Abstract
Purpose
The purpose of this study was to investigate the effect of bone-like hydroxyapatite/polyamino acid (BHA/PAA) in the osteogenesis and reconstruction of long segmental bone defects.
Methods
In vitro, MG63 cells were cultured with BHA/PAA. The osteoinductive activity of the BHA/PAA material was evaluated using inverted microscopy, scanning electron microscopy, MTT proliferation assay, and the determination of alkaline phosphatase activity and Ca2+ content. In vivo, the radial bone defect was made in 20 New Zealand White rabbits, and then these animal were randomly divided into two groups (n=10), the experimental group (with BHA/PAA) and the control group (without BHA/PAA). Postoperatively, the osteogenesis effect of BHA/PAA was evaluated through X-ray, hematoxylin–eosin staining, observation of the gross bone specimen, immunohistochemistry, and fluorescent confocal scanning microscopy.
Results
In vitro, BHA/PAA promoted the adhesion, growth, and calcium nodule formation of MG63 cells, and it had good osteogenesis activity. In vivo, with BHA/PAA material degradation and absorption, the new bone gradually formed, and the bone defect gradually recovered in the experimental group. In the control group, a limited bone formation was found at the bone broken ends, and the bone defect was obviously visible.
Conclusion
In vitro and in vivo, we confirmed that BHA/PAA was effective in inducing osteogenesis and reconstructing a long segmental bone defect.
Keywords: BHA/PAA, osteogenesis induction, reconstruction, bone defect
Introduction
With the development of municipal construction and an increase in the number of motor vehicles, the incidences of complex fractures, bone defects, and bone nonunions due to the high-energy trauma of motor vehicle accidents are increasing. The treatment of large segmental bone defects is still an unresolved clinical challenge to orthopedic surgeons. For the treatment of a bone defect, the best choice is an autologous bone graft, which is the gold standard.1 However, there are many disadvantages. It complicates a trauma surgery and increases the patient’s pain. Furthermore, sources for the graft are limited, and there are complications when taking bone from its original site, which greatly limits its application.2 Although the allograft bone graft and the xenograft bone graft overcome the shortcomings of autogenous bone grafts, they come from a different immune source, and a rejection reaction and the potential spreading of disease are significant possibilities.1,3 Furthermore, in the process of processing the graft, the bone cell activity and the osteogenic inducer factor may inactivate, decreasing the capacity of osteogenic induction, which also limits their application. To overcome these shortcomings, considerable research has focused on bone-like materials, which have good biological compatibility and biological activity, are biodegradable, induce osteogenesis, and have physicochemical properties similar to natural bone. There is no undesired immunological response and risk of infection. Finally, they are easy to process and are low cost. With the advance of biological tissue engineering and the development of forging technology, many bone graft materials have been developed, which have good biocompatibility, are biodegradable, and are capable of osteogenesis. Examples include hydroxyapatite (HA), nano-hydroxyapatite (n-HA), calcium phosphate, and artificial polymers. HA is the main component of bone and has good biocompatibility and osteogenic activity. It is widely being used as an alternative material in bone tissue repair.4,5 Bone-like hydroxyapatite (BHA), being similar to normal bone structure, is carbonated n-HA, and its activity is stronger and its degradation is faster than HA. It has good performance with regard to degradation and biological compatibility, does not elicit an immune response, and has a higher biological activity.6–8 It is also easier to combine with the host bone and to induce new bone formation when it is implanted in the body. The molecular structure of polyamino acid (PAA) is similar to collagen protein, and PAA has good biocompatibility and biodegradability,9 as well as good mechanical properties and processability. BHA/PAA combines the good mechanical properties of PAA with the ability of BHA to induce osteogenesis and bone conduction. One can also adjust the proportion of the two in order to adjust the speed of its degradation, which gives it great potential for a bone repair material.
This study used BHA/PAA as the bone graft material for the reconstruction of a radial bone defect in rabbits. Using in vitro and in vivo studies, it was evaluated for its osteoinductive activity and its ability to effectively reconstruct a bone defect. These findings provide a new approach for the clinical treatment and reconstruction of large segmental bone defects.
Materials and methods
Preparation of materials
Materials were prepared using the standard atmospheric pressure solution method.10 The cylindrical-shaped BHA/PAA materials were prepared with a size of 15×5×5 mm3 and a weight of 620.50±8.54 mg. Using the same method, the wafer-shaped materials were prepared with a diameter of 8 mm, a thickness of 1 mm, and a weight 100.48±1.45 mg. The aperture of the two materials was 100–500 μm. Materials were provided by the National Nano-material Company of Sichuan University, and the physical and chemical properties of the materials were detected by the Material Analysis and Testing Center of Sichuan University. The aforementioned materials were disinfected when they were prepared.
In vitro experiment
Osteoblast-like MG63 cell culture and identification
Approximately −70°C frozen human osteogenesis-like MG63 cells (provided by Sichuan University Analysis Testing Centre) were thawed in a 37°C water bath, with rapid rewarming and melting, washed with 1640 culture liquid, and centrifuged three times at 1,000 rpm, every 5 minutes. No ethics statement was required from the institutional review board for the use of this cell line. They were then formulated at a cell concentration of approximately 1×104/mL, inoculated in culture flasks, and 1640 culture solution (containing 10% fetal bovine serum, FBS) was added. Under standard conditions (37°C, 5% CO2, 95% air), MG63 cells were cultured, and the culture fluid was replaced every other day. When the MG63 cells grew in a single layer and the adherent growth of MG63 cells was more than 80% in culture flask, cells were passaged. The experimental cells were cultured in the logarithmic growth of the third-generation cells. Using an inverted microscope, the morphology of MG63 cells was observed to be spindle and polygonal, with an obvious nucleolus. After 2 days of culturing MG63 cells, we stained them with alizarin red and looked for red-stained calcium nodule formations. If formations were observed, it was confirmed that the MG63 cells were osteoblast-like cells and had good osteogenic activity, and these cells were used as the experimental cells.
The determination of osteogenic ability
The BHA/PAA wafer materials, washed three times with phosphate buffer solution (PBS), were placed in a 24-well culture plate in a total of nine holes. No material was designated as the control group. Approximately 1 mL of 1640 culture solution was used to soak the BHA/PAA material for 12 hours, and then the culture solution was washed off. Osteoblast-like MG63 cells (concentration 3×104/mL) were inoculated in each well (1 mL/well) under the standard conditions, and the culture solution was replaced every other day. MG63 cell morphology, adhesion, and growth were observed on the culture plate using an inverted microscope. Meanwhile, on culture day 7, three wafer materials and slides were taken from the experimental group and the control group, respectively, and processed as follows: 2% glutaraldehyde fixation, ethanol dehydration, substitution, critical point drying, and gold spraying. Finally, under the conditions of high vacuum and 15.0 kV, and using a scanning electron microscope (SEM), MG63 cells were evaluated for adhesion, growth, and the formation of calcium nodules on the material and slide surface.
MTT cell proliferation assay
The processing of the BHA/PAA wafer materials, the culturing of MG63 cells, and the dividing groups were the same as for the previous experiment, while the only difference was that there was no slide in the control group. Three wells were selected in each group on culture day 2, 5, and 7. Approximately 40 μL MTT liquid was added per well under a constant temperature of 37°C and allowed incubated for 4 hours. Then, the culture was terminated, and the 1640 and MTT mixture solution in each well was removed. Approximately 450 μL dimethyl sulfoxide (DMSO) per well was added, slightly shaken for 5–10 minutes, and then placed at 37°C for 30 minutes. Using a porous plate analyzer (HTS7000 plus type) at 570 nm wavelength, the optical density (OD) was determined for each well, and the average OD value of cell proliferation was calculated in each group at different time points.
The detection of alkaline phosphatase (ALP) activity and Ca2+ content
The processing of the BHA/PAA wafer materials, the culturing of MG63 cells, and the dividing groups were the same as for the previous experiment, while the only difference was that there was no slide in the control group. On cell culture days 2, 5, and 7, the materials from three wells were taken from experiment group and washed three times with PBS. MG63 cells were removed from the materials, and the adhesive and unadhesive MG63 cells from the culture plate wells were collected. Then, MG63 cells were digested with 0.25% trypsin for 5 minutes, and the digestion was terminated with 1640 culture fluid. MG63 cells were collected by centrifugation, and the supernatant was removed. MG63 cells were centrifuged three times to terminate the trypsin effect. Adding 100 μL PBS to the tube, the cell suspension was dissociated, sealed with EP tube, and placed in a −70°C freezer. Then, MG63 cells were rapidly frozen and thawed three times to rupture the osteoblast-like cell, forming an MG63 cell-freeze-thawing solution. The freeze-thawing solution (at 4°C) was centrifuged at 15,000 rpm for 15 minutes, and the supernatant was moved into a new test tube; this now becoming the test sample. The ALP activity and Ca2+ content was determined by the enzyme label, according to the manufacturer’s protocol. The experiment was repeated three times in each group, and the mean value was taken.
In vivo
The construction and treatment of an animal model of a radial bone defect
In our study, the animal model of a radial defect was constructed in 20 New Zealand White rabbits that were 4 months old and weighed 2.5–3.0 kg (2.73 kg on average). All surgical procedures were approved by the Animal Care Committee of Chongqing Medical University, Chongqing, People’s Republic of China. New Zealand White rabbits, bred and observed for 5 days, were put on the operating table and given pentobarbital (30 mg/kg) anesthesia intravenously. The right forearm, from elbow joint to wrist joint, was shaved and disinfected with polypovidone iodine solution. In the middle radius, an approximately 3 cm longitudinal incision was made to expose the radial diaphyseal, and an approximately 15 mm long radial cut was made with a micro-oscillating saw to create the radial defect. Then, the animals were randomly divided into two groups (n=10): the experimental group (treated with material with the size of 15×5×5 mm3 BHA/PAA) and the control group (without material). Finally, the wounds were washed with saline and closed with sutures layer by layer. Postoperatively, animals were kept in individual cages, fed a routine diet, were free to move about, and injected with penicillin 50 U/kg intramuscularly three times per day for 3 consecutive days, to prevent infection.
Imaging examination
Preoperatively, on day 1 and at 4, 8, and 12 weeks, positive lateral X-ray film (exposure conditions: 42 kV, 100 mA/s, 1.2 m) of the rabbit right forearm was taken. To ensure that the imaging results were true and reliable, two radiologists independently evaluated the radiographs in a double-blind manner. Images were evaluated for BHA/PAA materials degradation and absorption, new bone formation, bone connection, and bone defect recovery using the Lane and Sandhu scoring system11 (Table 1).
Table 1.
Lane and Sandhu modified radiation scoring system
| Bone formation | |
| No evidence of bone formation | 0 |
| Bone formation occupying 25% of bone defects | 1 |
| Bone formation occupying 50% of bone defects | 2 |
| Bone formation occupying 75% of bone defects | 3 |
| Bone formation occupying 100% of bone defects | 4 |
| Union (proximal and distal separately) | |
| Nonunion | 0 |
| Possible union | 1 |
| Radiographic union | 2 |
| Reconstruction | |
| No evidence of reconstruction | 0 |
| Bone marrow cavity reconstruction | 1 |
| Cortical reconstruction | 2 |
| Possible total score per class | |
| Bone formation | 4 |
| Proximal union | 2 |
| Distal union | 2 |
| Reconstruction | 2 |
| Maximum score | 10 |
Gross observation of bone specimen
Postoperatively, at 12 weeks, three rabbits were sacrificed in each group, their forearm muscle was completely removed, and the length of the whole radius was exposed. They were then observed for the degradation of the BHA/PAA materials, the formation of new bone, the bone connection, and the recovery of the bone defect.
The detection of pathology and immunohistochemistry
Postoperatively, at 12 weeks, three rabbits were sacrificed in each group, and bone tissue samples were collected. Samples were processed as described. The samples were fixed in 10% formalin, decalcified, embedded in paraffin, and cut into 5 μm thick sections for hematoxylin–eosin (HE) staining and for labeling the collagen fibers for immunohistochemistry. To ensure that the results were true and reliable, two pathologists independently evaluated the results in a double-blind manner to understand the formation of new bone and collagen fibers.
In vivo osteogenesis confocal scanning microscopy
After 12 weeks of treatment, two rabbits were sacrificed in each group (these rabbits were intramuscularly injected with 0.5% calcium calcein solution at a dosage of 5 mg/kg 1 week earlier). After taking samples of bone tissue and making hard tissue slices, these slices were observed for the formation of new bone using a confocal laser scanning microscope.
Statistical analysis
The results of the experimental data were processed via SPSS 18.0 statistical software (SPSS Inc., Chicago, IL, USA) and expressed as the mean ± standard deviation ( ). The differences between groups were analyzed using Student’s t-test, and P-values <0.05 were considered to be statistically significant.
Results
In vitro experiments
The observation of MG63 cells cultured with materials
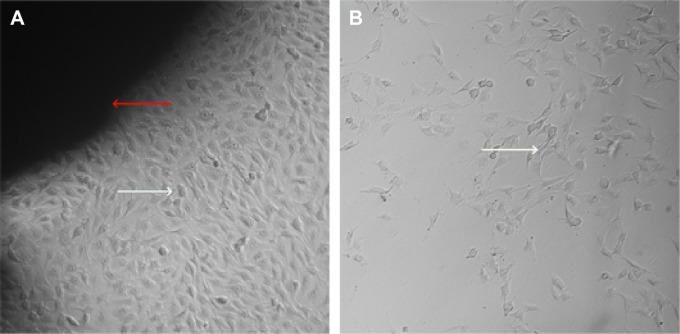
MG63 cells were cultured with BHA/PAA materials at different growth times were observed using an inverted microscope. We observed that around the material, osteoblast-like MG63 cells grew well in the experiment group compared with the control group, and the difference was significant (Figure 1). With time, the number of cells and nuclei increased, and some cells had two to three nuclei, which showed that the materials had good biocompatibility and osteogenic induction ability.
Figure 1.
MG63 cells were cultivated in vitro for 5 days.
Notes: Inverted microscopy observation (×100): a large amount of MG63 cells grew around the material in the experimental group, and the cell number was significantly fewer in the control group; the red arrow – BHA/PAA material, the white arrow – MG63 cell. (A) Experimental group; (B) control group.
Abbreviation: BHA/PAA, bone-like hydroxyapatite/polyamino acid.
The determination of osteogenic ability
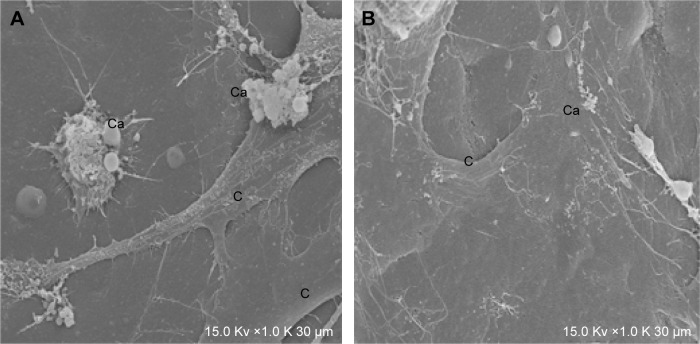
MG63 cells cultured with materials for 7 days were evaluated using a SEM. MG63 cells exhibited good adhesion and growth on the material surface and contacted each other to take on a network structure. Calcium nodules of varying sizes formed on the material surface in the experiment group; the formed ones were very few and were not obvious on the slide surface in the control group (Figure 2).
Figure 2.
MG63 cells cultured for 7 days observed by SEM (×1,000).
Notes: (A) In the experimental group, MG63 cells grew well, and lots of calcium nodules formed on the materials surface. (B) In the control group, very few calcium nodules formed on the slide surface.
Abbreviations: Ca, calcium nodule; C, MG63 cell; SEM, scanning electron microscope.
MTT cell proliferation assay
Within the first 3 days of culture, the growth of the MG63 cells was rapid. At days 4–5, the growth began to plateau and the growth reached a peak on the 5th day. Then it began to decline. Therefore, we chose the logarithmic growth period (day 2), the growth peak period (day 5), and the growth decreasing period (day 7) as the observation points. The cell proliferation of MG63 cells was detected in both the experimental group and control group using the MTT method (Table 2 and Figure 3). There was a significant difference in the experimental group compared with the control group at each time point (P<0.05).
Table 2.
MTT cell proliferation assay ( , n=3)
| Group | 2 d | 5 d | 7 d |
|---|---|---|---|
| Control group | 0.2597±0.0850 | 1.1222±0.4284 | 1.8746±0.5032 |
| Experimental group | 0.5285±0.0946a | 3.2618±0.6183a | 4.8462±0.8026a |
Note: Significant difference (P<0.05) was found between the experimental groupa and control group at each time point.
Abbreviations: SD, standard deviation; d, days.
Figure 3.
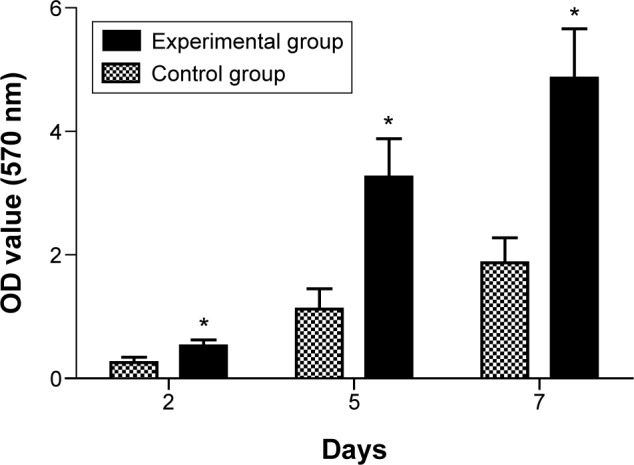
The result of MTT proliferation assay of MG63 culture with BHA/PAA material. Notes: The experimental group (*) versus the control group was significantly different (P<0.05).
Abbreviations: BHA/PAA, bone-like hydroxyapatite/polyamino acid; OD, optical density.
The ALP activity assay
ALP, an enzyme secreted by osteoblasts, is a specific marker of osteoblast differentiation. At different time points, by comparison, ALP activity of the experimental group was different from the control group (P<0.05). ALP activity of MG63 cells increased with the extension of culture time, and ALP activity increased more significantly in the experimental group than compared to the control group (Table 3 and Figure 4).
Table 3.
The ALP activity of MG63 cell at different culture periods ( , n=3, U/mL)
| Group | 2 d | 5 d | 7 d |
|---|---|---|---|
| Control group | 0.3114±0.0543 | 2.1225±0.4664 | 3.7235±0.6454 |
| Experimental group | 0.6446±0.1286a | 4.9525±0.8673a | 7.2644±1.2255a |
Note: Significant difference (P<0.05) was found between the experimental groupa and control group at each time point.
Abbreviations: SD, standard deviation; ALP, alkaline phosphatase; d, days.
Figure 4.
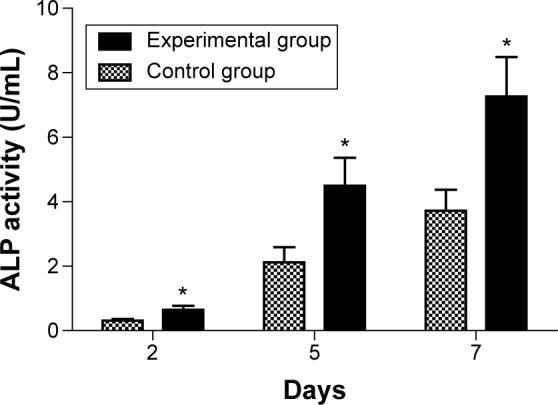
The result of ALP determination assay of MG63 at different time points.
Notes: The experimental group (*) versus the control group was significantly different (P<0.05).
Abbreviation: ALP, alkaline phosphatase.
Determination of calcium content
As shown in Table 4 and Figure 5, in the experimental group and the control group, the Ca2+ content of MG63 cells increased with the increasing culture time. At each time point, Ca2+ content was significantly different (P<0.05) between the experimental group and the control group.
Table 4.
Ca2+ content at different time points ( , n=3, μmol/mL)
| Group | 2 d | 5 d | 7 d |
|---|---|---|---|
| Control group | 0.0346±0.0128 | 0.1448±0.0443 | 0.2873±0.0636 |
| Experimental group | 0.0714±0.0194a | 0.3966±0.0754a | 0.5874±0.1075a |
Note: Significant difference (P<0.05) was found between the experimental groupa and control group at each time point.
Abbreviations: SD, standard deviation; d, days.
Figure 5.
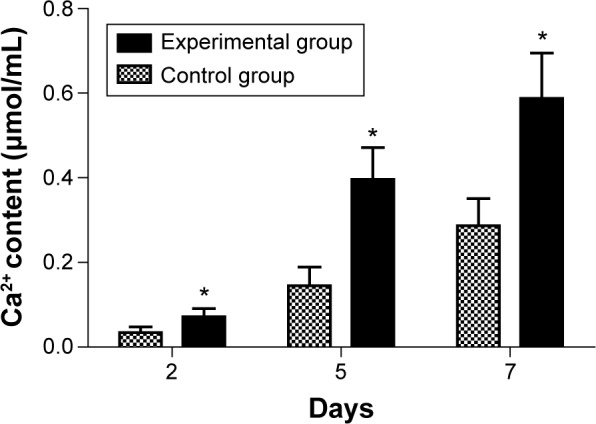
The result of the experiment studying calcium concentration of MG63 cells at different time points.
Notes: The experimental group (*) versus the control group was significantly different (P<0.05).
In vivo experiment
The observation of the experimental animals during the postoperative period
During the course of treatment, no animals died. Postoperative 1 week, animals suffered from limb hanging, lameness, poor spirit, eating less, and coarse hair. After 1 week, there was a gradual recovery, and there was no significant difference in the experimental group compared to the control group.
The observation of the gross bone specimen
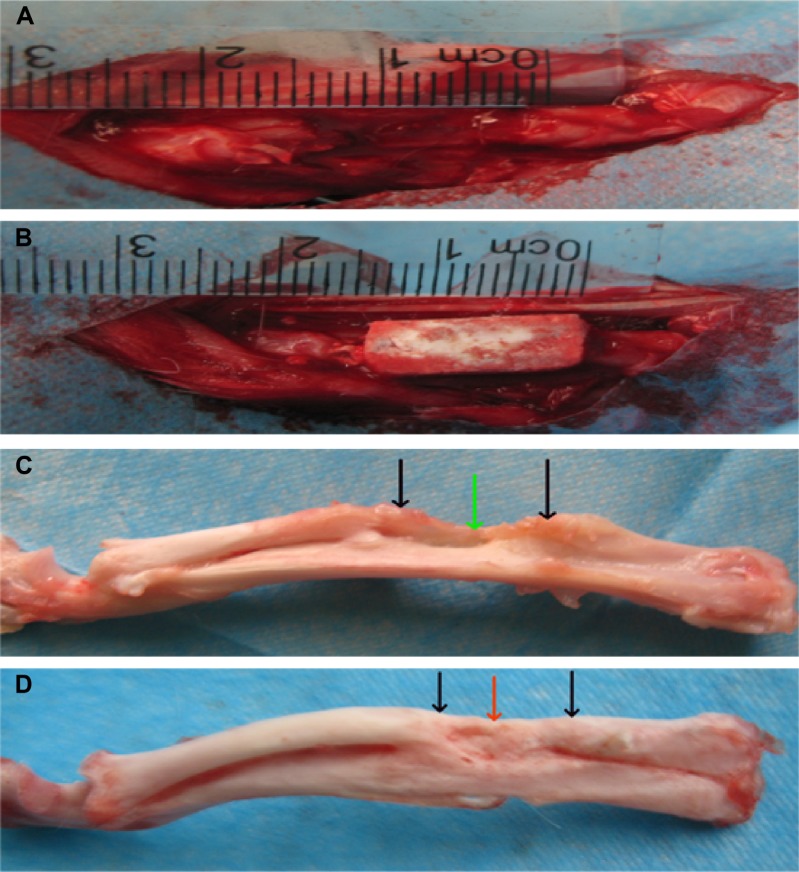
Approximately 12 weeks after surgery, experimental animals were sacrificed, and bone specimens were taken for gross observation. The implanted BHA/PAA materials completely fused with the host bone in the experimental group, and the material was covered with new bone, implicating a bone defect restoration. In the control group (no implanting material), a small amount of new bone formed at the broken bone ends; there was sclerosis around the edges, and bone defects were still present (Figure 6).
Figure 6.
The bone specimen 12 weeks after treatment.
Notes: In the perioperative photographs, (A) the control group and (B) the experimental group, with the BHA/PAA. After treatment for 12 weeks, (C) in the control group, bone defects still existed, there was marginal sclerosis, and the broken end was connected through the fiber; (D) in the experimental group, the BHA/PAA material was not visible, and new bone had formed, implicating that the bone defect was restored. Black arrow – bone defect edge, green arrow – fibrous tissue, and red arrow – new bone.
Abbreviation: BHA/PAA, bone-like hydroxyapatite/polyamino acid.
Radiological examination
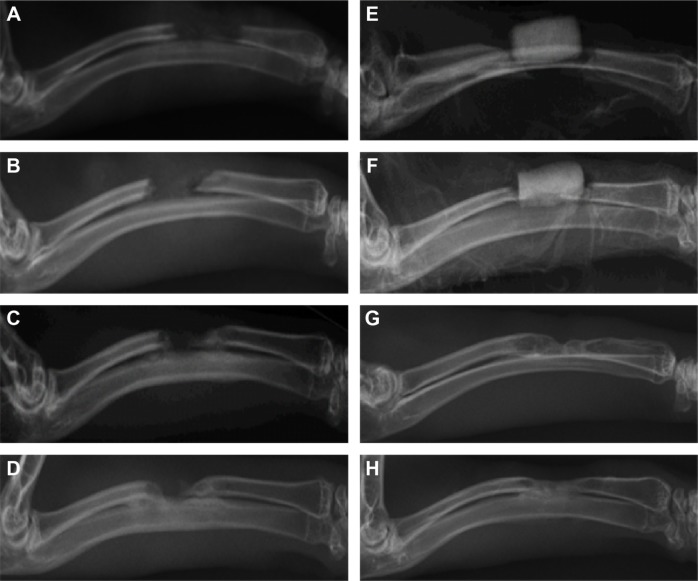
Postoperatively, on day 1 and at 4, 8, and 12 weeks, radiological examination revealed that, in the experimental group, the BHA/PAA material gradually degraded and was absorbed. Furthermore, trabecular bone and new bone formation was observed, and the radius recovered its normal structure. Much of the material had degraded and was absorbed, and was barely visible. In the control group, only a small amount of new bone had formed at the broken bone ends; there was also marginal sclerosis, and bone defects were visible. According to the Lane and Sandhu scoring system,11 the total score was 7–10 in the experimental group and 1–3 in the control group (Table 5 and Figure 7).
Table 5.
Modified Lane and Sandhu radiological scoring of bone formation, union, and remodeling (postoperatively)
| Time | Experimental group
|
Control group
|
||||
|---|---|---|---|---|---|---|
| Formation | Union | Reconstruction | Formation | Union | Reconstruction | |
| 0 d | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 wk | 1 | 1 | 0 | 1 | 0 | 0 |
| 8 wk | 2–3a | 2a | 1a | 1–2 | 0 | 0 |
| 12 wk | 3–4a | 2a | 1–2a | 1–2 | 0 | 0–1 |
Note: Significant difference (P<0.05) was found between the experimental groupa and control group at each time point.
Abbreviations: d, days; wk, weeks.
Figure 7.
Imaging examination at day 1 and 4, 8, and 12 weeks.
Notes: (A–D) In the control group, no new bone formation, and the bone defect was not restored. With the extension of time, the broken end gradually hardened. At 12 weeks, the broken ends sclerosed and closed. (E–H) In the experimental group, the material gradually degraded and was absorbed, and there was new bone formation. At 12 weeks, much of the material had degraded and was absorbed, and was only faintly visible with bone defect recovery.
Histopathology and Immunohistochemistry assay
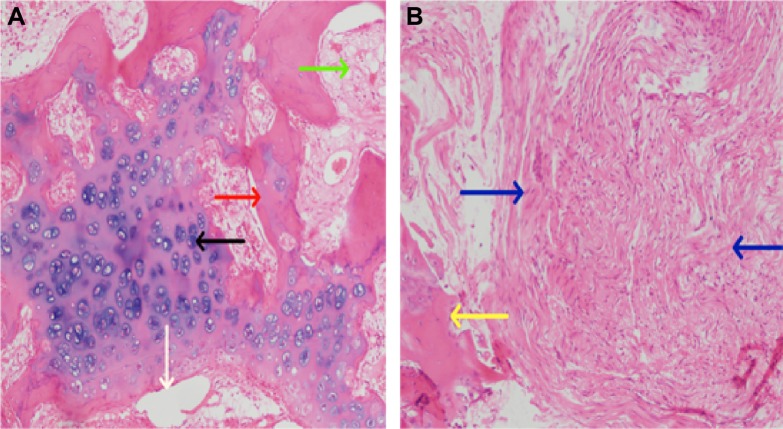
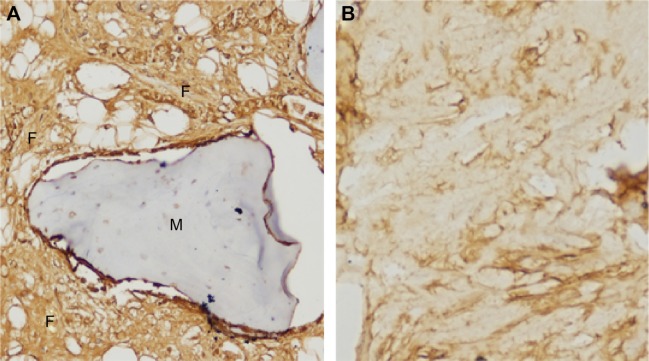
After treatment of the radial defect for 12 weeks, the bone tissue lesions were stained by HE. Much of material was degraded and absorbed, and new bone and cartilage formed in the experimental group. New bone and cartilage did not form in the control group, and massive fibrous tissue formed in the bone defect site (Figure 8). The bone tissue lesions were sliced and immunohistochemistry was performed against collagen I fibers. Collagen I fibers were visible and largely formed a weave-like structure in the experimental group. The formation of collagen I fibers was not clear or only a very small amount was formed in the control group. According to the Takikita et al12 semiquantitatively immunoreactive scoring (IRS) system, (the intensity of immunostaining as 0 [no immunostaining], 1 [weak immunostaining], 2 [moderate immunostaining], and 3 [strong immunostaining]), the total score was 3 in the experimental group and 0–1 in the control group (Figure 9).
Figure 8.
After 12 weeks, bone tissue pathology was evaluated using HE staining (×100).
Notes: (A) Experimental group: much of the material has degraded and been absorbed, and there is new bone formation. (B) Control group: there is a great deal of fibrous tissue in the bone defect site. The red arrow – new trabecular bone, the black arrow – chondrocytes and cartilage matrix, the white arrow – materials, the green arrow – bone marrow cavity, the blue arrow – fibrous tissue, and the yellow arrow – broken bone ends.
Abbreviation: HE, hematoxylin–eosin.
Figure 9.
At 12 weeks after treatment, immunohistochemical examination of collagen I fibers at the lesion (×200).
Notes: (A) Implanting group: a great deal of collagen fibers formed around the material. (B) Control group: collagen fiber formation was not notable.
Abbreviations: F, collagen I fiber; M, material.
Calcium calcein fluorescence labeling
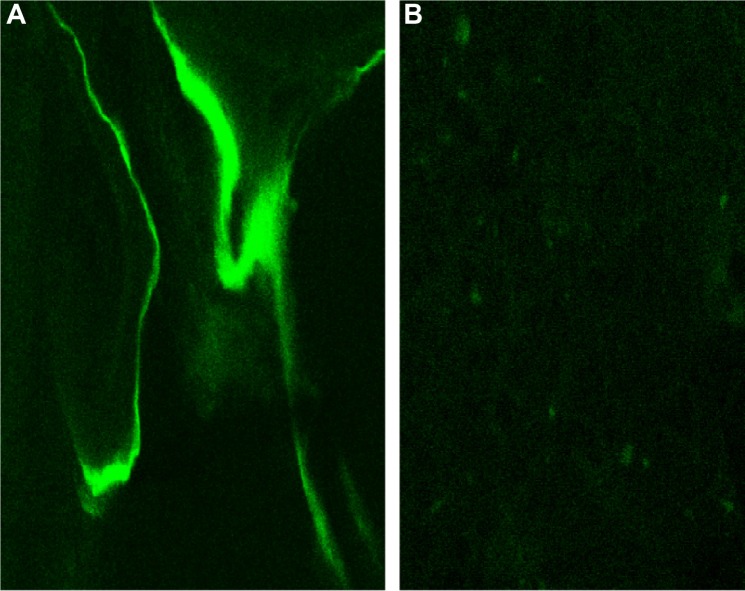
After treatment of the radial defect for 12 weeks, bone specimen sections were observed under laser confocal scanning microscopy using 0.5% calcium calcein labeling, and a strong green fluorescence was present in the material in the experimental group. Scattered, fuzzy fluorescence was observed in the control group. According to the Takikita et al12 semiquantitatively IRS system, the total score was 3 in the experimental group and 1 in the control group (Figure 10).
Figure 10.
After 12 weeks, lesion bone tissue was evaluated via fluorescent confocal scanning (×200).
Notes: (A) In the BHA/PAA material group, there is strong fluorescence in the material. (B) In control group, there was scattered, fuzzy fluorescence.
Abbreviation: BHA/PAA, bone-like hydroxyapatite/polyamino acid.
Discussion
Long segmental bone defects, especially those greater than 15 mm, have difficulty healing, and their treatment is very difficult for orthopedic surgeons in the clinical setting. Autologous bone grafts are the gold standard for the treatment of large, long segmental bone defects,1 but there is a limited amount of source material, and taking samples is painful for the patient, which limits its application.2 Although allografts and xenografts are widely used, they elicit an immune response and can potentially spread disease.1,3 During processing, their mechanical strength is decreased, and therefore, these factors greatly limit their scope of application. With the development of tissue engineering and forging technology, there are many composite artificial bone transplant materials, which have a good biocompatibility, degradability, absorb-ability, strong osteoinductive activity, mechanical strength, and easy processing molding properties, which overcome autogenous bone and allogenic bone defects.
This experiment used BHA/PAA as the bone filling material in the treatment of a rabbit radial bone defect. Through in vitro and in vivo experiments, it was evaluated for its biological biocompatibility and osteoinductive activity. BHA is carbonated HA that has been specially processed, and its composition and structure are similar to natural bone. This is due to the substitution of or OH− into the structure of HA, which causes HA lattice distortion and produces a great deal of defect and incomplete crystallization. Therefore, its activity is stronger, and it is more easily degraded and absorbed than HA. Furthermore, it has good biological compatibility and safety, and does not elicit an immune response. This retains the original biological compatibility, biological activity, and bone induction effects of HA,4,5 and greatly improves the surface activity and the degradation and absorbability.7 In vivo, Na+, K+, in BHA exchange with Ca2+, OH−, , in body fluid through BHA degradation, and then BHA unites with the host bone through hydrogen bonding as a whole. Therefore, BHA provides an excellent physiological scaffold for new bone formation and regeneration, and it gradually degrades during the bone healing process until it is completely replaced by autologous tissue. The material has a natural porous structure with a large internal surface area, which is conducive to the growth and formation of new vessels.13,14 It increases the ability of the local tissue blood supply and defends against inflammation, which is beneficial to the adhesion, growth, and proliferation of bone cells and bone marrow stem cells and new bone tissue formation on the material.15,16 Pure BHA, the same as HA, is not conducive to processing and plasticity. When it combines with PAA, its toughness and mechanical properties are enhanced, which has a good role in supporting bone defects. The molecular structure of PAA is similar to the collagen I molecular structure, with good biocompatibility and biodegradability,5 and its degradation product provides glycine, proline, and hydroxyproline, which promote the formation of collagen I fibers. Collagen I fibers are a scaffold for bone formation by attracting calcium deposition, to promote the new bone formation.
In vitro, the osteogenic induction activity of BHA/PAA was evaluated using an inverted microscope, SEM, MTT proliferation experiment, ALP activity, and calcium content assay.
The osteogenic MG63 cell strain was cultivated with BHA/PAA. Using an inverted microscope, we observed that the adhesion and growth of MG63 cells was significant around the material. With the extension of time, the cell number significantly increased, the nuclei grew, the nucleoli increased, and the nuclei divided. The cell adhesion and growth was significantly different in the experimental group compared to the control group (Figure 1). At the same time, a large degree of cell adhesion and growth were observed on the material surface. A large number of calcium nodules formed as observed by SEM detection (Figure 2). BHA/PAA has a good biological compatibility because it is a polar polymer, containing a large number of –OH, –NH2, and –COOH groups. These polar groups can promote the adhesion of organic components (laminin) on the material surface and promote the colonization of bone cells on the material.17 The porous network structure and surface roughness of BHA/PAA is beneficial to promote osteoblast adhesion, colonization, growth, proliferation, and promote the formation of new bone.18–20 The MTT proliferation experiment reflected cell proliferation in vitro (Table 2 and Figure 3). The experimental results showed that the osteoblast-like MG63 cell number increased with the extension of culture time in the experimental group and control group. Through statistical analysis, the cell proliferation was significantly different between the experimental group and the control group at each time point (P<0.05).
ALP is synthesized and secreted by osteoblasts. It is a good response to the differentiation and function of osteo-blasts. When ALP is highly expressed, it shows high activity, which is beneficial to the synthesis of collagen fibers, the formation of calcium nodules, and the maturation of bone mineralization.21–23 ALP level increases with the differentiation and maturation of osteoblasts, as an early marker of osteogenic differentiation.24 With the prolongation of culture time, the experimental study showed that the ALP activity increased at each time point and was significantly higher in the experimental group compared to the control group (Table 3 and Figure 4; P<0.05). Ca2+ is an important regulator of cellular physiological responses. Ca2+ can reflect the cellular functional state, which affects chemotaxis, adhesion, differentiation, maturation, and the cellular response to various growth factors and hormones. Ca2+ is closely related to the differentiation, maturation, collagen fiber formation, and new bone calcification of osteoblasts.25 In this experiment, the results of the Ca2+ content and ALP activity were consistent. The MG63 cells’ Ca2+ content was significantly increased in the experimental group compared to the control group (P<0.05), with the prolongation of culture time, and at each time point (Table 4 and Figure 5).
The in vitro experiments proved that BHA/PAA composite materials promoted the differentiation and maturation of osteoblast-like cells and promoted the formation of calcium nodules, indicating a good ability to induce bone formation.
We used an in vivo model to evaluate the degradation and absorption properties, as well as the osteogenic capability of BHA/PAA composite materials, using a gross bone specimen examination, imaging, pathology, immunohistochemistry, and confocal laser scanning microscopy.
For the imaging evaluation, the radial defect was treated in the experimental group with a BHA/PAA-implanted. At 12 weeks, the material was vaguely visible, and much of the material was degraded and absorbed. The radial defect had almost recovered to normal, and the bone defect was reconstructed. In the control group, the bone defect still existed at 12 weeks, and the broken end of the bone defect was hardened, and the medullary cavity was closed (Figure 7). After 12 weeks of treatment, HE staining of the lesion bone tissue showed that much of the material was degraded and absorbed in the experimental group, and new trabecular bone and cartilage had formed. In the control group, there was a great deal of fibrous tissue formation and no new bone formation (Figure 8). After 12 weeks of treatment, immunohistochemical examination of collagen I fibers showed that a great deal of collagen fibers formed in the experimental group, with a weave-like structure around the BHA/PAA material. In the control group, no or only a small amount of collagen I fibers formed (Figure 9). Collagen I fiber is a scaffold of calcium salt deposition on the new bone, and it is mainly secreted by osteoblasts. Many studies have shown that collagen I fibers are an important index that reflects bone formation and play an important role in osteoblast adhesion, differentiation, maturation, and calcium nodule formation.26
Approximately 12 weeks after surgery, slices were made from the bone tissue lesion, labeled with 0.5% calcium calcein, and observed using a confocal laser scanning microscope. A strong green fluorescent signal was detected in the BHA/PAA materials from the experimental group. Only scattered, fuzzy fluorescent spots were detected in the control group (Figure 10). Because calcium calcein has a special affinity to osteoblast cells, it will display a green fluorescence under a laser scanning confocal microscope. By detecting the intensity of calcium calcein fluorescence, one can estimate the degree of new bone mineralization and formation. A high fluorescent labeling rate indicates new bone formation.
The results of these in vivo experiments indicate that a BHA/PAA composite material has good biological activity and osteogenic induction ability. Furthermore, it can promote new bone formation, reconstruct a bone defect, and is capable of being degraded and absorbed.
Conclusion
In this study, using in vitro and in vivo models, we showed that a BHA/PAA composite material has good biological compatibility, is secure, is degradable and resorbable, and is capable of osteogenic induction. Furthermore, it reconstructed and recovered a radial defect in a rabbit model. Therefore, BHA/PAA is a good bone graft substitute material, and it is worth exploring in future research and popularizing in the clinical setting.
Acknowledgments
The research was financially supported by the National Natural Science Foundation Program of China (grant number 81171685).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.LeGeros RZ. Properties of osteoconductive biomaterials: calcium phosphates. Clin Orthop Relat Res. 2002;1(395):81–98. doi: 10.1097/00003086-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Jang ES, Park JW, Kweon H, et al. Restoration of peri-implant defects in immediateimplant installations by Choukroun platelet-rich fibrin and silk fibroin powder combination graft. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(6):831–836. doi: 10.1016/j.tripleo.2009.10.038. [DOI] [PubMed] [Google Scholar]
- 3.Moore WR, Graves SE, Bain GI. Synthetic bone graft substitutes. ANZ J Surg. 2001;71(6):354–361. [PubMed] [Google Scholar]
- 4.Gentile P, Chiono V, Boccafoschi F, et al. Composite films of gelatin and hydroxyapatite/bioactive glass for tissue-engineering applications. J Biomater Sci Polym Ed. 2010;21(8–9):1207–1226. doi: 10.1163/092050609X12481751806213. [DOI] [PubMed] [Google Scholar]
- 5.Roohani-Esfahani SI, Nouri-Khorasani S, Lu Z, Appleyard R, Zreiqat H. The influence hydroxyapatite nanoparticle shape and size on the properties of biphasic calcium phosphate scaffolds coated with hydroxyapatite-PCL composites. Biomaterials. 2010;31(21):5498–5509. doi: 10.1016/j.biomaterials.2010.03.058. [DOI] [PubMed] [Google Scholar]
- 6.Zhao W, Yu SF. Carbonate group (CO3) of the qualitative and quantitative analysis in dental apatites. J Zhongshan Med Univ. 2002;23(5S):7–8. [Google Scholar]
- 7.Bose S, Saha SK. Synthesis and characterization of hydroxyapatite nanopowders by emulsion technique. Chem Mater. 2003;15(23):4464–4469. [Google Scholar]
- 8.Wu Y, Bose S. Nanocrystalline hydroxyapatite: micelle templated synthesis and characterization. Langmuir. 2005;21(8):3232–3234. doi: 10.1021/la046754z. [DOI] [PubMed] [Google Scholar]
- 9.Matsumura Y. Poly (amino acid) micelle nanocarriers in preclinical and clinical studies. Adv Drug Deliv Rev. 2008;60(8):899–914. doi: 10.1016/j.addr.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Peng XL, Li YB, Wang XJ, et al. The study of medical nano hydroxyapatite/polyamide 66 composite in vitro immersion behavior. Functional Mater. 2004;2(35):253–255. [Google Scholar]
- 11.Lane JM, Sandhu HS. Current approaches to experimental bone grafting. Orthop Clin North Am. 1987;18(2):213–225. [PubMed] [Google Scholar]
- 12.Takikita M, Hu N, Shou JZ, et al. Fascin and CK4 as biomarkers for esophageal squamous cell carcinoma. Anticancer Res. 2011;31:945–952. [PMC free article] [PubMed] [Google Scholar]
- 13.Oudadesse H, Derrien AC, Lucas-Girot A. Statistical experimental design for studies of porosity and compressive strength in composite materials applied as biomaterials. Eur Phys J Appl Phys. 2005;31(3):217–223. [Google Scholar]
- 14.Chanda A, SinghaRoy R, Xue W, Bose S, Bandyopadhyay A. Bone cell–materials interaction on alumina ceramics with different grain sizes. Mater Sci Eng C. 2009;29(4):1201–1206. [Google Scholar]
- 15.Mastrogiacomo M, Scaglione S, Martinetti R, et al. Role of scaffold internal structure on in vivo bone formation in macroporous calcium phosphate bioceramics. Biomaterials. 2006;27(17):3230–3237. doi: 10.1016/j.biomaterials.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 16.Turco G, Marsich E, Bellomo F, et al. Alginate/hydroxyapatite biocomposite for bone ingrowth: a trabecular structure with high and isotropic connectivity. Biomacromolecules. 2009;10(6):1575–1583. doi: 10.1021/bm900154b. [DOI] [PubMed] [Google Scholar]
- 17.Bos R, Van der Mei HC, Busscher HJ. Physico-chemistry of initial microbial adhesive interactions its mechanisms and methods for study. FEMS Microbiol Rev. 1999;23:179–230. doi: 10.1111/j.1574-6976.1999.tb00396.x. [DOI] [PubMed] [Google Scholar]
- 18.Wei G, Ma PX. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials. 2004;25(19):4749–4757. doi: 10.1016/j.biomaterials.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Owen GR, Jackson J, Chehroudi B, Burt H, Brunette DM. A PLGA membrane controlling cell behaviour for promoting tissue regeneration. Biomaterials. 2005;26(35):7447–7456. doi: 10.1016/j.biomaterials.2005.05.055. [DOI] [PubMed] [Google Scholar]
- 20.Huang J, Zhao D, Dangaria SJ, et al. Combinatorial design of hydrolytically degradable, bone-like biocomposites based on PHEMA and hydroxyapatite. Polymer. 2013;54(2):909–919. doi: 10.1016/j.polymer.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang YG, Chen ZQ. Effect of hard tissue replacement materials on bone calcium protein and ALP levels of osteoblast. J Oral Med West China. 2000;18(3):192–194. [Google Scholar]
- 22.Liao H, Andersson AS, Sutherland D, Petronis S, Kasemo B, Thomsen P. Response of rat osteoblast-like cells to microstructured model surfaces in vitro. Biomaterials. 2003;24(4):649–654. doi: 10.1016/s0142-9612(02)00379-4. [DOI] [PubMed] [Google Scholar]
- 23.Dias AG, Lopes MA, Trigo Cabral AT, Santos JD, Fernandes MH. In vitro studies of calcium phosphate glass ceramics with different solubility with the use of human bone marrow cells. J Biomd Mater Res A. 2005;74(3):347–355. doi: 10.1002/jbm.a.30357. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Cooper PR, Barralet JE, Shelton RM. Influence of calcium phosphate crystal assemblies on the proliferation and osteogenic gene expression of rat bone marrow stromal cells. Biomaterials. 2007;28(7):1393–1403. doi: 10.1016/j.biomaterials.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 25.Hallab NJ, Bundy KJ, O’Connor K, Moses RL, Jacobs JJ. Evaluation of metallic and polymeric biomaterial surface energy and surface roughness characteristics for directed cell adhesion. Tissue Eng. 2001;7(1):55–69. doi: 10.1089/107632700300003297. [DOI] [PubMed] [Google Scholar]
- 26.Yang ZM, Yu XJ, Huang FG, et al. Exogenous type I collagen of embryonic periosteum ossification cell biological characteristics influence. J West China Major Med J. 2001;32:1. [Google Scholar]