Abstract
Palbociclib, an oral small-molecule inhibitor of cyclin-dependent kinases 4 and 6, was recently approved by the U.S. Food and Drug Administration in combination with letrozole for postmenopausal women with advanced hormone receptor–positive, her2-negative breast cancer. Patients with loss of CDKN2A (p16), an inherent negative regulator of cyclin-dependent kinases 4 and 6, were not separately studied because of the significant response of the patients selected based only on receptor status. Here, we report a patient with metastatic estrogen receptor– positive, her2-negative breast cancer with CDKN2A loss who experienced a clinical response to palbociclib.
Keywords: Medical oncology, breast cancer
INTRODUCTION
The paloma-1 trial of palbociclib, an oral small-molecule inhibitor of cyclin-dependent kinases 4 and 6 (cdk4/6), in combination with letrozole in postmenopausal women with advanced estrogen receptor (er)–positive, her2- negative breast cancer recently reported a significant progression-free survival (pfs) benefit in the palbociclib group1. The original design featured two cohorts: cohort 1 is based on receptor status, and cohort 2 is based on cyclin D1 (CCND1) amplification or p16 (INK4a or CDKN2A) loss. However, based on the significant response in cohort 1, the authors felt that further selection based on markers would be unlikely to improve outcomes, and so the two cohorts were combined. Here, we report a patient with metastatic er-positive, her2-negative breast cancer with CDKN2A loss who experienced a clinical response on palbociclib.
CASE DESCRIPTION
A 66-year-old woman with previously normal mammograms presented in March 2004 with a hard and firm left breast. Biopsy showed stage iv T3N3cM1 infiltrating ductal carcinoma, er-positive (50%), progesterone receptor– negative, and her2-negative, with a palpable solitary metastasis to a left cervical lymph node.
The patient was treated in March 2004 on a phase ii clinical trial with ixabepilone, progressing in February 2005 after 13 cycles. She underwent bilateral mastectomy and radiation to the left chest (5040 cGy), with a 900 cGy boost to the scar, 540 cGy boost to the axilla, 450 cGy boost to the supraclavicular region, and 720 cGy boost to the cervical nodes. She was treated with anastrozole from April 2005 to 2006, with imaging showing incidental liver lesions (felt to be unrelated to the breast cancer) and granulomatous disease of the lungs.
Because of patient preference, she was switched to fulvestrant and remained on that treatment until imaging in January 2014 showed progression of disease in the lung, shoulder, para-mediastinal, and supraclavicular regions. She also developed left eye ptosis, ultimately diagnosed as Horner syndrome, and worsening left arm brachial plexopathy, loss of arm function, and severe lymphedema despite treatment with hyperbaric oxygen, pentoxifylline, vitamin E, and a compression sleeve.
The patient’s course was complicated by a 1 cm hyper-pigmented lesion on her right chest wall that was concerning for melanoma. In February 2014, she underwent wide local excision, with pathology showing melanoma in situ with partial regression. Because of positive margins, she underwent a repeat excision, which did not show melanoma. With her worsening left arm swelling and pain, she ultimately underwent a forequarter amputation of the left upper extremity on 7 May 2014. Pathology showed meta-static adenocarcinoma consistent with the breast primary: er-positive, progesterone receptor–negative, and her2- negative. FoundationOne genomic testing (Foundation Medicine, Cambridge, MA, U.S.A.) of her resected tumour showed CDKN2A loss and normal RB1.
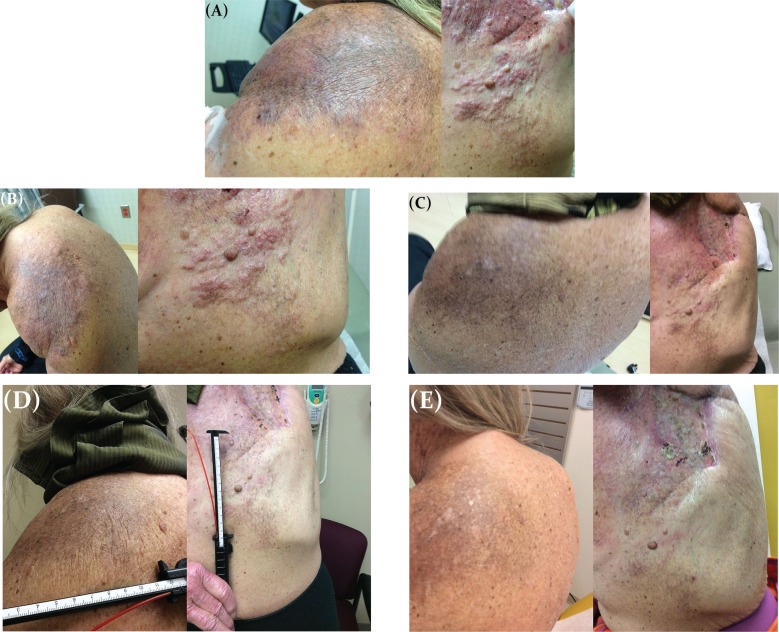
The patient was re-treated with anastrozole for 7 weeks before enrolling on a trial of capecitabine with or without ruxolitinib on 10 September 2014. However, after 8 weeks, she progressed, with extensive skin metastases. On 13 November 2014, she enrolled on a Pfizer-sponsored palbociclib expanded-access program and started palbociclib 125 mg daily for 21 days of a 28-day cycle and letrozole 2.5 mg daily. She completed cycle 1 on 3 December 2014 and, complicated by grade 3 febrile neutropenia, was delayed in starting cycle 2, during which her palbociclib dose was reduced to 100 mg. She started cycle 2 on 15 December 2014, and on day 15, her first clinical response was documented (Figure 1). She completed 4 cycles of palbociclib before developing worsening shortness of breath. Imaging on 11 March 2015 showed progression of the metastatic disease in the lung. She is currently on weekly paclitaxel.
FIGURE 1.
Clinical improvement on palbociclib. (A) Before palbociclib, 10 November 2014. (B) Cycle 1, day 12, 24 November 2014. (C) Cycle 2, day 15, 29 December 2014. (D) Cycle 3, day 1, 12 January 2015. (E) Cycle 4, day 1, 9 February 2015.
DISCUSSION AND CONCLUSIONS
The paloma-1 trial showed a significant pfs benefit of 10 months for treatment with palbociclib and letrozole compared with letrozole alone in breast cancer patients with hormone receptor (hr)–positive, her2-negative disease1. Recently, the results of paloma-3, a phase iii randomized placebo-controlled trial comparing palbociclib–fulvestrant with placebo–fulvestrant, were presented at the 2015 annual meeting of the American Society of Clinical Oncology and in the New England Journal of Medicine2.
The paloma-3 trial looked at 521 patients with advanced hr-positive, her2-negative disease who had progressed on prior endocrine therapy. Both pre- and postmenopausal patients were included, with pre- and perimenopausal patients also receiving goserelin. The primary endpoint was pfs. At its preplanned interim analysis, the study had met its primary endpoint: median pfs was 9.2 months for palbociclib–fulvestrant and 3.8 months for placebo– fulvestrant (p < 0.001). Subgroup analyses showed that pre-, peri-, and postmenopausal patients all benefitted from the addition of palbociclib. As was also seen in paloma-1, more adverse events—primarily myelosuppression—occurred in the palbociclib arm. The mechanism of action behind the myelosuppression is not fully understood, but mouse models suggest that cdk4/6 inhibitors such as palbociclib cause hematopoietic stem cells to enter a reversible quiescent phase; however, the cells do not undergo apoptosis3. As a result, the mature cells that ultimately circulate in the peripheral blood are reduced in number, thus causing myelosuppression. Once palbociclib is discontinued and the cdk4/6 inhibition is lifted, the quiescent hematopoietic stem cells are able to re-enter the cell cycle, thus restoring peripheral blood cell counts. This mechanism of action poses an interesting question about the role of granulocyte colony–stimulating factor, which inhibits apoptosis and increases hematopoietic stem-cell proliferation; studies are underway to investigate that role.
The interaction of cdk4/6 with cyclin D1 allows cell-cycle progression from G1 to S through the hyperphosphorylation of retinoblastoma protein, causing protein inactivation and release of transcription factor E2F. Cyclin D1 is amplified in approximately 15%–20% of breast cancers. Studies in mouse models have shown that the cdk4–cyclin D1 interaction is necessary for maintaining tumour cell proliferation; mice lacking cdk4 are resistant to cancers driven by certain oncogenes, such as her24. However, although cdk4 and cdk6 have 71% amino acid homology, a recent publication has suggested a new role for cdk6 in activating hematopoietic stem cells5. Thus, it could be hypothesized that the myelosuppression seen in palbociclib could result from an inhibition of cdk6 and, hence, hematopoiesis.
Abemaciclib is a new drug that, like palbociclib, inhibits G1 to S cell-cycle progression, but is a more potent inhibitor of cdk4–cyclin D1 rather than cdk6–cyclin D16. Phase i studies have determined a maximum tolerated dose of 200 mg every 12 hours for abemaciclib7, and currently, several open clinical trials for patients with hr-positive, her2-negative breast cancer are underway, looking at abemaciclib monotherapy (https://clinicaltrials.gov/ct2/show/NCT02102490), abemaciclib with fulvestrant (https://clinicaltrials.gov/ct2/show/NCT02107703), abemaciclib with aromatase inhibitors (https://clinicaltrials.gov/ct2/show/NCT02246621), and abemaciclib in the neoadjuvant setting (https://clinicaltrials.gov/ct2/show/NCT02441946).
Recently, another cdk 4/6 inhibitor, ribociclib, has been shown to have activity in neuroblastoma cell lines8. It is also being actively studied in patients with advanced hr-positive, her2-negative breast cancer in combination with letrozole, and in premenopausal women combined with goserelin plus tamoxifen compared with an aromatase inhibitor (https://clinicaltrials.gov/ct2/show/NCT01919229, https://clinicaltrials.gov/ct2/show/NCT01958021, https://clinicaltrials.gov/ct2/show/NCT02422615, and https://clinicaltrials.gov/ct2/show/NCT02278120). The results from those trials will not only shed light on the efficacy of the new cdk4/6 inhibitors in these various settings, but also their toxicity profiles.
Palbociclib, abemaciclib, and ribociclib are oral drugs that inhibit cdk4/6, but cells also possess an inherent negative regulator of the cdk4/6–cyclin D1 interaction, namely CDKN2A (p16). Studies have shown higher p16 expression in invasive ductal cancer than in ductal carcinoma in situ, and that expression is associated with triple-negative and high-grade breast cancers9–11. Interestingly, however, p16 expression has also been correlated with good response to chemotherapy, improved disease-free survival, improved breast cancer–specific survival, and a trend toward improved overall survival11,12. Furthermore, preclinical studies show that palbociclib inhibits the cell cycle and growth in cells with decreased p1613. Based on those pre-clinical data and our case, further studies of patients with CDKN2A loss would, perhaps, be of interest and benefit for that population.
ACKNOWLEDGEMENTS
This case report is published with the explicit permission and consent of the patient and of Pfizer.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: SMS’s institution has received research funding from Pfizer and Incyte. SMS is also a paid advisory board consultant for Pfizer. The other authors declare that they have no conflicts of interest. All authors contributed to the writing and revision of the report and approved the final version.
REFERENCES
- 1.Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, her2-negative, advanced breast cancer (paloma-1/trio-18): a randomized phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 2.Turner NC, Ro J, Andre F, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373:209–19. doi: 10.1056/NEJMoa1505270. [DOI] [PubMed] [Google Scholar]
- 3.Johnson SM, Torrice CD, Bell JF, et al. Mitigation of hematologic radiation toxicity in mice through pharmacological quiescence induced by cdk4/6 inhibition. J Clin Invest. 2010;120:2528–36. doi: 10.1172/JCI41402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu Q, Sicinska E, Geng Y, et al. Requirement for cdk4 kinase function in breast cancer. Cancer Cell. 2006;9:23–32. doi: 10.1016/j.ccr.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Scheicher R, Hoelbl-Kovacic A, Bellutti F, et al. cdk6 as a key regulator of hematopoietic and leukemic stem cell activation. Blood. 2015;125:90–101. doi: 10.1182/blood-2014-06-584417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gong X, Chiou LC, Lallena M, et al. Molecular features that determine the sensitivity of cancer cells to abemaciclib, an inhibitor of cdk4 and cdk6 [abstract 3104] Cancer Res. 2015;75:3104. doi: 10.1158/1538-7445.AM2015-3104. [DOI] [Google Scholar]
- 7.Patnaik A, Rosen LS, Tolaney SM, et al. Clinical activity of LY2835219, a novel cell cycle inhibitor selective for cdk4 and cdk6, in patients with metastatic breast cancer [abstract CT232] Cancer Res. 2014;74 [Google Scholar]
- 8.Rader J, Russell MR, Hart LS, et al. Dual cdk4/cdk6 inhibition induces cell-cycle arrest and senescence in neuroblastoma. Clin Cancer Res. 2013;19:6173–82. doi: 10.1158/1078-0432.CCR-13-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shan M, Zhang X, Liu X, et al. p16 and p53 play distinct roles in different subtypes of breast cancer. PLoS One. 2013;8:e76408. doi: 10.1371/journal.pone.0076408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JJ, Ko E, Cho J, et al. Methylation and immunoexpression of p16(INK4a) tumor suppressor gene in primary breast cancer tissue and their quantitative p16(INK4a) hypermethylation in plasma by real-time pcr. Korean J Pathol. 2012;46:554–61. doi: 10.4132/KoreanJPathol.2012.46.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bogina GS, Lunardi G, Marcolini L, et al. p16 but not retinoblastoma expression is related to clinical outcome in no-special-type triple-negative breast carcinomas. Mod Pathol. 2014;27:204–13. doi: 10.1038/modpathol.2013.137. [DOI] [PubMed] [Google Scholar]
- 12.Peurala E, Koivunen P, Haaspasaari KM, Bloigu R, Jukkola-Vuorinen A. The prognostic significance and value of cyclin D1, cdk4 and p16 in human breast cancer. Breast Cancer Res. 2013;15:R5. doi: 10.1186/bcr3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finn R, Dering J, Conklin D, et al. PD0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11:R77. doi: 10.1186/bcr2419. [DOI] [PMC free article] [PubMed] [Google Scholar]