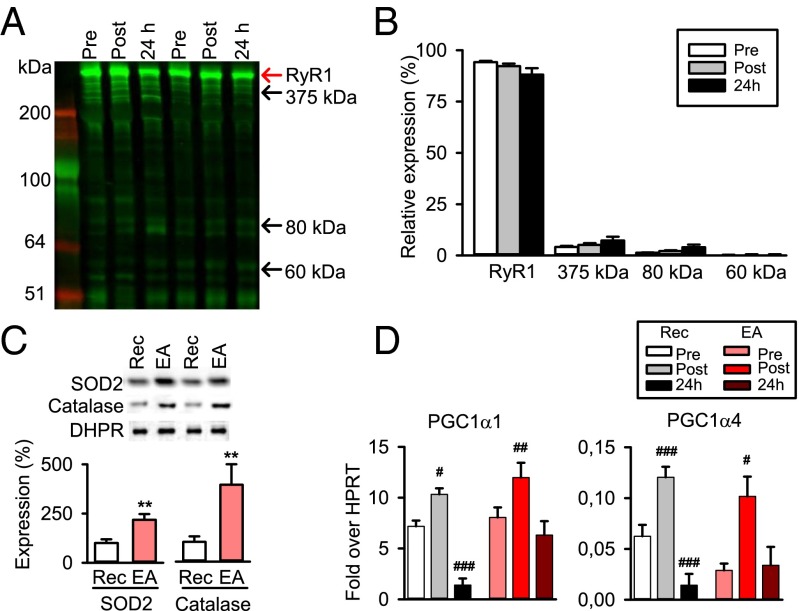
In the next set of experiments, we tested whether the HIIT exercise-induced RyR1 fragmentation also occurs in individuals with a high aerobic capacity. Fourteen elite endurance runners or road cyclists (SI Appendix, Table S1) performed the six bouts of 30-s all-out cycling. The mean power decreased as the series of cycling bouts progressed also in these athletes (SI Appendix, Fig. S10A), but the average decrease in the sixth bout was slightly smaller (∼15%) than in the recreationally active subjects (∼25%). Moreover, there was a marked decrease in electrically stimulated force production after exercise, especially at the low (10 Hz) stimulation frequency (SI Appendix, Fig. S10B). Intriguingly, Western blots showed no signs of increased RyR1 fragmentation after the HIIT exercise in the elite athletes (Fig. 2 A and B), which is in sharp contrast to the marked fragmentation observed in the recreationally active subjects.
Fig. 2.
HIIT exercise does not induce RyR1 fragmentation in elite endurance athletes. (A) Representative Western blots show no signs of RyR1 fragmentation after the cycling bouts in the elite athletes. Arrows indicate full-sized RyR1 (red arrow) and the location of ∼375-, 80-, and 60-kDa fragments (black arrows) observed 24 h after exercise in recreationally active subjects (Fig. 1A). (B) Mean data (± SEM) obtained from 14 elite athletes before (Pre) and ∼10 min (Post) and 24 h after exercise; total RyR1 expression was set to 100% at each time point in each subject. (C, Upper) Representative Western blots of SOD2, catalase, and DHPR from biopsies taken before the HIIT exercise in recreationally active subjects (Rec) and elite athletes (EA). DHPR did not differ between the two groups and was used as loading control. (C, Lower) Bar graphs show mean SOD2 and catalase expressions (± SEM; n = 7) relative to the mean in the Rec group, which was set to 100%. **P < 0.01 in unpaired t test. (D) Mean data (± SEM; n = 6–8) of the transcript levels of PGC-1α1 and -1α4 expressed relative to hypoxanthine guanine phosphoribosyl transferase (HPRT), which did not differ between the groups and was used as a housekeeping gene. #P < 0.05; ## P < 0.01; ### P < 0.001 vs. before exercise (one-way repeated measures ANOVA/Holm–Sidak post hoc test). PGC-1α4 was significantly higher before exercise in Rec than in EA (P < 0.05; unpaired t test).
Increased ROS production during exercise is classically linked to enhanced mitochondrial respiration, resulting in increased superoxide (O2−) production in complexes I and III of the electron transport chain (32). Superoxide dismutase 2 (SOD2) and catalase have key roles in cellular ROS metabolism by converting superoxide into hydrogen peroxide (H2O2) and H2O2 into water, respectively. We measured the protein expression of SOD2 and catalase in vastus lateralis muscle before the HIIT exercise and observed at least twice as high expression in the elite athletes as in the recreationally active subjects (Fig. 2C).
Changes in cellular Ca2+ handling can affect gene transcription and hence the adaptive response to physical exercise (33, 34). The peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) transcriptional coactivators have key roles for muscle adaptations, with PGC-1α1 being critically important for adaptations to endurance-type exercise and PGC-1α4 more important for resistance-type exercise (4, 35). The transcript levels for both these PGC-1α isoforms were significantly increased directly after the HIIT exercise in muscle biopsies from both recreationally active subjects and elite endurance athletes (Fig. 2D). Intriguingly, 24 h after the HIIT exercise, these transcripts were decreased by ∼80% in recreationally active subjects, whereas they were back at the pre-exercise level in the elite athletes. Moreover, transcripts of PGC-1α1–targeted genes encoding for mitochondrial proteins and several transcription factors that change in response to exercise also showed markedly decreased transcript levels 24 h after exercise only in recreationally active subjects (SI Appendix, Fig. S11). Thus, the HIIT exercise triggered prolonged changes in gene transcription in the recreationally active subjects, but not in the elite endurance athletes.
HIIT-Induced Fragmentation of RyR1 Is ROS-Dependent.
The absence of RyR1 changes combined with higher SOD2 and catalase protein expressions in the elite athletes suggests an involvement of ROS in the triggering of RyR1 fragmentation. Experiments on isolated mouse flexor digitorum brevis (FDB) muscle, which is a fast-twitch toe muscle containing mainly type IIa/IIx fibers (15), were performed to specifically study tentative ROS-induced modifications of RyR1. The mitochondrial ROS production was measured with the fluorescent indicator MitoSOX Red in single FDB fibers from sedentary control mice and mice that had free access to a running wheel in the cage. The latter mice performed voluntary endurance training by running ∼20 km each night for 40 d (SI Appendix, Fig. S12A). The isolated fibers were activated with electrical current pulses and a stimulation scheme mimicking the activation pattern during the all-out cycling bouts (six 30-s periods of 250 ms tetanic 100-Hz stimulation given every 500 ms with 4 min of rest between the stimulation periods). At 5 and 10 min after the simulated HIIT exercise, the MitoSOX Red fluorescence was increased by ∼200% in the sedentary control mice, whereas the increase was significantly smaller (by ∼80%) in the endurance-trained mice (P < 0.01; SI Appendix, Fig. S12B). The ROS-induced increase in MitoSOX Red fluorescence is not reversible. The stable fluorescence between 5 and 10 min after exercise therefore indicated that ROS production returned to a low baseline level once the HIIT-mimicking stimulation was stopped. Thus, there was a marked increase in mitochondrial ROS production during the simulated HIIT exercises, and this increase was attenuated with endurance training.
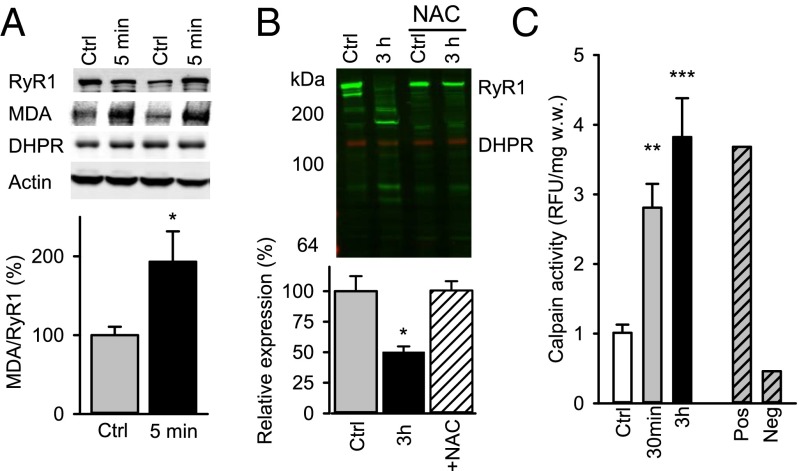
Next, intact, single-digit FDB muscles were activated with the HIIT-mimicking stimulation scheme, and Western blots were performed on muscles frozen 5 min after the last contraction displayed no signs of RyR1 degradation, and DHPR expression was similar to the control level. However, there was a doubling of RyR1 malondialdehyde (MDA) adducts (Fig. 3A). MDA protein adducts reflect the degree of lipid peroxidation and are frequently used as a biomarker of increased ROS production (36), and the RyR1 protein complex is known to be highly susceptible to ROS-induced modifications (37).
Fig. 3.
Simulated HIIT exercise causes a ROS-dependent RyR1 fragmentation in mouse muscle. (A) Representative Western blots of RyR1, MDA adducts on RyR1, DHPR, and actin obtained from single-digit FDB muscles 5 min after being fatigued by six bouts of 30-s simulated HIIT exercise or kept at rest (Ctrl). The contractions had no effect on RyR1, DHPR, and actin expression, but it approximately doubled the amount of MDA adducts on RyR1. (B) Western blot of RyR1 on single-digit FDB muscles snap-frozen either at rest (Ctrl) or 3 h after exercise, which was performed in the absence or presence of NAC (20 mM). Shown is relative expression of the full-size RyR1 with the average Ctrl set to 100% (n = 5–12 muscles). (C) Calpain activity in single-digit mouse FDB muscles before (Ctrl) and 30 min and 3 h after simulated HIIT exercise (n = 4–6). Positive and negative controls were obtained by adding fully active calpain and calpain inhibitor, respectively. Data are expressed as relative fluorescence units (RFU) divided by muscle wet weight (w.w.); the mean value in Ctrl was set to 1.0. All data are expressed as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001 with unpaired t test (A) or one-way ANOVA (B and C).
In the next series of experiments, FDB muscles were frozen 3 h after being exposed to the simulated HIIT exercise. Western blots from these displayed marked RyR1 fragmentation after the contractions when experiments were performed under control conditions (i.e., in standard Tyrode solution), whereas the fragmentation was completely blocked when muscles were exposed to the general antioxidant N-acetylcysteine (NAC; 20 mM) before and during the series of contractions (Fig. 3B). Thus, our results support a model where ROS induce modifications of RyR1 during the HIIT exercise, and these then trigger RyR1 fragmentation.
The distinct pattern of HIIT exercise-induced RyR1 fragmentation suggests that it involves an enzymatic cleavage process. Calpains are the likely candidates, and we measured the calpain activity in mouse FDB muscles before and after the simulated HIIT exercise. The results show a 3- to 4-fold higher calpain activity at 30 min and 3 h after the exercise (Fig. 3C).
The fragmentation of RyR1 after the simulated all-out cycling bouts might lead to dislocation of the protein. However, immunofluorescence RyR1 staining showed a similarly striated pattern before and 5 min and 3 h after the stimulation period, and this pattern was also observed when staining for the t-tubular voltage sensors DHPR (SI Appendix, Fig. S13). Noteworthy, the overall immunofluorescence staining for RyR1 was markedly decreased 3 h after the contractions, which probably reflects impaired antibody binding due to severe posttranslational modifications of RyR1 at this time point (cf Fig. 3B).
HIIT Induces a Prolonged Force Depression and an Increase in Resting [Ca2+]i.
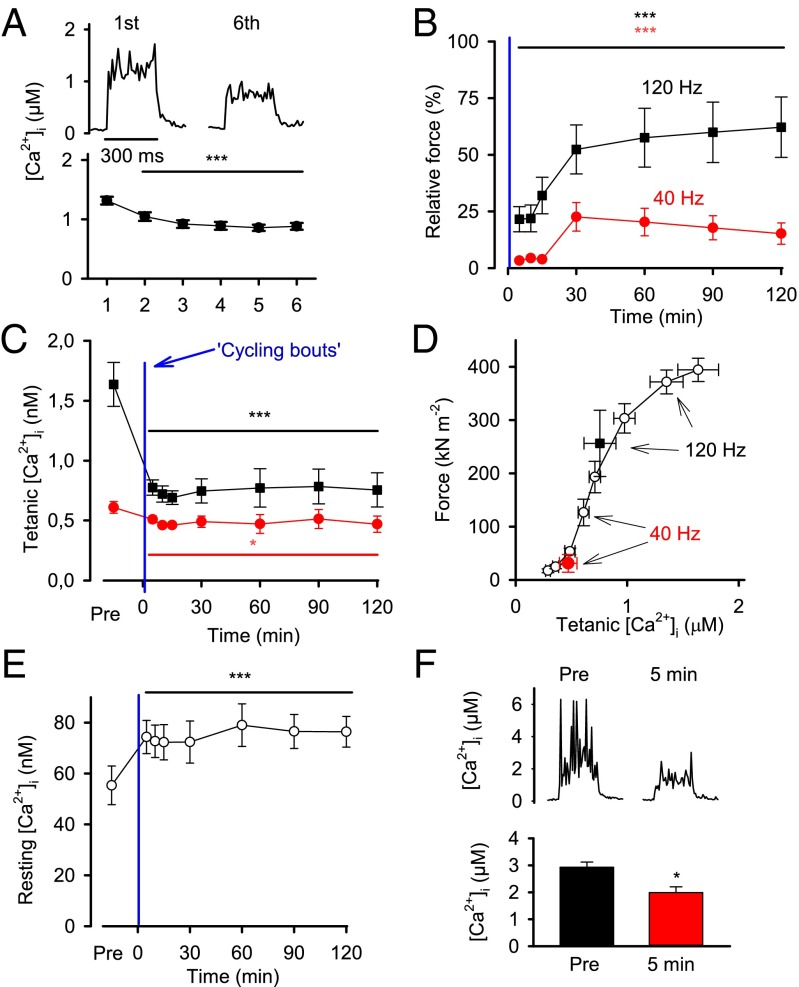
To assess the effect of RyR1 fragmentation on SR Ca2+ handling, we used mechanically dissected single mouse FDB fibers with intact tendons—i.e., a preparation that allows detailed measurements of [Ca2+]i as well as the resulting force (15, 38). [Ca2+]i during the initial 250 ms tetanic contraction of the simulated cycling bouts decreased with increasing number of bouts, being decreased by ∼35% at the start of the sixth bout (Fig. 4A); i.e., defects in SR Ca2+ release induced by the previous 30-s bouts of intense activation were not reversed during the 4-min rest periods between bouts.
Fig. 4.
Simulated HIIT exercise induces prolonged decrease in tetanic [Ca2+]i and increase in resting [Ca2+]i. (A, Upper) Representative records of [Ca2+]i during 100-Hz tetanic stimulation trains evoked at the start of the first and sixth simulated cycling bout in a single FDB fiber. (A, Lower) Mean [Ca2+]i in the initial tetanus of the six cycling bouts. (B) Force measured 5–120 min after the simulated HIIT exercise; data are expressed relative to the force before exercise, which in each fiber was set to 100% at both 40- and 120-Hz stimulation. (C) Tetanic [Ca2+]i before (PRE) and 5–120 min after exercise. (D) The relation between tetanic force and [Ca2+]i before (white circle; obtained by stimulating fibers at 15–150 Hz at 1-min intervals) and 120 min after exercise (black and red circles; data taken from B and C). (E) Resting [Ca2+]i before and 5–120 min after exercise. (F) Representative [Ca2+]i records and mean data obtained from 100-Hz tetanic stimulations in the presence of 5 mM caffeine produced before and 5 min after exercise. All data are expressed as mean ± SEM (n = 6–14 fibers). ***P < 0.001 vs. the first simulated cycling bout (A) or before exercise (B, C, and F) (one-way repeated measures ANOVA). *P < 0.05 in paired t test (F).
In agreement with the results of the above human experiments, isolated mouse FDB fibers entered a prolonged state of severely depressed force after the simulated HIIT exercise, especially at the lower (40 Hz) stimulation frequency (Fig. 4B); it should be noted that fusion occurs at higher frequencies in the mouse than in the human muscles, and 40 Hz stimulation of the mouse FDB fibers gave about the same proportion of the maximum force as 10 Hz for the human quadriceps muscle. Tetanic [Ca2+]i also displayed a prolonged decrease after the contraction bouts, but in this case the decrease was larger at 120-Hz than at 40-Hz stimulation (Fig. 4C). These seemingly conflicting results are explained by the shape of the force–[Ca2+]i relationship (5), and Fig. 4D shows that the force–[Ca2+]i relations in 40- and 120-Hz contractions produced 120 min after the simulated HIIT exercise overlap with the force–[Ca2+]i relationship under control conditions (obtained by producing 350-ms contractions at 15–150 Hz at 1-min interval in the same fibers before exercise).
Modified RyR1 can become leaky (6), which may result in an increase in resting [Ca2+]i. Accordingly, the simulated HIIT exercise induced a prolonged ∼40% increase in resting [Ca2+]i (Fig. 4E). Caffeine interacts with RyR1 to potentiate SR Ca2+ release (39). Fig. 4F shows [Ca2+]i records from 100 Hz tetani produced in an FDB fiber exposed to caffeine (5 mM) before and after the simulated HIIT exercise; mean data show ∼30% lower [Ca2+]i during caffeine tetani produced after the exercise (P < 0.05; Fig. 4F). These findings indicate that the simulated HIIT exercise induces RyR1 leakage, promoting Ca2+ fluxes from the SR toward the cytosol, which then results in increased resting [Ca2+]i while tetanic [Ca2+]i is reduced due to a decline in the releasable SR Ca2+ pool. It might also be noted that a prolonged increase in resting [Ca2+]i stimulates mitochondrial biogenesis and can thereby improve muscle endurance (14–16). Thus, the observed exercise-induced increase in resting [Ca2+]i provides a tentative trigger for HIIT-induced mitochondrial biogenesis (21).
Discussion
We show here that one short session of HIIT exercise (total exercise time ≤3 min) can induce an extensive fragmentation of the skeletal muscle SR Ca2+ release channel RyR1. Mechanistic experiments performed on isolated mouse muscle indicate that this fragmentation was triggered by ROS-dependent modifications of RyR1 as follows. (i) Mitochondrial ROS production increased substantially during the simulated HIIT exercise; in fact, the present increase in MitoSOX fluorescence in muscle fibers of control mice was ∼10 times larger than previously observed with a less demanding fatiguing stimulation protocol (20). (ii) There was a doubling of RyR1 MDA adducts, which reflect increased lipid peroxidation, 5 min after HIIT-mimicking exercise. (iii) A marked RyR1 fragmentation was present 3 h after exercise, and this fragmentation was prevented by the general antioxidant NAC. Furthermore, endurance training is known to improve muscular antioxidant capacity (32, 40). Accordingly, muscles of elite endurance athletes showed improved ROS defense by increased protein expression of SOD2 and catalase and no HIIT exercise-induced RyR1 fragmentation, and the exercise-induced increase in mitochondrial ROS production was significantly smaller in endurance-trained than in sedentary mice.
The HIIT exercise-induced RyR1 fragmentation showed a characteristic pattern with distinct bands on Western blots at ∼375, 80, and 60 kDa, which indicates a tightly controlled enzymatic cleavage process. Enzymes that might cause the RyR1 fragmentation include calpains and we observed a marked increase in total calpain activity in mouse FDB muscle after simulated HIIT exercise. Calpain-3, a muscle-specific member of the calpain family of nonlysosomal Ca2+-dependent proteases (41, 42), is particularly interesting in this respect because it has been shown to cleave the RyR1 monomer (565 kDa) into two fragments with molecular masses of ∼375 and 150 kDa without affecting other SR proteins (41, 43).
Intriguingly, the HIIT exercise resulted in prolonged low-frequency force depression (PLFFD) of similar magnitude in recreationally active subjects and elite endurance athletes, but only the former showed RyR1 fragmentation. We have previously shown that the mechanism behind PLFFD is shifted from decreased SR Ca2+ release to reduced myofibrillar Ca2+ sensitivity with either increased endogenous oxidant defense or exogenous application of antioxidants (20, 44, 45). For instance, PLFFD is caused by reduced myofibrillar Ca2+ sensitivity in mouse FDB fibers overexpressing SOD2, whereas it is due to decreased SR Ca2+ release in their wild-type counterparts (44). Accordingly, the expressions of SOD2 and catalase were at least twice as high in endurance athletes as in recreationally active subjects. Thus, our data fit with a model in which HIIT exercise-induced PLFFD in the recreationally active subjects relates to ROS-dependent RyR1 modifications, resulting in increased SR Ca2+ leak at rest and decreased SR Ca2+ release during contractions. Conversely, a more effective oxidant defense in the elite athletes would shift the cause of PLFFD to decreased myofibrillar Ca2+ sensitivity (45).
A prolonged alteration in muscle fiber [Ca2+]i homeostasis will affect cellular signaling and gene expression—e.g., induction of mitochondrial biogenesis via Ca2+–calmodulin protein kinase and calcineurin signaling (14–16, 33, 34)—whereas a change in myofibrillar Ca2+ sensitivity is less likely to have such effects. Major changes in RyR1 structure and in mRNA levels of proteins known to change with endurance training were observed 24 h after the HIIT exercise in the recreationally active subjects, but not in the elite athletes. This finding implies that prolonged Ca2+-dependent adaptations were triggered only in the recreationally active subjects, which fits with the general picture that HIIT exercise is less effective in well-trained subjects (21). However, the measured transcript levels related to mitochondrial biogenesis and endurance showed a general decrease—rather than the expected increase—24 h after the HIIT exercise. The training-induced increase in mitochondrial proteins appears to result from the cumulative effect of transient bursts of their mRNAs (46). Therefore, it might be that the decreased transcript levels 24 h after the HIIT exercise are the result of feedback from increases at earlier times; additional experiments are required to resolve this issue.
One conspicuous result of the present study is that the force produced in response to electrical nerve stimulation was close to normal 24 h after the HIIT exercise in recreationally active subjects, despite RyR1 showing major fragmentation at this time. Similarly, FDB fibers displayed decreased, but not absent, SR Ca2+ release in response to tetanic stimulation at the time when RyR1 was severely fragmented. The channel pore region of RyR1 is located close to the C-terminal of the protein, and even the smallest major fragments (60 kDa) observed 24 h after the HIIT exercise would include the pore (47, 48). Our immunostaining experiments on dissociated mouse FDB fibers showed a striated pattern of RyR1 staining at the time of fragmentation, hence indicating the continued presence of functional RyR1 Ca2+ pores in the SR membrane. The results of our measurements of [Ca2+]i in dissected mouse FDB fibers exposed to the simulated HIIT exercise imply that the fragmented RyR1s are leaky, resulting in the increased resting [Ca2+]i. Interestingly, these results fit with the finding that calpain-3–cleaved RyR1 became stabilized in an open subconducting state (41), which in the intact muscle fiber would lead to an increase in resting [Ca2+]i. Together, our results indicate that the fragmented RyR1s are leaky at rest, but they still provide a prompt SR Ca2+ release in response to action-potential-induced activation of the t-tubular voltage sensors.
In the present study, we demonstrate a fragmentation of RyR1 linking high-intensity exercise and increased ROS levels, via a prolonged increase in resting [Ca2+]i, to altered gene transcription and muscle adaptations. The induction of RyR1 fragmentation resulting in a long-lasting increase in resting [Ca2+]i provides a mechanism for how a short session of HIIT exercise (≤3 min) can be highly effective in triggering muscle adaptations. Moreover, the ROS dependency of RyR1 modifications offers a tentative explanation as to why an effective antioxidant treatment hampers beneficial adaptations induced by endurance training (17–19). Finally, destabilized RyR1 has predominantly been linked to muscle weakness in several pathological conditions as well as in normal aging (8–13), but here we show that RyR1 modifications can also have an integral role in physiological muscle adaptations.
Materials and Methods
Detailed materials and methods are described in SI Appendix, SI Materials and Methods.
Human Experiments.
Data were obtained from young (mean age 26 y) male subjects, who were either recreationally active or elite endurance athletes (SI Appendix, Table S1). The studies were approved by the local Ethics Committees and performed in accordance with the Helsinki Declaration. Each subject gave written informed consent before participation. Subjects performed one session of HIIT consisting of three to six 30-s all-out cycling bouts at 0.7 Nm per kg of body weight on a cycle ergometer, with a 4-min rest between tests (26). Force production and electromyography signals were measured before and up to 24 h after exercise. Muscle biopsies taken from the vastus lateralis muscle before and ∼10 min and 24 h after exercise were used for protein and mRNA analyses and measurements of myofibrillar function using skinned fibers.
Isolated Mouse Muscles.
All animal experiments complied with the Swedish Animal Welfare Act and the Swedish Welfare Ordinance. The study was approved by the Stockholm North Ethical Committee on Animal Experiments. Adult C57BL/6 mice were killed by cervical dislocation, and fast-twitch FDB muscles were removed. Force and [Ca2+]i were measured in mechanically dissected, intact single FDB fibers (38).
Statistical Analyses.
Statistically significant changes induced by the different types of exercise were assessed with unpaired t test, paired t test, one-way ANOVA, or one-way repeated-measure ANOVA as appropriate. The Holm–Sidak post hoc test was used to evaluate differences after vs. before exercise. The significance level was set to P < 0.05. All statistical analyses were conducted with SigmaPlot software for Windows (Systat).
Supplementary Material
Acknowledgments
We thank Sylvain Rayroud for technical assistance during cycling exercise sessions and Jui-Lin Fan for technical assistance with the VO2max data collection. This study was supported by grants from the Swedish Research Council (to H.W., J.L.R., and J.T.L.); the Swedish National Center for Sports Research (A.J.C. and H.W.); the Research Council of Lithuania (S.K., A.S., H.W., and M.B.); Novo Nordisk Fonden and Wenner-Gren Foundations (J.L.R.); and the Sir Jules Thorn Charitable Trust and the Chuard Schmid Foundation (N.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 15271.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1507176112/-/DCSupplemental.
References
- 1.Fielding RA, et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12(4):249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol. 2012;2(2):1143–1211. doi: 10.1002/cphy.c110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Westerblad H, Bruton JD, Katz A. Skeletal muscle: Energy metabolism, fiber types, fatigue and adaptability. Exp Cell Res. 2010;316(18):3093–3099. doi: 10.1016/j.yexcr.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Arany Z. PGC-1 coactivators and skeletal muscle adaptations in health and disease. Curr Opin Genet Dev. 2008;18(5):426–434. doi: 10.1016/j.gde.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: Cellular mechanisms. Physiol Rev. 2008;88(1):287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- 6.Bellinger AM, et al. Remodeling of ryanodine receptor complex causes “leaky” channels: A molecular mechanism for decreased exercise capacity. Proc Natl Acad Sci USA. 2008;105(6):2198–2202. doi: 10.1073/pnas.0711074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aydin J, et al. Nonshivering thermogenesis protects against defective calcium handling in muscle. FASEB J. 2008;22(11):3919–3924. doi: 10.1096/fj.08-113712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersson DC, et al. Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab. 2011;14(2):196–207. doi: 10.1016/j.cmet.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Umanskaya A, et al. Genetically enhancing mitochondrial antioxidant activity improves muscle function in aging. Proc Natl Acad Sci USA. 2014;111(42):15250–15255. doi: 10.1073/pnas.1412754111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamada T, et al. Nitrosative modifications of the Ca2+ release complex and actin underlie arthritis-induced muscle weakness. Ann Rheum Dis. 2015;74(10):1907–1914. doi: 10.1136/annrheumdis-2013-205007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reiken S, et al. PKA phosphorylation activates the calcium release channel (ryanodine receptor) in skeletal muscle: Defective regulation in heart failure. J Cell Biol. 2003;160(6):919–928. doi: 10.1083/jcb.200211012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lanner JT, et al. AICAR prevents heat-induced sudden death in RyR1 mutant mice independent of AMPK activation. Nat Med. 2012;18(2):244–251. doi: 10.1038/nm.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bellinger AM, et al. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat Med. 2009;15(3):325–330. doi: 10.1038/nm.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright DC, Geiger PC, Han DH, Jones TE, Holloszy JO. Calcium induces increases in peroxisome proliferator-activated receptor gamma coactivator-1α and mitochondrial biogenesis by a pathway leading to p38 mitogen-activated protein kinase activation. J Biol Chem. 2007;282(26):18793–18799. doi: 10.1074/jbc.M611252200. [DOI] [PubMed] [Google Scholar]
- 15.Bruton JD, et al. Increased fatigue resistance linked to Ca2+-stimulated mitochondrial biogenesis in muscle fibres of cold-acclimated mice. J Physiol. 2010;588(Pt 21):4275–4288. doi: 10.1113/jphysiol.2010.198598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Handschin C, Rhee J, Lin J, Tarr PT, Spiegelman BM. An autoregulatory loop controls peroxisome proliferator-activated receptor γ coactivator 1α expression in muscle. Proc Natl Acad Sci USA. 2003;100(12):7111–7116. doi: 10.1073/pnas.1232352100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ristow M, et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci USA. 2009;106(21):8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paulsen G, et al. Vitamin C and E supplementation hampers cellular adaptation to endurance training in humans: A double-blind, randomised, controlled trial. J Physiol. 2014;592(Pt 8):1887–1901. doi: 10.1113/jphysiol.2013.267419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomez-Cabrera MC, et al. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr. 2008;87(1):142–149. doi: 10.1093/ajcn/87.1.142. [DOI] [PubMed] [Google Scholar]
- 20.Cheng AJ, Bruton JD, Lanner JT, Westerblad H. Antioxidant treatments do not improve force recovery after fatiguing stimulation of mouse skeletal muscle fibres. J Physiol. 2015;593(2):457–472. doi: 10.1113/jphysiol.2014.279398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibala MJ, Little JP, Macdonald MJ, Hawley JA. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J Physiol. 2012;590(Pt 5):1077–1084. doi: 10.1113/jphysiol.2011.224725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Little JP, Safdar A, Wilkin GP, Tarnopolsky MA, Gibala MJ. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: Potential mechanisms. J Physiol. 2010;588(Pt 6):1011–1022. doi: 10.1113/jphysiol.2009.181743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gibala MJ, et al. Short-term sprint interval versus traditional endurance training: Similar initial adaptations in human skeletal muscle and exercise performance. J Physiol. 2006;575(Pt 3):901–911. doi: 10.1113/jphysiol.2006.112094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobs RA, et al. Improvements in exercise performance with high-intensity interval training coincide with an increase in skeletal muscle mitochondrial content and function. J Appl Physiol. 2013;115(6):785–793. doi: 10.1152/japplphysiol.00445.2013. [DOI] [PubMed] [Google Scholar]
- 25.Bacon AP, Carter RE, Ogle EA, Joyner MJ. VO2max trainability and high intensity interval training in humans: A meta-analysis. PLoS One. 2013;8(9):e73182. doi: 10.1371/journal.pone.0073182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Little JP, Safdar A, Bishop D, Tarnopolsky MA, Gibala MJ. An acute bout of high-intensity interval training increases the nuclear abundance of PGC-1α and activates mitochondrial biogenesis in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2011;300(6):R1303–R1310. doi: 10.1152/ajpregu.00538.2010. [DOI] [PubMed] [Google Scholar]
- 27.Guellich A, Seiler S, Emrich E. Training methods and intensity distribution of young world-class rowers. Int J Sports Physiol Perform. 2009;4(4):448–460. doi: 10.1123/ijspp.4.4.448. [DOI] [PubMed] [Google Scholar]
- 28.Laursen PB. Training for intense exercise performance: High-intensity or high-volume training? Scand J Med Sci Sports. 2010;20(Suppl 2):1–10. doi: 10.1111/j.1600-0838.2010.01184.x. [DOI] [PubMed] [Google Scholar]
- 29.Weston KS, Wisløff U, Coombes JS. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: A systematic review and meta-analysis. Br J Sports Med. 2014;48(16):1227–1234. doi: 10.1136/bjsports-2013-092576. [DOI] [PubMed] [Google Scholar]
- 30.Whyte LJ, Gill JM, Cathcart AJ. Effect of 2 weeks of sprint interval training on health-related outcomes in sedentary overweight/obese men. Metabolism. 2010;59(10):1421–1428. doi: 10.1016/j.metabol.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Proske U, Morgan DL. Muscle damage from eccentric exercise: Mechanism, mechanical signs, adaptation and clinical applications. J Physiol. 2001;537(Pt 2):333–345. doi: 10.1111/j.1469-7793.2001.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Powers SK, Jackson MJ. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88(4):1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tavi P, Westerblad H. The role of in vivo Ca²⁺ signals acting on Ca²⁺-calmodulin-dependent proteins for skeletal muscle plasticity. J Physiol. 2011;589(Pt 21):5021–5031. doi: 10.1113/jphysiol.2011.212860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gehlert S, Bloch W, Suhr F. Ca2+-dependent regulations and signaling in skeletal muscle: From electro-mechanical coupling to adaptation. Int J Mol Sci. 2015;16(1):1066–1095. doi: 10.3390/ijms16011066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruas JL, et al. A PGC-1α isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell. 2012;151(6):1319–1331. doi: 10.1016/j.cell.2012.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ho E, Karimi Galougahi K, Liu CC, Bhindi R, Figtree GA. Biological markers of oxidative stress: Applications to cardiovascular research and practice. Redox Biol. 2013;1(1):483–491. doi: 10.1016/j.redox.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lanner JT. Ryanodine receptor physiology and its role in disease. Adv Exp Med Biol. 2012;740(1):217–234. doi: 10.1007/978-94-007-2888-2_9. [DOI] [PubMed] [Google Scholar]
- 38.Andrade FH, Reid MB, Allen DG, Westerblad H. Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J Physiol. 1998;509(Pt 2):565–575. doi: 10.1111/j.1469-7793.1998.565bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allen DG, Westerblad H. The effects of caffeine on intracellular calcium, force and the rate of relaxation of mouse skeletal muscle. J Physiol. 1995;487(Pt 2):331–342. doi: 10.1113/jphysiol.1995.sp020883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gomez-Cabrera MC, Domenech E, Viña J. Moderate exercise is an antioxidant: Upregulation of antioxidant genes by training. Free Radic Biol Med. 2008;44(2):126–131. doi: 10.1016/j.freeradbiomed.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Shevchenko S, Feng W, Varsanyi M, Shoshan-Barmatz V. Identification, characterization and partial purification of a thiol-protease which cleaves specifically the skeletal muscle ryanodine receptor/Ca2+ release channel. J Membr Biol. 1998;161(1):33–43. doi: 10.1007/s002329900312. [DOI] [PubMed] [Google Scholar]
- 42.Sorimachi H, et al. Molecular cloning of a novel mammalian calcium-dependent protease distinct from both m- and μ-types. Specific expression of the mRNA in skeletal muscle. J Biol Chem. 1989;264(33):20106–20111. [PubMed] [Google Scholar]
- 43.Shoshan-Barmatz V, Weil S, Meyer H, Varsanyi M, Heilmeyer LM. Endogenous, Ca2+-dependent cysteine-protease cleaves specifically the ryanodine receptor/Ca2+ release channel in skeletal muscle. J Membr Biol. 1994;142(3):281–288. doi: 10.1007/BF00233435. [DOI] [PubMed] [Google Scholar]
- 44.Bruton JD, et al. Reactive oxygen species and fatigue-induced prolonged low-frequency force depression in skeletal muscle fibres of rats, mice and SOD2 overexpressing mice. J Physiol. 2008;586(1):175–184. doi: 10.1113/jphysiol.2007.147470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Westerblad H, Allen DG. Emerging roles of ROS/RNS in muscle function and fatigue. Antioxid Redox Signal. 2011;15(9):2487–2499. doi: 10.1089/ars.2011.3909. [DOI] [PubMed] [Google Scholar]
- 46.Perry CGR, et al. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J Physiol. 2010;588(Pt 23):4795–4810. doi: 10.1113/jphysiol.2010.199448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zalk R, et al. Structure of a mammalian ryanodine receptor. Nature. 2015;517(7532):44–49. doi: 10.1038/nature13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yan Z, et al. Structure of the rabbit ryanodine receptor RyR1 at near-atomic resolution. Nature. 2015;517(7532):50–55. doi: 10.1038/nature14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.