Abstract
Background
The liver is a common site of primary and metastatic cancer. Liver-directed therapies are commonly used to treat cancer involving the liver. We report on the patterns, predictors, and outcomes of liver-directed therapies in hospitalized cancer patients in the United States.
Methods
Data were obtained from all U.S. states that contributed to the Nationwide Inpatient Sample maintained by the Agency for Healthcare Research and Quality between 2006 and 2010. Univariate and multivariate testing was used to identify factors significantly associated with patient outcome.
Results
For the 5-year period of interest, 12,540 patient discharges were identified. Mean age in the sample was 60 years. Primary liver lesions (n = 8840) made up 26.9% of the sample; the remaining cases were metastases. Most procedures were performed in large (79%) urban (98%) hospitals and in patients with insurance (97.9%). The most common intervention was partial hepatectomy (42.7%), followed by open (9.9%), percutaneous (7.2%), and laparoscopic (5.04%) ablation of liver lesions; embolization (9.8%); and liver transplantation (2.64%). The incidence of in-hospital mortality was very low (2.4%), and the complication rate was 12.2%. Complications such as acute liver necrosis, ascites, hepatic coma, hepatorenal syndrome, liver abscess, and high number of comorbid illnesses (>8) accounted for 60% of the in-hospital mortality.
Conclusions
The low rate of morbidity and mortality associated with liver-directed therapies in hospitalized cancer patients supports the continuing utility of such procedures in the management of primary and metastatic liver cancer. The patterns of health disparities observed with respect to the use of liver-directed therapies are concerning.
Keywords: Liver, therapies, predictors, outcomes, hospitalization
INTRODUCTION
Liver involvement with cancer is a major cause of cancer-related morbidity and mortality worldwide1,2. Primary cancer of the liver is the 2nd leading cause of cancer death globally1, and estimates suggest that it will be responsible for 28,250 deaths in the United States in 2015. Those deaths increased at an annualized rate of 2.5% each year between 2007 and 20113, with a significant contribution by the epidemic of hepatitis C virus4. In addition, the liver is a frequent site for metastatic disease. More than 25% of metastases involve the liver, making it a common site for disease spread5.
Primary liver tumours with no extrahepatic metastasis are frequently treated with liver-directed therapies. Metastasis to the liver can be treated either with systemic chemo-therapy or, in certain clinical situations, with liver-directed therapies. “Liver-directed therapies” encompass a variety of procedures that include liver transplantation, surgical resection (open or laparoscopic), transcatheter therapy (bland embolization, chemoembolization, radioembolization), ablation (radiofrequency ablation, microwave thermotherapy, cryosurgery or cryotherapy, and ethanol or alcohol ablation), hepatic artery infusion, and stereotactic radiotherapy6,7.
Liver-directed therapies have been associated with increased survival in patients with primary or metastatic liver malignancies6,8,9. A retrospective analysis of the use of liver resection, ablation, or embolization in 1918 patients with metastatic colorectal cancer treated during 2000–2009 found an improved median overall survival of 28.4 months in patients treated with liver-directed therapies compared with 21.1 months in those not so treated (p < 0.0001)10. Another retrospective review of 254 patients diagnosed with hepatocellular carcinoma and treated with liver-directed therapies between 2003 and 2011 also reported a survival benefit with those interventions11.
Although liver-directed therapies have a clear role in the management of primary and metastatic cancer in the liver, those therapies have not been adequately compared in prospective randomized trials. Clinically, the choice of which liver-directed therapy to apply in a specific patient is based on patient characteristics, disease extent, liver function, and institutional preference. At the national level, data about the patterns of use of liver-directed therapies, as well as the predictors and outcomes of the relevant procedures, are limited. Because hospitalized patients are treated with or monitored after liver-directed therapies, we used the U.S. Nationwide Inpatient Sample (nis) database to describe the patterns of use of liver-directed therapies in patients with cancer. We also evaluated the patient and disease characteristics associated with the use each therapy type. Finally, we evaluated the in-hospital outcomes associated with each of the liver-directed therapies.
METHODS
For this study, we used the 2007–2010 nis datasets. The nis is the largest database of health care outcomes in hospitalized patients in the United States; it is maintained by the Healthcare Cost and Utilization Project of the Agency for Healthcare Research and Quality. The data, which are collected from more than 1000 U.S. hospitals, consist of the clinical and demographic information included in hospital discharge abstracts. For confidentiality reasons, patient identifiers are not released; the hospital discharge event is therefore the primary sampling unit in the present analysis. The nis database codes for diagnosis-related groups, procedures, and diagnostic indices are based on the International Classification of Diseases, Ninth Revision, Clinical Modification. The primary outcome was the type of liver-directed therapy that patients received. Selection criteria included hospital discharges with primary liver cancer or liver metastases from other primary cancers and at least 1 liver-directed therapy. Hematologic malignancies such as lymphomas and leukemia with hepatic involvement were excluded from the analyses. Data collected included age, insurance status, presence of meta-static disease, comorbid medical conditions (including liver disease other than malignancy), gastrointestinal (gi) primary tumour, complications, in-hospital mortality, and patient disposition.
Statistical Analysis
The clinical and demographic characteristics of the patients are presented as descriptive statistics appropriate to the variable type and distribution. Discharge weights were used to calculate national estimates. Univariate analysis of numerical covariates was performed using a logistic model, and a weighted chi-square test was used for categorical covariates. Sample stratification, clustering, and weighting were taken into account. To identify predictors of liver-directed therapies, multivariable analysis was conducted using a backward variable selection method with the alpha level of removal set at 0.1. A generalized linear model, with use of the generalized estimating equation, was used to simultaneously account for hospital-level and patient-level variation in each endpoint. The model accounted for data correlations by assuming exchangeability for admissions from the same hospital. All analyses were performed using the SAS 9.3 software application (SAS Institute, Cary, NC, U.S.A.) with a significance level of 0.05 and without adjustment for multiple comparisons.
RESULTS
Baseline Characteristics
The analyses included 12,540 patient discharges identified for the 5-year period covered by the study. Table i details the clinical and demographic characteristics of the patient discharges. Mean age was 60 ± 14 years (standard deviation), with a male-to-female ratio of 1.3:1. Most patients (60%) were white, and nearly all patients (98%) had health insurance coverage. Primary liver cancer was the diagnosis in about 27% of the patients (n = 3378). Of the 9162 patient discharges with metastasis to the liver, 1414 (15.4%) had a primary tumour of the gi tract. Most patients were treated in large urban hospitals. The median 8 comorbid medical conditions in the cohort included liver cirrhosis (10% of patients) with ascites (5.5%) and portal hypertension (3.2%).
TABLE I.
Descriptive statistics
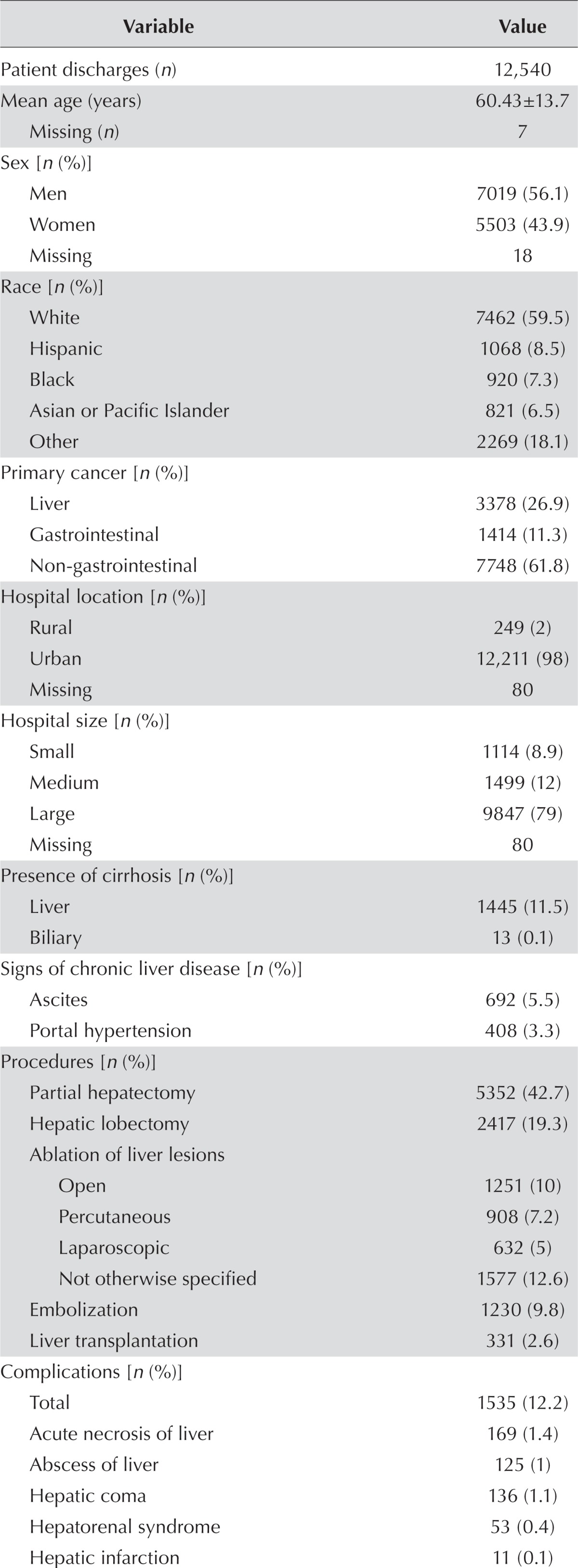
| Variable | Value | |
|---|---|---|
| Patient discharges (n) | 12,540 | |
| Mean age (years) | 60.43±13.7 | |
| Missing (n) | 7 | |
| Sex [n (%)] | ||
| Men | 7019 (56.1) | |
| Women | 5503 (43.9) | |
| Missing | 18 | |
| Race [n (%)] | ||
| White | 7462 (59.5) | |
| Hispanic | 1068 (8.5) | |
| Black | 920 (7.3) | |
| Asian or Pacific Islander | 821 (6.5) | |
| Other | 2269 (18.1) | |
| Primary cancer [n (%)] | ||
| Liver | 3378 (26.9) | |
| Gastrointestinal | 1414 (11.3) | |
| Non-gastrointestinal | 7748 (61.8) | |
| Hospital location [n (%)] | ||
| Rural | 249 (2) | |
| Urban | 12,211 (98) | |
| Missing | 80 | |
| Hospital size [n (%)] | ||
| Small | 1114 (8.9) | |
| Medium | 1499 (12) | |
| Large | 9847 (79) | |
| Missing | 80 | |
| Presence of cirrhosis [n (%)] | ||
| Liver | 1445 (11.5) | |
| Biliary | 13 (0.1) | |
| Signs of chronic liver disease [n (%)] | ||
| Ascites | 692 (5.5) | |
| Portal hypertension | 408 (3.3) | |
| Procedures [n (%)] | ||
| Partial hepatectomy | 5352 (42.7) | |
| Hepatic lobectomy | 2417 (19.3) | |
| Ablation of liver lesions | ||
| Open | 1251 (10) | |
| Percutaneous | 908 (7.2) | |
| Laparoscopic | 632 (5) | |
| Not otherwise specified | 1577 (12.6) | |
| Embolization | 1230 (9.8) | |
| Liver transplantation | 331 (2.6) | |
| Complications [n (%)] | ||
| Total | 1535 (12.2) | |
| Acute necrosis of liver | 169 (1.4) | |
| Abscess of liver | 125 (1) | |
| Hepatic coma | 136 (1.1) | |
| Hepatorenal syndrome | 53 (0.4) | |
| Hepatic infarction | 11 (0.1) | |
| Disposition [n (%)] | ||
| Home | 9455 (75.4) | |
| Death | 256 (2) | |
| Hospice | 74 (0.6) | |
| Others | 2755 (22) | |
| Insurance status [n (%)] | ||
| Insured | 12243 (97.9) | |
| Non-insured | 260 (2.1) | |
| Missing | 37 | |
| Diagnoses (n) | ||
| Mean | 8.78±4.7 | |
| Median | 8 | |
| Range | 1–31 | |
Type of Liver-Directed Therapy
A wide variety of liver-directed therapies were used during the identified hospitalizations. The most common procedure was partial hepatectomy (42.7%), followed by ablation (open: 9.98%; percutaneous: 7.24%; laparoscopic: 5.04%), transcatheter therapy (9.8%), and liver transplantation (2.64%). Partial hepatectomy was performed almost exclusively in patients without liver cirrhosis (94% vs. 6% in those with cirrhosis, p < 0.001). Partial hepatectomy was also more commonly performed in patients with gi primary tumours (61.7% vs. 38.26%, p < 0.001).
Among the ablative techniques, open ablation was the most commonly used procedure, followed by percutaneous and laparoscopic ablation. Open ablative techniques were also more likely in patients with gi primary tumours (12.2%). Percutaneous liver tumour ablation was more often used in patients with liver cirrhosis (14.6%); laparoscopy was more common in patients with portal hypertension (15.6%). Transcatheter therapies included transarterial chemoembolization and radioembolization with microspheres in 1086 patients (8.7%). Transarterial embolization was used in 144 patients (1.15%).
Liver transplantation was more commonly performed in patients without liver cirrhosis (67.7% vs. 32.3%, p = 0.012). Of the 331 patients who received a liver graft, 37 (11%) had metastatic disease at the time of transplantation (p < 0.001).
Complications and In-Hospital Mortality
Across all procedures, complications were observed in 12.2% of patient discharges.
A higher risk for complications was observed for partial hepatectomy [odds ratio (or): 1.47; 95% confidence interval (ci): 1.28 to 1.70; p < 0.001], a gi primary (or: 1.59; 95% ci: 1.21 to 2.07; p < 0.001), and metastatic disease (or: 1.83; 95% ci: 1.56 to 2.16; p < 0.001). Laparoscopic ablation (or: 0.53; 95% ci: 0.41 to 0.67; p < 0.001) and embolization (or: 0.51; 95% ci: 0.34 to 0.77; p < 0.001) were associated with a lower risk of complications. Observed complications in patients undergoing partial hepatectomy included acute liver necrosis (1.3%), liver abscess (1%), and hepatorenal syndrome (0.2%). Compared with the less-invasive ablative techniques (percutaneous and laparoscopic), open ablation was more commonly complicated by liver abscess (8.7%) and in-hospital mortality (7.2%). Overall, liver transplantation was associated with a lower incidence of complications (or: 0.28; 95% ci: 0.2 to 0.41; p < 0.001).
The 303 patients who died in the hospital after liver-directed therapy made for an in-hospital mortality rate of 2.4%. In-hospital mortality was significantly associated with acute liver necrosis (p < 0.001), ascites (n = 60, p < 0.001), hepatic coma (p < 0.001), hepatorenal syndrome (p < 0.001), liver abscess (p = 0.012), and a higher number of comorbid medical illnesses (15 vs. 8, p < 0.001). Together, the foregoing factors accounted for more than 60% of the mortality rate. Site of the primary tumour (gi vs. non-gi), insurance status, and presence of metastatic disease or liver cirrhosis did not influence mortality. Higher mortality was also observed in patients who underwent partial hepatectomy (40%), which constituted 2.3% of the partial hepatectomy group (Table ii). Primary cancer of the liver was associated with a lower risk of in-hospital mortality (or: 0.33; 95% ci: 0.26 to 0.43; p < 0.001). The mortality rate from complicated transcatheter therapies was 2.9%, and compared with the uncomplicated procedures, complicated ones were associated with an almost doubled risk of inhospital mortality (or: 1.93; 95% ci: 1.12 to 3.33; p = 0.018). Although 16 patients in the liver transplantation group died in the hospital (4.63%), liver transplantation (compared with other liver-directed therapies) showed no statistically significant association with in-hospital mortality.
TABLE II.
Multivariable analyses of covariates associated with complications and in-hospital mortality
| Covariate | OR | 95% CI | p Value | |
|---|---|---|---|---|
| Complication presenta | ||||
| Partial hepatectomy | Yes | 1.47 | 1.28 to 1.70 | <0.001 |
| No | Reference | |||
| Total hepatectomy or liver transplantation | Yes | 0.28 | 0.20 to 0.41 | <0.001 |
| No | Reference | |||
| Laparoscopic ablation of liver lesions | Yes | 0.53 | 0.41 to 0.67 | <0.001 |
| No | Reference | |||
| Embolization | Yes | 0.51 | 0.34 to 0.77 | 0.001 |
| No | Reference | |||
| Primary tumour type | Gastrointestinal | 1.59 | 1.21 to 2.07 | <0.001 |
| Non-gastrointestinal | Reference | |||
| Metastatic cancer | Present | 1.83 | 1.56 to 2.16 | <0.001 |
| Not present | Reference | |||
| Died during hospitalizationa,b | ||||
| Hepatic lobectomy | Yes | 0.54 | 0.43 to 0.69 | <0.001 |
| No | Reference | |||
| Embolization | Yes | 1.93 | 1.12 to 3.33 | 0.018 |
| No | Reference | |||
| Liver primary | Yes | 0.33 | 0.26 to 0.43 | <0.001 |
| No | Reference | |||
| Number of diagnoses | <8 | 0.84 | 0.82 to 0.86 | <0.001 |
Of 12,540 discharges, 12,503 were used. Backward selection with an alpha level of removal of 0.1 was used.
On multivariable analysis, insurance was marginally significantly related to in-hospital mortality.
OR = odds ratio; CI = confidence interval.
DISCUSSION
Liver-directed therapies have a central role in the curative and palliative treatment of patients with primary or metastatic cancer involving the liver. In the present study, we systematically evaluated a large database of hospitalized U.S. patients with cancer involving the liver who were treated with liver-directed therapies. Specific factors such as the type of procedure performed, primary site of the malignancy, comorbid illnesses at the time of hospitalization, discharge disposition, and in-hospital mortality were used to characterize management patterns and predictors of outcome in the patients.
Liver-directed therapies are more frequently used for metastatic disease involving the liver than for primary hepatic tumours—a pattern that reflects the higher incidence of liver metastasis. Of the liver-directed therapies, partial hepatectomy is the procedure most commonly used in hospitalized patients. Ablation, stereotactic radiosurgery, and transcatheter therapies are commonly performed in the outpatient setting and thus might be underrepresented in the source database.
Factors associated with the choice of liver-directed therapy include the type of malignancy and the patient’s underlying liver function. Partial hepatectomy was significantly associated with gi primaries and an absence of underlying cirrhosis. Similarly, open ablation was preferentially used in patients with primary gi tumours and in the absence of cirrhosis. Laparoscopic and percutaneous ablations were more likely to be used in patients with portal hypertension, ascites, or cirrhosis. The latter selection is driven by the high operative morbidity and mortality associated with advanced liver disease13 and was an expected finding. Compared with partial hepatectomy, liver transplantation was performed in a higher proportion of patients with cirrhosis.
Most liver-directed therapies were performed in large urban hospitals; fewer than 2% of the therapies were performed in rural settings. That finding suggests that patients being treated at smaller hospitals and in rural areas are not being offered these specialized therapies despite their proven benefit in cancer control14. More concerning is the observation that only 2% of patients undergoing liver-directed procedures had no health insurance. Those data suggest that uninsured patients with cancer are less likely to be offered invasive procedures despite clinical evidence of benefit. The resulting patterns raise a concern about disparities in the utilization of, and access to, liver-directed therapies. Future prospective trials should evaluate the access to liver-directed therapies for uninsured patients or patients treated in rural areas and smaller hospitals. If the discrepancies are real, it could be important to target this gap with the goal of improving cancer outcomes by improving access to available treatments.
Overall, in-hospital morbidity and mortality were low despite the invasive nature of the procedures and the high level of comorbidities seen in our cohort of patients. The relatively high proportion of patients receiving partial hepatectomy who died in the hospital is most likely a reflection of the invasive nature of a procedure that is being performed in patients who have an underlying cancer. That observation highlights the importance of proper patient selection. Previous reports have noted that liver lobectomy in patients undergoing colon resection was associated with a prolonged length of stay, the highest complication rates, and an unadjusted mortality rate almost double the rate in patients who underwent colon resection but other forms of liver resection12. Most complications associated with liver-directed therapies were related to liver injury, including acute liver necrosis, liver abscess, hepatic infarction, hepatic coma, and hepatorenal syndrome.
The two main limitations of the present study are the retrospective nature of the nis database and the inclusion of hospitalized patients only. The retrospective nature of the study limits the ability to control for confounding variables and potential bias. Specifically, the absence of performance status, extent of cancer in the liver, prior treatment, and lack of quantification of liver reserve for the treated patients limits the analysis. The in-hospital nature of the database excludes liver-directed procedures that are commonly performed in the outpatient setting: percutaneous ablation, stereotactic body radiation therapy, and 90Y radioembolization. The absence of outpatient data also limits the ability to determine the effects of such treatments on long-term disease control and survival. Nonetheless, our report carefully examined the pattern and predictors of liver-directed therapies in a large representative cohort of hospitalized cancer patients throughout the United States. The associations between key clinical, treatment, and mortality data reported in the analysis provide a very strong rationale for well-designed prospective studies to establish a casual association.
CONCLUSIONS
Our study confirms the safety and low complication rates associated with liver-directed therapies in hospitalized patients with primary or metastatic cancer involving the liver. Application of the various liver-directed therapies appears to be determined by the type of malignancy and any underlying liver cirrhosis. Concerning patterns of health care disparity are apparent in the use of liver-directed therapies and warrant further confirmatory research.
ACKNOWLEDGEMENTS
OBA is supported by U.S. National Institutes of Health grant T32 CA160040-01A1 (principal investigator: Dong M. Shin). TKO and BFE are Georgia Cancer Coalition Distinguished Cancer Scholars.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Stewart BW, Wild CP, editors. World Cancer Report 2014. Lyon, France: International Agency for Research on Cancer; 2015. [PubMed] [Google Scholar]
- 2.Ananthakrishnan A, Gogineni V, Saeian K. Epidemiology of primary and secondary liver cancers. Semin Intervenl Radiol. 2006;23:47–63. doi: 10.1055/s-2006-939841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 4.Ward JW. The hidden epidemic of hepatitis C virus infection in the United States: occult transmission and burden of disease. Top Antivir Med. 2013;21:15–19. [PMC free article] [PubMed] [Google Scholar]
- 5.Abbruzzese JL, Abbruzzese MC, Lenzi R, Hess KR, Raber MN. Analysis of a diagnostic strategy for patients with suspected tumors of unknown origin. J Clin Oncol. 1995;13:2094–103. doi: 10.1200/JCO.1995.13.8.2094. [DOI] [PubMed] [Google Scholar]
- 6.Clark ME, Smith RR. Liver-directed therapies in metastatic colorectal cancer. J Gastrointest Oncol. 2014;5:374–87. doi: 10.3978/j.issn.2078-6891.2014.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aliberti C, Tilli M, Benea G, Fiorentini G. Trans-arterial chemoembolization (tace) of liver metastases from colorectal cancer using irinotecan-eluting beads: preliminary results. Anticancer Res. 2006;26:3793–5. [PubMed] [Google Scholar]
- 8.Bester L, Meteling B, Boshell D, Chua TC, Morris DL. Transarterial chemoembolisation and radioembolisation for the treatment of primary liver cancer and secondary liver cancer: a review of the literature. J Med Imaging Radiat Oncol. 2014;58:341–52. doi: 10.1111/1754-9485.12163. [DOI] [PubMed] [Google Scholar]
- 9.Barman PM, Sharma P, Krishnamurthy V, et al. Predictors of mortality in patients with hepatocellular carcinoma undergoing transarterial chemoembolization. Dig Dis Sci. 2014;59:2821–5. doi: 10.1007/s10620-014-3247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vargas GM, Parmar AD, Sheffield KM, Tamirisa NP, Brown KM, Riall TS. Impact of liver-directed therapy in colorectal cancer liver metastases. J Surg Res. 2014;191:42–50. doi: 10.1016/j.jss.2014.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho EY, Cozen ML, Shen H, et al. on behalf of the hovas Group (Hepatocellular Carcinoma Treatment Outcome at VA San Francisco) Expanded use of aggressive therapies improves survival in early and intermediate hepatocellular carcinoma. HPB (Oxford) 2014;16:758–67. doi: 10.1111/hpb.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abbott AM, Parsons HM, Tuttle TM, Jensen EH. Short-term outcomes after combined colon and liver resection for synchronous colon cancer liver metastases: a population study. Ann Surg Oncol. 2013;20:139–47. doi: 10.1245/s10434-012-2515-z. [DOI] [PubMed] [Google Scholar]
- 13.Ko CJ, Lin PY, Lin KH, Lin CC, Chen YL. Presence of fibrosis is predictive of postoperative survival in patients with small hepatocellular carcinoma. Hepatogastroenterology. 2014;61:2295–300. [PubMed] [Google Scholar]
- 14.Singla S, Hochwald SN, Kuvshinoff B. Evolving ablative therapies for hepatic malignancy. Biomed Res Int. 2014;2014:230174. doi: 10.1155/2014/230174. [DOI] [PMC free article] [PubMed] [Google Scholar]


