Abstract
Reactivation of hepatitis B virus (hbv) is a reported complication for patients undergoing chemotherapy, particularly immunochemotherapy with anti-CD20 agents such as rituximab. However, as the use of molecularly targeted agents increases, the risk of viral reactivation is less clearly defined. Here, we present the case of a 62-year-old woman with newly diagnosed EGFR mutation–positive metastatic non-small-cell lung cancer (nsclc).
Per interview, our patient had a remote history of hbv infection. She was started on erlotinib and developed profound diarrhea leading to renal failure that required hospital admission and temporary discontinuation of erlotinib. At 8 days after erlotinib cessation, she had a marked spike in her liver function tests, with viral serologies that were consistent with hbv reactivation. Although erlotinib and other tyrosine kinase inhibitors (tkis) are not classically associated with hbv reactivation, hbv reactivation can occur even in the setting of tki withdrawal. Before tki initiation, careful patient screening in those at risk for hbv should be performed to attenuate preventable hepatotoxicity and to differentiate between other causes of hepatotoxicity (for example, drug-induced toxicity).
Keywords: Non-small-cell lung cancer, erlotinib, hepatitis B reactivation, EGFR mutation
INTRODUCTION
Lung cancer is the 2nd most common malignancy in the United States and the leading cause of cancer-related deaths1. Non-small-cell lung cancer (nsclc) is the most common subtype of lung cancer. When nsclc is diagnosed in the metastatic stage, prognosis is dismal, with 1-year and 5-year survivals of only 15.9% and 1.5% respectively2. Recently, however, significant developments have occurred in targeting mutations, most notably the epidermal growth factor receptor (EGFR) mutation. In the first-line setting in EGFR-mutated nsclc, tyrosine kinase inhibitors (tkis) such as erlotinib have significantly improved progression-free survival from a median of about 5.6 months with chemotherapy to 11 months, thus establishing egfr inhibitors as a standard of care for EGFR-mutated nsclc3. In addition, in comparison with non-EGFR-mutant nsclc treated with chemotherapy, the EGFR mutation appears to confer a survival advantage with targeted treatment, improving overall survival from just over 1 year to upwards of 2–3 years4.
Reactivation of the hepatitis B virus (hbv) has been well described in the literature in cancer patients receiving chemotherapy, immunosuppressive therapy, or steroids. In patients receiving standard chemotherapy, hbv reactivation occurs in about 20%–50% of patients positive for hepatitis B surface antigen (hbsag)5–7. Although any cytotoxic chemotherapy agent can lead to hbv reactivation, the risk is even greater with anti-CD20 monoclonal antibodies such as rituximab. Reactivation of hbv has been added to the existing U.S. Food and Drug Administration black-box warning on the rituximab label. The warning recommends that, before the start of treatment with rituximab, all prospective patients be screened for hbv infection by measurement of hbsag and hepatitis B core antibody8. A few case reports of hbv reactivation with the use of small-molecule tkis such as sorafenib, imatinib, nilotinib, and ruxolitinib have also been noted in the literature9–11. Interestingly, hbv reactivation can also occur upon chemotherapy withdrawal and is thought to be a result of increased replication of hbv during immunosuppression and rebound hepatic damage after immune reconstitution12,13.
No published literature has described induction of hbv reactivation with erlotinib, an egfr-targeted tki. In the present case report, we describe a patient with hbv reactivation after withdrawal of erlotinib treatment for EGFR-mutant metastatic nsclc.
CASE DESCRIPTION
In a 62-year-old woman with a 40 pack–year smoking history, screening chest radiography being done as part of preoperative workup for a coronary artery bypass procedure showed increased opacification of the right middle lobe (rml). Positron-emission tomography showed a 3.9×2.6-cm thick-walled cavitary mass in the right lower lobe; a hypermetabolic lesion near the distal bronchus intermedius, occluding the rml bronchus and causing complete rml collapse; and numerous hypermetabolic mediastinal lymph nodes. Subsequent magnetic resonance imaging of the brain showed numerous (>20) brain metastases, with the largest one (12 mm) in the left inferior cerebellum being associated with mild left cerebellar tonsillar herniation. Biopsies taken from the rml lesion and the 4L lymph node showed different histologies: squamous cell carcinoma and adenocarcinoma respectively. The biopsy from the rml was positive for the EGFR exon 19 deletion. The patient’s disease was staged as an EGFR-mutated stage iv adenocarcinoma of the lung.
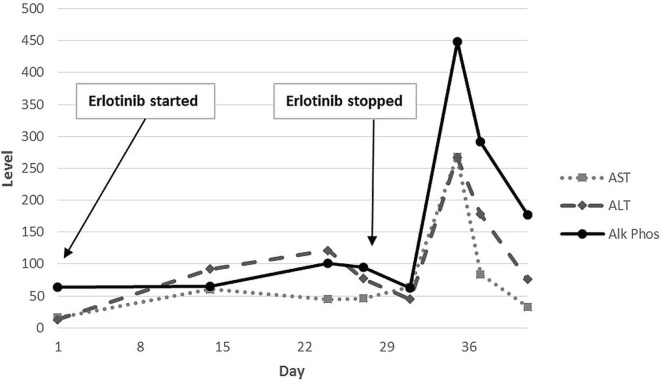
Given the numerous brain metastases (with one causing tonsillar herniation), the patient received whole-brain radiation therapy. She was started on concurrent erlotinib (150 mg standard daily dosing) based on the safety and favourable objective response rate noted in a recent phase ii trial14. The laboratory workup before therapy was unremarkable: creatinine 0.93 mg/dL (reference range: 0.4–1.2 mg/dL), blood urea nitrogen 14 mg/dL (reference range: 8–23 mg/dL), aspartate transaminase (ast) 16 IU/L (reference range: 10–30 IU/L), alanine transaminase (alt) 13 IU/L (reference range: 11–45 IU/L), and alkaline phosphatase (alp) 64 IU/dL (reference range: 30–130 IU/dL). Figure 1 presents a graph of the patient’s liver function tests (lfts).
FIGURE 1.
Liver function tests as erlotinib was started and stopped. AST = aspartate transaminase; ALT = alanine transaminase; Alk Phos = alkaline phosphatase.
Laboratory values checked 2 weeks after initiation of erlotinib showed a slight increase in creatine to 1.33 mg/dL and a notable new mild transaminitis (ast 60 IU/L, alt 92 IU/L, and alp 65 IU/dL). The patient had been complaining of 2–3 episodes of diarrhea daily. Loperamide was prescribed, with plans for weekly chemistries and lfts. The patient’s next laboratory values showed a blood urea nitrogen of 193 mg/dL and a creatinine of 16.64 mg/dL. Her ast and alt values had increased mildly to 45 IU/L and 121 IU/L. A recommendation was made that she stop the erlotinib and come to the emergency room immediately.
On admission to the intensive care unit, the patient was found to be profoundly hypotensive, and she was given aggressive fluid resuscitation. On interview, she reported large-volume diarrhea over the preceding week. She responded well to fluids alone, showing no major electrolyte imbalances. Erlotinib was held during her admission. Her diarrhea eventually subsided while she was off erlotinib. On discharge, her creatinine was 1.22 mg/dL, and her lfts had trended down (ast 64 IU/L, alt 45 IU/L, and alp 62 IU/dL).
Repeat laboratory values at 8 days after discontinuation of erlotinib showed a normal serum creatine at 1.03 mg/dL; however, marked transaminitis was present at ast 268 IU/L and alt 267 IU/L, and alp was elevated at 448 IU/dL. Liver ultrasonography was normal, showing normal common and intrahepatic bile ducts, no mass lesions, and unremarkable echogenicity. Hepatitis serologies showed positivity for hbsag, for total hepatitis B core antibody, and for hepatitis Be antibody; and negativity for hepatitis B surface antibody and for hepatitis Be antigen. Polymerase chain reaction for hepatitis B dna was 307 IU/mL. Hepatitis A immunoglobulin M antibody and hepatitis C antibody were negative. On further questioning, the patient reported having been diagnosed with hbv in 1980 when she was in the navy, but having received no follow-up after that diagnosis. On chart review, her lfts had never registered elevated as far back as 2003.
At the patient’s next clinic visit, her lfts had trended down without intervention. Entecavir was started, together with dose-reduced erlotinib (100 mg). The most recent polymerase chain reaction for hbv dna, at 4 months after initiation of therapy, was undetectable.
DISCUSSION
Here, we describe a case of acute renal failure because of erlotinib treatment, with subsequent hbv reactivation after erlotinib withdrawal. To the best of our knowledge, no prior case reports of erlotinib-withdrawal-induced or erlotinib-induced hbv reactivation have been published. In the original egfr tki trials, no hepatitis reactivation was reported, given that chronic hepatitis B and C infections were exclusionary criteria. In post-marketing data, multiple case reports of the rare adverse event of hepatic failure, some with fatal outcomes, have been published15–17. None of the cases of hepatic failure have been linked to hepatitis B or C reactivation.
In our patient, lfts rose markedly within a week after erlotinib discontinuation and then improved without any intervention. It is unclear whether that pattern represents an acute reactivation of previously cleared hepatitis B or exacerbation of a chronic underlying hepatitis B, because no viral serologies to document hbsag seroconversion had been obtained before initiation of chemotherapy. Regardless, the flare of this patient’s lfts indicated that she had a clinically significant hepatitis B infection that necessitated antiviral treatment.
In 2010, the American Society of Clinical Oncology published a provisional clinical opinion article about screening for chronic hbv in patients receiving chemotherapy18. The article concluded that the evidence for routine screening was insufficient, but advocated for physician judgment when considering screening, especially for individuals at high risk for chronic hbv infection or those being considered for highly immunosuppressive therapies. Recently released clinical practice guidelines from the American Society of Clinical Oncology note that providers should screen patients about to receive highly immunosuppressive therapy (such as hematopoietic-cell transplantation and regimens including rituximab) and patients with risk factors for hbv infection19.
Small-molecule tkis are not classically associated with immunosuppression because they do not directly cause leucopenia, nor do they target immune-system players such as B lymphocytes. However, as noted earlier, the literature contains case reports describing hbv reactivation with a variety of tkis such as sorafenib, imatinib, nilotinib, and ruxolitinib9–11. Erlotinib might have to be included in that group of tkis, and we suggest that additional study into whether the risk of hbv reactivation is increased with erlotinib is warranted. Based on our experience, we favour hbv screening in patients at risk before treatment with erlotinib is initiated. If positivity for hbsag or hepatitis B core antibody is found, we recommend treatment with antiviral agents in consultation with the Infectious Diseases or Hepatology service.
SUMMARY
We present a patient experiencing acute renal failure from diarrhea secondary to erlotinib initiation, and acute hbv reactivation after erlotinib withdrawal. Although tkis are not generally associated with a risk of viral reactivation, we advocate risk stratification to identify patients with chronic hbv infection before treatment is initiated. Stratification will help both to attenuate preventable hepatotoxicity and to differentiate between other causes of hepatotoxicity (for example, drug-induced toxicity).
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.American Cancer Society . Cancer Facts and Figures 2013. Atlanta, GA: American Cancer Society; 2013. [Google Scholar]
- 2.Cetin K, Ettinger DS, Hei YJ, O’Malley CD. Survival by histologic subtype in stage iv nonsmall cell lung cancer based on data from the Surveillance, Epidemiology and End Results Program. Clin Epidemiol. 2011;3:139–48. doi: 10.2147/CLEP.S17191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sebastian M, Schmittel A, Reck M. First-line treatment of EGFR-mutated nonsmall cell lung cancer: critical review on study methodology. Eur Respir Rev. 2014;23:92–105. doi: 10.1183/09059180.00008413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang S, Wang Z, Guo J, et al. Correlation between EGFR mutation status and response to first-line platinum-based chemotherapy in patients with advanced non-small cell lung cancer. Onco Targets Ther. 2014;7:1185–93. doi: 10.2147/OTT.S63665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeo W, Chan PK, Zhong S, et al. Frequency of hepatitis B virus reactivation in cancer patients undergoing cytotoxic chemotherapy: a prospective study of 626 patients with identification of risk factors. J Med Virol. 2000;62:299–307. doi: 10.1002/1096-9071(200011)62:3<299::AID-JMV1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Lok AS, Liang RH, Chiu EK, Wong KL, Chan TK, Todd D. Reactivation of hepatitis B virus replication in patients receiving cytotoxic therapy. Report of a prospective study. Gastroenterology. 1991;100:182–8. doi: 10.1016/0016-5085(91)90599-g. [DOI] [PubMed] [Google Scholar]
- 7.Lau GK, Leung YH, Fong DY, et al. High hepatitis B virus (hbv) dna viral load as the most important risk factor for hbv reactivation in patients positive for hbv surface antigen undergoing autologous hematopoietic cell transplantation. Blood. 2002;99:2324–30. doi: 10.1182/blood.V99.7.2324. [DOI] [PubMed] [Google Scholar]
- 8.Mitka M. fda: increased hbv reactivation risk with of atumumab or rituximab. JAMA. 2013;310:1664. doi: 10.1001/jama.2013.281115. [DOI] [PubMed] [Google Scholar]
- 9.Shiba S, Kondo S, Ueno H, Morizane C, Ikeda M, Okusaka T. Hepatitis B virus reactivation during treatment with multi-tyrosine kinase inhibitor for hepatocellular carcinoma. Case Rep Oncol. 2012;5:515–19. doi: 10.1159/000342913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai GM, Yan SL, Chang CS, Tsai CY. Hepatitis B reactivation in chronic myeloid leukemia patients receiving tyrosine kinase inhibitor. World J Gastroenterol. 2013;19:1318–21. doi: 10.3748/wjg.v19.i8.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen CH, Hwang CE, Chen YY, Chen CC. Hepatitis B virus reactivation associated with ruxolitinib. Ann Hematol. 2014;93:1075–6. doi: 10.1007/s00277-013-1936-5. [DOI] [PubMed] [Google Scholar]
- 12.Thung SN, Gerber MA, Klion F, Gilbert H. Massive hepatic necrosis after chemotherapy withdrawal in a hepatitis B virus carrier. Arch Intern Med. 1985;145:1313–14. doi: 10.1001/archinte.1985.00360070195034. [DOI] [PubMed] [Google Scholar]
- 13.Lau JY, Lai CL, Lin HJ, et al. Fatal reactivation of chronic hepatitis B virus infection following withdrawal of chemotherapy in lymphoma patients. Q J Med. 1989;73:911–17. [PubMed] [Google Scholar]
- 14.Welsh JW, Komaki R, Amini A, et al. Phase ii trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J Clin Oncol. 2013;31:895–902. doi: 10.1200/JCO.2011.40.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramanarayanan J, Scarpace SL. Acute drug induced hepatitis due to erlotinib. JOP. 2007;8:39–43. [PubMed] [Google Scholar]
- 16.Saif MW. Erlotinib-induced acute hepatitis in a patient with pancreatic cancer. Clin Adv Hematol Oncol. 2008;6:191–9. [PubMed] [Google Scholar]
- 17.Liu W, Makrauer FL, Qamar AA, Jänne PA, Odze RD. Fulminant hepatic failure secondary to erlotinib. Clin Gastroenterol Hepatol. 2007;5:917–20. doi: 10.1016/j.cgh.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Artz AS, Somerfield MR, Feld JJ, et al. American Society of Clinical Oncology provisional clinical opinion: chronic hepatitis B virus infection screening in patients receiving cytotoxic chemotherapy for treatment of malignant diseases. J Clin Oncol. 2010;28:3199–202. doi: 10.1200/JCO.2010.30.0673. [DOI] [PubMed] [Google Scholar]
- 19.Hwang JP, Somerfield MR, Alston-Johnson DE, et al. Hepatitis B virus screening for patients with cancer before therapy: American Society of Clinical Oncology provisional clinical opinion update. J Clin Oncol. 2015;33:2212–20. doi: 10.1200/JCO.2015.61.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]