Abstract
The voltage‐operated Ca2+ channels (VOCC), which allow Ca2+ influx from the extracellular space, are inhibited by anti‐hypertensive agents such as verapamil and nifedipine. The Ca2+ entering from outside into the cell triggers Ca2+ release from the sarcoplasmic reticulum (SR) stores. To refill the depleted Ca2+ stores in the SR, another type of Ca2+ channels in the cell membrane, known as store‐operated Ca2+ channels (SOCC), are activated. These SOCCs are verapamil and nifedipine resistant, but are SKF 96465 (SK) and gadolinium (Gd3+) sensitive. Both SK and Gd3+ have been shown to reduce [Ca2+]i in the smooth muscle, but their effects on blood pressure have not been reported. Our results demonstrated that both SK and Gd3+ produced a dose‐dependent reduction in blood pressure in rat. The combination of SK and verapamil produced an additive action in lowering the blood pressure. Furthermore, SK, but not Gd3+ suppressed proliferation of vascular smooth muscle cells in the absence or presence of lysophosphatidic acid (LPA). SK decreased the elevation of [Ca2+]i induced by LPA, endothelin‐1 (ET‐1) and angiotensin II (Ang II), but did not affect the norepinephrine (NE)‐evoked increase in [Ca2+]i. On the other hand, Gd3+ inhibited the LPA and Ang II induced change in [Ca2+]i, but had no effect on the ET‐1 and NE induced increase in [Ca2+]i. The combination of verapamil and SK abolished the LPA‐ or adenosine‐5′‐triphosphate (ATP)‐induced [Ca2+]i augmentation. These results suggest that SOCC inhibitors, like VOCC blocker, may serve as promising drugs for the treatment of hypertension.
Keywords: Ca2+ antagonists, antihypertensive agents, intracellular Ca2+, cell proliferation, hypertension
Introduction
In view of the effect of different vasoactive agents such as Ang II, NE, ET‐1 and LPA on blood pressure 1, 2, 3, a wide variety of receptor‐blocking drugs are being used for the treatment of hypertension. As the vasoactive hormones and agents are known to promote Ca2+ entry into the smooth muscle cells 4, 5, several VOCC antagonists or L‐type Ca2+ channel antagonists including verapamil are also known to produce beneficial effect in hypertensive cases6. The increase in intracellular Ca2+ concentration ([Ca2+]i) in smooth muscle cells is not only dependent upon the entry of extracellular Ca2+ but Ca2+ released from the intracellular store has also been demonstrated to play a critical role 7. The release of Ca2+ from the intracellular Ca2+ stores not only contributes to the increase of [Ca2+]i directly, but also causes Ca2+ influx through voltage‐independent Ca2+ channels, which are called Ca2+ release‐activated currents or SOCC 8. Although SOCC blockers such as SKF 96365 (SK) and Gd3+ have been shown to reduce [Ca2+]i in vascular smooth muscle cells (VSMC) 9, the effects of these agents on blood pressure as well as their actions on LPA‐induced elevation of blood pressure have not been reported previously. Although SK has been shown to reduce the ET‐1‐induced vasoconstriction of rat cerebral arteries 10, the effects of this agent on VSMC with respect to cell proliferation and changes in [Ca2+]i because of different agonists have not been studied. In view of the fact that hypertension is associated with an increase in smooth muscle cell proliferation and [Ca2+]i 11, this study was undertaken to test the action of SK and Gd3+ on blood pressure, cell proliferation and [Ca2+]i. The specificity of the effect of SK was also examined by using various agents such as Ang II, ET‐1, NE and LPA, which are known to increase the [Ca2+]i and blood pressure. Some experiments were carried out to investigate the effect of SOCC blocker, SK in combination with VOCC antagonist, verapamil on blood pressure and [Ca2+]i.
Materials and methods
The use of animals and experimental protocol were according to the guidelines of the Canadian Council of Animal Care as approved by the Animal Care Committee of the University of Manitoba. SKF 96365 was purchased from Biomol Research Laboratory (Plymouth Meeting, PA, USA). Gadolinium, L‐α‐LPA and verapamil were obtained from Sigma Chemicals (Oakville, ON, Canada); Fura‐2 acetoxymenthyl/ester (Fura 2‐AM) was purchased from Molecular Probes (Eugene, OR, USA); [3H] ‐ thymidine was purchased from Amersham (Oakville, ON. Canada). DMEM and foetal bovine serum (FBS) were obtained from Invitrogen (Burlington, ON, Canada), whereas the A10 VSMC line was from the American Type Culture Collection (Manassas, VA, USA).
Measurement of blood pressure
Male Sprague Dawley rats weighing 250–300 g were anaesthetized by an intraperitoneal injection of ketamine (90 mg/kg) and xylazine (10 mg/kg) mixture. The right carotid artery was exposed and cannulated with a microtip pressure transducer (model SPR‐249; Millar Instruments, Houston, TX, USA). The catheter was inserted carefully into the lumen of the carotid artery, then catheter was secured with a silk ligature around the artery and blood pressure values were recorded using the computer programme AcqKnowledge for Windows 3.5 (Biopac Systems Inc., Goleta, CA, USA). Arterial systolic and diastolic pressures were measured simultaneously 12.
Cell number count
The cultured A10 VSMC were treated with 0.25% trypsin‐1 mM ethylenediaminetetraacetic acid (EDTA) for 2 min. and were then collected and centrifuged at 240 × g for 5 min. at room temperature. The supernatant was removed and the cells were suspended in N‐2‐hydroxyethylpiperazine‐N′‐2‐ethane sulphonic acid (HEPES) buffer; 0.1 ml of 0.4% Trypan blue was added to 0.5 ml cell suspension and kept for 5 min. at room temperature. About 20 μl of cell suspension was filled in the haemocytometer and the non‐stained cells were counted under microscope.
Measurement of DNA synthesis
A10 VSMC were cultured in DMEM containing 10% FBS and 0.01 mg/ml gentamicin (Gibco, Burlington, ON, Canada) at 37°C with 95% air and 5% CO2. DNA synthesis in A10 VSMC was measured by [3H]‐thymidine incorporation into the DNA of the cells 13; cells were cultured in 12‐well plates. Before the experiments, cells were incubated in serum‐free DMEM for 20 hrs. Lysophosphatidic acid was added 10 min. after the addition of different inhibitors and after incubation for 4 hrs, 1 μCi [3H]‐thymidine was added. The reaction was terminated after 22 hrs by keeping the cell culture plates on the ice and removing the culture medium. The cells were washed three times with 1 ml HEPES buffer (mM/l: NaCl 145, KCl 4.5, CaCl2 1.0, MgSO4·7H2O 1.0, HEPES 10, glucose 5, bovine serum albumin 0.1%, KH2PO4 1.0, pH 7.4). These cells were then incubated for 1 hr in cold 5% trichloroacetic acid on the ice, washed two more times with 0.5 ml HEPES buffer, and incubated with 0.2 ml NaOH (0.5 N) for 1 hr. The aliquots were transferred to scintillation vial. The radioactivity was counted in a Beckman LS 6500 scintillation counter after the addition of 10 ml Cytoscint‐ES (MP Biomedicals, Santa Ana, CA, USA).
Measurement of [Ca2+]i
The cultured A10 VSMC were incubated with 0.25% trypsin‐1 mM EDTA for 2 min. and then the cells were harvested and centrifuged at 240 × g for 5 min. at room temperature. The supernatant was discarded and the cells were incubated with 10 μM Fura 2‐AM in HEPES buffer for 40 min. at 37°C. The cells were then washed twice with HEPES buffer and the cell number was adjusted to 0.3 × 106 cells/ml by adding HEPES buffer. The fluorescence intensity of Fura‐2 was determined by a SLM DMX–1100 dual‐wavelength spectrofluorometer (SLM Instruments, Inc, Urbana, IL, USA); the ratio (R) of fluorescence signal at 340/380 (nM) was calculated automatically. The Rmax and Rmin values were determined by the addition of 40 μl Triton X‐100 (10%) and 20 μl EGTA (40 mM) to a cuvette with 2 ml cell suspension respectively. The [Ca2+]i was calculated according to the following formula: [Ca2+]i = 224 × [(R − Rmin)/(Rmax − R)] × Sf2/Sb2, where Sf2 and Sb2 are the fluorescence proportionality coefficients obtained at 380 nm under Rmin and Rmax conditions respectively 14.
Statistical analysis
The data are expressed as mean ± S.E.M. Statistical analysis was performed with the Microcal Origin Version 6 (Microcal Software Inc., Northampton, MA, USA). The data analysis was carried out by one‐way anova analysis, the comparison of mean values of the two groups was performed by Student's t‐test. P values less than 0.05 were considered to be significantly different.
Results
SOCC blockers on blood pressure
The effects of SOCC blockers on blood pressure in rats were tested by injecting different doses of SK or Gd3+ intravenously. The blood pressure was monitored before and after treatment. In preliminary experiments, both SK (45–450 μg/100 g b.w.) and Gd3+ (16–160 μg/100 g b.w.) were found to lower blood pressure in a dose‐dependent manner. As shown in Figure 1, both agents induced dose‐ and time‐dependent reductions in systolic (25% by SK and 23% by Gd3+, Figures 1A and 1C, respectively) and diastolic (35% by SK and 33% by Gd3+, Figures 1B and 1C, respectively) blood pressures. The maximum effects were achieved within 30 sec. of injection. Both systolic blood pressure and diastolic blood pressure were still significantly lower at 60 sec. of the injection of SK; however, there is no significant difference in the systolic blood pressure at 60 sec. following Gd3+ treatment.
Figure 1.
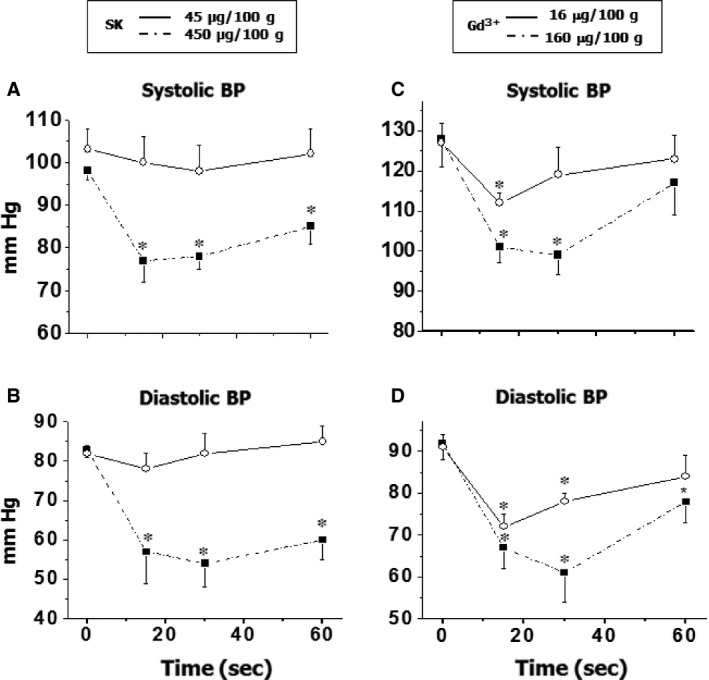
The effect of SK and Gd3+ on blood pressure. The blood pressure was recorded from the carotid artery before and after treatment by a microtip pressure transducer and computer programme Acqknowledge for Windows 3.5. *P < 0.05 compared with basal value; n = 6.
In view of the importance of LPA in the development of hypertension 3, the effect of SOCC blockers on the LPA‐induced elevation of blood pressure were tested by giving different doses of SK (4.5–45 μg/100 g b.w.; i.v.) 30 sec. prior to the treatment with LPA (5.6 μg/100 g b.w.). It was observed that SK pre‐treatment caused a dose‐dependent inhibition of the LPA‐induced change in blood pressure; higher dose of SK (45 μg/100 g b.w.) abolished the effect of LPA (Fig. 2). As VOCC antagonists are commonly used for the control of blood pressure, it was planned to test if the combination use of VOCC and SOCC blockers can produce an additive effect. The blood pressure was monitored before and after injection of SK (45 μg/100 g b.w.), verapamil (15 μg/100 g b.w.) or combination of these two agents. It is pointed out that the use of verapamil in combination with SK was based on the fact that verapamil is well known to block VOCC in both the VSMC and the heart 6, 15 and such a combination could be expected to reduce the side effects of this agent. As shown in Table 1, the combination of SK and verapamil produced a stronger effect on the diastolic blood pressure in comparison to the treatments with SK or verapamil alone.
Figure 2.
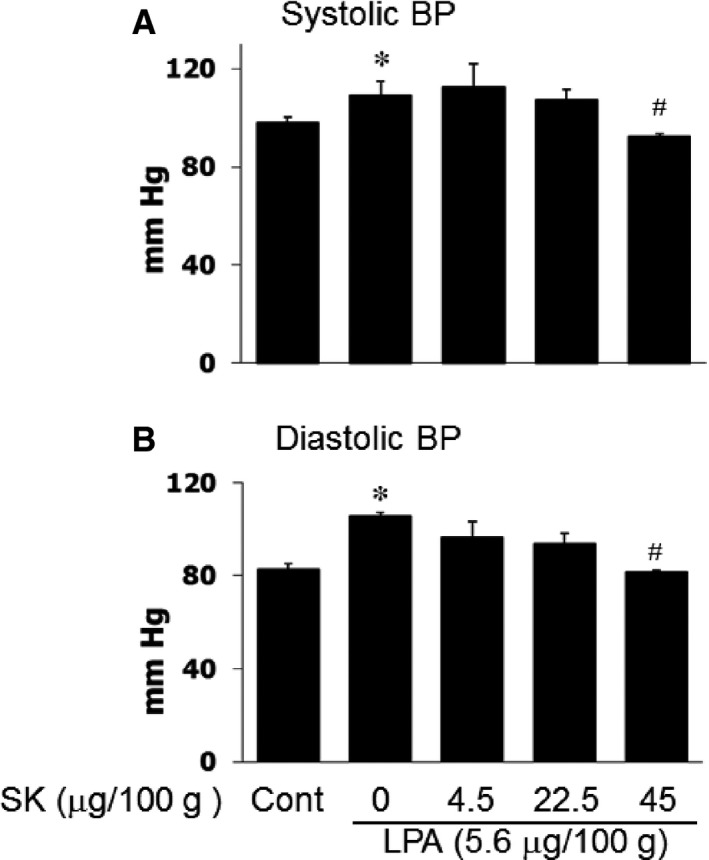
The effect of SK on LPA‐induced blood pressure elevation. The blood pressure was recorded from carotid artery prior to and after treatment by a microtip pressure transducer and computer programme Acqknowledge for Windows 3.5. *P < 0.05 compared to control (Cont) values (basal line), # P < 0.05 compared to LPA injection group but without SK; n = 6.
Table 1.
Effects of SK (SK&F 96365), verapamil and SK+verapamil combination on blood pressure in rats
| Time | SK (N = 4) | Verapamil (N = 5) | Combination (N = 8) | |||
|---|---|---|---|---|---|---|
| SBP | DBP | SBP | DBP | SBP | DBP | |
| 0 | 129 ± 11 | 85 ± 6 | 124 ± 7 | 87 ± 4 | 134 ± 4 | 89 ± 2 |
| 10 sec. | 113 ± 4 | 74 ± 10 | 98 ± 3a | 67 ± 3a | 96 ± 2a | 54 ± 2a , b |
| 30 sec. | 120 ± 9 | 77 ± 7 | 97 ± 3a | 66 ± 4a | 92 ± 2a | 47 ± 2a , b |
| 1 min. | 139 ± 12 | 90 ± 7 | 99 ± 2a | 72 ± 1a | 99 ± 4a | 61 ± 4a , b |
| 5 min. | 142 ± 12 | 91 ± 13 | 113 ± 10 | 78 ± 4 | 128 ± 6 | 83 ± 4 |
P < 0.05 compared with the respective control value (before the treatment).
P < 0.05 compared with respective values of verapamil (15 μg/100 g) group and SK (45 μg/100 g) group.
SBP: systolic blood pressure (mm Hg); DBP: diastolic blood pressure (mm Hg).
SOCC blockers on cell proliferation
The ratio of arterial lumen and wall thickness is an essential factor for the regulation of blood pressure 11. As cell proliferation plays critical role in the thickness of blood vessel, the effect of SOCC blockers on cell proliferation was tested in cultured A10 VSMC. As shown in Figure 3, SK caused a dose‐dependent inhibition of cell proliferation in the absence or presence of LPA as reflected by the change in cell numbers; however, Gd3+ treatment had no significant effect on cell number. To confirm this finding, [3H] thymidine incorporation, an index of DNA synthesis and cell proliferation, was examined in cultured A10 VSMC. As shown in Figure 4, [3H] thymidine incorporation with or without LPA was significantly inhibited by SK treatment, meanwhile Gd3+ had no significant effect on [3H] thymidine incorporation.
Figure 3.
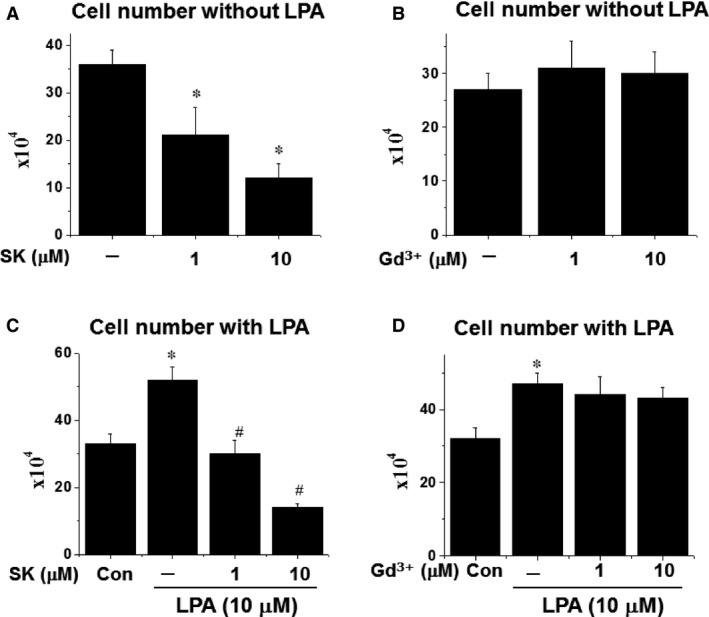
The effect of SK or Gd3+ on cell proliferation in the presence or absence of LPA. Prior to treatment, the cells were cultured in serum free medium for 20 hrs, then different concentrations of SK were added to different wells and 10 min. later, LPA was added to all the wells except for control (Con) group. After continuing culture for 24 hrs, the cell number was counted. *P < 0.05 compared to control value; # P < 0.05 compared to the group with LPA but no SK or Gd3+; n = 6.
Figure 4.
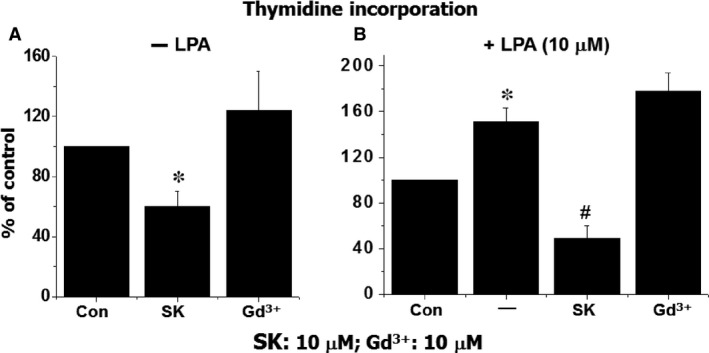
The effect of SK or Gd3+ on DNA synthesis in the presence or absence of LPA. Prior to treatment, the cells were cultured in serum free medium for 20 hrs, then different concentration of SK or Gd3+ were added to different wells and 10 min. later, LPA was added to all the wells except for control (Con) group. After incubation for 4 hrs, 3H‐thymidine was added, then reaction was terminated 20 hrs later. *P < 0.05 compared to control value; # P < 0.05 compared to the group with LPA but no SK or Gd3+; n = 6.
Store‐operated Ca2+ channel blocker on intracellular Ca2+
Intracellular Ca2+ concentration is a key factor for the control of vascular tone as well as cell proliferation 16. To examine the effects of SOCC blockers on intracellular calcium mobilization, the effects of SK and Gd3+ on the various vasoactive agonists (such as LPA, ATP, NE, Ang II and ET‐1)‐evoked changes in [Ca2+]i were examined in cultured A10 VSMC. Figure 5 demonstrated that SK and Gd3+ had no significant effect on basal [Ca2+]i; however, LPA‐induced increase in [Ca2+]i was inhibited by both SK and Gd3+ in a concentration‐dependent manner. But these antagonists had no significant effect on NE‐induced increase in [Ca2+]i (Fig. 6). For ET‐1‐induced elevation of [Ca2+]i, SK demonstrated a concentration‐dependent suppressive effect; whereas, Gd3+ had no significant action (Fig. 7). On the other hand, for Ang II‐induced increase in [Ca2+]i, both SK and Gd3+ exerted inhibitory effects.
Figure 5.
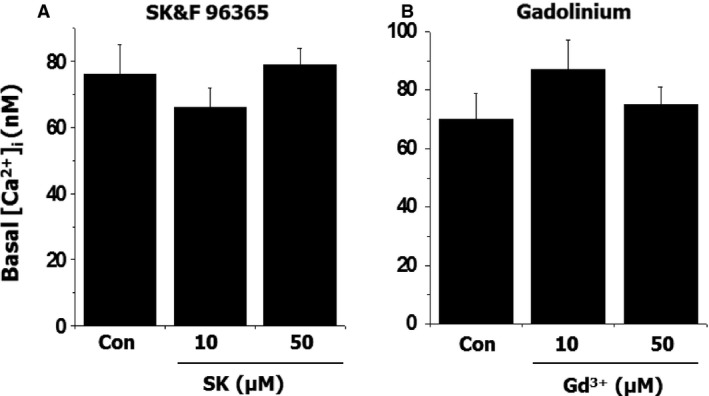
The effect of SK or Gd3+ on intracellular free Ca2+ concentration in vascular smooth muscle cells. The cells were incubated with 10 μM Fura 2‐AM in HEPES buffer for 30 min. The intensity of fluorescence before and after treatment was recorded by a SLM DMX ‐1100 dual wavelength spectrofluorometer; n = 6.
Figure 6.
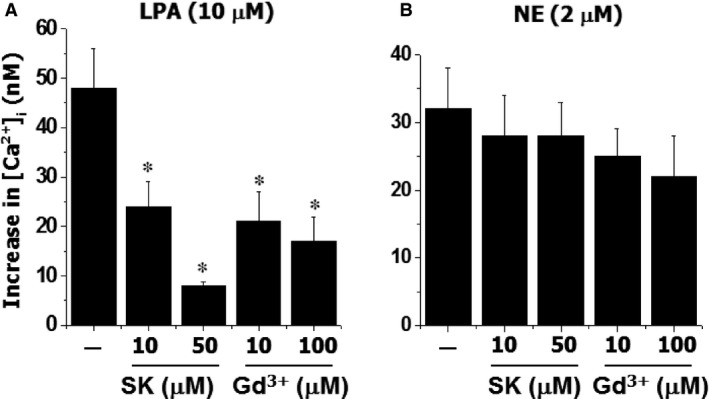
The effect of SK or Gd3+ on LPA or NE‐induced change in intracellular free Ca2+ concentration in vascular smooth muscle cells. The cells labelled with Fura 2‐AM were incubated with different concentrations of SK or Gd3+ for 30 sec. prior to the challenging with LPA or NE. *P < 0.05 compared to control value with LPA or NE alone; n = 6.
Figure 7.
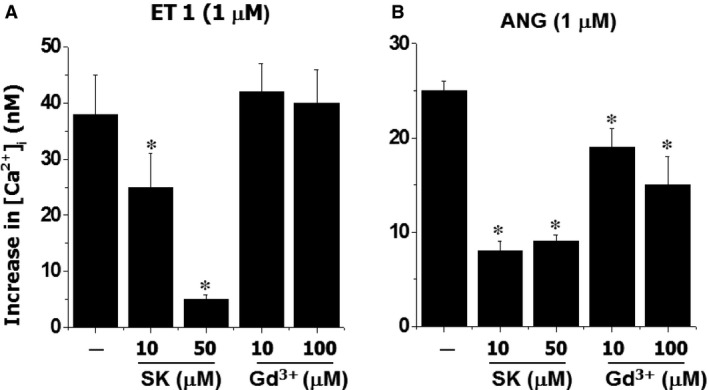
The effect of SK or Gd3+ on ET 1 or ANG‐induced change in intracellular free Ca2+ concentration in vascular smooth muscle cells. The cells labelled with Fura 2‐AM were incubated with different concentrations of SK or Gd3+ for 30 sec. prior to the challenging with ET 1 or ANG. *P < 0.05 compared to control value with ET 1 or ANG alone; n = 6.
Combination of store‐operated Ca2+ channel blocker and voltage‐dependent Ca2+ channel blocker on [Ca2+]i
The mobilization of intracellular Ca2+ is elicited by Ca2+ influx through VOCC, SOCC, receptor operated Ca2+ channel (ROCC) and Ca2+ release from intracellular store 17. To examine if the combination of SOCC and VOCC blockers is more effective than the individual agent, the actions of SK and verapamil alone or in combination were examined on the LPA‐ or ATP‐induced increase in [Ca2+]i (Fig. 8). Both SK and verapamil suppressed the LPA‐ or ATP‐induced elevation of [Ca2+]i in A10 VSMC significantly; the combination of SK and verapamil demonstrated a stronger inhibitory effect; the LPA‐induced response was inhibited by 85%, 51% and 95% following the treatment with SK, verapamil and combination of SK and verapamil respectively. For the ATP‐induced response, the inhibitory actions were: 49% by SK, 62% by verapamil and 91% by SK plus verapamil. The reason for choosing LPA and ATP as agonists in this experiment is based on the fact that these agents are broadly used for Ca2+ mobilization in VSMC 14.
Figure 8.
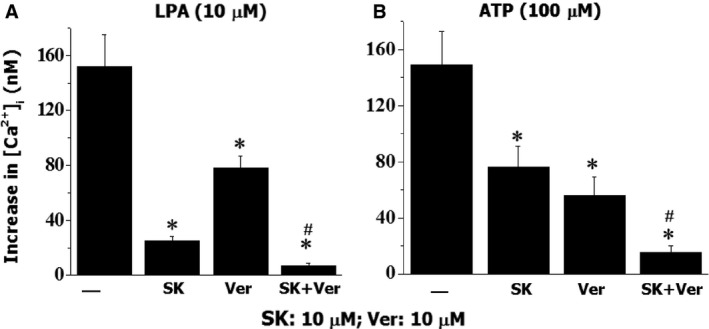
The effect of SK, verapamil (Ver) or SK + Ver on LPA‐ or ATP‐induced changes in intracellular free Ca2+ concentration in vascular smooth muscle cells. The cells labelled with Fura 2‐AM were incubated with SK, Ver or SK+Ver for 30 sec. prior to the challenging with LPA or ATP. *P < 0.05 compared to control value with LPA or ATP alone without inhibitors, # P < 0.05 compared to other groups; n = 6.
Discussion
Hypertension is a major cause of many cardiovascular diseases such as stroke, heart attack, atherosclerosis, chronic heart failure and kidney failure. These cardiovascular diseases affect more than 65 million Americans 18. Voltage‐operated Ca2+ channels blockers, including verapamil, nifedipine and diltiazem are useful agents for the reduction of blood pressure in the majority of population; however, some patients do not respond to these drugs. In addition, the major side effect of VOCC blockers is the increase of heart failure incidence as the heart beat is dependent on the Ca2+ influx from extracellular space through VOCC. Blocking these channels would reduce cardiac contractility and eventually lead to heart failure 15 and this effect may limit their clinical use in hypertension. On the other hand, Ca2+ influx through SOCC is triggered by hormones, growth factors and neurotransmitters. The route of Ca2+ influx through SOCC provides a minimal contribution to the beat‐to‐beat Ca2+ transients in the heart, but plays a major role in the increase of [Ca2+]i in VSMC 19, 20. Thus, SOCC blockers may have less side effects on cardiac contractility when used for the treatment of hypertensive patients.
It is known that Ang II, ET‐1 and LPA are involved in the development of hypertension and atherosclerosis 3. Voltage‐operated Ca2+ channels blockers have only a partial inhibitory effect on ET‐1‐induced increase in [Ca2+]i and cell contraction 21. Arun et al. 22 have reported that verapamil and diltiazem do not inhibit the Ang II‐induced contraction of vascular smooth muscle, indicating that L‐type Ca2+ channels are not involved in this response. In an experiment using rat tail artery, the Ang II‐ and ET‐1‐induced contraction was found to be insensitive to L‐type Ca2+ channel antagonist, nifedipine, but blocked by SK, indicating that SK may be useful for the treatment of hypertension in patients insensitive to L‐type calcium channel blocker 23. Bova et al. 24 demonstrated that norbormide, a vasoconstrictor agent for rat small artery, caused a concentration‐dependent contraction, which was not sensitive to verapamil, but was almost completely inhibited by 30 μM SK. These reports suggest that SOCC blockers may be superior to VOCC antagonists in certain cases.
In this study, we have observed for the first time that SOCC blocker SK reduced blood pressure significantly, and SK suppressed the LPA‐induced elevation of blood pressure. We have also shown the reduction of blood pressure by Gd3+, another SOCC blocker 9. These results indicate that SOCC blockers may be used for the control of blood pressure. It should be noted that because the ET‐1‐induced pulmonary contraction was partially blocked by SK and Gd3+, different investigators 25, 26 have reported the role of SOCCs in the pathogenesis of pulmonary hypertension. However, some caution should be exercised about this viewpoint regarding the use of SOCC blockers in the treatment of pulmonary hypertension because Gd3+, unlike SK, was unable to block the ET‐1‐induced increase in [Ca2+]i in VSMC. It is also pointed out that SK and Gd3+ produced toxic effects in the body 27, thus SOCC blockers with low toxic and less side effects need to be developed. Zuo et al. 28 have demonstrated that tyrosine kinase inhibitors depressed the SOCC activity and it is possible to use tyrosine kinase inhibitors to reduce blood pressure. Accordingly, it would be interesting to investigate the effects of SK and Gd3+ on the tyrosine kinase activity in VSMC.
It has been shown that SOCCs play an important role in mitogenic response to growth factor in VSMC 20, and the effect of L‐type Ca2+ channel blocker in this regard are controversial. Xiao et al. 29 reported that ET‐1 caused a concentration‐dependent increase in cell count and [3H] thymidine incorporation in VSMC; both nifedipine and SK inhibited these responses. However, Kawanabe et al. 30 reported that nifedipine had no effect on ET‐1‐induced augmentation of [Ca2+]i in sustained phase and ET‐1‐stimulated cell proliferation. As SK significantly inhibited both changes in [Ca2+]i and cell growth, it appears that SOCC inhibitors may prevent remodelling of VSMC. This view is consistent with the observations of Leung et al. 20, who have shown the role of SOCC in VSMC proliferation. Although both SK and Gd3+ produce a reduction in blood pressure, these agents were found to exert different actions with respect to VSMC proliferation and [Ca2+]i changes. In this regard, it should be noted that there are three important components in SOCC, namely, a stromal interaction molecule (which is Ca2+ sensor), a pore forming protein (which allows Ca2+ influx) and a transient receptor (which regulates the function of SOCC) 31, 32, 33. As all three components of SOCC are important in the control of Ca2+ influx in VSMC 32, 33, it is likely that the difference in the actions of SK and Gd3+ on cell proliferation and [Ca2+]i observed in this study may be as a result of their effects on different targeting sites in SOCC. Because the expression of the transient receptor component of SOCC is up‐regulated in the vasculature of hypertensive rats 33, future studies should be carried out to investigate the effects of different SOCC blockers on this component.
It is noteworthy that both SK and Gd3+ have been shown to block Ca2+ influx through SOCC in VSMC and their inhibitory effects are agonist and cell‐type dependent. For instance, in arteriolar smooth muscle cells 34, 35, Gd3+ produced a concentration‐dependent inhibition of SOCC‐mediated Ca2+ entry; however, the effects of SK on cyclopiazonic acid and thapsigargin depletion‐induced SOCC activation were different. In our study, both SK and Gd3+ inhibited LPA‐induced alteration of [Ca2+]i as observed previously 7; however, it had no significant effect on the increase in [Ca2+]i, induced by NE. This is because NE evoked the increase in [Ca2+]i mainly by cell membrane depolarization 36 and thus VOCC were activated 37. On the other hand, ET‐1 has been shown to increase [Ca2+]i through SOCC which was blocked by SK 38; the results in this study are consistent with this observation. Furthermore, Gd3+ produced no effect on ET‐1‐induced [Ca2+]i change in our experiments, indicating the distinct effect of these two blockers on SOCC in VSMC. Taken together, LPA, Ang II, NE and ET‐1, were all observed to increase in [Ca2+]i, but the mechanisms seemed to be different.
We have shown that both SK and verapamil, when used in combination, produced a synergistic effect on LPA‐ and ATP‐induced [Ca2+]i increase in VSMC. Other investigators have reported an additive inhibitory effect on ET‐1‐induced vasoconstriction in cerebral arteries by using SK and nifedipine (VOCC blocker) 10. These observations suggest that the use of SOCC blockers in combination with VOCC inhibitor may be more effective in lowering the blood pressure. However, extensive studies by using other agonists including ET‐1, Ang II and NE are required for establishing the beneficial effects of the combination of SOCC and VOCC blockers. Another limitation of this study is concerned with the specificity of SK and Gd3+ on SOCC. In this regard, it has been reported that these two agents in higher concentrations may also inhibit ROCC and other Ca2+ channels 39, 40, 41. Thus more specific chemical inhibitors or specific antibodies are needed for further study. Nevertheless, on the basis of the results described in this study, it is suggested that SOCC blockers may be considered as a new category of antihypertensive agents and SOCC may be a molecular target for drug development.
Conflicts of interest
The authors declare no conflict of interest with any grant funding agency.
Acknowledgements
The infrastructure support for this study was provided by St. Boniface Hospital Research Foundation.
References
- 1. Kohan DE, Rossi NF, Inscho EW, et al Regulation of blood pressure and salt homeostasis by endothelin. Physiol Rev. 2011; 91: 1–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Unger T, Paulis L, Sica DA. Therapeutic perspectives in hypertension: novel means for rennin‐angiotensin‐aldosterone system modulation and emerging device‐based approaches. Eur Heart J. 2011; 32: 2739–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xu YJ, Aziz OA, Bhugra P, et al Potential role of lysophosphatidic acid in hypertension and atherosclerosis. Can J Cardiol. 2003; 19: 1525–36. [PubMed] [Google Scholar]
- 4. Schiffrin EL. Vascular protection with newer antihypertensive agents. J Hypertens Suppl. 1998; 16: S25–9. [PubMed] [Google Scholar]
- 5. Schiffrin EL. Intracellular signal transduction for vasoactive peptides in hypertension. Can J Physiol Pharmacol. 1994; 72: 954–62. [DOI] [PubMed] [Google Scholar]
- 6. Elliott WJ, Ram CVS. Calcium channel blockers. J Clinic Hyperten. 2011; 13: 687–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hill‐Eubanks DC, Werner ME, Heppner TJ, et al Calcium signaling in smooth muscle. Cold Spring Harb Perspect Biol. 2011; 3: a004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nauli SM, Kawanabe Y. Involvement of extracellular Ca2+ influx through voltage‐independent Ca2+ channels in endothelin‐1 function. Cell Signal. 2005; 17: 911–6. [DOI] [PubMed] [Google Scholar]
- 9. Poburko D, Lhote P, Szado T, et al Basal calcium entry in vascular smooth muscle. Eur J Pharmacol. 2004; 28: 19–29. [DOI] [PubMed] [Google Scholar]
- 10. Mamo YA, Angus JA, Ziogas J, et al The role of voltage‐operated and non‐voltage‐operated calcium channels in endothelin‐induced vasoconstriction of rat cerebral arteries. Eur J Pharmacol. 2014; 742: 65–73. [DOI] [PubMed] [Google Scholar]
- 11. Houssaini A, Abid S, Mouraret N, et al Rapamycin reverses pulmonary artery smooth muscle cell proliferation in pulmonary hypertension. Am J Respir Cell Mol Biol. 2013; 48: 568–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xu YJ, Rathi SS, Zhang M, et al Mechanism of the positive inotropic effect of lysophosphatidic acid in rat heart. Cardiovasc Pharmacol Ther. 2002; 7: 109–15. [DOI] [PubMed] [Google Scholar]
- 13. Xu YJ, Rathi SS, Chapman DC, et al Mechanisms of lysophosphatidic acid‐induced DNA synthesis in vascular smooth muscle cells. J Cardiovasc Pharmacol. 2003; 41: 381–7. [DOI] [PubMed] [Google Scholar]
- 14. Xu YJ, Saini HK, Cheema SK, et al Mechanisms of lysophosphatidic acid‐induced increase in intracellular calcium in vascular smooth muscle cells. Cell Calcium. 2005; 38: 569–79. [DOI] [PubMed] [Google Scholar]
- 15. Colovina VA. Cell proliferation in associated with enhanced capacitative Ca2+ entry in human arterial myocytes. Am J Physiol (Cell Physiol). 1999; 277: C343–9. [DOI] [PubMed] [Google Scholar]
- 16. Jackson WF. Ion channels and vascular tone. Hypertension. 2000; 35: 173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. DeSimone ME, Crowe A. Nonpharmacological approaches in the management of hypertension. J Am Acad Nurse Pract. 2009; 21: 189–96. [DOI] [PubMed] [Google Scholar]
- 18. Colucci WS, Fifer MA, Lorell BH, et al Calcium channel blockers in congestive heart failure: theoretic consideration and clinical experience. Am J Med. 1985; 78: 9–17. [DOI] [PubMed] [Google Scholar]
- 19. Guibert C, Ducret T, Savineau JP. Voltage‐independent calcium influx in smooth muscle. Prog Biophys Mol Biol. 2008; 98: 10–23. [DOI] [PubMed] [Google Scholar]
- 20. Leung FP, Yung LM, Yao X, et al Store‐operated calcium entry in vascular smooth muscle. Br J Pharmacol. 2008; 153: 846–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McNair LL, Salamanca DA, Khalil RA. Endothelin‐1 promotes Ca2+ antagonist‐insensitive coronary smooth muscle contraction via activation of epsilon‐protein linase C. Hypertension. 2004; 43: 897–904. [DOI] [PubMed] [Google Scholar]
- 22. Arun KHS, Kaul CL, Ramarao P. AT1 receptors and L‐type calcium channels: functional coupling in supersensitivity to angiotensin II in diabetic rats. Cardiovas Res. 2005; 65: 374–86. [DOI] [PubMed] [Google Scholar]
- 23. Jiang Y, Trigggle CR. Lack of involvement of endothelin‐1 in angiotensin II‐induced contraction of the lsolated rat tail artery. Brit J Pharmacol. 2000; 131: 1055–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bova S, Trevisl L, Cima S, et al Signaling mechanisms for selective vasocontractor effect of norbormide on the rat small arteries. J Pharmacol Experim Therapeu. 2000; 296: 458–63. [PubMed] [Google Scholar]
- 25. Firth AL, Won JY, Park WS. Regulation of Ca2+ signaling in pulmonary hypertension. Korean J Physiol Pharmacol. 2013; 17: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang K, Lu W, Jiang Q, et al PPAPγ inhibits hypoxia‐induced store‐operated calcium entry by suppressing caveolin‐1. Am J Respir Cell Mol Biol. 2015; Doi:10.1165/rcmb.2015‐0002OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bernstein EJ, Schmidt‐Lauber C, Kay J. Nephrogenic system fibrosis: a system fibrosing resulting from gadolinium exposure. Best Pract Res Lin Rheumatol. 2012; 26: 489–503. [DOI] [PubMed] [Google Scholar]
- 28. Zuo WL, Du JY, Huang JH, et al Tyrosine phosphorylation modulates store‐operated calcium entry in cultured rat epididymal basal cells. J Cell Physiol. 2011; 226: 1069–73. [DOI] [PubMed] [Google Scholar]
- 29. Xiao G‐N, Guan Y‐Y, He H. Effect of Cl‐ channels on endothelin‐1‐induced proliferation of rat vascular smooth muscle cells. Life Sci. 2002; 70: 2233–41. [DOI] [PubMed] [Google Scholar]
- 30. Kawanabe Y, Hashimoto N, Masaki T. Characterization of Ca2+ channels involved in endothelin‐1‐induced contraction of rabbit basilar artery. J Cardiovas Pharmacol. 2002; 40: 438–47. [DOI] [PubMed] [Google Scholar]
- 31. Giachini FRC, Chiao C‐W, Carneiro FS, et al Increased activation of stromal interaction molecular‐1/Orai‐1 in aorta from hypertensive rats: a novel insight into vascular dysfunction. Hypertension. 2009; 53: 409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eder P, Poteser M, Groschner K. TRPC3: a multifunctional, pore‐forming signaling molecule. Handb Exp Pharmacol. 2007; 179: 77–92. [DOI] [PubMed] [Google Scholar]
- 33. Liu D, Yang D, He H, et al Increased transient receptor potential canonical type 3 channels in vasculature from hypertensive rats. Hypertension. 2009; 53: 70–6. [DOI] [PubMed] [Google Scholar]
- 34. Flemming R, Xu SZ, Beech DJ. Pharmacological profile of store‐operated channels in cerebral arteriolar smooth muscle cells. Br J Pharmacol. 2003; 139: 955–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nilsson H, Videbaek LM, Toma C, et al Role of intracellular calcium for noradrenaline‐induced depolarization in rat mesenteric small arteries. J Vasc Res. 1998; 35: 36–44. [DOI] [PubMed] [Google Scholar]
- 36. Yousif MH, Williams KI, Oriowo MA. Source(s) of activator calcium for noradreneline‐induced vasoconstriction in the perfused rabbit isolated ovarian vascular bed: a role for tyrosine kinase. Gen Pharmacolol. 1999; 32: 563–79. [DOI] [PubMed] [Google Scholar]
- 37. Liskova S, Petrova M, Karen P, et al Contribution of Ca2+‐dependent Cl‐ channels to norepinephrine‐induced contraction of femoral artery is replaced by increasing EDCF contribution during ageing. Biomed Res Int. 2014; 2014: 289361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McFadzean I, Gibson A. The developing relationship between receptor‐operated and store‐operated calcim channels in smooth muscle. Br J Pharmacol. 2002; 135: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Singh A, Hildebrand ME, Garcia E, et al The transient receptor potential channel antagonist SKF96365 is a potent blocker of low‐voltage‐activated T‐type calcium channels. Bt J Pharmacol. 2010; 160: 1464–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Song M, Chen D, Yu SP. The TRPC channel blocker SKF96365 inhibits glioblastoma cell growth by enhancing reverse mode of the Na+/Ca2+ exchanger and increasing intracellular Ca2+ . Br J Pharmacol. 2014; 171: 3432–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen KH, Liu H, Yang L, et al SKF‐96365 strongly inhibits voltage‐gated sodium current in rat ventricular myocytes. Pflugers Arch. 2014; 467: 1227–36. [DOI] [PubMed] [Google Scholar]


