Abstract
Background
We estimated the relations of sociodemographic, organizational, disease, and treatment variables with the risk of death from colorectal cancer (crc) in a Quebec population-based sample of patients with locally advanced crc (lacrc) who underwent tumour resection with curative intent.
Methods
Information from medical records and administrative databases was obtained for a random sample of 633 patients surgically treated for stages ii–iii rectal and stage iii colon cancer and declared to the Quebec cancer registry in 1998 and 2003. We measured personal, disease, and clinical management characteristics, relative survival, and through multivariate modelling, relative excess rate (rer) of death.
Results
The relative 5- and 10-year survivals in this cohort were 67.7% [95% confidence interval (ci): 65.8% to 69.6%] and 61.2% (95% ci: 58.3% to 64.0%) respectively. Stage T4, stage N2, and emergency rather than elective surgery affected 18%, 24% and 10% of patients respectively. Those disease progression characteristics each independently increased the rer of death by factors of 2 to almost 5. Grade, vascular invasion, and tumour location were also significantly associated with the rer for death. Receiving guideline-adherent treatment was associated with a 60% reduction in the rer for death (0.41; 95% ci: 0.28 to 0.61), an effect that was consistent across age groups. Clear margins (proximal–distal, radial) and clinical trial enrolment were each associated with a nonsignificant 50% reduction in the rer. Of patients less than 70 years of age and 70 years of age and older, 81.3% and 42.0% respectively received guideline-adherent treatment.
Conclusions
This study is the first Quebec population-based examination of patients with lacrc and their management, outcomes, and outcome determinants. The results can help in planning crc control strategies at a population level.
Keywords: Colorectal neoplasms, population-based studies, multivariate analyses, relative survival, relative excess hazard rate for death, treatment outcomes, medical records, registries
INTRODUCTION
Colorectal cancer (crc) is the second most frequent cause of cancer death in Quebec1. Given that the peak incidence of crc occurs in individuals 70–80 years of age2, the burden of crc will increase as the population ages. It is only in stage ii–iii rectum and stage iii colon cancers that chemotherapy, radiotherapy, or chemoradiation have been demonstrated to reduce cancer recurrence and death3–7. In this subgroup of crc patients, referred to here as having locally advanced crc (lacrc), cure is therefore usually attempted through tumour resection with clear margins, complemented by radiotherapy or systemic cancer therapy. When the primary tumour is found to adhere to adjacent organs, invasive en bloc multi-visceral resection is required8.
Patients with lacrc represent 40% of all crc cases in Quebec. From a population perspective, optimal management of this subgroup of crc patients constitutes an important part of crc control, but a considerable fraction are elderly individuals for whom “best management” retains considerable clinical uncertainty. Thus, an increase in the knowledge about both the population-based distributions of treatments and prognostic characteristics in lacrc and the effect of those prognostic characteristics and of current clinical management practices on patient outcomes should help stakeholders to plan more effective cancer control strategies.
To advance such knowledge, we documented the socio-demographic, disease, and clinical management characteristics of a population-based sample of lacrc patients from two age groups (<70 years and ≥70 years) who underwent tumour resection with curative intent, and we estimated the independent effects of various characteristics on the 5-year risk of crc death. We also assessed 5- and 10-year crc survival in this population.
METHODS
Study Population
Briefly, this population-based study used a 22% random sample of all patients with invasive crc declared to the Quebec cancer registry (qcr) in 1998 or 20039. Patients were identified in a multistage process that first took a random sampling of Quebec hospitals and then took a random sampling of crc patients declared by the selected hospitals. Hospitals with an annual cancer caseload of fewer than 5 individuals, who collectively reported fewer than 1% of crc cases, were excluded, as were patients with a prior crc history and those who had been identified through a death certificate or at autopsy. Until 2010, the qcr was based on Quebec’s registry of hospital discharge diagnoses. In 1996, the completeness of the declaration of crc cancer cases to the qcr was estimated to be 97.1%10.
To be eligible for this particular study, patients from the previously identified group had to have TNM stage ii– iii rectal [T3–4N0M0 and T(any)N+M0; International Classification of Diseases, Ninth Revision, codes 154.0, 154.1, 154.8] or stage iii colon [T(any)N+M0; International Classification of Diseases codes 153.0–153.9] and had to have undergone tumour resection. Patients whose tumour resection was not curative in intent were excluded.
Data Collection
Study data were obtained from patient medical records and, using anonymous linkage, from two Régie de l’assurance maladie du Québec (Quebec’s public population-based health insurance) databases: the health insurance population registry (the most complete database of the Quebec population) and the physician claims database. Information was also retrieved from the databases of the 12 radiotherapy centres in Quebec. Two cancer registrars extracted information from the medical records using a pre-coded computerized form. We limited data collection to the first course of treatment, and we considered the patient’s treating hospital to be the one that declared the case to the qcr. For cases in which no first pathology report confirmed the cancer diagnosis, the date of first endoscopy or first imaging revealing the cancer was taken as the date of diagnosis.
Characteristics Studied
Our study considered these variables: year of diagnosis (1998, 2003), sex, age (<70, ≥70), the treating hospital’s annual crc caseload (5–39, 40–89, and 90–188 patients), enrolment in a clinical trial, tumour site (proximal, distal, unknown or overlapping sub-sites of colon, and rectum), T stage (1–2, 3, 4), N stage (0, 1, 2), grade (well and moderately differentiated, poorly differentiated and undifferentiated, unknown), vascular and nervous invasion (no, yes, unknown), emergency surgery, proximal–distal margin and radial margin status [negative (R0), positive (R1–R2), unknown], number of lymph nodes examined (<12, ≥12, unknown), positive lymph node ratio (<75th percentile, ≥75th percentile, unknown), standard or multi-visceral tumour resection, and adherence to treatment guidelines. Patients were considered to have received treatment adherent to lacrc guidelines recognized in North America11–13 whenever chemotherapy, radiotherapy, or chemotherapy and radiation had been given in the adjuvant or neoadjuvant setting. Starting date of radiotherapy was based first on information from the radiotherapy centre, second on information from the medical record, and third on information from physician claims. Starting date of chemotherapy was based first on physician claims and second on information in the medical record. “Proximal colon” was considered to encompass the cecum, appendix, ascending colon, hepatic flexure, and transverse colon; “distal colon” was considered to encompass the splenic flexure and the descending and sigmoid colon. Emergency surgery was defined as surgery performed within 48 hours of presentation with an occlusion or perforation, or performed after introduction of a self-expanding metallic stent. “Positive lymph node ratio” is the ratio of lymph nodes invaded to lymph nodes examined. We considered a patient to have undergone multi-visceral resection whenever the surgical protocol or medical notes mentioned simultaneous resection of the primary tumour and adjacent organs or anatomic structures.
With priority given to pathology information, cancer stage was reconstituted from medical record information about bowel-wall, lymph-node, and distant invasion and from mentions of TNM, Dukes, or modified Astler–Coller stage. Stage was considered to be missing when information to determine it was insufficient. Agreement between the reconstituted stage and the stage mentioned in the medical record, when present, was 90%. Stage reconstitution was based on the American Joint Committee on Cancer (5th edition) rules14.
Statistical Analyses
Descriptive information about the distributions of patients according to sociodemographic and disease characteristics and treatments received are presented by age group. We calculated relative survival starting at 30 days after surgery and continuing for 120 months. Relative survival was calculated as the ratio of observed survival to expected survival in the absence of disease and can be interpreted as the probability of survival for crc patients in the hypothetical situation in which crc would be the only cause of death15,16. Relative survival and its 95% confidence interval were estimated using the Ederer ii method17. The background mortality in the general population used to estimate expected survival was based on data from the Quebec death registry. We used the Wilmoth method18 to construct life tables of all-cause death rates by sex, age (in single years), and calendar year (1981–2008). The Ewbank method19 was used to smooth the single-year life tables. Vital status was documented from the health insurance population registry to June 2009, with survivors at the end of follow-up censored at the date of their last contact with the Régie de l’assurance maladie du Québec. Because survival was equivalent for patients diagnosed in 1998 and 2003, data from the two years were pooled.
We used multivariable modelling of the relative excess hazard rate (rer) of death to quantify the independent statistical associations between crc death risk and each of the sociodemographic, disease, and clinical management characteristics, starting at 30 days after surgery and continuing for 60 months. The excess hazard rate for death is the instantaneous risk of dying from crc, over and above the expected risk of dying from all other causes. It is the mortality counterpart of net crc survival derived from relative survival ratios20. The rer estimates and their 95% confidence intervals are based on a generalized linear model with a Poisson type error15. All available characteristics were entered into the model except those thought to be correlated with others (TNM stage with T stage and N stage; number of lymph nodes examined with positive lymph node ratio; multi-visceral resection with margin status). After confirming negligible differences between weighted and unweighted estimates, sampling design was ignored in all estimate and variance calculations.
We performed additional analyses to explore the effects of potential immortal person-time bias on our results. For 90% of the patients who received adjuvant therapy, that therapy started during the first 12 weeks after surgery. Because survival follow-up began at day 30 after surgery (before the end of the 12-week window), immortal person-time could bias results if patients dying during the exposure window were more likely to be non-exposed subjects. Thus, outcome in the non-treatment group could be worsened. To explore that possibility, we therefore considered the 12 weeks after surgery to be the exposure window, and to evaluate the effect on the rer, we restricted our analyses to the population of patients surviving until 3 months after surgery.
The study protocol was approved by the Commission d’accès à l’information du Québec and by the director of professional services at each hospital selected.
RESULTS
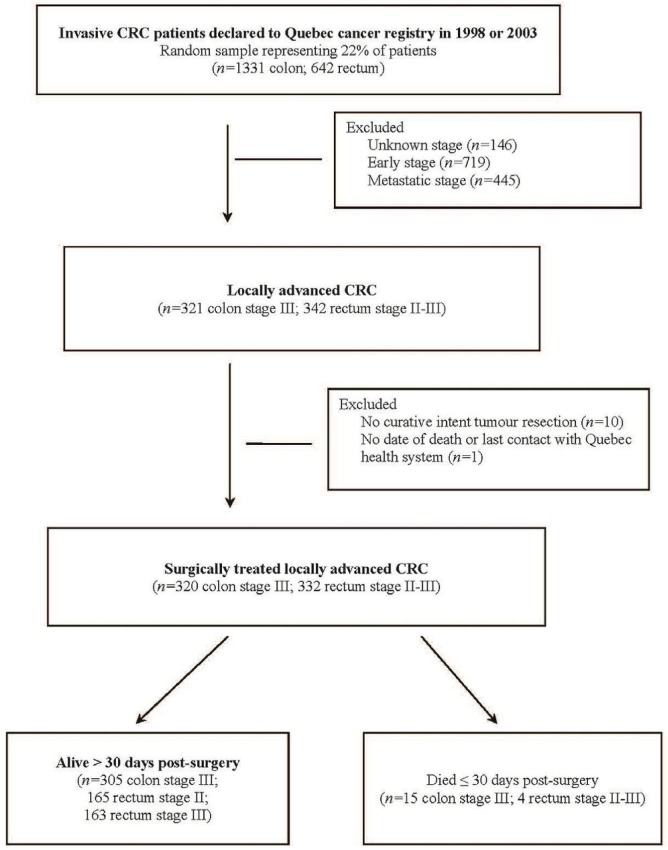
At the outset, 1973 patients among the 8808 with invasive crc declared to the qcr in 1998 and 2003 were selected. Application of the criteria for lacrc resulted in the identification of 663 patients (Figure 1). Fewer than 2% were excluded because they did not undergo curative-intent tumour resection (n = 10) or because their date of death or last contact with the Quebec health care system could not be found (n = 1). Of the remaining 652 patients with lacrc, 19 died within the 30 days after surgery (17 after standard resection, 2 after multi-visceral resection).
FIGURE 1.
Study cohort ascertainment. CRC = colorectal cancer.
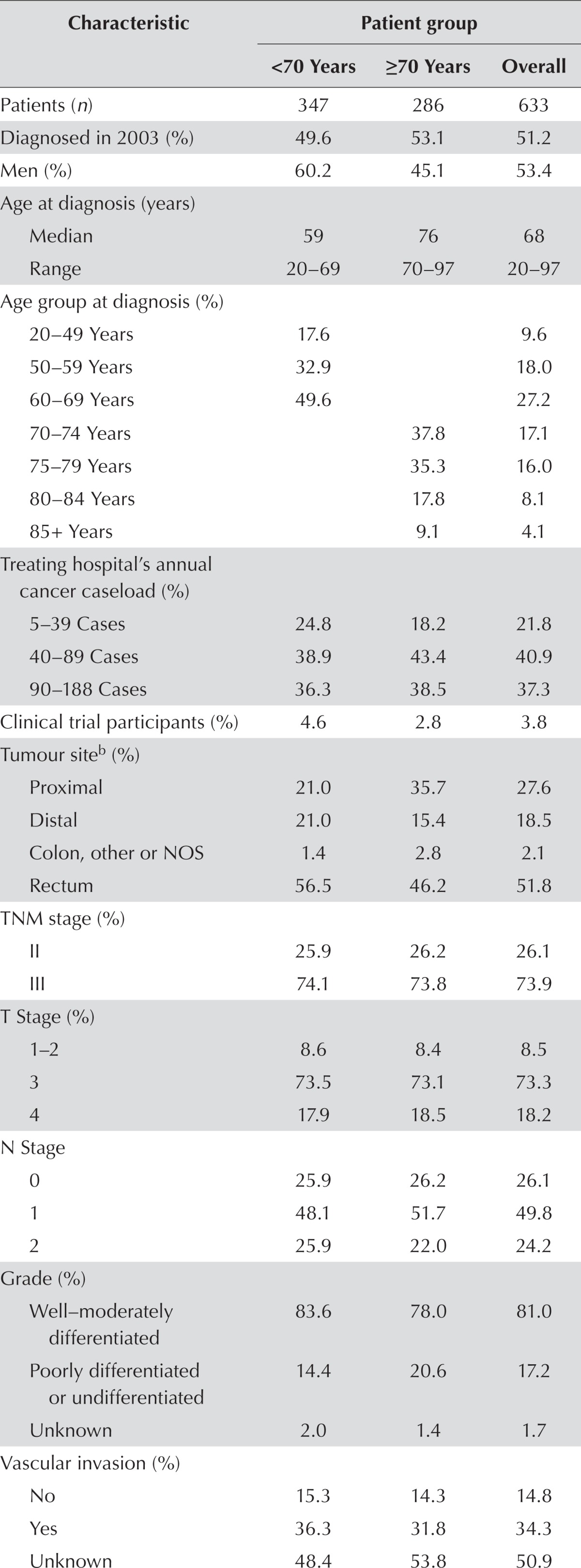
Of the 633 patients who survived for at least 30 days after surgery, 286 (45.2%) were 70 or more years of age. Older patients were more likely to be women and to have a proximal colon cancer (Table i). The 3.3% of patients who underwent multi-visceral resection constituted 4.0% of the group less than 70 years of age and 2.5% of the group 70 or more years of age. Among patients receiving standard surgery, all underwent radical resection, except for 3 who underwent local excision.
TABLE I.
Characteristics of patients with locally advanced colorectal cancera who underwent tumour resection with curative intent
| Characteristic | Patient group | ||
|---|---|---|---|
|
| |||
| <70 Years | ≥70 Years | Overall | |
| Patients (n) | 347 | 286 | 633 |
| Diagnosed in 2003 (%) | 49.6 | 53.1 | 51.2 |
| Men (%) | 60.2 | 45.1 | 53.4 |
| Age at diagnosis (years) | |||
| Median | 59 | 76 | 68 |
| Range | 20–69 | 70–97 | 20–97 |
| Age group at diagnosis (%) | |||
| 20–49 Years | 17.6 | 9.6 | |
| 50–59 Years | 32.9 | 18.0 | |
| 60–69 Years | 49.6 | 27.2 | |
| 70–74 Years | 37.8 | 17.1 | |
| 75–79 Years | 35.3 | 16.0 | |
| 80–84 Years | 17.8 | 8.1 | |
| 85+ Years | 9.1 | 4.1 | |
| Treating hospital’s annual cancer caseload (%) | |||
| 5–39 Cases | 24.8 | 18.2 | 21.8 |
| 40–89 Cases | 38.9 | 43.4 | 40.9 |
| 90–188 Cases | 36.3 | 38.5 | 37.3 |
| Clinical trial participants (%) | 4.6 | 2.8 | 3.8 |
| Tumour site b (%) | |||
| Proximal | 21.0 | 35.7 | 27.6 |
| Distal | 21.0 | 15.4 | 18.5 |
| Colon, other or NOS | 1.4 | 2.8 | 2.1 |
| Rectum | 56.5 | 46.2 | 51.8 |
| TNM stage (%) | |||
| II | 25.9 | 26.2 | 26.1 |
| III | 74.1 | 73.8 | 73.9 |
| T Stage (%) | |||
| 1–2 | 8.6 | 8.4 | 8.5 |
| 3 | 73.5 | 73.1 | 73.3 |
| 4 | 17.9 | 18.5 | 18.2 |
| N Stage | |||
| 0 | 25.9 | 26.2 | 26.1 |
| 1 | 48.1 | 51.7 | 49.8 |
| 2 | 25.9 | 22.0 | 24.2 |
| Grade (%) | |||
| Well–moderately differentiated | 83.6 | 78.0 | 81.0 |
| Poorly differentiated or undifferentiated | 14.4 | 20.6 | 17.2 |
| Unknown | 2.0 | 1.4 | 1.7 |
| Vascular invasion (%) | |||
| No | 15.3 | 14.3 | 14.8 |
| Yes | 36.3 | 31.8 | 34.3 |
| Unknown | 48.4 | 53.8 | 50.9 |
| Neural invasion (%) | |||
| No | 14.7 | 15.0 | 14.9 |
| Yes | 13.0 | 8.0 | 10.7 |
| Unknown | 72.3 | 76.9 | 74.4 |
| Had emergency surgery (%) | 8.9 | 10.5 | 9.6 |
| Margin status (%) | |||
| Negative | 91.6 | 91.6 | 91.6 |
| Positive | 1.4 | 1.7 | 1.6 |
| Unknown | 6.9 | 6.6 | 6.8 |
| Radial margin status (%) | |||
| Negative | 16.7 | 13.6 | 15.3 |
| Positive | 2.9 | 2.1 | 2.5 |
| Unknown | 80.4 | 84.3 | 82.1 |
| Nodes examined (%) | |||
| <12 | 35.7 | 44.4 | 39.7 |
| ≥12 | 53.0 | 47.2 | 50.4 |
| Unknown | 11.2 | 8.4 | 10.0 |
| 75th percentile PLNR,c ×100 (%) | 28.6 | 33.3 | 31.4 |
| Multi-visceral resection | 4.0 | 2.5 | 3.3 |
| Received guideline-adherent treatmentd | 81.3 | 42.0 | 63.5 |
| Died during follow-up | |||
| 1 Month to 1 year | 2.9 | 13.3 | 7.6 |
| 1 Month to 2 years | 13.0 | 29.0 | 20.2 |
| 1 Month to 3 years | 20.7 | 37.8 | 28.4 |
| 1 Month to 4 years | 28.8 | 45.8 | 36.5 |
| 1 Month to 5 years | 33.1 | 50.7 | 41.1 |
| 1 Month to 10 years | 40.3 | 61.2 | 49.8 |
Rectum stages II–III, colon stage III.
“Proximal” includes cecum, appendix, ascending colon, hepatic flexure, and transverse colon; “distal” includes splenic flexure, descending colon, and sigmoid.
Ratio of nodes invaded to nodes examined. Measured in the 89.9% of patients with a known ratio (88.8% in the <70 group, and 91.3% in the ≥70 group).
Adherence was considered positive whenever chemotherapy, radiotherapy, or radiochemotherapy was given in adjuvant or neoadjuvant setting.
NOS = not otherwise specified; PLNR = positive lymph node ratio.
Patients who underwent some form of neoadjuvant or adjuvant cancer therapy were considered to have received guideline-adherent treatment. Such treatment was received by 63.5% of patients overall, by 81.3% of patients less than 70 years of age, and by 42.0% of patients 70 or more years of age (63.9%, 40.6%, 19.6%, and 0% in patients 70–74, 75–79, 80–84, and 85 or more years of age respectively). Median time from diagnosis to surgery was 113 days in those receiving neoadjuvant therapy and 17 days in those not receiving such therapy (data not shown). Median delay from surgery to start of adjuvant therapy was 43 days (41 days in the <70 age group, 47 in the ≥70 age group; data not shown). Whether they had colon or rectum cancer, patients had very similar probabilities of receiving neoadjuvant or adjuvant therapy (63.0% vs. 64.0%) and similar delays between surgery and start of adjuvant therapy (42 days vs. 43 days; data not shown). For details about specific cancer therapies administered, see Table ii.
TABLE II.
Type and setting of adjuvant management received by patients with locally advanced colorectal cancera who underwent curative-intent tumour resection
| Variable | Patient group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Overall | Colon cancer | Rectal cancer | |||||||
|
|
|
|
|||||||
| <70 Years | ≥70 Years | All ages | <70 Years | ≥70 Years | All ages | <70 Years | ≥70 Years | All ages | |
| Patients (n) | 282 | 120 | 402 | 126 | 66 | 192 | 156 | 54 | 210 |
| Type of treatment (%) | |||||||||
| Chemotherapy only | 51.8 | 62.5 | 55.0 | 94.4 | 100.0 | 96.4 | 17.3 | 16.7 | 17.1 |
| Radiotherapy only | 5.0 | 13.3 | 7.5 | — | — | — | 9.0 | 29.6 | 14.3 |
| Chemoradiation | 43.3 | 24.2 | 37.6 | 5.6 | — | 3.7 | 73.7 | 53.7 | 68.6 |
| Treatment setting (%) | |||||||||
| Neoadjuvant only | 3.9 | 11.7 | 6.2 | — | — | — | 7.1 | 25.9 | 11.9 |
| Adjuvant only | 91.1 | 87.5 | 90.1 | 100.0 | 100.0 | 100.0 | 84.0 | 72.2 | 81.0 |
| Both | 5.0 | 0.8 | 3.7 | — | — | — | 9.0 | 1.9 | 7.1 |
Rectum stages II–III, colon stage III.
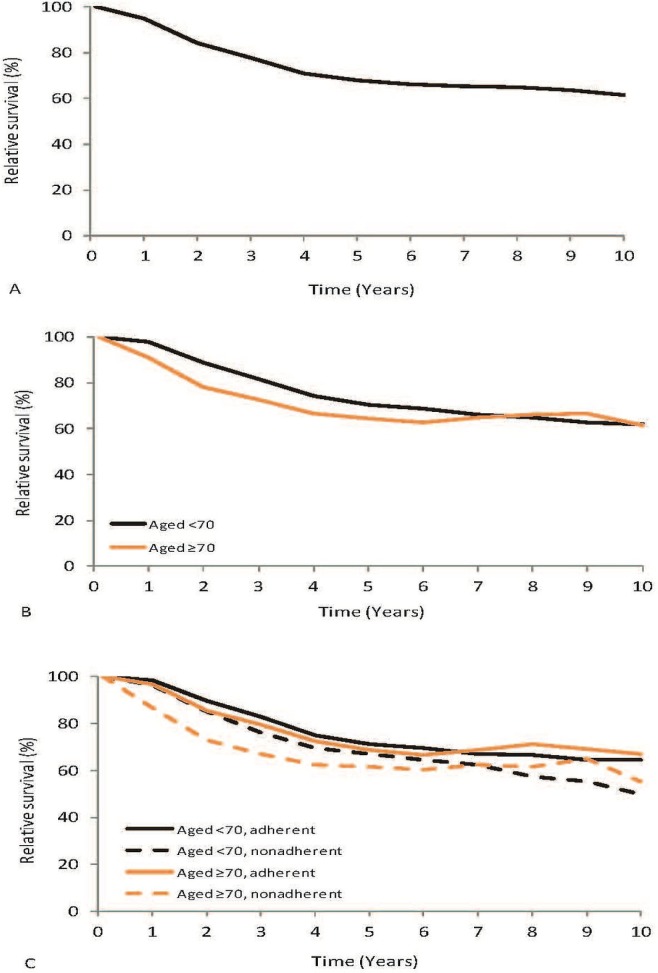
The 5-year relative survival of lacrc patients was 67.7% (95% ci: 65.8% to 69.6%), and the 10-year relative survival was 61.2% [95% ci: 58.3% to 64.0%; Figure 2(A)]. By age group, 5-year survival was 70.4% (95% ci: 68.0% to 72.6%) in patients less than 70 years of age and 64.6% (95% ci: 61.3% to 67.8%) in patients 70 or more years of age [Figure 2(B)]. However, those age differences were no longer apparent 10 years after surgery. For patients less than 70 years of age, 5-year relative survival was 71.1% (95% ci: 68.4% to 73.6%) in the group receiving guideline-adherent treatment and 67.2% (95% ci: 62.5% to 71.6%) in the group whose treatment was not guideline-adherent. For patients 70 or more years of age, the corresponding relative survivals were 68.8% (95% ci: 63.6% to 73.7%) and 61.6% [95% ci: 57.3% to 65.8%; Figure 2(C)].
FIGURE 2.
Relative survival of patients with locally advanced colorectal cancer (rectum stages II–III, colon stage III) who underwent tumour resection with curative intent: (A) all patients diagnosed in 1998 or 2003, (B) all patients according to age at diagnosis, and (C) all patients according to age at diagnosis and adherence to treatment guidelines.
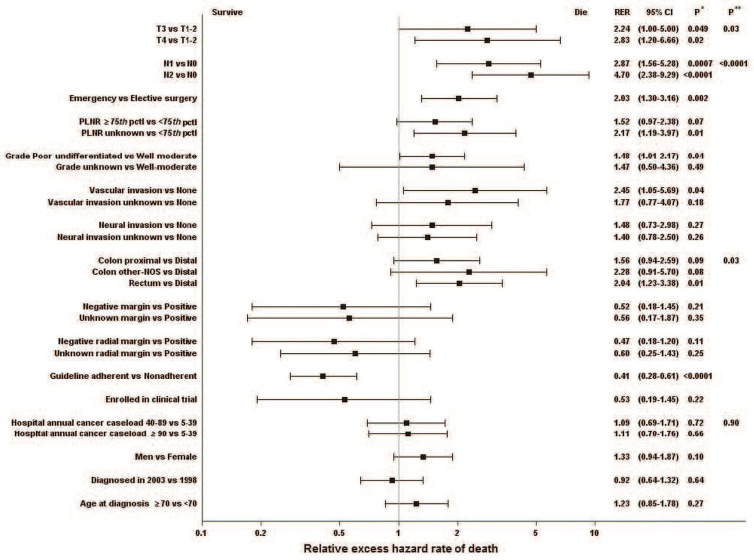
In the multivariate analysis that simultaneously included all characteristics assessed, three characteristics related to disease progression were independently associated with the rer for death (Figure 3). Compared with T stages 1–2, stages 3 and 4 were associated with rers for death of 2.24 (95% ci: 1.00 to 5.00) and 2.83 (95% ci: 1.20 to 6.66) respectively. Compared with N stage 0, N stages 1 and 2 were associated with rers for death of 2.87 (95% ci: 1.56 to 5.28) and 4.70 (95% ci: 2.38 to 9.29) respectively. Need for emergency surgery was associated with a rer for death of 2.03 (95% ci: 1.30 to 3.16).
FIGURE 3.
Multivariate analysis of the relative excess hazard rate for death during the 60-month period after surgery for patients with locally advanced colorectal cancer [LACRC (rectum stages II–III, colon stage III)] who underwent tumour resection with curative intent. * Difference between the “exposed” and reference categories. ** Overall difference between categories for characteristics classified into 3 or more categories without a category for missing values. RER = relative excess hazard rate for death; CI = confidence interval; PLNR = ratio of the number of invaded to examined nodes; pctl = percentile; NOS = not otherwise specified.
Tumour characteristics that were independently associated with an excess hazard for death were grade (poorly differentiated or undifferentiated vs. well or moderately differentiated: rer: 1.48; 95% ci: 1.01 to 2.17) and vascular invasion (invasion versus no invasion: rer: 2.45; 95% ci: 1.05 to 5.69). Tumour location in the rectum, compared with the distal colon, was associated with a rer for death of 2.04 (95% ci: 1.23 to 3.38).
With respect to management, receipt of guideline-adherent treatment—measured here as receipt of either neoadjuvant or adjuvant therapy (including chemo-therapy, radiotherapy, or both)—was, compared with not receiving such treatment, associated with a 60% reduction in excess hazard for death (rer: 0.41; 95% ci: 0.28 to 0.61). Having clear (R0) surgical margins, either proximal–distal or radial, was associated with a 50% reduction in the excess hazard for death, but the differences were not statistically significant (proximal–distal rer: 0.52; 95% ci: 0.18 to 1.45; radial rer: 0.47; 95% ci: 0.18 to 1.20). Enrolment in a clinical trial was also associated with a statistically nonsignificant reduction in the hazard for death (rer: 0.53; 95% ci: 0.19 to 1.45). None of the organizational or sociodemographic characteristics considered affected the excess hazard for death. The rer for death associated with guideline adherence was 0.44 (95% ci: 0.25 to 0.78) for patients less than 70 years of age and 0.38 (95% ci: 0.22 to 0.66) for patients 70 or more years of age.
The restriction of the study population to patients surviving 3 months after surgery to explore the potential for immortal person-time-bias scarcely changed the results (data not shown).
DISCUSSION
In this Quebec population-based sample of surgically treated stages ii–iii rectum and stage iii colon cancer patients (that is, patients with locally advanced disease), 10-year cancer survival reached 61%. Certain characteristics related to disease progression were strongly associated with the 5-year risk for death from crc: T4 stage (representing 18% of the patients), N2 stage (representing 24%), and need for emergency surgery (occurring in 10%) each independently increased the excess hazard for death by factors of 2 to almost 5. Using our definition of guideline-adherent treatment (treatment that included neoadjuvant or adjuvant radiation therapy, chemotherapy, or both), 81% of patients less than 70 years of age and 42% of those 70 or more years of age were treated according to guidelines. Treatment that followed guidelines was associated with a 60% reduction in the excess hazard for death, although that finding must be interpreted with caution, as will be discussed shortly. Achieving clear proximal–distal or radial surgical margins was also associated with a better outcome, but those associations were not statistically significant. As well, participating in a clinical trial was associated with a 50% reduction in the excess hazard for death, but again, the reduction was not statistically significant. Only 4% of patients were enrolled in a trial. Sex, age, year of diagnosis, and hospital cancer caseload were not associated with the excess hazard for death.
Results from this analysis are consistent with those from other multivariate analyses of disease and treatment characteristics related to patient outcome in lacrc21–23. In a recent meta-analysis, Böckelman et al.23 demonstrated the independent prognostic value of T stage, N stage, vascular invasion, neural invasion, positive lymph node ratio, and grade on the 5-year risk of cancer recurrence among stage iii colon cancer patients.
Caution is needed when interpreting the estimated 60% mortality reduction associated with guideline adherence. Because no statistical adjustment was made for the underlying health and functional status of the patients, that finding should be interpreted to reflect both the effect of guideline adherence and the effect of the underlying general health and functioning in those patients, such that those receiving guideline-adherent treatment were also more likely to be in better overall health than patients not so treated21,24,25. Furthermore, some overestimation of the effect of guideline adherence could result from the intrinsic nature of the rer measure, in which the population-matched mortality rates used to estimate the expected-hazard death rates do not account for differences in general health and functioning because the rates are matched solely on age, sex, and calendar year. The cancer-specific mortality reduction associated with guideline adherence in lacrc patients generally ranges from 30% to 40% in observational studies, and most of those studies are also affected by some degree of confounding by underlying health status2,21,25–28. A landmark pooled analysis of trials of fluorouracil-based adjuvant chemotherapy in stage iii colon cancer patients published in 2001 reported a 24% overall mortality reduction across all age groups29. The magnitude of the guideline-adherence disparity observed in the present study between patients less than 70 years of age and those 70 or more years of age appears comparable to disparities noted elsewhere in Canada for similar years30–34.
Our study has several strengths. Its population-based design means that results can be generalized to real-world practice. Also, the systematic medical record review coupled with linkage to administrative health care databases means that we were able to reconstitute disease stage and minimize missing values for that characteristic. Information from the medical record also meant that we were able to take into account the most commonly measured and recognized prognostic characteristics in crc.
Study limitations include low statistical power (resulting from the small sample size and a non-negligible proportion of missing information for some variables) to detect some clinically important differences. The lack of power was particularly true for the status of the radial margin, which was unknown for more than 80% of patients. Although some recently discovered tumour markers were not taken into account, all the disease and treatment characteristics examined in this study are still in use, because the newer markers have not yet been proved to better discriminate between patients35. Other known crc prognostic factors that could not be accounted for in the present study are pre-treatment levels of carcinoembryonic antigen, response to neoadjuvant therapy, and type of surgery (standard vs. total mesorectal excision). However, pathologic T and N stage are recognized as good surrogates of both response to neoadjuvant therapy and pre-treatment stage22.
By considering the various factors that, from a population-based perspective, affect the outcome of lacrc, our study highlights potentially promising control strategies in crc. First, the pathologic and management characteristics associated with disease progression were the ones that most affected the 5-year risk of crc death, and substantial fractions of both younger and older patients had such characteristics. That finding underscores the need for population-based strategies in addition to organized screening programs to accelerate detection and management of subclinical and clinical crc cases. Such strategies should include enhancements to symptom awareness, encouragement of timely referral for suspicious cases, and assurance of rapid progress through the investigation phase and the full spectrum of care36. Second, our results provide the first evidence of an age-related discrepancy in treatment in this group of patients in Quebec. That observation highlights the importance of monitoring and understanding the nature of lacrc patient management to achieve assurance that all age groups receive optimal treatment, particularly now that several consensus statements have addressed crc management in elderly patients2,37,38. Third, there is reason to think that surgical approaches in crc have evolved considerably in Quebec since 2003, and our results provide a baseline for monitoring changes in surgical practice. For example, in many countries, use of en bloc multi-visceral resection has been rising steadily since the mid-1990s, with some institutions reporting rates in the range of 6%–12% for T3–4 patients39,40. In our population sample, with its almost 92% of T3–4 tumours, multi-visceral resection was performed in 3% of patients. Fourth, the quality of reporting on characteristics such as neural and vascular invasion and margin status might have improved since 2003 and should be assessed. Finally, our findings suggest that a better understanding of the factors that favour enrolment of cancer patients in Quebec in clinical trials is needed.
CONCLUSIONS
This population-based portrait of patients with stage ii–iii rectum and stage iii colon cancers, their management, and their survival and determinants of survival suggests that the use of screening and additional population-based strategies to tackle the extent of disease progression in preclinical and clinical lacrc, thus accelerating detection and treatment, will likely be the most promising avenues for making gains in crc control. The age discrepancy in the treatment of lacrc in Quebec, as elsewhere, is an issue that has to be addressed to ensure care equity and possibly to make further gains in crc control.
ACKNOWLEDGEMENTS
We acknowledge the Régie de l’assurance maladie du Québec and the Ministère de la santé et des services sociaux du Québec for their support in the creation of the sample database. We are also grateful to directors of departments of Professional Services and of Health Records of participating hospitals for their ongoing support throughout data collection from medical records.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Canadian Cancer Society’s Advisory Committee on Cancer Statistics . Canadian Cancer Statistics 2014. Toronto, ON: Canadian Cancer Society; 2014. [Google Scholar]
- 2.Papamichael D, Audisio R, Horiot JC, et al. Treatment of the elderly colorectal cancer patient: siog expert recommendations. Ann Oncol. 2009;20:5–16. doi: 10.1093/annonc/mdn532. [DOI] [PubMed] [Google Scholar]
- 3.Petersen SH, Harling H, Kirkeby LT, Wille-Jorgensen P, Mocellin S. Postoperative adjuvant chemotherapy in rectal cancer operated for cure. Cochrane Database Syst Rev. 2012;3:CD004078. doi: 10.1002/14651858.CD004078.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German cao/aro/aio-94 randomized phase iii trial after a median follow-up of 11 years. J Clin Oncol. 2012;30:1926–33. doi: 10.1200/JCO.2011.40.1836. [DOI] [PubMed] [Google Scholar]
- 5.Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991;324:709–15. doi: 10.1056/NEJM199103143241101. [DOI] [PubMed] [Google Scholar]
- 6.Gill S, Loprinzi CL, Sargent DJ, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage ii and iii colon cancer: who benefits and by how much? J Clin Oncol. 2004;22:1797–806. doi: 10.1200/JCO.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 7.Sargent D, Sobrero A, Grothey A, et al. Evidence for cure by adjuvant therapy in colon cancer: observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2009;27:872–7. doi: 10.1200/JCO.2008.19.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson H, Petrelli N, Carlin A, et al. on behalf of the National Cancer Institute Expert Panel Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst. 2001;93:583–96. doi: 10.1093/jnci/93.8.583. [DOI] [PubMed] [Google Scholar]
- 9.Perron L, Major D, Brisson J. Survie relative et portrait des soins et services reçus par les hommes et femmes du Québec avec un cancer colorectal déclaré en 1998 et en 2003. Québec, QC: l’Institut national de santé publique du Québec; 2014. [Google Scholar]
- 10.Brisson J, Major D, Pelletier E. Évaluation de l’exhaustivité du fichier des tumeurs du Québec. Québec, QC: l’Institut national de santé publique du Québec; 2003. [Google Scholar]
- 11.Engstrom PF, Benson AB, 3rd, Saltz L, on behalf of the National Comprehensive Cancer Network Colon cancer. Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2003;1:40–53. doi: 10.6004/jnccn.2003.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engstrom PF, Benson AB, 3rd, Saltz L, on behalf of the National Comprehensive Cancer Network Rectal cancer. Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2003;1:54–63. doi: 10.6004/jnccn.2003.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NIH consensus conference Adjuvant therapy for patients with colon and rectal cancer. JAMA. 1990;264:1444–50. doi: 10.1001/jama.1990.03450110090034. [DOI] [PubMed] [Google Scholar]
- 14.Fleming ID, Cooper JS, Henson DE, et al., editors. AJCC Cancer Staging Manual. 5th ed. New York, NY: Lippincott–Raven Publishers; 1997. [Google Scholar]
- 15.Dickman PW, Slggett A, Hills M, Hakulinen T. Regression models for relative survival. Stat Med. 2004;23:51–64. doi: 10.1002/sim.1597. [DOI] [PubMed] [Google Scholar]
- 16.Ellison LF, Wilkins K. An update on cancer survival. Health Rep. 2010;21:55–60. [PubMed] [Google Scholar]
- 17.Ederer F, Axtell LM, Cutler SJ. The relative survival rate: a statistical methodology. Natl Cancer Inst Monogr. 1961;6:101–21. [PubMed] [Google Scholar]
- 18.Wilmoth JR, Andreev K, Jdanov D, Glei DA. Methods Protocol for the Human Mortality Database. Berkeley, CA, and Rostock, Germany: University of California–Berkeley and Max Planck Institute for Demographic Research; 2007. [Available online at: http://www.mortality.org/Public/Docs/MethodsProtocol.pdf; cited 9 April 2014] [Google Scholar]
- 19.Ewbank DC, Gomez De Leon JC, Stoto MA. A reducible four-parameter system of model life tables. Popul Stud (Camb) 1983;37:105–27. doi: 10.1080/00324728.1983.10405927. [DOI] [PubMed] [Google Scholar]
- 20.Perme MP, Stare J, Esteve J. On estimation in relative survival. Biometrics. 2012;68:113–20. doi: 10.1111/j.1541-0420.2011.01640.x. [DOI] [PubMed] [Google Scholar]
- 21.Potosky AL, Harlan LC, Kaplan RS, Johnson KA, Lynch CF. Age, sex, and racial differences in the use of standard adjuvant therapy for colorectal cancer. J Clin Oncol. 2002;20:1192–202. doi: 10.1200/JCO.20.5.1192. [DOI] [PubMed] [Google Scholar]
- 22.Das P, Crane CH. Staging, prognostic factors, and therapy of localized rectal cancer. Curr Oncol Rep. 2009;11:167–74. doi: 10.1007/s11912-009-0025-3. [DOI] [PubMed] [Google Scholar]
- 23.Böckelman C, Engelmann BE, Kaprio T, Hansen TF, Glimelius B. Risk of recurrence in patients with colon cancer stage ii and iii: a systematic review and meta-analysis of recent literature. Acta Oncol. 2015;54:5–16. doi: 10.3109/0284186X.2014.975839. [DOI] [PubMed] [Google Scholar]
- 24.Abraham A, Habermann EB, Rothenberger DA, et al. Adjuvant chemotherapy for stage iii colon cancer in the oldest old: results beyond clinical guidelines. Cancer. 2013;119:395–403. doi: 10.1002/cncr.27755. [DOI] [PubMed] [Google Scholar]
- 25.Sanoff HK, Carpenter WR, Sturmer T, et al. Effect of adjuvant chemotherapy on survival of patients with stage iii colon cancer diagnosed after age 75 years. J Clin Oncol. 2012;30:2624–34. doi: 10.1200/JCO.2011.41.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sundararajan V, Mitra N, Jacobson JS, Grann VR, Heitjan DF, Neugut AI. Survival associated with 5-fluorouracil-based adjuvant chemotherapy among elderly patients with node-positive colon cancer. Ann Intern Med. 2002;136:349–57. doi: 10.7326/0003-4819-136-5-200203050-00007. [DOI] [PubMed] [Google Scholar]
- 27.Iwashyna TJ, Lamont EB. Effectiveness of adjuvant fluorouracil in clinical practice: a population-based cohort study of elderly patients with stage iii colon cancer. J Clin Oncol. 2002;20:3992–8. doi: 10.1200/JCO.2002.03.083. [DOI] [PubMed] [Google Scholar]
- 28.Jessup JM, Stewart A, Greene FL, Minsky BD. Adjuvant chemotherapy for stage iii colon cancer: implications of race/ethnicity, age, and differentiation. JAMA. 2005;294:2703–11. doi: 10.1001/jama.294.21.2703. [DOI] [PubMed] [Google Scholar]
- 29.Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001;345:1091–7. doi: 10.1056/NEJMoa010957. [DOI] [PubMed] [Google Scholar]
- 30.Eldin NS, Yasui Y, Scarfe A, Winget M. Adherence to treatment guidelines in stage ii/iii rectal cancer in Alberta, Canada. Clin Oncol (R Coll Radiol) 2012;24:e9–17. doi: 10.1016/j.clon.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Maddison AR, Asada Y, Urquhart R, Johnston G, Burge F, Porter G. Inequity in access to guideline-recommended colorectal cancer treatment in Nova Scotia, Canada. Healthc Policy. 2012;8:71–87. [PMC free article] [PubMed] [Google Scholar]
- 32.Winget M, Hossain S, Yasui Y, Scarfe A. Characteristics of patients with stage iii colon adenocarcinoma who fail to receive guideline-recommended treatment. Cancer. 2010;116:4849–56. doi: 10.1002/cncr.25250. [DOI] [PubMed] [Google Scholar]
- 33.Rayson D, Urquhart R, Cox M, Grunfeld E, Porter G. Adherence to clinical practice guidelines for adjuvant chemotherapy for colorectal cancer in a Canadian province: a population-based analysis. J Oncol Pract. 2012;8:253–9. doi: 10.1200/JOP.2012.000578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cree M, Tonita J, Turner D, et al. Comparison of treatment received versus long-standing guidelines for stage iii colon and stage ii/iii rectal cancer patients diagnosed in Alberta, Saskatchewan, and Manitoba in 2004. Clin Colorectal Cancer. 2009;8:141–5. doi: 10.3816/CCC.2009.n.023. [DOI] [PubMed] [Google Scholar]
- 35.Dahl O, Pfeffer F. Twenty-five years with adjuvant chemo-therapy for colon cancer—a continuous evolving concept. Acta Oncol. 2015;54:1–4. doi: 10.3109/0284186X.2014.958533. [DOI] [PubMed] [Google Scholar]
- 36.Del Giudice ME, Vella ET, Hey A, Simunovic M, Harris W, Levitt C. Guideline for referral of patients with suspected colorectal cancer by family physicians and other primary care providers. Can Fam Physician. 2014;60:717–23. e383–90. [PMC free article] [PubMed] [Google Scholar]
- 37.Vickers M, Samson B, Colwell B, et al. Eastern Canadian Colorectal Cancer Consensus Conference: setting the limits of resectable disease. Curr Oncol. 2010;17:70–7. doi: 10.3747/co.v17i3.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pallis AG, Papamichael D, Audisio R, et al. eortc Elderly Task Force experts’ opinion for the treatment of colon cancer in older patients. Cancer Treat Rev. 2010;36:83–90. doi: 10.1016/j.ctrv.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 39.Lehnert T, Methner M, Pollok A, Schaible A, Hinz U, Herfarth C. Multivisceral resection for locally advanced primary colon and rectal cancer: an analysis of prognostic factors in 201 patients. Ann Surg. 2002;235:217–25. doi: 10.1097/00000658-200202000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yun SH, Yun HR, Lee WS, Cho YB, Lee WY, Chun HK. The clinical outcome and prognostic factors after multi-visceral resection for advanced colon cancer. Eur J Surg Oncol. 2009;35:721–7. doi: 10.1016/j.ejso.2008.01.024. [DOI] [PubMed] [Google Scholar]