Abstract
Introduction
Stage iii lung cancer is the most advanced stage of lung cancer for which the potential of curative treatment is often discussed. However, a large proportion of patients are treated with palliative intent. An understanding of the time-dependent and -independent factors contributing to the choice of palliative-intent treatment is needed to help optimize patient outcomes.
Methods
This retrospective cohort study of patients with stage iii non-small-cell lung cancer (nsclc) newly diagnosed between 1 January 2008 and 31 December 2012 at the Cancer Centre of Southeastern Ontario collected data including patient demographics, clinical characteristics, tumour characteristics, treatment, and outcomes.
Results
Of 237 patients with stage iii nsclc included in the study, 130 were not treated with radical or curative intent (55%). Major time-independent variables cited for palliative-intent treatment included extreme age (5%), comorbidity (27%), patient choice (5%), and poor lung function (5%). Time-dependent variables included tumour progression on imaging (15%), weight loss (33%), performance status (32%), and the occurrence of a major complication such as hemoptysis, lung collapse, or pulmonary embolism (7%). A significant number of patients (20%) experienced a decline in performance status—to 2, 3, or 4 from 0 or 1—over the course of the diagnostic journey, and 12% experienced a transition from no weight loss to more than 10% weight loss.
Conclusions
A significant proportion of patients receive palliative therapy for stage iii nsclc because of changes that occur during the diagnostic journey. Shortening or altering that pathway to avoid tumour growth or patient deterioration during care could allow for more patients to be treated with curative intent.
Keywords: Chemotherapy, patient pathways, lung cancer
BACKGROUND
Stage iii represents the most advanced stage of non-small-cell lung cancer (nsclc) for which the possibility of cure is often discussed, and approximately 20%–25% of newly diagnosed nsclc is stage iii1,2. Standard treatment options vary considerably throughout the world and include bi- and trimodality therapy, consisting of either surgery followed by chemotherapy, chemotherapy concurrent with radiation therapy alone, neoadjuvant concurrent chemotherapy and radiation therapy, or neoadjuvant chemotherapy alone followed by surgery3.
Although bimodality therapy of some form is recognized as standard treatment for stage iii nsclc in all guidelines, the guidelines are based on clinical trials that have included patients with a good performance status and minor weight loss—a population that is generally younger and has fewer comorbidities than the general population of lung cancer patients. In interpreting the trials, guidelines often limit their recommendations for multimodality therapy to this set of “good risk” patients and recommend palliative treatment only for patients with stage iii nsclc with excessive volume of disease, excessive weight loss, or poor performance status4.
Patients with stage iii nsclc and significant weight loss or borderline performance status—Eastern Cooperative Oncology Group (ecog) 2 or higher—are at increased risk of toxicity and at higher risk of early death and progression5. Weight loss suggests an increased risk of systemic failure and local progression, and therefore aggressive local therapy might not be indicated. Concerns about both toxicity and effectiveness in these patients mean that palliative therapies are often recommended. To improve survival for all patients with stage iii nsclc, the criteria for choosing palliative-intent treatments over radical-intent (potentially curative) treatments have to be evaluated, and opportunities to increase the number of patients treated with radical intent have to be realized.
Criteria for palliative treatment include those that are time-dependent and -independent. Time-independent criteria are those patient or tumour characteristics that are expected to vary minimally over the patient’s disease course and diagnostic workup period. Time-dependent criteria are those that could change in status over a short period and that likely depend on the biology of the tumour and host, and the elapsed time. For instance, age is a relatively time-independent variable—unless the workup takes years. In contrast, weight loss is a time-dependent variable: with aggressive tumours, a patient’s weight can deteriorate over a relatively short time.
Time-independent criteria that can affect the choice between palliative and radical or curative therapies include patient age, patient values or choice, medical comorbidities, and lung function. Time-dependent criteria can include weight loss, performance status, and tumour size or volume of the radiation field. Sudden secondary complications of stage iii nsclc—such as pulmonary embolism, pulmonary obstruction, and pneumonia—can also be time-dependent.
For time-dependent criteria, changes in timelines can significantly affect treatment options and outcomes. Time from the development of symptoms to chest radiography or computed tomography (ct) imaging; time from chest radiography to ct imaging; time from ct imaging to pathology confirmation; and time from pathology confirmation to decision and initiation of treatment all contribute to the total pre-treatment time. As those timelines increase, the chances for tumour growth, progressive weight loss, deterioration of performance status, and an interval complication such as an embolus, pneumonia, collapsed lung, or disease progression can increase as well.
We undertook a review of patients diagnosed with stage iii nsclc and registered at an academic cancer centre in southeastern Ontario with the goal of answering these questions:
■ Of patients with stage iii nsclc, what proportion are treated with palliative intent?
■ What are the documented criteria for administration of palliative treatments?
■ In patients with criteria that depend on both tumour biology and elapsed time, what is the incidence of time-dependent changes during the diagnostic or referral process?
METHODS
Cohort Definition
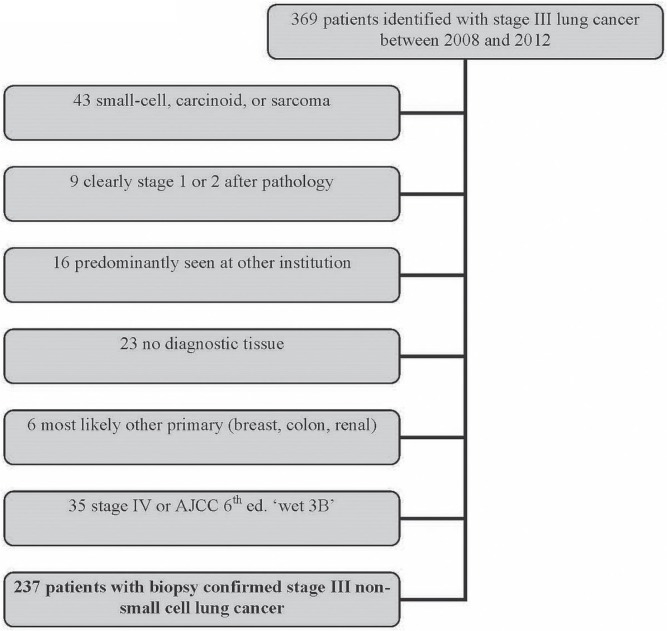
Patients with stage iii lung cancer diagnosed between 1 January 2008 and 31 December 2012 were identified through the cancer centre database (Figure 1). Patients were excluded if they were treated predominantly elsewhere (that is, clinical data were not available), if their histology was other than nsclc, if they were not stage iii according to the 7th edition of the American Joint Committee on Cancer staging system, if they lacked biopsy confirmation of nsclc, or if they were not followed more than once.
FIGURE 1.
Flowchart of patient identification for the study.
Clinical and Treatment Characteristics
Diagnosis date, age, and sex were extracted from the clinical database. Histology, T stage, N stage, and radiation and chemotherapy treatments received were extracted from the cancer centre database and crosschecked with the clinical notes and medication administration record. Date of death and last date known to be alive were extracted from the clinical database and supplemented by online obituary records. Survival was calculated from date of pathology confirmation to death or censoring.
Dates of appointments, imaging tests, pathology tests, and treatments were extracted from the medical record directly. If the exact date was unclear, but the month was known, then the date was attributed to the 15th day of that month. For patients who underwent multiple chest ct examinations before further investigations were done (such as follow-up of a questionable finding), the date of the confirmatory ct examination before the pathology diagnosis was used.
Palliative radiation treatments included intended radiation treatments in the “low-dose” range, such as 20 Gy or less in 1 to 5 fractions, or the “high-dose palliation” range, such as 30 Gy or more in 10 or more fractions. Radical or potentially curative therapies included all patients who received intended radiation doses of 60 Gy or more, or planned surgical resection, or a planned radiation dose of more than 45 Gy given with neoadjuvant intent.
Data about weight loss and performance status were extracted from the consultation notes of any or all of the radiation oncologist, medical oncologist, respirologist, or thoracic surgeon. Weight loss data were analyzed as categories: less than 5%, 5%–10%, more than 10%, or unknown. If the absolute weight loss value was recorded, the percentage was calculated directly.
Performance status was recorded as ecog performance status. If the patient’s performance status was given as a range (for example, ecog 2–3), the higher performance status (that is, 3) was used. If two oncologists assessed the patient within 1 week, the first score was used.
Smoking status was reviewed from the medical record and was classified as never or light (<2 pack–years), former (>2 pack–years, but quit >6 months earlier), or current or recent.
Method of diagnosis confirmation was classified as bronchoscopy, radiology-guided biopsy, or other (expectorated tissue, or surgical or transesophageal biopsy). Method of lymph node staging was classified as imaging only [positron-emission tomography (pet) or combined ct/pet] or tissue (endoluminal ultrasound biopsy, mediastinoscopy, or thoracotomy).
The reason or reasons for palliation were extracted from any combination of clinical notes (radiation oncologist and medical oncologist notes) and the tumour board note. Reasons included patient choice, nonpulmonary medical comorbidity, lung-function or pulmonary comorbidity, tumour size (burden of intrathoracic disease) or location, weight loss, complications (obstruction, pneumonia, collapse), chronologic age, and performance status. Patients could have been ineligible for radical treatment for multiple reasons.
A change in the status of a time-dependent variable during medical workup as the predominant reason for palliation was ascribed if these conditions were met:
■ The reason for palliation was explicitly stated (radical radiation no longer feasible because of a change in scan or planning scan), or
■ weight loss or performance status were cited as the reason, and a weight or performance status that would not have excluded the patient from radical therapy had been documented by the same or a different physician at an earlier visit.
Statistics are presented as proportions. Kaplan–Meier estimates were used to calculate median survival.
RESULTS
Of 369 patients with stage iii lung cancer extracted from the clinical database, 43 with other diagnoses (carcinoid, small-cell carcinoma, or hemangiosarcoma) were excluded. Another 9 patients who were clearly at an earlier stage were also excluded. For 16 patients predominantly seen elsewhere, only minimal clinical information was available. No tissue confirmation could be found for 23 patients, and 6 patients were subsequently shown to most likely have another primary. After completion of staging investigations, 35 patients were upstaged to stage iv according to the 7th edition of the American Joint Committee on Cancer staging system. Of the remaining 237 patients with biopsy-confirmed clinical or pathologic stage iii nsclc, 130 (55%) received palliative treatments, and 107 (45%) received curative-intent therapy.
Patient Characteristics
Table i presents patient and treatment characteristics for the entire cohort. As can be seen, the number of nonsmokers is disproportionately low. That observation could be the result of either a very high penetration of smoking (such that the “former smoker” population is quite high) or a different biology of lung cancer (if nonsmokers are more likely to have a different stage distribution, for instance). The high number of squamous cell cancers (43%) and central tumours staged at stage iii might be partly explained by the low nonsmoker rates.
TABLE I.
Characteristics of patients with stage III lung cancer, 2009–2013
| Characteristic | Treatment group | p Value | ||
|---|---|---|---|---|
|
| ||||
| Overall | Palliative | Radical | ||
| Patients (n) | 237 | 130 | 107 | |
| Patient-related | ||||
| Median age (years) | 68 | 72 | 64 | 0.001 |
| Sex [n (%)] | 0.06 | |||
| Women | 119 (50) | 70 (54) | 49 (46) | |
| Men | 118 (50) | 60 (46) | 58 (54) | |
| Smoking status (n) | 1.0 | |||
| Never | 7 | 4 | 3 | |
| Former | 111 | 61 | 50 | |
| Current | 116 | 62 | 54 | |
| Unknown | 3 | 3 | 0 | |
| Performance status (n) | 0.006 | |||
| 0–1 | 157 | 65 | 92 | |
| 2 | 42 | 33 | 9 | |
| 3–4 | 27 | 27 | 0 | |
| Unknown | 11 | 5 | 6 | |
| Weight loss (n) | 0.18 | |||
| <5% | 132 | 52 | 80 | |
| 5–10% | 35 | 24 | 11 | |
| >10% | 59 | 45 | 14 | |
| Unknown | 11 | 9 | 2 | |
| Tumour-related | ||||
| Histology (n) | 0.001 | |||
| Squamous | 103 | 72 | 31 | |
| Adenocarcinoma | 60 | 25 | 35 | |
| NOS | 74 | 33 | 41 | |
| T Stage (n) | 0.001 | |||
| Tx | 11 | 10 | 1 | |
| T1–2 | 66 | 25 | 41 | |
| T3 | 54 | 25 | 29 | |
| T4 | 106 | 70 | 36 | |
| N Stage (n) | 0.12 | |||
| Nx | 18 | 15 | 3 | |
| N0 | 26 | 13 | 13 | |
| N1 | 17 | 10 | 7 | |
| N2 | 149 | 75 | 74 | |
| N3 | 27 | 17 | 10 | |
NOS = no otherwise specified.
Compared with the curative-intent group, the group that received palliative therapy had a significantly higher median age and significantly greater proportions of individuals with poor performance status or weight loss. The two groups showed no significant differences in histologic type. Because of the high degree of collinearity between factors such as weight loss and performance status, a multivariate analysis was not performed.
Reasons for Palliative Treatment
Table ii shows the reasons cited for palliative treatment. A significant proportion of the reasons for choosing palliative treatment included time-independent variables such as nonpulmonary comorbidity, poor lung function, or patient choice, but a large number of the cited reasons were time-dependent: weight loss, poor performance status, and a large volume or distribution of disease. In the entire cohort of stage iii patients, a “change in scan” was documented as the reason or a major reason for palliative therapy in 20 (approximately 8%).
TABLE II.
Reasons cited for a palliative approach in patients with stage III non-small-cell lung cancer
| Reason | Patient group (%) | |
|---|---|---|
|
| ||
| Overall | Palliative | |
| Time-independent | ||
| Extreme age | 3 | 5.0 |
| Comorbidities | 15 | 27 |
| Patient choice | 3 | 6 |
| Pulmonary function tests | 3 | 6 |
| Time dependent | ||
| Hemoptysis | 1 | 2 |
| Recurrent pneumonia | 3 | 5 |
| Size or location of tumour, volume of radiation | 21 | 40 |
| Weight loss | 18 | 33 |
| Performance status | 17 | 32 |
| “Significant change” on imaging | 8 | 15 |
Time-Dependent Changes During the Diagnostic Journey
Table iii identifies patients whose weight loss and performance status were documented at the time of a respiratory medicine or thoracic surgery consultation and again at the oncology consultation. In that subset of patients, 18 of the 92 (20%) whose weight loss was documented as less than 5% at the time of the initial respiratory medicine diagnosis were subsequently found to have a weight loss of more than 5% at the time of oncology consultation. In 11 patients (12%), the subsequently recorded weight loss was more than 10%.
TABLE III.
Performance status and weight loss at the time of oncology consultation for patients with performance status and weight loss documented at respiratory medicine and oncology consultations
| Status documented at respiratory medicine consultation | Pts (n) | Performance status (n pts) documented at oncology consultation | Status documented at respiratory medicine consultation | Pts (n) | Weight loss (n pts) documented at oncology consultation | ||||
|---|---|---|---|---|---|---|---|---|---|
| 0–1 | 2 | 3 or 4 | <5% | 5%–10% | >10% | ||||
| Performance status | Weight loss | ||||||||
| 0–1 | 123 | 99 | 15 | 9 | <5% | 92 | 74 | 7 | 11 |
| 2 | 15 | 2 | 10 | 3 | 5%–10% | 27 | 5 | 10 | 12 |
| 3–4 | 4 | 0 | 1 | 3 | >10% | 24 | 2 | 0 | 22 |
For patients with a significant weight change, the median time from ct imaging to oncology consultation was 72.5 days; it was 65 days for patients without a significant weight change. That difference was nonsignificant by Kruskal–Wallis test. For the same patients, the median times from respirology medicine consultation to oncology consultation were 54 and 45 days respectively. The shortest interval during which a patient progressed from no weight loss to more than 5% weight loss was 34 days.
A similar trend was noticed for performance status. Of patients with an ecog performance status documented at the time of respiratory medicine consultation, 123 had a performance status of 0 or 1, and 19 had a performance status of 2 or greater. At the time of oncology consultation, 24 patients whose initial performance status was 0 or 1 now had a performance status of 2 or greater. For this subset of patients with documentation of performance status, the median times from respirology medicine consultation to first oncology consultation were 65 days for those with a change in performance status and 66 days for those without such a change. Interestingly, of 26 patients whose time from ct imaging to radiation oncology appointment exceeded 100 days, only 3 experienced a significant change in documented performance status. Of 22 patients for whom the time from respirology medicine consultation to oncology consultation exceeded 3 months, only 2 experienced a deterioration in performance status.
Survival
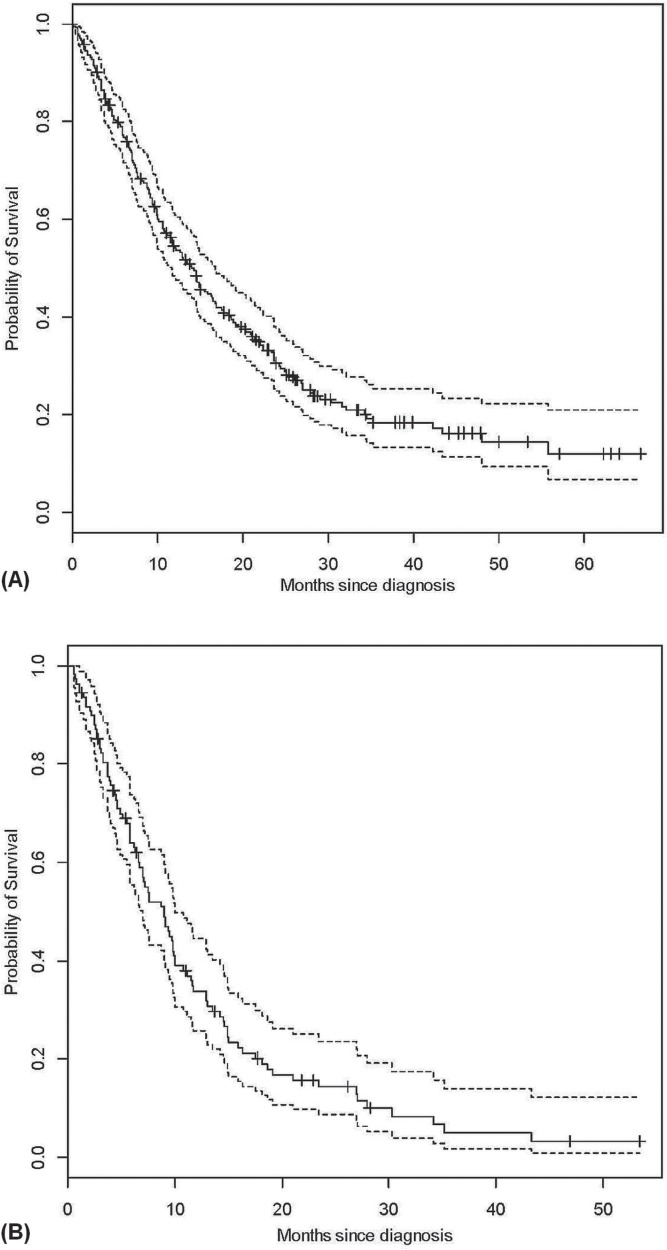
Figure 2 shows survival for our entire cohort of patients diagnosed with stage iii nsclc. Median survival was 14 months overall; in patients treated with palliative intent, it was 9 months. The median survival of patients treated with radical intent (not shown) was 22.5 months.
FIGURE 2.
Overall survival for (A) all patients and (B) palliatively- treated patients.
DISCUSSION
Key Findings
Median survival for our patients undergoing curative treatment with radical radiation or surgery is within the range of other contemporary series describing treatment with combined chemoradiation. However, more than half the patients with stage iii nsclc did not begin radical-intent therapy; they were treated with palliative intent. Reasons for palliative treatment varied, but a substantial proportion of patients treated with palliative intent were so treated because of changes that occurred during the diagnostic journey.
Improvement in outcomes for patients with stage iii nsclc has often focused on clinical trials for patients with good performance status, minimal weight loss, good pulmonary function, and minimal comorbidity. With respect to patients who do not fit those criteria, guideline recommendations vary from combined-modality therapy for patients with a performance status of 2 or significant weight loss (American College of Chest Physicians, level 2C) to combined-modality therapy for those with minimal weight loss and a good performance status only3,4.
To increase the number of patients being appropriately treated with curative-intent therapy, two major options are available. The first is to re-examine eligibility criteria and to include borderline patients among those treated with radical therapy. However, whether patients with a performance status of 2, weight loss of more than 5%–10%, poor pulmonary function, or multiple comorbidities benefit from radical concurrent chemotherapy and radiation has not been clearly established, as evidenced by the level 2C recommendation from the American College of Chest Physicians. The second method is to expedite evaluation and diagnostic and therapeutic decision-making to reduce the number of patients who lose eligibility because of symptomatic or radiologic progression. The present single-institution review suggests that the proportion of patients who deteriorate during that period is not insignificant.
Whether rapid evaluation and start of treatment in these patients will improve outcomes in stage iii lung cancer is not clear. It is possible that patients who “select out” of radical treatment are also destined to do poorly and not to benefit from radical therapy—that is, those who deteriorate during the workup phase might have inherently bad biology. Although that hypothesis is possibly true, such reasoning should be questioned to ensure that it is not simply a rationalization for wait times.
A recently reported review of 122 stage iii patients from Hamilton, Ontario, had a similar 50%:50% ratio of radical and palliative therapy6. In that study, weight loss, performance status, and a combination of the two were the predominant reasons for choosing palliative therapy. A small proportion of the patients were excluded from radical therapy because of comorbid conditions. Work published by the same investigators in a separate paper revealed a long period from symptoms to treatment for all patients7, which might partly explain the similar proportion of patients who were ineligible for radical treatments.
Other work has focused on changes in imaging, including pet imaging, for this group of patients. A recent paper from Geiger et al.8 in Pennsylvania showed that 30% of their patients who underwent repeat pet/ct imaging had developed pet evidence of stage iv disease. In 2000, O’Rourke and Edwards9 showed that, in a small set of patients, more than 20% showed stage evolution from their diagnostic to their radiotherapy planning scans. Other work has consistently found that, on repeat scans, more than 30% of patients progress to the point of incurability.
The fact that most of our patients who waited more than 100 days did not experience progressive weight loss or that only a small proportion of patients with more than 100 days between imaging and oncology consultation deteriorated in terms of performance status is likely a reflection of depletion bias. Patients with tumour–host biology that results in significant weight loss are unlikely to still be stage iii at 100 days. Similarly, previous work that seems to show no effect of time to treatment on outcome in stage iii lung cancer10 might be subject to the same bias. Indeed, studies that report outcomes based on time to treatment, but that adjust for performance status, weight loss, tumour size, and palliative or radical intent, will very likely show that time is not an independent factor. Given that the factors being adjusted for—such as performance status, weight loss, tumour size, and intent—are themselves partly time-dependent, those studies are subject to confounding.
One implication of the present work could be that cross-institutional comparisons of radically treated patients will have to account for differences in health service factors that result in variable proportions of patients being treated with radical therapy. Compared with a centre having slower evaluation times, a centre having rapid evaluation and treatment of stage iii nsclc might be biased toward worse outcomes for radically treated stage iii patients simply because the centre whose process encompasses more delays will “select out” the patients with aggressive-biology disease. If comparisons of outcomes in radically treated stage iii nsclc are to be made between centres, a “long workup bias” probably has to be acknowledged.
Conversely, if radically treated patients in institutions that have higher proportions of patients treated radically were to report outcomes similar to those in health systems with lower proportions of radically treated patients, it could be argued that the percentage of patients treated with radical intent is a marker for quality care—perhaps not the quality of care once patients cross the barrier to an oncology consultation, but the quality of the system and patient factors that lead up to treatment and the number of patients who deteriorate before decisions about the appropriateness of radical therapy are made.
SUMMARY
Improving outcomes in the treatment of stage iii nsclc will involve optimization of medical systems and public education, such that the number of patients who fulfil eligibility criteria for radical treatment is optimized, and the number of patients who experience progressive weight loss, decline in performance status, stage evolution, and serious intercurrent events during the diagnostic and evaluation process is minimized.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Groome PA, Bolejack V, Crowley JJ, et al. on behalf of the iaslc International Staging Committee; Cancer Research and Biostatistics; observers to the committee; and participating institutions The iaslc Lung Cancer Staging Project: validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:694–705. doi: 10.1097/JTO.0b013e31812d05d5. [DOI] [PubMed] [Google Scholar]
- 2.Aberle DR, DeMello S, Berg CD, et al. on behalf of the National Lung Screening Trial Research Team Results of the two incidence screenings in the National Lung Screening Trial. N Engl J Med. 2013;369:920–31. doi: 10.1056/NEJMoa1208962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vansteenkiste J, De Ruysscher D, Eberhardt WE, et al. on behalf of the esmo Guidelines Working Group Early and locally advanced non-small-cell lung cancer (nsclc): esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi89–98. doi: 10.1093/annonc/mdt241. [DOI] [PubMed] [Google Scholar]
- 4.Ramnath N, Dilling TJ, Harris LJ, et al. Treatment of stage iii non–small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(suppl):e314S–40S. doi: 10.1378/chest.12-2360. [DOI] [PubMed] [Google Scholar]
- 5.Werner-Wasik M, Scott C, Cox JD, et al. Recursive partitioning analysis of 1999 Radiation Therapy Oncology Group (rtog) patients with locally-advanced non-small-cell lung cancer (la-nsclc): identification of five groups with different survival. Int J Radiat Oncol Biol Phys. 2000;48:1475–82. doi: 10.1016/S0360-3016(00)00801-4. [DOI] [PubMed] [Google Scholar]
- 6.Al-Shamsi HO, Al Farsi A, Ellis PM. Stage iii non-small-cell lung cancer: establishing a benchmark for the proportion of patients suitable for radical treatment. Clin Lung Cancer. 2014;15:274–80. doi: 10.1016/j.cllc.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Ellis PM, Vandermeer R. Delays in the diagnosis of lung cancer. J Thorac Dis. 2011;3:183–8. doi: 10.3978/j.issn.2072-1439.2011.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geiger GA, Kim MB, Xanthopoulos EP, et al. Stage migration in planning pet/ct scans in patients due to receive radio-therapy for non-small-cell lung cancer. Clin Lung Cancer. 2014;15:79–85. doi: 10.1016/j.cllc.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 9.O’Rourke N, Edwards R. Lung cancer treatment waiting times and tumour growth. Clin Oncol (R Coll Radiol) 2000;12:141–4. doi: 10.1053/clon.2000.9139. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Correa CR, Hayman JA, et al. Time to treatment in patients with stage iii non–small cell lung cancer. Int J Radiat Oncol Biol Phys. 2009;74:790–5. doi: 10.1016/j.ijrobp.2008.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]