Abstract
Background
Patient function is a key part of the clinical decision to offer chemotherapy and has, in earlier studies, been associated with chemotherapy toxicity. Objective testing might be more accurate than patient-reported or physician-assessed physical function, and thus might be a stronger predictor of chemotherapy toxicity in older adults.
Methods
Patients, 70 years of age and older, with thoracic or colorectal cancer were recruited. Three physical tests were performed before commencement of a new line of chemotherapy: grip strength, 4-m walk test, and the Timed Up and Go (tug). Our pilot study explored the association between those tests and chemotherapy toxicity.
Results
The 24 patients recruited had a median age of 74.5 years (range: 70–84 years), and 54.2% had an Eastern Cooperative Oncology Group performance status of 0 or 1. Median score on the Charlson comorbidity index was 1 (range: 0–4). Almost two thirds had metastatic disease, 70% were chemonaïve, and 83.3% were about to receive polychemotherapy. Patients had a mean tug of 13.2 ± 5.7 s and a mean gait speed of 0.74 ± 0.24 m/s; 50% had a grip strength test in the lowest 20th percentile. Grades 3–5 chemotherapy toxicities occurred in 34.7% of the patients; two thirds required a dose reduction or delay; and one third discontinued chemotherapy because of toxicity. Hospitalization attributable to chemotherapy was uncommon (12.5%). A trend toward increased severe chemotherapy toxicity with slower gait speed was observed (p = 0.049).
Conclusions
Abnormalities in objective markers of physical function are common in older adults with cancer, even in those deemed fit for chemotherapy. However, those abnormalities were not associated with an increased likelihood of chemotherapy toxicity in the population included in this small pilot study.
Keywords: Aging, older adults, elderly people, neoplasms, objective markers, physical health, chemotherapy toxicity
INTRODUCTION
Adults 70 years of age and older constitute 43% of all newly diagnosed cancer patients, and that proportion is growing1. Aging, however, is a heterogeneous process. As a result, chronologic age is not always equal to functional or physiologic age. Furthermore, multiple studies have shown that chronologic age alone is not an accurate predictor of prognosis or chemotherapy tolerance2–6.
Given the heterogeneity of aging, identifying patient factors that predict for chemotherapy toxicity or tolerance has been of great interest. Two large prospective studies developed models to predict chemotherapy toxicity in older adults with cancer6,7. Although the predictive markers identified in those two studies differ somewhat, the common factors were chemotherapy characteristics (type, number of agents, and dose) and dependence for instrumental activities of daily living (instrumental adls), a measure of patient function. Several other studies of older cancer patients have also shown that functional status appears to predict severe chemotherapy toxicity, treatment interruption, and dose reduction2,4,8, suggesting that patient function is a key factor in predicting toxicity from chemotherapy.
Patient function can be ascertained either subjectively or objectively. Commonly used subjective measures of patient function include the physician-rated Eastern Cooperative Oncology Group (ecog) performance status (ps) or Karnofsky ps and the patient-reported need for assistance with adls (bathing, dressing, eating, toileting, transferring) and instrumental adls (cooking, chores, transportation, medication administration)9. However, subjective methods of functional assessment have several limitations. Traditional oncologic assessment using the ecog ps and Karnofsky ps is often insensitive to underlying functional impairment in older adults. Although 80% of older cancer patients have an ecog ps of 0 or 1 (fully active with no or mild restrictions on strenuous tasks), up to 50% of such patients require assistance with their instrumental adls9. Even asking about dependence for adls or instrumental adls might not be accurate, because, compared with family members or caregivers, older patients often rate their own functioning more highly10. In addition, questionnaire-based assessments only partially correlate with objective markers of function9, and so an objective test of patient function might be more accurate and potentially a better predictor of chemotherapy toxicity in the older adult.
Objective tests of function include the Timed Up and Go (tug) test11 and the 4-m walk test12, assessments of gait and mobility, and the grip-strength test. Objective markers of function have been found to be predictive of risk of falls13, disability12,14,15, hospitalization12,16, and mortality16–18 in older adults. Furthermore, compared with subjective testing, objective measures can better identify subtle differences in patient function and can detect physical impairments, with fewer patients scoring at the highest threshold of testing (that is, there is less of a ceiling effect)19. Use of objective measures could be important, given that older adults offered chemotherapy are already highly selected based on their fitness. An objective measure of function might help oncologists to appreciate subtle differences in the functional status of older patients being considered for chemotherapy and to better select patients who will tolerate chemotherapy with less toxicity. Furthermore, the inclusion of testing that is quick and easy to perform and to interpret might be more appealing to oncologists to include in their assessments of older adults.
We therefore conducted a pilot study to explore the association between 3 objective markers of function (assessments of mobility, gait speed, and strength) and toxicity in older adults receiving chemotherapy.
METHODS
Patients 70 years of age and older with either thoracic or colorectal cancer who were being seen at a major Canadian cancer centre were approached before they started a new line of chemotherapy. Patients with all stages of cancer were included. Patients who were receiving concurrent radiation or who had already started chemotherapy were excluded, as were patients unable to ambulate. Patients gave informed consent, and the study was approved by the institutional research ethics board.
Patient and Treatment Characteristics
Baseline patient characteristics—including demographics, ecog ps, body mass index (bmi), unintentional weight loss, blood pressure, cancer type, cancer stage, and prior treatments—were obtained by chart review. Baseline laboratory values were recorded. Baseline treatment characteristics, including drugs administered and initial treatment doses, were also obtained.
Objective Markers of Function
Before initiation of chemotherapy, the 3 objective tests of function were administered to patients by a trained research assistant.
The tug test is a commonly used, validated, and standardized measure of functional mobility11. It has good inter- and intra-observer reliability and has been shown to correlate with the Berg Balance Scale (r = −0.81), gait speed (r = −0.61), and the Barthel Index of adls (r = −0.78)11. Patients are asked to rise from a seated position, walk 3 m at their usual pace, and then return to the chair and sit. Patients are allowed to use any assisted ambulatory device that they usually use. The time taken by patients to complete the test is recorded. Normal values for this test are 9.2 s (95% confidence interval: 8.2 s to 10.2 s) for patients 70–79 years of age and 11.3 s (95% confidence interval: 10.0 s to 12.7 s) for patients 80–99 years of age20. A time of 12 s or less is considered normal in community-dwelling older adults21.
Gait speed was measured using the 4-m walk test12,22,23. Patients were timed while walking 4 m at their usual pace from a standing position. Results were converted into a gait speed of metres per second by dividing the distance (4 m) by the recorded time. Gait speed has been correlated with survival in community-dwelling older adults. Even small changes in gait speed of 0.1 m/s are clinically significant across a wide range of gait speeds18.
Grip strength was tested using a Jamar handgrip dynamometer (Lafayette Instrument, Lafayette, IN, U.S.A.), which has good validity with weights (r = 0.96 to 0.9998) and high test–retest (r > 0.80) and inter-rater reliability (intraclass correlation coefficient: 0.85 to 0.98)24–26. Patients were asked to squeeze the dynamometer as hard as possible with their dominant or strongest hand for 3 s. Three trials were performed, with the highest value being used. In the general geriatric population, low handgrip strength has been linked to poorer overall survival and increased disability27. Based on its use in earlier studies as an indicator of frailty, grip strength in the lowest 20th percentile by sex and bmi was classified as abnormal28.
Outcomes
Chemotherapy toxicity was graded according to the U.S. National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 4.0. Grades 3–5 toxicities attributable to chemotherapy and chemotherapy toxicity resulting in dose reductions, treatment delays, treatment discontinuation, emergency room (er) visits, hospitalizations and death were captured in the chart review. Chemotherapy toxicities were captured from initiation of chemotherapy until 30 days after the last dose of chemotherapy received. All events were captured once per patient and are reported using the highest grade of toxicity. The number of cycles of chemotherapy received and the date of death, if available, were also recorded.
Statistical Analysis
Patient, cancer, and treatment characteristics are summarized using descriptive statistics. Associations between the 3 objective markers of function and chemotherapy toxicity (dose reductions or delays, treatment discontinuation because of toxicity, er visits or hospitalizations because of toxicity) were determined using the Fisher exact test and the Mann–Whitney test. Associations between ecog ps and chemotherapy toxicity were tested using the Cochran– Armitage exact trend test. Grip strength was analyzed as a categorical variable, and gait speed, as a continuous variable. The tug test was analyzed as a continuous variable, but an exploratory analysis using 12 s as a cut-off (considered abnormal in older adults) was also conducted. Because of the exploratory nature of our pilot study, no adjustment for multiple testing was performed.
RESULTS
Patient Characteristics
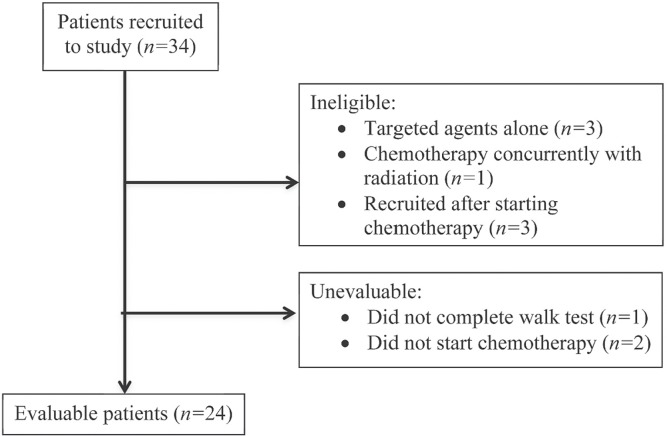
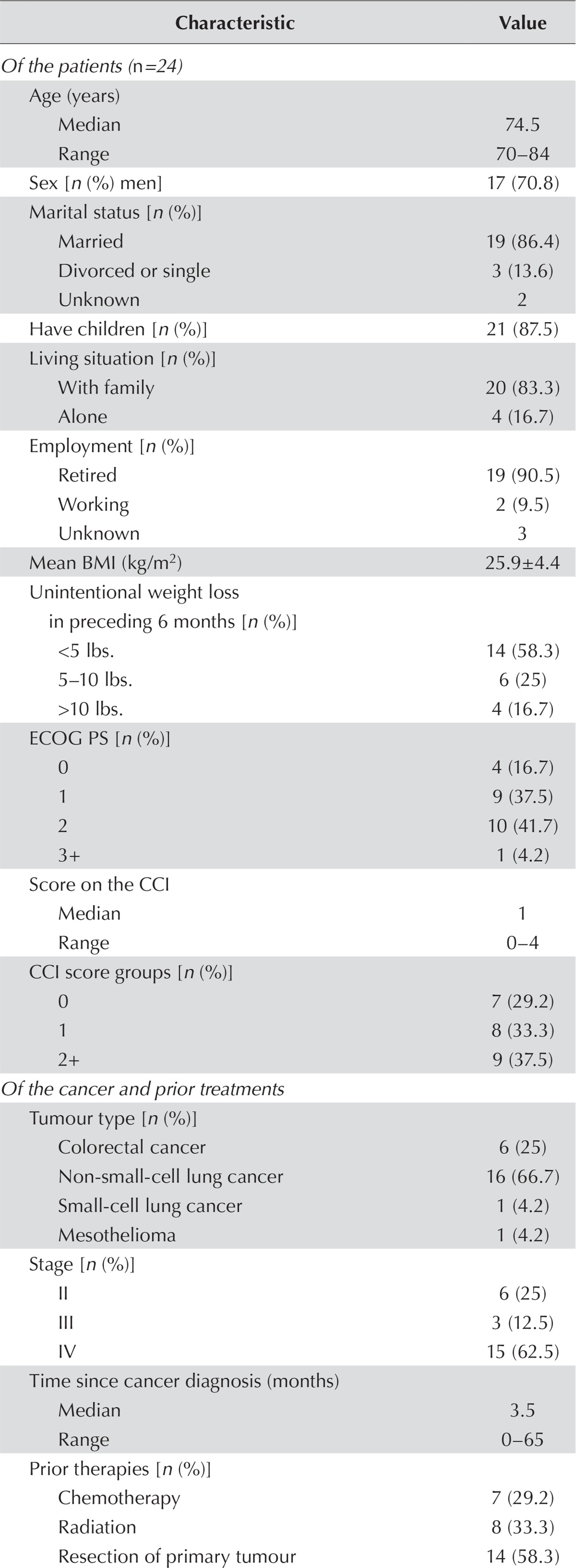
During a 7-month period from 2011 to 2012, a convenience sample of 24 patients was recruited (Figure 1). Patients had a median age of 74.5 years (range: 70–84 years), were predominantly men (70.8%), were married (86.4%), and lived with their family (83.3%, Table i). Most had an ecog ps of 0 or 1 (54.2%). Almost 70% had at least 1 comorbidity, and more than one third had a score of 2 or more on the Charlson comorbidity index. Most patients (58.3%) had not experienced significant weight loss, and the average bmi (± standard deviation) was 25.9 ± 4.4 kg/m2.
FIGURE 1.
CONSORT diagram of patients included in the study.
TABLE I.
Characteristics of the study cohort
| Characteristic | Value |
|---|---|
| Of the patients (n=24) | |
| Age (years) | |
| Median | 74.5 |
| Range | 70–84 |
| Sex [n (%) men] | 17 (70.8) |
| Marital status [n (%)] | |
| Married | 19 (86.4) |
| Divorced or single | 3 (13.6) |
| Unknown | 2 |
| Have children [n (%)] | 21 (87.5) |
| Living situation [n (%)] | |
| With family | 20 (83.3) |
| Alone | 4 (16.7) |
| Employment [n (%)] | |
| Retired | 19 (90.5) |
| Working | 2 (9.5) |
| Unknown | 3 |
| Mean BMI (kg/m 2) | 25.9±4.4 |
| Unintentional weight loss in preceding 6 months [n (%)] | |
| <5 lbs. | 14 (58.3) |
| 5–10 lbs. | 6 (25) |
| >10 lbs. | 4 (16.7) |
| ECOG PS [n (%)] | |
| 0 | 4 (16.7) |
| 1 | 9 (37.5) |
| 2 | 10 (41.7) |
| 3+ | 1 (4.2) |
| Score on the CCI | |
| Median | 1 |
| Range | 0–4 |
| CCI score groups [n (%)] | |
| 0 | 7 (29.2) |
| 1 | 8 (33.3) |
| 2+ | 9 (37.5) |
| Of the cancer and prior treatments | |
| Tumour type [n (%)] | |
| Colorectal cancer | 6 (25) |
| Non-small-cell lung cancer | 16 (66.7) |
| Small-cell lung cancer | 1 (4.2) |
| Mesothelioma | 1 (4.2) |
| Stage [n (%)] | |
| II | 6 (25) |
| III | 3 (12.5) |
| IV | 15 (62.5) |
| Time since cancer diagnosis (months) | |
| Median | 3.5 |
| Range | 0–65 |
| Prior therapies [n (%)] | |
| Chemotherapy | 7 (29.2) |
| Radiation | 8 (33.3) |
| Resection of primary tumour | 14 (58.3) |
| Lines of prior systemic therapy (n) | |
| Median | 0 |
| Range | 0–5 |
| Prior systemic therapy groups [n (%)] | |
| 0 Lines | 18 (75) |
| 1 Lines | 3 (12.5) |
| 2+ Lines | 3 (12.5) |
| Of current chemotherapy | |
| Agents received [n (%)] | |
| 1 | 4 (16.7) |
| 2+ | 20 (83.3) |
| Initial dose [n (%)] | |
| Standard | 15 (62.5) |
| Dose-reduced | 9 (37.5) |
BMI = body mass index; ECOG PS = Eastern Cooperative Oncology Group performance status; CCI = Charlson comorbidity index.
Most patients were newly diagnosed and chemonaïve, and had metastatic disease, with non-small-cell lung cancer being the most common tumour type (Table i). Almost 60% had undergone resection of the primary tumour, and one third had received radiation.
Results of Objective Testing
Patients underwent objective testing at a median of 2 days before initiation of chemotherapy (interquartile range: 1–9 days; range: 0–74 days). The average result on the tug test was 13.2 ± 5.7 s, with 33.3% of the patients taking more than 12 s (Table ii). Mean gait speed was 0.76 ± 0.24 m/s, and 91.7% of the patients had a gait speed of less than 1.0 m/s. Mean hand grip in the group was 25.8 ± 5.9 kg, with half of the patients scoring in the lowest 20th percentile by sex and bmi.
TABLE II.
Results of objective testing in 24 patients
| Test | Result |
|---|---|
| Score on Timed Up and Go Test (s) | |
| Mean | 13.2±5.7 |
| Median | 10.9 |
| Range | 8.6–35 |
| Score groups, Timed Up and Go Test [n (%)] | |
| >12 s | 8 (33.3) |
| >20 s | 1 (4.2) |
| Gait speed (m/s) | |
| Mean | 0.76±0.24 |
| Median | 0.78 |
| Range | 0.29–1.33 |
| Hand grip strength (kg) | |
| Mean | 25.8±5.9 |
| Median | 24.5 |
| Range | 16–36 |
| Hand grip strength in lowest 20th percentile [n (%)] | 12 (50) |
Treatment Characteristics and Chemotherapy Toxicity
Most patients (83.3%) were about to received polychemotherapy, and almost two thirds started at standard doses (Table i), with a median starting dose intensity of 100% (range: 44.3%–100%). Patients received a median of 4 cycles of chemotherapy (range: 1–12 cycles). More than 40% completed all planned cycles of chemotherapy, although one quarter discontinued because of progression.
Grade 3 or greater hematologic and nonhematologic toxicities were common (16.7% and 20.8% respectively, Table iii). One third of patients experienced grade 2 toxicities (most being nonhematologic) as the most severe grade of toxicity from chemotherapy. Most patients (70.8%) required a dose delay or dose reduction. The mean dose reduction was 10.4% ± 15.0% (median: 0%; range: 0%–50%). One third of patients discontinued chemotherapy because of toxicity, all because of nonhematologic toxicities [grades 2 (n = 4), 3 (n = 2), or 4 (n = 2)], predominantly fatigue and infection.
TABLE III.
Chemotherapy characteristics in 24 patients
| Characteristic | Value |
|---|---|
| Toxicity (grades 3–5) | |
| Hematologic | 4 (16.7) |
| Nonhematologic | 5 (20.8) |
| Chemotherapy delivery | |
| Completed full course | 10 (41.7) |
| Discontinued for progression | 6 (25.0) |
| Discontinued for toxicity | 8 (33.3) |
| Dose alteration (at least 1) | |
| Reduction | 11 (45.8) |
| Delay | 13 (54.2) |
| Hospitalization | |
| For chemotherapy toxicity | 3 (12.5) |
| For cancer symptoms | 2 (8.3) |
| ER visit without hospitalization | 6 (25) |
| Death | |
| From chemotherapy toxicity | 0 |
| Within 6 months of chemotherapy initiation | 10 (41.7) |
| Within 12 months of chemotherapy initiation | 14 (58.3) |
ER = emergency room.
One third of patients visited the er or were hospitalized in relation to chemotherapy toxicity, with er visits not requiring hospitalization being more common (Table iii). No deaths attributable to chemotherapy occurred. Except for 1 er visit related to chemotherapy toxicity, all toxicities leading to er visits or hospitalization occurred within 3 months of chemotherapy initiation.
Mortality
More than 40% of the patients died within 6 months of chemotherapy initiation, and nearly 60% died within 12 months (Table iii). All deaths were related to disease progression.
Association Between Objective Markers of Function and Outcomes
We observed no significant differences in tug test results or percentage of patients with the lowest grip strength with respect to the presence of chemotherapy toxicity (grades 3–5 toxicity, delays and dose reductions, chemotherapy discontinuations, or hospitalizations and er visits; Table iv). A trend toward more grades 3–5 toxicities was observed with slower gait speed (0.6 ± 0.2 s vs. 0.8 ± 0.2 s, p = 0.049). No association between gait speed and other outcomes of chemotherapy treatment were noted. Furthermore, we observed no association between the number of abnormal test results for an individual patient and the likelihood of chemotherapy toxicity. Similarly, no association between ecog ps and any chemotherapy toxicity outcome was observed.
TABLE IV.
Association of chemotherapy toxicity with objective test results and performance status
| Variable | Grade 3–5 toxicities (hematologic and nonhematologic) | Dose reduction or delay because of chemotherapy toxicity | Chemotherapy discontinuation because of toxicity | Hospitalization or ER visit because of chemotherapy | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| No (n=15) | Yes (n=8) | p Value | No (n=8) | Yes (n=16) | p Value | No (n=16) | Yes (n=8) | p Value | No (n=16) | Yes (n=8) | p Value | |
| Grip strength in bottom 20th percentile [n (%)] | 7 (46.7) | 4 (50.0) | 1.00 | 6 (75.0) | 6 (37.5) | 0.19 | 7 (43.8) | 5 (62.5) | 0.67 | 9 (56.3) | 3 (37.5) | 0.67 |
| Mean gait speed (m/s) | 0.8±0.2 | 0.6±0.2 | 0.049 | 0.7±0.3 | 0.8±0.2 | 0.30 | 0.8±0.2 | 0.7±0.2 | 0.54 | 0.8±0.3 | 0.7±0.2 | 0.90 |
| Mean Timed Up and Go Test (s) | 11.9±3.0 | 16.0±8.6 | 0.37 | 14.6±8.5 | 12.5±3.8 | 0.69 | 11.9±3.1 | 15.8±8.6 | 0.34 | 14.0±6.4 | 11.6±3.7 | 0.30 |
| ECOG PS [n (%)] | ||||||||||||
| 0 | 2 (13.3) | 2 (25.0) | 0.80 | 0 | 4 (25.0) | 0.29 | 2 (12.5) | 2 (25.0) | 1.00 | 3 (18.8) | 1 (12.5) | 0.60 |
| 1 | 7 (46.7) | 2 (25.0) | 3 (37.5) | 6 (37.5) | 7 (43.8) | 2 (25.0) | 6 (37.5) | 3 (37.5) | ||||
| 2 | 6 (40.0) | 3 (37.5) | 5 (62.5) | 5 (31.3) | 7 (43.8) | 3 (37.5) | 7 (43.8) | 3 (37.5) | ||||
| 3 | 0 | 1 (12.5) | 0 | 1 (6.3) | 0 | 1 (12.5) | 0 | 1 (12.5) | ||||
ER = emergency room; ECOG PS = Eastern Cooperative Oncology Group performance status.
DISCUSSION
Despite the increasing incidence of cancer, chemotherapy utilization rates decline markedly as patients age. One of the driving factors is concern about chemotherapy toxicity29–31. Our results suggest that severe chemotherapy toxicity occurs in 34% of older adults (70 years of age and older) with thoracic or gastrointestinal malignancies and that dose delays and reductions are a common result of chemotherapy toxicity, with one third of patients stopping chemotherapy as a result. Visits to the er because of chemotherapy-related toxicity are not uncommon, but hospitalizations are rare, as are deaths attributable to chemotherapy toxicity. A trend toward more grades 3–5 chemotherapy toxicities with slower gait speed was noted. No other associations between objective markers of physical health and chemotherapy toxicity were seen.
Participants in the present study consisted of a select population of older adults who were eligible and willing to undergo chemotherapy. Nevertheless, a large proportion of patients had abnormal physical function as measured by objective tests. Half the patients had a grip strength in the lowest 20th percentile, one third had a tug test result of more than 12 s, and average gait speed was 0.76 ± 0.24 m/s. Rates of physical impairment were higher than those seen in other studies of community-dwelling healthy older adults. The proportion of patients with low grip strength was higher than that seen in the Cardiovascular Health Study (20%)28, and average gait speed was slower than speeds measured for community-dwelling older adults in all but one study in a large systematic review18. Compared with other studies of older cancer patients, our study had a larger proportion of patients with low grip strength (50% vs. 21.4%)32 and a higher proportion with a gait speed less than 1.0 m/s (91.7% vs. 54.4%)32, but a smaller proportion with a prolonged time on the tug test7,33–38. Because gait speed was not reported in the latter studies, it is difficult to know whether average gait speed was slower in their patients than in ours. Differences in objective physical test results could be a result of differences in the participating patients with respect to age (65+ years vs. 70+ years), proportion with metastatic disease, tumour types, number and severity of comorbidities, and nutrition status.
We found no associations of the 3 objective markers of physical function with chemotherapy toxicity. The only exception was a small trend in the association between gait speed and grades 3–5 chemotherapy toxicity. Some studies have reported an association between grip strength and grades 3–5 chemotherapy toxicities32. No association between gait speed and chemotherapy toxicity has previously been shown32,39, but several studies have noted an association between a tug test result exceeding 20 s and death within 6 months34, functional decline38, and early chemotherapy discontinuation37. The association that we observed between gait speed and chemotherapy toxicity requires confirmation in a larger study.
Given that chemotherapy toxicities were collected retrospectively, we focused on moderate-to-severe toxicities (grades 3–5) in our analysis, an approach similar to that used by other studies in this area6,7,32. Our aim was to minimize the likelihood of missing data, given that milder toxicities might be underreported by patients or physicians, particularly if they occurred earlier in the cycle. However, our results suggest that less severe toxicities could still be clinically relevant to older adults and clinicians. Severe toxicities occurred in about one third of patients; however, about half the patients required dose delays and reductions. Half of all chemotherapy discontinuations for toxicity involved grade 2 toxicities. That finding highlights the potential importance of less severe toxicities in older adults; future studies in older adults should consider the frequency and impact of less severe toxicities, and not just grade 3 or higher adverse events.
Our study has several limitations. First, the small sample size in this pilot study limited our ability to detect potentially weaker associations between the selected objective markers of physical health and chemotherapy toxicity. Our findings, both positive and negative, are also prone to random variation. In addition, because of the small sample size and the resulting potential for spurious results with multiple testing, we did not test for associations with other relevant variables such as initial chemotherapy dose intensity, number of chemotherapy agents used, and patient characteristics. We did not capture other measures of physical health such lean muscle mass, which might also relate to chemotherapy toxicity and which might be a potential confounder40–42. Furthermore, we made use of a convenience sample, which could potentially bias the results, because clinicians might have referred healthier patients for participation in the study. In addition, we did not exclude patients with a significant delay between objective testing and initiation of chemotherapy. It is possible that the predictive value of baseline objective markers of physical health for chemotherapy toxicity could decline with time43. Although few toxicities occurred more than 3 months after chemotherapy initiation, standardizing the time for observation of toxicities (using, for example, 3 months) might be important in future studies examining the relationship between baseline objective testing and chemotherapy toxicity.
Lastly, given that we were interested in whether simple tests could enhance the traditional oncology clinical assessment, we did not use specific measures to test for cognition, falls, or other domains typically captured in a comprehensive geriatric assessment. That omission limits our ability to identify subtle differences between this pilot project and other studies, which might explain differences in the objective testing results and the chemotherapy toxicity rates observed. Furthermore, we are unable to compare the objective tests with other chemotherapy toxicity prediction tools such as the Chemotherapy Risk Assessment Scale for High-Age Patients6 and the model developed by the Cancer and Aging Research Group7.
CONCLUSIONS
Our results indicate that, in carefully selected patients 70 years of age and older with gastrointestinal and thoracic cancers, chemotherapy can be safely administered, although dose delays and reductions are common. As indicated by objective testing, abnormal physical function is more common in older adults with cancer than in older patients without cancer. However, in our study, no association of the objective tests with chemotherapy toxicity was found, although a trend for slower gait speed to be associated with severe chemotherapy toxicities was noted. Further investigation is required to confirm our findings. It does not appear that the addition of the objective tests of physical functioning to the usual oncologic clinical assessment provides additional information about chemotherapy toxicity risk in this cohort of patients assessed by physicians to be fit enough for chemotherapy. Although several models for chemotherapy toxicity based on a geriatric assessment are available, further studies exploring how to incorporate those models into the oncology clinic or developing new, simpler models are needed to increase awareness and uptake.
ACKNOWLEDGEMENTS
This project was supported by a Physicians’ Services Incorporated Foundation Resident Research Grant and a Comprehensive Research Experience for Medical Students Grant, as well as by the Division of Medical Oncology, Princess Margaret Cancer Centre.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Canadian Cancer Society’s Advisory Committee on Cancer Statistics . Canadian Cancer Statistics 2014. Toronto, ON: Canadian Cancer Society; 2014. [Google Scholar]
- 2.Freyer G, Geay JF, Touzet S, et al. Comprehensive geriatric assessment predicts tolerance to chemotherapy and survival in elderly patients with advanced ovarian carcinoma: a gineco study. Ann Oncol. 2005;16:1795–800. doi: 10.1093/annonc/mdi368. [DOI] [PubMed] [Google Scholar]
- 3.Extermann M, Chen H, Cantor AB, et al. Predictors of tolerance to chemotherapy in older cancer patients: a prospective pilot study. Eur J Cancer. 2002;38:1466–73. doi: 10.1016/S0959-8049(02)00090-4. [DOI] [PubMed] [Google Scholar]
- 4.Garg P, Rana F, Gupta R, Buzaianu EM, Guthrie TH. Predictors of toxicity and toxicity profile of adjuvant chemotherapy in elderly breast cancer patients. Breast J. 2009;15:404–8. doi: 10.1111/j.1524-4741.2009.00745.x. [DOI] [PubMed] [Google Scholar]
- 5.Clough-Gorr KM, Stuck AE, Thwin SS, Silliman RA. Older breast cancer survivors: geriatric assessment domains are associated with poor tolerance of treatment adverse effects and predict mortality over 7 years of follow-up. J Clin Oncol. 2010;28:380–6. doi: 10.1200/JCO.2009.23.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (crash) score. Cancer. 2012;118:3377–86. doi: 10.1002/cncr.26646. [DOI] [PubMed] [Google Scholar]
- 7.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29:3457–65. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marinello R, Marenco D, Roglia D, et al. Predictors of treatment failures during chemotherapy: a prospective study on 110 older cancer patients. Arch Gerontol Geriatr. 2009;48:222–6. doi: 10.1016/j.archger.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol. 2007;25:1824–31. doi: 10.1200/JCO.2007.10.6559. [DOI] [PubMed] [Google Scholar]
- 10.Rubenstein LZ, Schairer C, Wieland GD, Kane R. Systematic biases in functional status assessment of elderly adults: effects of different data sources. J Gerontol. 1984;39:686–91. doi: 10.1093/geronj/39.6.686. [DOI] [PubMed] [Google Scholar]
- 11.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–8. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 12.Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–22. doi: 10.1046/j.1532-5415.2003.51104.x. [DOI] [PubMed] [Google Scholar]
- 13.Viccaro LJ, Perera S, Studenski SA. Is timed up and go better than gait speed in predicting health, function, and falls in older adults? J Am Geriatr Soc. 2011;59:887–92. doi: 10.1111/j.1532-5415.2011.03336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–61. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rantanen T, Guralnik JM, Foley D, et al. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281:558–60. doi: 10.1001/jama.281.6.558. [DOI] [PubMed] [Google Scholar]
- 16.Legrand D, Vaes B, Mathei C, Adriaensen W, Van Pottelbergh G, Degryse JM. Muscle strength and physical performance as predictors of mortality, hospitalization, and disability in the oldest old. J Am Geriatr Soc. 2014;62:1030–8. doi: 10.1111/jgs.12840. [DOI] [PubMed] [Google Scholar]
- 17.Cesari M, Onder G, Zamboni V, et al. Physical function and self-rated health status as predictors of mortality: results from longitudinal analysis in the ilsirente study. BMC Geriatr. 2008;8:34. doi: 10.1186/1471-2318-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–8. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brach JS, VanSwearingen JM, Newman AB, Kriska AM. Identifying early decline of physical function in community-dwelling older women: performance-based and self-report measures. Phys Ther. 2002;82:320–8. [PubMed] [Google Scholar]
- 20.Bohannon RW. Reference values for the Timed Up and Go test: a descriptive meta-analysis. J Geriatr Phys Ther. 2006;29:64–8. doi: 10.1519/00139143-200608000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Bischoff HA, Stahelin HB, Monsch AU, et al. Identifying a cutoff point for normal mobility: a comparison of the Timed “Up and Go” test in community-dwelling and institutionalised elderly women. Age Ageing. 2003;32:315–20. doi: 10.1093/ageing/32.3.315. [DOI] [PubMed] [Google Scholar]
- 22.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.M85. [DOI] [PubMed] [Google Scholar]
- 23.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–31. doi: 10.1093/gerona/55.4.M221. [DOI] [PubMed] [Google Scholar]
- 24.Roberts HC, Denison HJ, Martin HJ, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40:423–9. doi: 10.1093/ageing/afr051. [DOI] [PubMed] [Google Scholar]
- 25.Bohannon RW, Schaubert KL. Test–retest reliability of grip-strength measures obtained over a 12-week interval from community-dwelling elders. J Hand Ther. 2005;18:426–7. doi: 10.1197/j.jht.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Peolsson A, Hedlund R, Oberg B. Intra- and inter-tester reliability and reference values for hand strength. J Rehabil Med. 2001;33:36–41. doi: 10.1080/165019701300006524. [DOI] [PubMed] [Google Scholar]
- 27.Ling CH, Taekema D, de Craen AJ, Gussekloo J, Westendorp RG, Maier AB. Handgrip strength and mortality in the oldest old population: the Leiden 85-plus study. CMAJ. 2010;182:429–35. doi: 10.1503/cmaj.091278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fried LP, Tangen CM, Walston J, et al. on behalf of the Cardiovascular Health Study Collaborative Research Group Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 29.Yee KWL, Pater JL, Pho L, Zee B, Siu LL. Enrollment of older patients in cancer treatment trials in Canada: why is age a barrier? J Clin Oncol. 2003;21:1618–23. doi: 10.1200/JCO.2003.12.044. [DOI] [PubMed] [Google Scholar]
- 30.Hurria A, Wong FL, Villaluna D, et al. Role of age and health in treatment recommendations for older adults with breast cancer: the perspective of oncologists and primary care providers. J Clin Oncol. 2008;26:5386–92. doi: 10.1200/JCO.2008.17.6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kornblith AB, Kemeny M, Peterson BL, et al. on behalf of the Cancer and Leukemia Group B Survey of oncologists’ perceptions of barriers to accrual of older patients with breast carcinoma to clinical trials. Cancer. 2002;95:989–96. doi: 10.1002/cncr.10792. [DOI] [PubMed] [Google Scholar]
- 32.Puts MT, Monette J, Girre V, et al. Are frailty markers useful for predicting treatment toxicity and mortality in older newly diagnosed cancer patients? Results from a prospective pilot study. Crit Rev Oncol Hematol. 2011;78:138–49. doi: 10.1016/j.critrevonc.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Bellera CA, Rainfray M, Mathoulin-Pelissier S, et al. Screening older cancer patients: first evaluation of the G-8 geriatric screening tool. Ann Oncol. 2012;23:2166–72. doi: 10.1093/annonc/mdr587. [DOI] [PubMed] [Google Scholar]
- 34.Soubeyran P, Fonck M, Blanc-Bisson C, et al. Predictors of early death risk in older patients treated with first-line chemotherapy for cancer. J Clin Oncol. 2012;30:1829–34. doi: 10.1200/JCO.2011.35.7442. [DOI] [PubMed] [Google Scholar]
- 35.Huisman MG, van Leeuwen BL, Ugolini G, et al. “Timed Up & Go”: a screening tool for predicting 30-day morbidity in onco-geriatric surgical patients? A multicenter cohort study. PLoS One. 2014;9:e86863. doi: 10.1371/journal.pone.0086863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liuu E, Canoui-Poitrine F, Tournigand C, et al. on behalf of the elcapa Study Group Accuracy of the G-8 geriatric-oncology screening tool for identifying vulnerable elderly patients with cancer according to tumour site: the elcapa-02 study. J Geriatr Oncol. 2014;5:11–19. doi: 10.1016/j.jgo.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Kim JW, Kim YJ, Lee KW, et al. The early discontinuation of palliative chemotherapy in older patients with cancer. Support Care Cancer. 2014;22:773–81. doi: 10.1007/s00520-013-2033-y. [DOI] [PubMed] [Google Scholar]
- 38.Hoppe S, Rainfray M, Fonck M, et al. Functional decline in older patients with cancer receiving first-line chemotherapy. J Clin Oncol. 2013;31:3877–82. doi: 10.1200/JCO.2012.47.7430. [DOI] [PubMed] [Google Scholar]
- 39.Puts MT, Monette J, Girre V, et al. Does frailty predict hospitalization, emergency department visits, and visits to the general practitioner in older newly-diagnosed cancer patients? Results of a prospective pilot study. Crit Rev Oncol Hematol. 2010;76:142–51. doi: 10.1016/j.critrevonc.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 40.Tan BH, Brammer K, Randhawa N, et al. Sarcopenia is associated with toxicity in patients undergoing neo-adjuvant chemotherapy for oesophago-gastric cancer. Eur J Surg Oncol. 2015;41:333–8. doi: 10.1016/j.ejso.2014.11.040. [DOI] [PubMed] [Google Scholar]
- 41.Barret M, Antoun S, Dalban C, et al. Sarcopenia is linked to treatment toxicity in patients with metastatic colorectal cancer. Nutr Cancer. 2014;66:583–9. doi: 10.1080/01635581.2014.894103. [DOI] [PubMed] [Google Scholar]
- 42.Antoun S, Borget I, Lanoy E. Impact of sarcopenia on the prognosis and treatment toxicities in patients diagnosed with cancer. Curr Opin Support Palliat Care. 2013;7:383–9. doi: 10.1097/SPC.0000000000000011. [DOI] [PubMed] [Google Scholar]
- 43.Kasymjanova G, Correa JA, Kreisman H, et al. Prognostic value of the six-minute walk in advanced non-small cell lung cancer. J Thorac Oncol. 2009;4:602–7. doi: 10.1097/JTO.0b013e31819e77e8. [DOI] [PubMed] [Google Scholar]