Abstract
Objective
Administrative data are used to describe the pancreatic cancer (pcc) population. The analysis examines demographic details, incidence, site, survival, and factors influencing mortality in a cohort of individuals diagnosed with pcc.
Methods
Incident cases of pcc diagnosed in Ontario between 1 January 2004 and 31 December 2011 were extracted from the Ontario Cancer Registry. They were linked by encrypted health card number to several administrative databases to obtain demographic and mortality information. Descriptive, bivariate, and survival analyses were conducted.
Results
During the period of interest, 9221 new cases of pcc (4548 in men, 4673 in women) were diagnosed, for an age-adjusted standardized annual incidence in the range of 8.6–9.5 per 100,000 population. Mean age at diagnosis was 70.3 ± 12.5 years (standard deviation). Five-year survival was 7.2% (12.8% for those <60 years of age and 3.6% for those >80 years of age). Survival varied by sex, older age, rural residence, lower income, site of involvement in the pancreas, and presence of comorbidity.
Conclusions
The mortality rate in pcc is exceptionally high. With an increasing incidence and a mortality positively associated with age, additional support will be needed for this highly fatal disease as demographics in Ontario continue to trend toward a higher proportion of older individuals.
Keywords: Pancreatic adenocarcinoma, retrospective analyses, population studies, outcomes
INTRODUCTION
Cancer of the pancreas is rare, and yet it accounts for the 4th largest number of cancer deaths in Canada each year. In 2014, 4700 new cases of pancreatic cancer (pcc) in Canada (9.3 per 100,000 population) were projected. Once a patient is diagnosed, prognosis is poor, with the number of deaths from pcc in 2014 estimated to be 4400 (8.6 per 100,000 population) 1. The relative survival ratio for pcc is the lowest among the common malignancies and has been estimated to be 21% at 1 year and 6%–8% at 5 years 1,2. The mortality subsequent to most other cancers has declined since 2000, but no corresponding mortality improvement in pcc has occurred3.
Because of the absence of symptoms associated with early-stage pcc and a lack of cost-effective screening strategies, the disease is often detected at later stages4,5. In most cases, pcc is not discovered until after it has metastasized to adjacent organs, such as the liver. For patients with metastatic pcc, life expectancy approximates 2.5 months with best supportive care 6,7. New chemo-therapy regimens such as folfirinox and nab-paclitaxel appear to offer longer life expectancy (up to a median of 11 months), but the improvement is accompanied by deleterious side effects and complications 8.
Ontario is the largest province in Canada and has the greatest incidence of pcc patients in the country by virtue of its population size 1. However, little is known about the overall epidemiology of the disease and the factors influencing survival in this group of patients 9. In the present analysis, we examined a cohort of patients with a diagnosis of pcc and their survival at 5 years.
METHODS
This population-based retrospective cohort study used administrative datasets housed at the Institute for Clinical Evaluative Sciences, a prescribed entity under Ontario’s Personal Health Information Protection Act, which allows use of individual patient-level data for the purpose of research. Approval was obtained from the research ethics board at Sunnybrook Health Sciences Centre.
Incident cases of pcc diagnosed between 1 January 2004 and 31 December 2011 were extracted from the Ontario Cancer Registry (ocr). The ocr captures information about all Ontario residents who have been newly diagnosed with cancer or who have died of cancer. Previous validation studies have demonstrated that the ocr is a valid data source, with high sensitivity and specificity for identifying cancer patients 10. Malignant neoplasms of the pancreas were identified using International Classification of Diseases version 9 codes (1570, 1571, 1572, 1573, 1574, 1578, and 1579). In the resulting dataset, the pancreatic adenocarcinoma cohort was identified by ocr histology codes (8000, 8001, 8010, 8020, 8021, 8031, 8035, 8140, 8144, 8145, 8255, 8340, 8341, 8344, 8440, 8442, 8470, 8481, 8490, 8500, 8560, 8570, 8574, 8575, 9990). The site of the cancer was determined by International Classification of Diseases version 9 codes: head of the pancreas (1570), tail of the pancreas (1572), and other (or unspecified) locations (1571, 1573, 1574, 1578, 1579).
The patients were linked by encrypted health card number to other administrative datasets housed at the Institute for Clinical Evaluative Sciences. Demographic and mortality information were obtained from the Registered Persons Database, which provides basic demographic information such as birth date, death date, and postal code of residence for all residents with an Ontario health card number. All patients were followed from their date of diagnosis to their date of death, to 5 years after diagnosis, or to 31 December 2013, whichever came first.
Geographic area of residence for the individual patients was linked to Canadian census data by geocoding postal codes into dissemination areas (the smallest unit of census geography), and neighbourhood-level information on median family income (a household size–adjusted measure of household income) was obtained 11.
We further linked the patient cohort to the Canadian Institute for Health Information’s Discharge Abstract Database, which provides detailed diagnostic information for each hospital admission. For each patient, we used data from hospitalizations occurring in the 2 years before the pcc diagnosis to calculate a score on Charlson comorbidity index. For patients with a Charlson score of 0, we assigned a comorbidity status of “no”; for those with a score equal to or greater than 1, we assigned a comorbidity status of “yes.”
We obtained Ontario population information during the study period from the Ontario Population Estimates and Projections, which are the intercensal and postcensal estimates of the Ontario population by sex, age, and geographic area. Those estimates are produced by Statistics Canada.
Statistical Analysis
We describe patient demographics, disease characteristics, and length of follow-up by patient age at diagnosis. Comparisons between age groups were made using oneway analysis of variance for continuous variables and chi-square tests for categorical variables.
We then calculated the crude incidence rate of pcc for men and women by age group for each year during the study period. For a specific year, the incidence rate was calculated by dividing the number of pcc cases by the yearly population size in each age and sex stratum.
We used the life-table method and dates of death according to the Registered Persons Database as of 31 December 2013 to estimate 5-year survival probabilities. Kaplan–Meier survival analyses were used to describe the survival probability for patients in various age groups, disease site groups, and diagnostic periods. Age at diagnosis was adjusted when computing the survival probability for various disease sites and diagnostic periods.
Cox regression was used to examine factors associated with survival. Variable selection was based on both clinical relevance and influence on risk estimates or statistical significance. The variables examined were sex, age, site of cancer, residence, income, year of diagnosis, and comorbidity. To determine whether the selected variables met the proportional hazards assumption, we generated time-dependent covariates by creating interactions of the predictors and a function of survival time, and included them in the model. If any of the time-dependent covariates were significant, then those predictors were considered not to be proportional 12.
All analyses were performed using the SAS 9.2 software application (SAS Institute, Cary, NC, U.S.A.). Statistical significance was set at p < 0.05.
RESULTS
New cases of pcc diagnosed between 2004 and 2011 numbered 9221 (4548 cases in men, 4673 cases in women). During that 8-year period, the age-adjusted standardized annual incidence rate fluctuated in the range 8.57–9.47 per 100,000 population. The most frequent site of pcc was the head of the pancreas (42%); 9.1% of pccs were reported to have occurred in the tail. The remaining cases (48.9%) were in other locations (including multiple sites) or were not specified in the ocr.
Mean age at diagnosis was 70.3 ± 12.5 years (standard deviation). Women were older (72.3 ± 12.8 years) than men (68.1 ± 11.8 years) at the time of diagnosis. Of these individuals with pcc, 15% were rural residents (rural residents represented 14% of the general population in 2011). Distribution by income quintile was equal across the cohort (Table i). In the younger age groups, the incidence of pcc was lower in women than in men. The female incidence then steadily increased with age and, in 5 of the 8 years, overtook the male incidence in the 81 years and older category (Table ii).
TABLE I.
Demographic details of patients with pancreatic cancer in Ontario, 2004–2011
| Variable | Patient group | p Valuea | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| ≤60 Years | 61–70 Years | 71–80 Years | ≥81 Years | Overall | ||
| Patients (n) | 2096 | 2264 | 2750 | 2111 | 9221 | |
| Year of diagnosis [n (%)] | ||||||
| 2004 | 226 (10.8) | 273 (12.1) | 327 (11.9) | 233 (11.0) | 1059 (11.5) | 0.075 |
| 2005 | 235 (11.2) | 281 (12.4) | 344 (12.5) | 249 (11.8) | 1109 (12.0) | |
| 2006 | 243 (11.6) | 270 (11.9) | 315 (11.5) | 236 (11.2) | 1064 (11.5) | |
| 2007 | 297 (14.2) | 255 (11.3) | 377 (13.7) | 275 (13.0) | 1204 (13.1) | |
| 2008 | 295 (14.1) | 268 (11.8) | 356 (12.9) | 261 (12.4) | 1180 (12.8) | |
| 2009 | 261 (12.5) | 293 (12.9) | 354 (12.9) | 295 (14.0) | 1203 (13.0) | |
| 2010 | 273 (13.0) | 279 (12.3) | 332 (12.1) | 287 (13.6) | 1171 (12.7) | |
| 2011 | 266 (12.7) | 345 (15.2) | 345 (12.5) | 275 (13.0) | 1231 (13.3) | |
| Sex [n (%)] | ||||||
| Women | 857 (40.9) | 987 (43.6) | 1436 (52.2) | 1393 (66.0) | 4673 (50.7) | <0.001 |
| Men | 1239 (59.1) | 1277 (56.4) | 1314 (47.8) | 718 (34.0) | 4548 (49.3) | |
| Rural [n (%)] | ||||||
| Missing | <6 | <6 | <6 | <6 | 12 (0.1) | 0.002 |
| No | 1781 (85.0) | 1904 (84.1) | 2295 (83.5) | 1850 (87.6) | 7830 (84.9) | |
| Yes | 310 (14.8) | 357 (15.8) | 453 (16.5) | 259 (12.3) | 1379 (15.0) | |
| Income quintile [n (%)] | ||||||
| Missing | 13 (0.6) | 16 (0.7) | 8 (0.3) | 15 (0.7) | 52 (0.6) | 0.466 |
| 1 (lowest) | 437 (20.8) | 460 (20.3) | 585 (21.3) | 451 (21.4) | 1933 (21.0) | |
| 2 | 426 (20.3) | 478 (21.1) | 586 (21.3) | 426 (20.2) | 1916 (20.8) | |
| 3 | 401 (19.1) | 422 (18.6) | 537 (19.5) | 447 (21.2) | 1807 (19.6) | |
| 4 | 435 (20.8) | 454 (20.1) | 538 (19.6) | 395 (18.7) | 1822 (19.8) | |
| 5 (highest) | 384 (18.3) | 434 (19.2) | 496 (18.0) | 377 (17.9) | 1691 (18.3) | |
| Site [n (%)] | ||||||
| Head of pancreas | 974 (46.5) | 1013 (44.7) | 1144 (41.6) | 744 (35.2) | 3875 (42.0) | <0.001 |
| Tail of pancreas | 255 (12.2) | 236 (10.4) | 239 (8.7) | 110 (5.2) | 840 (9.1) | |
| Others | 867 (41.4) | 1015 (44.8) | 1367 (49.7) | 1257 (59.5) | 4506 (48.9) | |
| Comorbidityb [n (%)] | ||||||
| No | 1879 (89.6) | 1869 (82.6) | 2151 (78.2) | 1568 (74.3) | 7467 (81.0) | <0.001 |
| Yes | 217 (10.4) | 395 (17.4) | 599 (21.8) | 543 (25.7) | 1754 (19.0) | |
| Died within 5 years’ follow-up [n (%)] | ||||||
| No | 286 (13.6) | 219 (9.7) | 159 (5.8) | 82 (3.9) | 746 (8.1) | <0.001 |
| Yes | 1810 (86.4) | 2045 (90.3) | 2591 (94.2) | 2029 (96.1) | 8475 (91.9) | |
| Follow-up days (n) | ||||||
| Mean | 431.72±529.65 | 351.76±467.22 | 259.35±405.20 | 180.21±351.75 | 303.10±450.51 | <0.001 |
| Median | 207 | 159 | 97 | 49 | 113 | <0.001 |
| Interquartile range | 74–525 | 51–429 | 33–279 | 17–158 | 35–340 | |
Reflects significance of variation between age groups.
Based on score on the Charlson comorbidity index (0=no, ≥1=yes) up to 2 years before the diagnosis with pancreatic cancer.
TABLE II.
Incidence of pancreatic cancer per 100,000 population by year of diagnosis, age, and sex
| Year of diagnosis | Sex | Patient age group | |||
|---|---|---|---|---|---|
|
| |||||
| ≤60 | 61–70 | 71–80 | ≥81 | ||
| 2004 | Women | 1.69 | 22.31 | 45.59 | 69.11 |
| Men | 2.66 | 35.21 | 47.26 | 62.78 | |
| 2005 | Women | 1.73 | 22.07 | 47.36 | 70.93 |
| Men | 2.75 | 35.51 | 49.93 | 62.4 | |
| 2006 | Women | 1.79 | 21.46 | 41.85 | 60.36 |
| Men | 2.8 | 32.18 | 46.89 | 62.4 | |
| 2007 | Women | 2.35 | 21.13 | 45.19 | 72.79 |
| Men | 3.24 | 27.68 | 61.09 | 59.05 | |
| 2008 | Women | 2.02 | 20.51 | 47.58 | 61.85 |
| Men | 3.51 | 27.96 | 50.53 | 61.98 | |
| 2009 | Women | 2.27 | 21.5 | 46.64 | 68.97 |
| Men | 2.6 | 28.88 | 49.75 | 63.95 | |
| 2010 | Women | 2.3 | 22.5 | 42.67 | 66.48 |
| Men | 2.79 | 25.34 | 47.95 | 63.31 | |
| 2011 | Women | 2.08 | 24.98 | 43.91 | 61.51 |
| Men | 2.88 | 34.38 | 50.35 | 64.58 | |
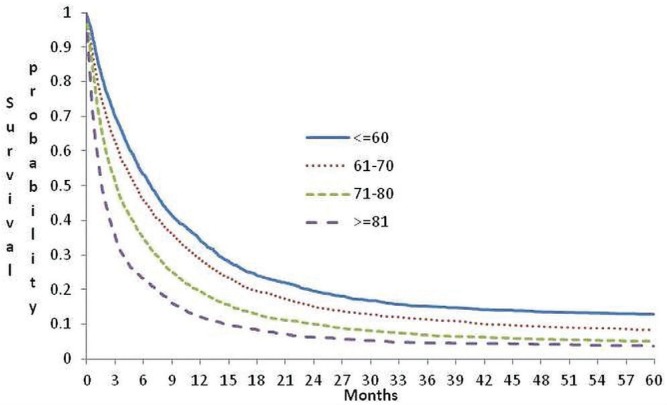
In this cohort, 92% died during follow-up. The overall survival within 5 years of follow-up was 7.2%. The mean and median follow-up times were 303 ± 451 days and 113 days (interquartile range: 35–340 days) respectively. At 1 month after diagnosis, 78% of the cohort members were alive; at 1 year, only 23.5% were still living. The probability of survival decreased as age at diagnosis increased. The 30-day and 1-year survival rates for affected individuals 60 years of age or less were 88.6% and 34.4% respectively; for those more than 80 years of age, the equivalent rates were 62.2% and 12.1%. Median survival for individuals 60 years of age or younger at diagnosis was 207 days; it was 49 days for individuals more than 80 years of age (Figure 1).
FIGURE 1.
Probability of survival for patients with pancreatic cancer in Ontario by age at diagnosis.
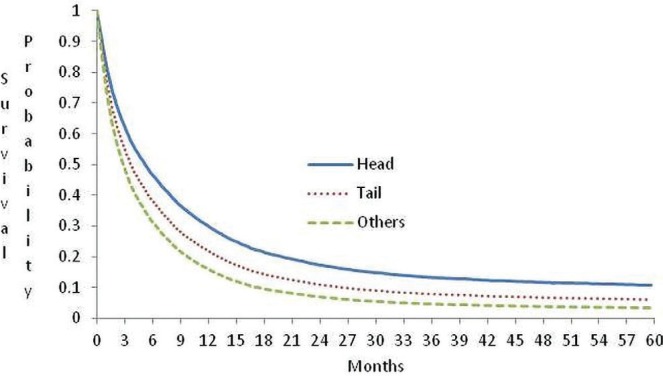
With respect to site of the primary cancer, median survival was 5.0 months in individuals with a diagnosis of carcinoma in the head of pancreas; 3.2 months in those with a diagnosis of carcinoma in the tail of pancreas; and 3.0 months for other sites (Figure 2).
FIGURE 2.
Probability of survival for patients with pancreatic cancer in Ontario by site in the pancreas, adjusted for age.
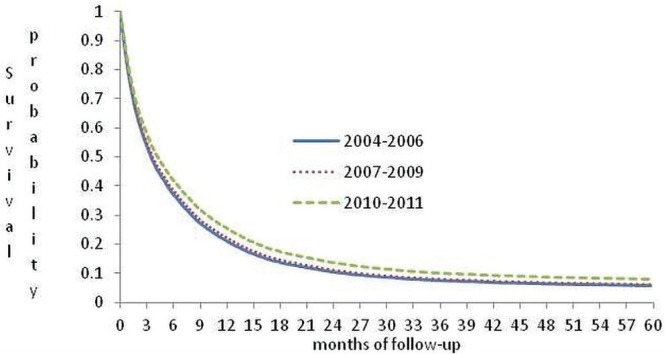
All variables included in the Cox regression met the proportional hazards assumption. Male sex, older age, presence of cancer in sites other than the head of pancreas, rural residence, low income, and presence of comorbidity were associated with lower survival. Survival seemed to improve with more recent diagnosis (Table iii, Figure 3).
TABLE III.
Multivariate Cox regression survival analysis of risk factors in patients diagnosed with pancreatic cancer in Ontario
| Parameter | Reference group | Comparison group | HR | 95% CI |
|---|---|---|---|---|
| Age group | ≤60 Years | 61–70 | 1.089 | 1.043 to 1.138 |
| 71–80 | 1.196 | 1.123 to 1.1275 | ||
| ≥81 | 1.531 | 1.44 to 1.627 | ||
| Sex | Female | Male | 2.076 | 1.943 to 2.217 |
| Rural | No | Yes | 1.076 | 1.005 to 1.152 |
| Income quintile | 5 (highest) | 1 (lowest) | 1.076 | 1.005 to 1.152 |
| 2 | 1.086 | 1.013 to 1.164 | ||
| 3 | 1.01 | 0.943 to 1.083 | ||
| 4 | 1.045 | 0.984 to 1.11 | ||
| Site | Head | Others | 1.424 | 1.361 to 1.49 |
| Tail | 1.339 | 1.238 to 1.449 | ||
| Score on the CCI | 0 | ≥1 | 1.081 | 1.023 to 1.143 |
| Year of diagnosis | 2004–2006 | 2007–2009 | 0.969 | 0.922 to 1.018 |
| 2010–2011 | 0.877 | 0.829 to 0.927 |
HR = hazard ratio; CI = confidence interval; CCI = Charlson comorbidity index.
FIGURE 3.
Probability of survival for patients with pancreatic cancer in Ontario by year of diagnosis, adjusted for age at diagnosis.
DISCUSSION
The present work provides an overview of a contemporary cohort of the entire patient population with pcc in Ontario. Survival in that cohort is in line with pcc survival in other jurisdictions, with one quarter still living at 12 months after diagnosis 2 and 7.2% still living at 5 years. Our 5-year survival findings are similar to the survival reported in Ireland (5%) 6 and the relative survival reported in the United States (6%) 4. A report by Cancer Research UK indicated that the relative 5-year survival was 3.3% for patients diagnosed with pcc during 2010–2011 in England and Wales 13. All numbers show extremely poor survival with this disease.
Other reports show a relative survival rate of 10.9% for pcc patients in Ontario 14,15. Cancer Care Ontario 16 and several Canadian government agencies 3 report an 8% 5-year relative survival for pcc in the Canadian population. Because the relative survival is the ratio of the observed survival for a group of persons diagnosed with cancer to the survival expected for people in the entire population, it differs slightly from the result found in an actual 5-year survival analysis, as estimated for the present study.
Health system administrative data was used for patient follow-up. Ontario has a publicly funded single-payer system that covers medically necessary health care costs for all eligible residents. It is unlikely that pcc patients would pursue health care elsewhere; however, the main loss to follow-up in our cohort would have been caused by patients moving out of the province. We had no information on such movements. However, we did ascertain patient eligibility for Ontario Health Insurance Plan coverage at the end of follow-up, and found that only 90 patients (0.98%) had an invalid health card number. It is possible that we lost follow-up for those 90 patients. The proportion of the overall cohort that those patients represent is very low and unlikely to bias our estimates of survival probability.
Our study reports a relatively equal distribution of new pcc cases for the two sexes, but some studies have reported a higher incidence among men 17,18. In our analyses, survival was decreased for men compared with women despite an equal sex distribution. The literature shows that increasing age is associated with a higher incidence of pcc 19. That association is important in light of an aging population. Our results confirmed the association and also showed that older age at diagnosis is associated with much lower survival.
Our study suggests that tumour location is associated with patient outcomes, which reflects other published data 20,21. Unfortunately, derivation of tumour location from the administrative data was not possible for 49% of our cohort. The literature suggests that only 10% of pancreatic tumours are multifocal, with 65% being located in the head of the pancreas, and 15% being located in the tail of the pancreas 22. We assume a similar distribution in Ontario patients.
In a study based in the United States, Mack and Paganini-Hill 23 showed that pcc was not evenly distributed by social class. By contrast, we found rather uniform rates by income quintile. However, we did observe decreased survival in the lowest income quintiles and in patients residing in rural areas, which is consistent with other available data 24. An American population study published in 2006 by Cress et al. 24 showed a relationship of socioeconomic status with the proportion of patients receiving surgical resection, with access to high-volume care centres suggested to be the main cause of that disparity. The finding that patients in our cohort from the lowest income quintiles and from rural regions experienced relatively lower survival might indicate that there are barriers to appropriate care in Ontario.
Comorbidity status also appeared to have a significant effect on patient survival in our cohort. That observation supports findings in the existing literature that higher scores on the Charlson comorbidity index might be associated with lower rates of surgical resection 25,26, less-aggressive palliative chemotherapy 27, and relatively worse outcomes with gemcitabine-based chemotherapy 28.
Survival was observed to be greater for patients in our cohort who were diagnosed in 2010–2011 than in 2004–2006. One possible explanation for that finding is the cumulative effect of gradual increases in chemotherapy uptake in the palliative setting 29. Use of recently developed chemotherapy regimens such as folfirinox (approved in Ontario in 2011) continues to increase for pcc patients 30, and survival for individuals diagnosed after 2011 is expected to reflect the effect of those regimens.
Although the ocr does not contain full staging information, the literature suggests that at least half of all pcc patients will have metastatic disease at presentation. Consequently, the effects of the newer chemotherapeutic options on survival at a population scale should be investigated.
The strength of our study is its inclusion of all cases of pcc in the province. The single-payer nature of health care in the province, regardless of health care provider, ensures that data are collected for all patients.
Our study is limited because of the type of information available in the administrative databases. Stage and genetic factors provide clinicians with information about appropriate management and can affect survival. Similarly, the availability of information related to treatment regimens would add considerable insight. In some provinces, collaborative staging permits assignment of an American Joint Committee on Cancer stage based on the best available information. Although some collaborative staging information is available in the ocr, staging information was unknown for 70% of the patients in our cohort for 2004; that proportion improved to only approximately 50% for the years 2007–2011. Details of treatment information such as receipt of chemotherapy, radiation therapy, or surgical therapy are also not collected by the ocr. To obtain those details, it would have been necessary establish links with other databases, which was out of scope for the objectives of this epidemiologic report.
Published studies have examined the effect of risk factors for the development of pcc and factors that affect survival (including smoking, caffeine use, alcohol consumption, comorbidity, occupational exposure, and diet 17,19,31–38), although some of the risks are not clear 39,40. Unfortunately, information on the foregoing risk factors are not available in provincial administrative databases; however, a review of this cohort’s pathology reports to look at the effect of stage on survival is underway.
CONCLUSIONS
Pancreatic cancer, although infrequent, has an exceptionally high mortality rate. Here, we have provided a population- based analysis of pcc from the perspective of patient demographics and survival. Results show the effects of age, sex, income, residence, location of cancer, comorbidity, and year of diagnosis on survival. Future research by anyone using our results as a base case should focus on shifting the survival curve to the right.
ACKNOWLEDGEMENTS
The investigators thank Nelson Chong, Institute for Clinical Evaluative Sciences (ices) Programmer, and Rene Robitaille, Cancer Care Ontario (cco) Programmer for their help in untangling and linking the datasets. We are grateful to Peggy Kee, Thi Ho, and Katrina Chan for administrative insights.
This study was supported through provision of data by ices and cco and through an annual grant by the Ontario Ministry of Health and Long-Term Care and the Ontario Institute for Cancer Research (oicr) that provides funding support to ices. The opinions, results, and conclusions reported in this paper are those of the authors and independent from the funding sources. No endorsement by ices, cco, oicr, or the Government of Ontario is intended or should be inferred. NC is supported through the Sherif and MaryLou Hanna Chair in Surgical Oncology Research.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Canadian Cancer Society’s Advisory Committee on Cancer Statistics . Canadian Cancer Statistics 2014. Toronto, ON: Canadian Cancer Society; 2014. [Google Scholar]
- 2.Ellison LF, Wilkins K. An update on cancer survival. Health Rep. 2010;21:55–60. [PubMed] [Google Scholar]
- 3.Canadian Cancer Society’s Advisory Committee on Cancer Statistics . Canadian Cancer Statistics 2013. Toronto, ON: Canadian Cancer Society; 2013. [Google Scholar]
- 4.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 5.DaCosta Byfield S, Nash Smyth E, Mytelka D, Bowman L, Teitelbaum A. Healthcare costs, treatment patterns, and resource utilization among pancreatic cancer patients in a managed care population. J Med Econ. 2013;16:1379–86. doi: 10.3111/13696998.2013.848208. [DOI] [PubMed] [Google Scholar]
- 6.Sharp L, Carsin AE, Cronin-Fenton DP, O’Driscoll D, Comber H. Is there under-treatment of pancreatic cancer? Evidence from a population-based study in Ireland. Eur J Cancer. 2009;45:1450–9. doi: 10.1016/j.ejca.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 7.Krzyzanowska MK, Weeks JC, Earle CC. Treatment of locally advanced pancreatic cancer in the real world: population-based practices and effectiveness. J Clin Oncol. 2003;21:3409–14. doi: 10.1200/JCO.2003.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Conroy T, Desseigne F, Ychou M, et al. on behalf of the Groupe Tumeurs Digestives of Unicancer and the prodige Intergroup folfirinox versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 9.Michaud D. Epidemiology of pancreatic cancer. Minerva Chir. 2004;59:99–111. [PubMed] [Google Scholar]
- 10.Brenner D, Tammemägi M, Bull S, Pinnaduwaje D, Andrulis I. Using cancer registry data: agreement in cause-of-death data between the Ontario Cancer Registry and a longitudinal study of breast cancer patients. Chronic Dis Can. 2009;30:16–19. [PubMed] [Google Scholar]
- 11.Wilkins R. PCCF+ Version 5E User’s Guide. Automated Geographic Coding Based on the Statistics Canada Postal Code Conversion Files, Including Postal Codes through March 2009. Ottawa, ON: Health Analysis Division, Statistics Canada; 2009. [Google Scholar]
- 12.University of California–Los Angeles (ucla) Institute for Digital Research and Education . Supplemental notes to Applied Survival Analysis [Web page] Los Angeles, CA: UCLA Statistical Consulting Group; n.d. [Available at: http://www.ats.ucla.edu/stat/examples/asa/test_proportionality.htm; cited 4 May 2015] [Google Scholar]
- 13.Quaresma M, Coleman MP, Rachet B. 40-Year trends in an index of survival for all cancers combined and survival adjusted for age and sex for each cancer in England and Wales, 1971–2011: a population-based study. Lancet. 2015;385:1206–18. doi: 10.1016/S0140-6736(14)61396-9. [DOI] [PubMed] [Google Scholar]
- 14.Hurton S, MacDonald F, Porter G, Walsh M, Molinari M. The current state of pancreatic cancer in Canada: incidence, mortality, and surgical therapy. Pancreas. 2014;4:879–85. doi: 10.1097/MPA.0000000000000147. [DOI] [PubMed] [Google Scholar]
- 15.Canadian Partnership Against Cancer (cpac) The 2014 Cancer System Performance Report. Toronto, ON: CPAC; 2014. [Google Scholar]
- 16.Bahl S, Theis B, Nishri D, Chin Cheong S, Marrett L. Cancer in Ontario: Overview. A Statistical Report. Toronto, ON: Cancer Care Ontario; 2010. [Google Scholar]
- 17.Boyle P, Hsieh CC, Maisonneuve P, et al. Epidemiology of pancreas cancer (1988) Int J Pancreatol. 1989;5:327–46. doi: 10.1007/BF02924298. [DOI] [PubMed] [Google Scholar]
- 18.Shaib YH, Davila JA, El-Serag HB. The epidemiology of pancreatic cancer in the United States: changes below the surface. Aliment Pharmacol Ther. 2006;24:87–94. doi: 10.1111/j.1365-2036.2006.02961.x. [DOI] [PubMed] [Google Scholar]
- 19.Lowenfels AB, Maisonneuve P. Epidemiology and risk factors for pancreatic cancer. Best Pract Res Clin Gastroenterol. 2006;20:197–209. doi: 10.1016/j.bpg.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Artinyan A, Soriano PA, Prendergast C, Low T, Ellenhorn JD, Kim J. The anatomic location of pancreatic cancer is a prognostic factor for survival. HPB (Oxford) 2008;10:371–6. doi: 10.1080/13651820802291233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lau MK, Davila JA, Shaib YH. Incidence and survival of pancreatic head and body and tail cancers: a population-based study in the United States. Pancreas. 2010;39:458–62. doi: 10.1097/MPA.0b013e3181bd6489. [DOI] [PubMed] [Google Scholar]
- 22.Ghaneh P, Costello E, Neoptolemos JP. Biology and management of pancreatic cancer. Gut. 2007;56:1134–52. doi: 10.1136/gut.2006.103333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mack TM, Paganini-Hill A. Epidemiology of pancreas cancer in Los Angeles. Cancer. 1981;47(suppl):1474–84. doi: 10.1002/1097-0142(19810315)47:6+<1474::AID-CNCR2820471406>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 24.Cress RD, Yin D, Clarke L, Bold R, Holly EA. Survival among patients with adenocarcinoma of the pancreas: a population-based study (United States) Cancer Causes Control. 2006;17:403–9. doi: 10.1007/s10552-005-0539-4. [DOI] [PubMed] [Google Scholar]
- 25.Sandroussi C, Brace C, Kennedy ED, Baxter NN, Gallinger S, Wei AC. Sociodemographics and comorbidities influence decisions to undergo pancreatic resection for neoplastic lesions. J Gastrointest Surg. 2010;14:1401–8. doi: 10.1007/s11605-010-1255-2. [DOI] [PubMed] [Google Scholar]
- 26.Bilimoria KY, Bentrem DJ, Ko CY, Stewart AK, Winchester DP, Talamonti MS. National failure to operate on early stage pancreatic cancer. Ann Surg. 2007;246:173–80. doi: 10.1097/SLA.0b013e3180691579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho TH, Barbera L, Saskin R, Lu H, Neville BA, Earle CC. Trends in the aggressiveness of end-of-life cancer care in the universal health care system of Ontario, Canada. J Clin Oncol. 2011;29:1587–91. doi: 10.1200/JCO.2010.31.9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakai Y, Isayama H, Sasaki T, et al. Comorbidity, not age, is prognostic in patients with advanced pancreatic cancer receiving gemcitabine-based chemotherapy. Crit Rev Oncol Hematol. 2011;78:252–9. doi: 10.1016/j.critrevonc.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Oberstein PE, Hershman DL, Khanna LG, Chabot JA, Insel BJ, Neugut AI. Uptake and patterns of use of gemcitabine for metastatic pancreatic cancer: a population-based study. Cancer Invest. 2013;31:316–22. doi: 10.3109/07357907.2013.789904. [DOI] [PubMed] [Google Scholar]
- 30.Cartwright TH, Ginsburg A, Wilfong LS, Harrell RK, Hoverman JR. Use of first-line chemotherapy for advanced pancreatic cancer: folfirinox versus gemcitabine-based therapy [abstract 4132] J Clin Oncol. 2014. p. 32. [Available online at: http://meetinglibrary.asco.org/content/132567-144; cited 30 September 2015]
- 31.Andreotti G, Silverman DT. Occupational risk factors and pancreatic cancer: a review of recent findings. Mol Carcinog. 2012;51:98–108. doi: 10.1002/mc.20779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ben Q, Xu M, Ning X, et al. Diabetes mellitus and risk of pancreatic cancer: a meta-analysis of cohort studies. Eur J Cancer. 2011;47:1928–37. doi: 10.1016/j.ejca.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Coughlin SS, Calle EE, Patel AV, Thun MJ. Predictors of pancreatic cancer mortality among a large cohort of United States adults. Cancer Causes Control. 2000;11:915–23. doi: 10.1023/A:1026580131793. [DOI] [PubMed] [Google Scholar]
- 34.Li D. Diabetes and pancreatic cancer. Mol Carcinog. 2012;51:64–74. doi: 10.1002/mc.20771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lucenteforte E, La Vecchia C, Silverman D, et al. Alcohol consumption and pancreatic cancer: a pooled analysis in the International Pancreatic Cancer Case–Control Consortium (PanC4) Ann Oncol. 2012;23:374–82. doi: 10.1093/annonc/mdr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paluszkiewicz P, Smolinska K, Debinska I, Turski WA. Main dietary compounds and pancreatic cancer risk. The quantitative analysis of case–control and cohort studies. Cancer Epidemiol. 2012;36:60–7. doi: 10.1016/j.canep.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Talamini R, Polesel J, Gallus S, et al. Tobacco smoking, alcohol consumption and pancreatic cancer risk: a case–control study in Italy. Eur J Cancer. 2010;46:370–6. doi: 10.1016/j.ejca.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Wynder EL, Mabuchi K, Maruchi N, Fortner JG. Epidemiology of cancer of the pancreas. J Natl Cancer Inst. 1973;50:645–67. doi: 10.1093/jnci/50.3.645. [DOI] [PubMed] [Google Scholar]
- 39.Genkinger JM, Li R, Spiegelman D, et al. Coffee, tea, and sugar-sweetened carbonated soft drink intake and pancreatic cancer risk: a pooled analysis of 14 cohort studies. Cancer Epidemiol Biomarkers Prev. 2012;21:305–18. doi: 10.1158/1055-9965.EPI-11-0945-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inoue-Choi M, Flood A, Robien K, Anderson K. Nutrients, food groups, dietary patterns, and risk of pancreatic cancer in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2011;20:711–14. doi: 10.1158/1055-9965.EPI-11-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marcaccio M, Langer B, Rumble B, et al. on behalf of the Expert Panel on hpb Surgical Oncology . Hepatic, Pancreatic, and Biliary Tract (HPB) Surgical Oncology Standards. Toronto, ON: Cancer Care Ontario; 2006. [Google Scholar]