Abstract
Background
Treatment of malignant pericardial effusion remains controversial, because no randomized controlled trials have been conducted to determine the best approach, and results of retrospective studies have been inconsistent. The objective of the present study was to compare pericardiocentesis and pericardiotomy with respect to efficacy for preventing recurrence, and to determine, for those two procedures, diagnostic yields, complication rates, and effects on survival. We also aimed to identify clinical and procedural factors that could predict effusion recurrence.
Methods
We retrospectively assessed 61 patients who underwent a procedure for treatment of a malignant pericardial effusion at the Institut universitaire de cardiologie et de pneumologie de Québec between February 2004 and September 2013.
Results
Pericardiocentesis was performed in 42 patients, and pericardiotomy, in 19 patients. The effusion recurrence rate was significantly higher in patients treated with pericardiocentesis than with pericardiotomy (31.0% vs. 5.3%, p = 0.046). The diagnostic yield of the procedures was not significantly different (92.9% vs. 86.7%, p = 0.6). The overall rate of complications was similar in the two groups, as was the median overall survival (2.4 months vs. 2.6 months, p = 0.5). In univariate analyses, the procedure type was the only predictor of recurrence that approached statistical significance. Age, sex, type of cancer, presence of effusion at the time of cancer diagnosis, prior chest irradiation, tamponade upon presentation, and total volume of fluid removed did not influence the recurrence rate.
Conclusions
Compared with pericardiocentesis, pericardiotomy had higher success rate in preventing recurrence of malignant pericardial effusion, with similar diagnostic yields, complication rates, and overall survival.
Keywords: Malignant pericardial effusion, pericardiocentesis, pericardiotomy, recurrence, complications, diagnostic yield, survival
INTRODUCTION
Malignant pericardial effusion is a common problem in oncology, and the primary tumour that most frequently involves the pericardium is lung cancer1. Patients are often asymptomatic, but they can also present with cardiorespiratory symptoms (for example, dyspnea, cough, chest pain), clinical signs (tachycardia, for instance), echocardiographic features of right heart compromise, and possibly life-threatening cardiac tamponade requiring emergency drainage2. Malignancies can involve the pericardium by four mechanisms: direct extension or metastatic spread via lymphatics or blood, chemotherapeutic toxicity, radiation toxicity, and opportunistic infections related to immunosuppressive therapies3. Despite treatment, median overall survival in patients with malignant pericardial effusion is reported to be in the range of 2–4 months and is influenced mainly by the nature of the underlying malignancy, lung cancer being a poor prognostic factor4.
For patients with advanced cancer involving the pericardium, the goals of treatment should be to use a minimally invasive procedure with a good safety profile to achieve symptom relief, improvement in quality of life, and prevention of recurrence. Several approaches can be used to treat malignant pericardial effusion, but no randomized controlled trials have been conducted to compare their risk–benefit ratio; no “gold standard” has been established; and practices differ widely. Many authors believe that treatment must be individualized according to the patient’s condition and tumour type, and to the success rates and risks of the various options, local availability, and expertise5.
One consensus is that cardiac tamponade is a clear indication for urgent pericardiocentesis. For effusions without tamponade, guidelines from the European Society of Cardiology state that pericardiocentesis can relieve symptoms and establish diagnosis, and that systemic antineoplastic treatment and intrapericardial instillation of a cytostatic or sclerosing agent can prevent recurrence6. Subxiphoid pericardiotomy is indicated when pericardiocentesis cannot be performed. Extended catheter drainage after pericardiocentesis, percutaneous balloon pericardiotomy, and various other surgical approaches are also possible options to prevent recurrence. They have been compared, with inconsistent results, in many retrospective studies7–11. Recently, a retrospective analysis in 88 patients showed that, compared with surgical pericardiotomy, pericardiocentesis with extended drainage had the same diagnostic yield, the same recurrence rate, and fewer complications12; and a systematic review including 1399 patients concluded that surgical drainage is superior to nonsurgical approaches in terms of symptom relief, effusion recurrence, and morbidity13.
The objective of our study was to compare pericardiocentesis with pericardiotomy in respect of their efficacy for preventing recurrence in patients with malignant pericardial effusion, and to determine the diagnostic yields, complication rates, and effect on survival of the two procedures. We also aimed to identify clinical and procedural factors that could predict effusion recurrence.
METHODS
Patients
We retrospectively reviewed the medical records of all patients who underwent a procedure for malignant pericardial effusion at the Institut universitaire de cardiologie et de pneumologie de Québec between February 2004 and September 2013. The study was carried out with the approval of the institute’s ethics committee review board (CÉR 21060). The study population was divided into two groups, and patients treated with pericardiocentesis under local anesthesia and ultrasound guidance were compared with patients who underwent surgical or percutaneous balloon pericardiotomy. Because this study was retrospective, the choice of the therapeutic intervention was at the treating physician’s discretion.
Data Extraction
The medical records of the patients were searched for patient age and sex, type of cancer, presence of pericardial effusion at the time of cancer diagnosis, prior chest irradiation, tamponade upon presentation, and type of procedure (including volume of pericardial fluid removed and cytology results). Clinical outcomes included length of stay in hospital, need for intensive care, complications of interventions, recurrence of effusion, and mortality.
Statistical Analysis
Descriptive statistics (proportions, means, standard deviations, and ranges) are used to describe the study population. Clinical characteristics of the patients treated with pericardiocentesis and those treated with pericardiotomy were compared using chi-square tests for dichotomous variables and unpaired t-tests for continuous variables. We used univariate analyses to test the influence of each baseline clinical characteristic and treatment (that is, pericardiocentesis or pericardiotomy) on risk of recurrence. Fine–Gray Cox models were constructed to account for death as a competing risk event for recurrence14. For both groups, Kaplan–Meier estimates were used to construct survival curves, and the log-rank test was used for between-group comparisons. In all analyses, statistical significance (p value) was set at the 0.05 level.
RESULTS
Patients
Of the 61 patients included in the study, 42 (69%) were treated with pericardiocentesis, and 19 (31%) underwent pericardiotomy (7 by the subxiphoid approach, 5 by percutaneous balloon procedure, 4 by left mini-thoracotomy, 1 by left video-assisted thoracic surgery, 1 by laparoscopy, and 1 by sternotomy). Most of the patients (n = 52, 85%) had lung cancer (46 adenocarcinomas, 2 squamous cell cancers, 2 small-cell lung cancers, 2 non-small-cell lung cancers not otherwise specified). Other diagnoses included breast cancer in 5 patients, and single cases of mesothelioma, uterine cancer, bladder cancer, and adenocarcinoma of unknown origin.
Table i shows the baseline characteristics of the patients. The only significant difference was that the pericardiocentesis group contained a higher proportion of patients with pulmonary neoplasms. Almost all the patients treated with pericardiocentesis underwent prolonged drainage, with the pericardial catheter left in place for at least 24 hours and up to 7 days, until drainage stopped. Only 1 patient underwent local sclerotherapy with bleomycin.
TABLE I.
Baseline characteristics of the study patients
| Characteristic | Patient group | p Value | ||
|---|---|---|---|---|
|
| ||||
| Overall | Pericardiocentesis | Pericardiotomy | ||
| Patients (n) | 61 | 42 | 19 | — |
| Age (years) | ||||
| Mean | 61±11 | 62±11 | 59±11 | 0.3 |
| Range | 38–83 | 41–83 | 38–80 | |
| Sex [n (%) men] | 28 (45.9) | 17 (40.5) | 11 (57.9) | 0.2 |
| Lung cancer [n (%)] | 52 (85.3) | 40 (95.2) | 12 (63.2) | 0.003 |
| Effusion at the time of cancer diagnosis [n (%)] | 21 (34.4) | 15 (35.7) | 6 (31.6) | 0.8 |
| Prior chest radiation [n (%)] | 9 (14.8) | 5 (11.9) | 4 (21.1) | 0.4 |
| Tamponade upon presentation [n (%)] | 41 (67.2) | 29 (69.1) | 12 (63.2) | 0.7 |
| Total volume of fluid removed (mL) | ||||
| Mean | 584±299 | 611±315 | 498±235 | 0.2 |
| Range | 70–1650 | 70–1650 | 100–930 | |
| Patients tested (n) | 53 | 40 | 13 | |
Clinical Outcomes
The overall rate of pericardial effusion recurrence was 23%, and the rate was significantly different in the two groups (31.0% pericardiocentesis vs. 5.3% pericardiotomy, p = 0.046; Table ii). In the univariate analyses, procedure type was the only predictor of recurrence that approached the level of statistical significance (hazard ratio: 0.154; p = 0.08). Age, sex, type of cancer, presence of effusion at the time of cancer diagnosis, prior chest irradiation, tamponade on presentation, and total volume of fluid removed had no influence on the recurrence rate. The mean interval between the procedure and effusion recurrence was 90 days. Of the 13 patients with recurrence after pericardiocentesis, 9 were subsequently treated with pericardiotomy, 3 underwent repeat pericardiocentesis, and 1 received no further treatment. The 1 patient with recurrence after pericardiotomy was a 57-year-old woman with breast adenocarcinoma, initially treated with a pericardiotomy by left video-assisted thoracic surgery. She experienced a recurrent pericardial effusion 2 days later and underwent another pericardiotomy by the subxiphoid approach.
TABLE II.
Outcomes in the study patients
| Outcome | Patient group | p Value | ||
|---|---|---|---|---|
|
| ||||
| Overall (n=61) | Pericardiocentesis (n=42) | Pericardiotomy (n=19) | ||
| Positive cytology [n (%)] | 52 (85.3) | 39 (92.9) | 13/15a (86.7) | 0.6 |
| Length of stay (days) | ||||
| Mean | 11.4±8.6 | 11.2±8.9 | 11.6±8.1 | 0.9 |
| Range | (2–45) | (3–45) | (2–34) | |
| ICU admission [n (%)] | 23 (37.7) | 13 (31.0) | 10 (52.6) | 0.11 |
| Recurrence of effusion [n (%)] | 14 (23.0) | 13 (31.0) | 1 (5.3) | 0.046 |
Cytology was not performed in 4 patients.
ICU = intensive care unit.
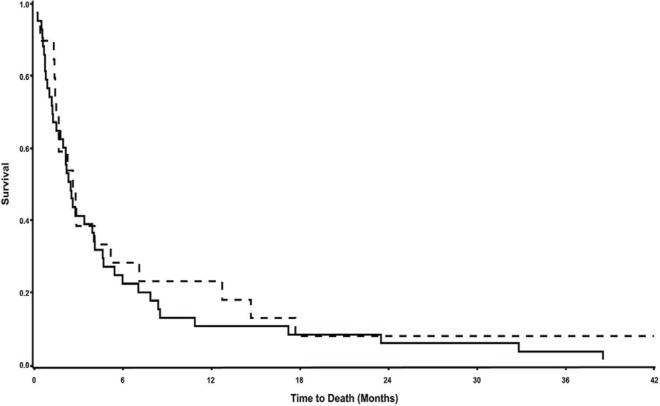
Median survival in the entire cohort was 2.5 months: 2.4 months in the pericardiocentesis group and 2.6 months in the pericardiotomy group, with no statistical difference (p = 0.5), as shown in Figure 1.
FIGURE 1.
Survival curves for the study population. Median survival was 2.5 months for the entire cohort, 2.4 months for the pericardiocentesis group (solid line), and 2.6 months for the pericardiotomy group (dashed line), p = 0.5.
Complications of Procedures
As Table iii shows, 36.1% of the patients experienced at least 1 complication from the procedure, most frequently atrial fibrillation or flutter. Two patients (3.3% of the cohort) died within 7 days of the procedure: one died from auricular fibrillation and respiratory distress 7 days after pericardiocentesis, and the other patient died 48 hours after pericardiotomy because of hemodynamic instability. There was no difference between the patient groups in terms of complications.
TABLE III.
Complications related to the procedure
| Complication | Patient group [n (%)] | p Value | ||
|---|---|---|---|---|
|
| ||||
| Overall (n=61) | Pericardiocentesis (n=42) | Pericardiotomy (n=19) | ||
| Patients with at least 1 complicationa | 22 (36.1) | 15 (35.7) | 7 (36.8) | 0.9 |
| Atrial fibrillation or flutter | 14 (23) | 10 (24) | 4 (21) | |
| Atrioventricular block needing pacemaker | 1 (2) | 1 (5) | ||
| Elevated cardiac enzymes | 1 (2) | 1 (2) | ||
| Prolonged intubation | 1 (2) | 1 (5) | ||
| Bronchospasm | 2 (3) | 1 (2) | 1 (5) | |
| Pneumothorax | 1 (2) | 1 (2) | ||
| Pulmonary edema | 1 (2) | 1 (2) | ||
| Acute renal failure | 4 (7) | 2 (5) | 2 (11) | |
| Hyperkalemia | 2 (3) | 2 (5) | ||
| Elevated liver enzymes | 3 (5) | 2 (5) | 1 (5) | |
| Bacteremia | 1 (2) | 1 (5) | ||
| Pulmonary infection | 1 (2) | 1 (2) | ||
| Delirium | 1 (2) | 1 (5) | ||
| Deep venous thrombosis | 1 (2) | 1 (5) | ||
| Death within 7 days | 2 (3) | 1 (2) | 1 (5) | |
Some patients had more than 1 complication.
Cytology was performed for all patients undergoing pericardiocentesis and for 15 of the 19 patients undergoing pericardiotomy. Results were positive for 39 patients of the 42 in the pericardiocentesis group (92.9%) and for 13 of the tested 15 in the pericardiotomy group (86.7%), a difference that was nonsignificant (p = 0.6). The proportion of intensive care admissions and the duration of hospitalizations were similar in the two groups.
DISCUSSION
In this cohort of patients with malignant pericardial effusion, recurrence rates were higher with pericardiocentesis than with pericardiotomy by the surgical or percutaneous approach (31.0% vs. 5.3%). Diagnostic yield (92.9% vs. 86.7%), complication rates (35.7% vs. 36.8%), and overall survival (2.4 months vs. 2.6 months) were not statistically different between the groups.
The success rates for pericardiocentesis (69.0%) and pericardiotomy (94.7%) in preventing effusion recurrence were comparable to the rates reported in the most recently published systematic review (61.8% for pericardiocentesis and 93.5% for surgical approaches)13. The complication rate in our cohort (36.1%) was higher than rates previously reported (between 5% and 32% for the various procedures), mainly attributable to minor complications such as atrial arrhythmias, which might have been underreported in other studies. In our study, the diagnostic yield from pericardiocentesis (92.9%) was similar to the expected yield, which was 92% in a larger series including 165 patients15. The diagnostic value of pericardiotomy is less well described in the literature, but was reported to be 53% in a recent retrospective comparison, with no difference when compared with pericardiocentesis (44%)12. The yields with both techniques were very low in the latter study; hence, the diagnostic yield of 86.7% that we observed seems more accurate. Finally, the median survival of 2.5 months with both techniques is comparable to that in previous reports4.
Our study has a number of potential limitations. First, it is a retrospective study in which therapeutic decisions were at the discretion of the primary oncologist, causing a possible selection bias. The patients chosen for more-invasive approaches might have been healthier, with a better performance status—a data point that we were unable to extract from the files because of too much missing information. We also could not compare quality of life between the two groups after the procedure. Furthermore, the proportions of lung cancer cases in the two groups were unbalanced, and patients with lung cancer are known to have a poorer prognosis. Second (and again because of the retrospective nature of the study), the procedures performed were very heterogeneous: pericardiotomy was performed using a variety of approaches, and use of extended drainage with pericardiocentesis was inconsistent. Also, because a large proportion of the patients in our cohort had pulmonary neoplasms, our results might not be generalizable to all patients with malignant pericardial effusion.
CONCLUSIONS
In this retrospective study of 61 patients with malignant pericardial effusion, we observed that the success rate in preventing recurrence was significantly higher with pericardiotomy than with pericardiocentesis (94.7% vs. 69.0%), with similar diagnostic yields, complication rates, and effect on overall survival. It is our view that pericardiotomy should be offered to all patients with malignant pericardial effusion who are hemodynamically stable and who have a good performance status and an estimated life expectancy of at least 3 months (the time at which recurrences tend to appear). However, adequately controlled trials should be conducted to confirm the superiority of pericardiotomy over pericardiocentesis.
ACKNOWLEDGEMENTS
The authors acknowledge Serge Simard, msc stats, from the Centre de recherche de l’Institut universitaire de cardiologie et de pneumologie de Québec, Université Laval, Québec, for statistical analyses, and Sylvie Martin for editorial assistance.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Abraham KP, Reddy V, Gattuso P. Neoplasms metastatic to the heart: review of 3314 consecutive autopsies. Am J Cardiovasc Pathol. 1990;3:195–8. [PubMed] [Google Scholar]
- 2.Thurber DL, Edwards JE, Achor RW. Secondary malignant tumors of the pericardium. Circulation. 1962;26:228–41. doi: 10.1161/01.CIR.26.2.228. [DOI] [PubMed] [Google Scholar]
- 3.Refaat MM, Katz WE. Neoplastic pericardial effusion. Clin Cardiol. 2011;34:593–8. doi: 10.1002/clc.20936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cullinane CA, Paz IB, Smith D, Carter N, Grannis FW., Jr Prognostic factors in the surgical management of pericardial effusion in the patient with concurrent malignancy. Chest. 2004;125:1328–34. doi: 10.1378/chest.125.4.1328. [DOI] [PubMed] [Google Scholar]
- 5.Vaitkus PT, Herrmann HC, LeWinter MM. Treatment of malignant pericardial effusion. JAMA. 1994;272:59–64. doi: 10.1001/jama.1994.03520010071035. [DOI] [PubMed] [Google Scholar]
- 6.Maisch B, Seferović PM, Ristić AD, et al. on behalf of the Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology Guidelines on the diagnosis and management of pericardial diseases executive summary. Eur Heart J. 2004;25:587–610. doi: 10.1016/j.ehj.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Rafique AM, Patel N, Biner S, et al. Frequency of recurrence of pericardial tamponade in patients with extended versus nonextended pericardial catheter drainage. Am J Cardiol. 2011;108:1820–5. doi: 10.1016/j.amjcard.2011.07.057. [DOI] [PubMed] [Google Scholar]
- 8.Celik S, Lestuzzi C, Cervesato E, et al. Systemic chemotherapy in combination with pericardial window has better outcomes in malignant pericardial effusions. J Thorac Cardiovasc Surg. 2014;148:2288–93. doi: 10.1016/j.jtcvs.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 9.Tsang TS, Seward JB, Barnes ME, et al. Outcomes of primary and secondary treatment of pericardial effusion in patients with malignancy. Mayo Clin Proc. 2000;75:248–53. doi: 10.1016/S0025-6196(11)65028-3. [DOI] [PubMed] [Google Scholar]
- 10.Allen KB, Faber LP, Warren WH, Shaar CJ. Pericardial effusion: subxiphoid pericardiostomy versus percutaneous catheter drainage. Ann Thorac Surg. 1999;67:437–40. doi: 10.1016/S0003-4975(98)01192-8. [DOI] [PubMed] [Google Scholar]
- 11.Laham RJ, Cohen DJ, Kuntz RE, Baim DS, Lorell BH, Simons M. Pericardial effusion in patients with cancer: outcome with contemporary management strategies. Heart. 1996;75:67–71. doi: 10.1136/hrt.75.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel N, Rafique AM, Eshaghian S, et al. Retrospective comparison of outcomes, diagnostic value, and complications of percutaneous prolonged drainage versus surgical pericardiotomy of pericardial effusion associated with malignancy. Am J Cardiol. 2013;112:1235–9. doi: 10.1016/j.amjcard.2013.05.066. [DOI] [PubMed] [Google Scholar]
- 13.Jama GM, Scarci M, Bowden J, Marciniak SJ. Palliative treatment for symptomatic malignant pericardial effusion. Interact Cardiovasc Thorac Surg. 2014;19:1019–26. doi: 10.1093/icvts/ivu267. [DOI] [PubMed] [Google Scholar]
- 14.Fine JP, Gray RJ. A proportional hazards model for the sub-distribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. doi: 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 15.Meyers DG, Meyers RE, Prendergast TW. The usefulness of diagnostic tests on pericardial fluid. Chest. 1997;111:1213–21. doi: 10.1378/chest.111.5.1213. [DOI] [PubMed] [Google Scholar]