Abstract
The Mediator multiprotein complex physically links transcription factors to RNA polymerase II and the basal transcription machinery. While the Mediator complex has been shown to be required for transcriptional initiation and elongation, the understanding of its interplay with histone modifying enzymes and post‐translational modifications remains elusive. In this issue of The EMBO Journal, Yao et al (2015) report that the MED23 subunit of the Mediator complex physically associates with the heterodimeric RNF20/40 E3‐ligase complex to facilitate the monoubiquitylation of histone H2B on gene bodies of actively transcribed genes.
Subject Categories: Chromatin, Epigenetics, Genomics & Functional Genomics; Post-translational Modifications, Proteolysis & Proteomics; Transcription
Mediator is a multiprotein complex essential in all cell types to maintain gene expression patterns. It orchestrates various aspects of RNA polymerase II transcription including initiation, re‐initiation, pausing and elongation as well as the spatial organisation of chromatin (Yin & Wang, 2014; Allen & Taatjes, 2015). One of its key functions is to mediate transcription by acting as a physical bridge between cell type‐specific transcription factors bound at enhancer elements and RNA Pol II together with basal transcription factors bound at gene promoters (Kagey et al, 2010). In this issue, Yao et al (2015) characterise a new role for the Mediator complex component MED23 in promoting monoubiquitylation of histone H2B at lysine 120 (H2BK120ub), a mark that localises on the gene bodies of actively transcribed genes and is tightly associated with transcriptional elongation (Pavri et al, 2006; Minsky et al, 2008).
Yao et al (2015) began their study by performing an unbiased screen of 22 different histone PTMs looking for changes between wild‐type and MED23 null mouse embryonic fibroblasts (MEFs). They observed a striking global decrease of H2BK120ub in the absence of MED23. Following this discovery, they pursued several biochemical, transcriptomic and functional approaches in an attempt to explain this observation. Firstly, they showed that MED23 physically associates with the heterodimeric E3‐ligase RNF20/40 which is known to function in concert with the super elongation complex (SEC) and polymerase‐associated factor (PAF) complexes to mediate H2BK120ub (Zhu et al, 2005; Pavri et al, 2006). Next they used cell‐free ubiquitylation assays to show that both the full Mediator complex and isolated MED23 are capable of enhancing RNF20/40‐mediated monoubiquitylation of histone H2B in cooperation with the PAF complex. Chromatin immunoprecipitation (ChIP) analyses on wild‐type versus MED23 null MEFs confirmed that MED23 is required in vivo for both RNF20/40 and PAF1 binding at the promoter and gene body of the Egr1 gene. Moreover, Western blot analysis revealed that RNF20 protein levels were reduced on chromatin in MED23 null MEFs, likely explaining the decreased H2BK120ub in these cells. Importantly, the authors extended these observations by providing elegant genomewide binding analysis of the global effects of MED23 loss on H2BK120ub and RNA polymerase II binding. This showed that H2BK120ub levels were dramatically reduced along the gene bodies of about 900 genes whose expression was down‐regulated in MED23 null cells. In addition, the authors found decreased binding of RNA Pol II on both promoters and gene bodies of these genes in MED23 null cells. Taken together, these data implicate MED23 as being required not only for transcription initiation, but also for transcriptional elongation by promoting H2BK120ub. It suggests a model in which MED23 mediates the targeting of the RNF20/40 complex to gene bodies, possibly as part of the Mediator complex (Fig 1).
Figure 1. The link between the Mediator subunit MED23 and H2B monoubiquitylation.
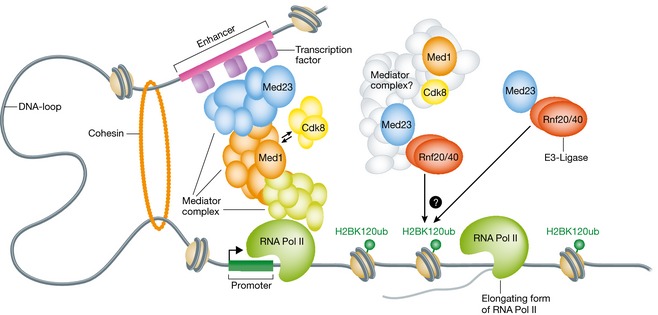
The Mediator core complex, which is composed of 26 subunits, stabilises promoter/enhancer loops by physically bridging transcription factors bound at enhancer elements with the RNA polymerase II transcription machinery at core promoter regions, thereby coordinating transcription initiation events. The Mediator subunit MED23, either alone or in a specialised mediator complex, associates with the E3‐ligase RNF20/40 to promote the H2BK120ub mark along the gene body of an actively transcribed gene, thereby promoting transcriptional elongation.
A question remains as to whether MED23 functions to mediate H2BK120ub as part of the Mediator complex, on its own, or as part of a different, more specialised complex. While previous reports did not identify RNF20/40 in purifications of other Mediator complex components (Ebmeier & Taatjes, 2010; Huang et al, 2012), Yao et al (2015) provide evidence that at least the CDK8 and MED1 subunits of the Mediator complex can co‐immunoprecipitate with RNF20/40 in HeLa cells. In line with this, they show that the complete Mediator complex is more effective at increasing the activity of RNF20/40 in in vitro ubiquitylation assays compared to MED23 alone. However, in contrast to MED23, the knockdown of several other Mediator complex subunits, including MED6, MED12, MED14 and MED24, led to an increase of H2BK120ub levels, suggesting that Mediator complexes with varying compositions may have different roles. Taken together, the results presented by Yao et al (2015) leave open the question whether MED23 acts alone or in a specialised form of the Mediator complex to promote H2BK120ub. Furthermore, it is unclear whether MED23 or other Mediator complex subunits have roles in the monoubiquitylation of histone H2B in cell types other than MEFs. Towards addressing this, it will be important to perform systematic genomewide mapping and proteomic analyses of MED23, other Mediator components and RNF20/40 in different cell types. Regardless of the work still to be done, the current study by Yao et al (2015) demonstrates for the first time that a Mediator subunit directly promotes a histone post‐translational modification on active genes, potentially providing new mechanistic insights into the regulation of transcriptional elongation.
See also: X Yao et al (December 2015)
References
- Allen BL, Taatjes DJ (2015) The Mediator complex: a central integrator of transcription. Nat Rev Mol Cell Biol 16: 155–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebmeier CC, Taatjes DJ (2010) Activator‐Mediator binding regulates Mediator cofactor interactions. Proc Natl Acad Sci USA 107: 11283–11288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Li W, Yao X, Lin QJ, Yin JW, Liang Y, Heiner M, Tian B, Hui J, Wang G (2012) Mediator complex regulates alternative mRNA processing via the MED23 subunit. Mol Cell 45: 459–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagey MH, Newman JJ, Bilodeau S, Zhan Y, Orlando DA, van Berkum NL, Ebmeier CC, Goossens J, Rahl PB, Levine SS, Taatjes DJ, Dekker J, Young RA (2010) Mediator and cohesin connect gene expression and chromatin architecture. Nature 467: 430–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minsky N, Shema E, Field Y, Schuster M, Segal E, Oren M (2008) Monoubiquitinated H2B is associated with the transcribed region of highly expressed genes in human cells. Nat Cell Biol 10: 483–488 [DOI] [PubMed] [Google Scholar]
- Pavri R, Zhu B, Li G, Trojer P, Mandal S, Shilatifard A, Reinberg D (2006) Histone H2B monoubiquitination functions cooperatively with FACT to regulate elongation by RNA polymerase II. Cell 125: 703–717 [DOI] [PubMed] [Google Scholar]
- Yao X, Tang Z, Fu X, Yin J, Liang Y, Li C, Li H, Tian Q, Roeder RG, Wang G (2015) The Mediator subunit MED23 couples H2B mono‐ubiquitination to transcriptional control and cell fate determination. EMBO J 34: 2885–2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin JW, Wang G (2014) The Mediator complex: a master coordinator of transcription and cell lineage development. Development 141: 977–987 [DOI] [PubMed] [Google Scholar]
- Zhu B, Zheng Y, Pham AD, Mandal SS, Erdjument‐Bromage H, Tempst P, Reinberg D (2005) Monoubiquitination of human histone H2B: the factors involved and their roles in HOX gene regulation. Mol Cell 20: 601–611 [DOI] [PubMed] [Google Scholar]


