Abstract
IMPORTANCE
The risk of cardiovascular disease (CVD) after infection is poorly understood.
OBJECTIVE
To determine whether hospitalization for pneumonia is associated with an increased short-term and long-term risk of CVD.
DESIGN, SETTINGS, AND PARTICIPANTS
We examined 2 community-based cohorts: the Cardiovascular Health Study (CHS, n = 5888; enrollment age, ≥65 years; enrollment period, 1989–1994) and the Atherosclerosis Risk in Communities study (ARIC, n = 15 792; enrollment age, 45-64 years; enrollment period, 1987–1989). Participants were followed up through December 31, 2010. We matched each participant hospitalized with pneumonia to 2 controls. Pneumonia cases and controls were followed for occurrence of CVD over 10 years after matching. We estimated hazard ratios (HRs) for CVD at different time intervals, adjusting for demographics, CVD risk factors, subclinical CVD, comorbidities, and functional status.
EXPOSURES
Hospitalization for pneumonia.
MAIN OUTCOMES AND MEASURES
Incident CVD (myocardial infarction, stroke, and fatal coronary heart disease).
RESULTS
Of 591 pneumonia cases in CHS, 206 had CVD events over 10 years after pneumonia hospitalization. Compared with controls, CVD risk among pneumonia cases was highest during the first year after hospitalization and remained significantly higher than among controls through 10 years. In ARIC, of 680 pneumonia cases, 112 had CVD events over 10 years after hospitalization. After the second year, CVD risk among pneumonia cases was not significantly higher than among controls.
|
Pneumonia Cases |
Controls | HR (95% CI) | |
|
CHS | |||
| No. of participants | 591 | 1182 | |
| CVD events | |||
| 0-30 d | 54 | 6 | 4.07 (2.86-5.27) |
| 31-90 d | 11 | 9 | 2.94 (2.18-3.70) |
| 91 d-1 y | 22 | 55 | 2.10 (1.59-2.60) |
| 9-10 y | 4 | 12 | 1.86 (1.18-2.55) |
|
ARIC | |||
| No. of participants | 680 | 1360 | |
| CVD events | |||
| 0-30 d | 4 | 3 | 2.38 (1.12-3.63) |
| 31-90 d | 4 | 0 | 2.40 (1.23-3.47) |
| 91 d-1 y | 11 | 8 | 2.19 (1.20-3.19) |
| 1-2 y | 8 | 7 | 1.88 (1.10-2.66) |
CONCLUSIONS AND RELEVANCE
Hospitalization for pneumonia was associated with increased short-term and long-term risk of CVD, suggesting that pneumonia may be a risk factor for CVD.
Cardiovascular disease (CVD) is the principal cause of morbidity and mortality worldwide.1 In the United States, there are about 725 000 new cases of myocardial infarction and 795 000 new cases of stroke,2 and 264 000 people die due to these conditions each year.1 Therefore, characterizing the risk factors for CVD is important to design optimal preventive strategies.
Infections can induce demand ischemia, endothelial dysfunction, procoagulant changes in blood, and inflammatory changes in atherosclerotic plaques.3,4 Alone or in combination, these effects can increase the short-term risk of cardiovascular events.3,4 However, because heightened systemic inflammatory and procoagulant activity can persist long after infections resolve,5,6 the effect of infections on CVD risk could also extend for several months or years.
Several clinical studies have documented a 2-fold to 8-fold increase in the risk of CVD within the first 30 days after respiratory infections.3 A few studies also examined the association between infection and subsequent long-term risk of CVD and their results have been conflicting.7-10 Characterizing the short-term and long-term risk of CVD after infection is important because it could suggest that infection is a risk factor for CVD and explain why patients with infections have increased short-term and long-term morbidity and mortality.11,12
In this study, we analyzed data from participants enrolled in 2 community-based, observational, multicenter cohorts to determine the risk of CVD. We determined whether the risk of CVD varies over a 10-year period after pneumonia and whether the association persists after adjusting for traditional and novel cardiovascular risk factors. We chose pneumonia because respiratory tract infections have been consistently associated with increased risk of CVD,3,7 pneumonia is a well-characterized infectious syndrome that affects 1.2% of the population in the northern hemisphere each year,13 and it is the most common medical diagnosis responsible for hospitalizations in the United States.14
Methods
Study Design
We conducted a matched-cohort study nested within 2 population-based, multicenter, observational cohorts that were followed up for over 21 years: the Cardiovascular Health Study (CHS, n = 5888) cohort and the Atherosclerosis Risk in Communities (ARIC, n = 15 792) cohort. The institutional review boards at each CHS and ARIC study site approved the studies. All participants gave informed consent. We identified pneumonia hospitalizations within the first 15 years. Within each cohort, we used incidence density sampling to match participants hospitalized with pneumonia to 2 controls. We examined risk of CVD at various time intervals in the first 10 years after pneumonia occurred. We did not combine the cohorts due to differences in baseline clinical characteristics and risk of CVD among CHS and ARIC participants.15,16 We first examined CHS and then replicated our analyses in ARIC.
Participants and Data Collection
The CHS and ARIC studies recruited noninstitutionalized community-dwelling adults between 1987 and 1989 (ARIC) and between 1989 and 1994 (CHS).15,16 At enrollment, participants were 65 years or older in CHS and aged 45 to 64 years in ARIC.15,16 Details of eligibility criteria and study procedures in each cohort have been described elsewhere (eMethods 1 in the Supplement).15,16 We chose these cohorts because they were designed to understand the incidence, risk factors, and progression of CVD15,16; their methodology for the ascertainment of cardiovascular events was rigorous, comparable, and validated17-20; and the age range of their participants would maximize the generalizability of our results.
In both cohorts, baseline evaluation included standardized physical examination, diagnostic testing, laboratory evaluation, and questionnaires on health status, medical history, and lifestyle habits to determine cardiovascular risk factors. Thereafter, regular study visits and telephone follow ups were conducted to update cardiovascular risk factors.15,16 Participants were followed up through December 31, 2010.
A combination of annual study visits, telephone follow ups, review of Medicare Provider Utilization files (CHS only), local hospitalization records, death certificates from state vital statistics offices, and local newspaper obituaries were used for identification of new cardiovascular events, hospital admissions, and mortality.15,16
Adjudication of Pneumonia Hospitalizations
Hospital discharge abstracts were obtained for all hospitalizations. We used previously validated International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes 480 through 487 in the first 5 discharge diagnoses fields to identify pneumonia hospitalizations.21,22 We reviewed the medical charts of 158 pneumonia admissions identified by this approach in CHS. Clinical diagnoses of pneumonia were present in 89% of cases and in 88% of cases for radiographic diagnoses.23 We identified severe pneumonia (pneumonia with organ dysfunction) using previously validated ICD-9-CM codes for severe sepsis.24
Cardiovascular Disease
Our primary outcome was incident (new-onset) CVD, including myocardial infarction, stroke, and fatal coronary heart disease. All CVD events were centrally adjudicated using standardized clinical, laboratory, and radiological criteria as previously described (eTable 1 in the Supplement).15-18
Selection of the Analysis Cohorts
We excluded participants who had CVD at study entry, developed CVD before pneumonia occurred, or had missing information on CVD. Among the remaining eligible participants, we built nested cohorts of patients with and without pneumonia within CHS and ARIC using the “incidence density sampling without replacement of controls” approach because it produces unbiased estimates.25 Briefly, we identified participants who were hospitalized with pneumonia in each cohort and determined the time (in days) from their enrollment to first hospitalization with pneumonia (ie, time to pneumonia). We then matched each participant with pneumonia by age (±5 years) to 2 controls randomly selected from participants who were alive and actively followed in the study at least for the same period (time to pneumonia) but did not have pneumonia or CVD during that time. Once chosen, the participants could not be sampled again. Participants initially selected as a control could be hospitalized with pneumonia at a later time.
Covariates
Depending on availability in each study, covariates (eTable 2 in the Supplement) included demographics (age, sex, and race), cardiovascular risk factors (hypertension; diabetes mellitus; total, high-density lipoprotein, and low-density lipoprotein cholesterol; smoking [history of smoking and pack-years smoked]; alcohol abuse;26 atrial fibrillation; body mass index [BMI, calculated as weight in kilograms divided by height in meters squared]; chronic kidney disease; and serum levels of C-reactive protein);27,28 subclinical CVD (presence of subclinical disease based on measurements in various vascular beds in CHS and in individual measures of vascular disease in ARIC);29 lung function (percentage of the predicted forced expiratory volume in the first second of expiration [FEV1]); measures of functional status (trajectories of activities of daily living [ADL] and independent activities of daily living [IADL] over time);30 and cognition (trajectories of modified mini-mental status examination scores over time) (eMethods 2 in the Supplement).30 In CHS, covariates were longitudinally updated for the first 10 years of the study. We used the most recent value or trajectories of longitudinal data before participant’s inclusion in the nested analysis cohort. In ARIC, only baseline values were included. We considered, but did not include, statin usage before pneumonia hospitalization because its prevalence at baseline was very low and similar (2.3% in CHS) in participants with and without pneumonia and it remained similar between the 2 groups over the first 10 years. Less than 5% of data for each covariate was missing.
Statistical Analyses
We compared distributions of categorical and continuous variables using χ2 tests, 2-sample t tests, or Wilcoxon 2-sample tests, as appropriate.
To examine the association between pneumonia and subsequent risk of CVD in the nested analysis cohorts, we used the Gray piecewise constant time-varying coefficients model31 to estimate the hazard ratios (HRs) and their 95% CIs at prespecified postpneumonia intervals (0-30 days, 31-90 days, 91 days-1 year, and then yearly up to year 10) based on prior studies.7,8,32 We chose the Gray model for 2 reasons. First, the Gray model does not assume time-invariant effects of the exposure (as opposed to traditional models such as Cox proportional hazards model).31 This is important because the association between pneumonia and CVD varies over time.7,8,32 Second, the Gray model accounts for differences in exposure time. This is important because participants with pneumonia had shorter survival times compared with controls within each time interval. Participants were censored at the time of their first CVD event, death, or loss to follow up. Using the Gray model, we conducted unadjusted and adjusted analyses adjusting for the covariates described above. The HRs for the covariates did not vary over time after pneumonia and thus a single HR was estimated for each covariate. We show the risk of CVD for common traditional risk factors, including hypertension, smoking, and diabetes.
We also constructed failure plots using the Gray model. We computed these curves using the covariates described above. For each covariate, we computed the 25th, 50th, and 75th percentiles and then used these values to obtain the corresponding curves from the Gray model for participants with and without pneumonia.
We conducted stratified analysis to assess whether the association between pneumonia and CVD was observed for severe and nonsevere pneumonia cases. In addition, we conducted 3 sensitivity analyses. First, we explored whether the association between pneumonia and CVD in our primary analysis was specific to this infection or whether it was also present with other acute illnesses that lead to hospitalization. We therefore repeated our primary analysis but matched each participant hospitalized with pneumonia to a participant hospitalized for other reasons (excluding hospitalizations for CVD and infections). Second, we restricted our analysis to include only pneumonia cases that had pneumonia recorded in the primary (first) discharge diagnosis field of their hospitalization along with their corresponding controls. This stringent definition reduces the sensitivity of capturing hospitalizations with pneumonia but maximizes the probability that pneumonia was the primary reason for these admissions.22 Third, we repeated our primary analysis excluding hospitalizations in which both heart failure and pneumonia were recorded. We excluded these cases because these individuals could have had undiagnosed CVD (which may manifest as heart failure), or these hospitalizations may have been solely due to heart failure rather than pneumonia (as it is often difficult to clinically distinguish between them).
The cumulative risk of CVD and mortality was calculated for both cohorts using all participants hospitalized with pneumonia. We used a complete case approach (ie, observations of participants with missing data were omitted) to estimate the number of cases and controls that were at risk and that developed CVD at each time interval as well as the corresponding HRs for CVD between the 2 groups. All tests were 2-sided and a P value of less than .05 was considered statistically significant. All statistical analyses were performed using SAS (SAS Institute), version 9.2, and R statistical software, version 2.1.
Results
Cardiovascular Health Study
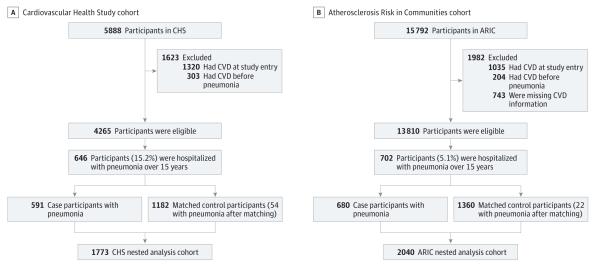
There were 4265 eligible participants. After matching, the analysis cohort nested within CHS consisted of 1773 (591 pneumonia cases and 1182 controls) participants (Figure 1A). Of note, 54 controls (4.5%) developed pneumonia after they were identified as a control using incidence density sampling; however, they were still considered controls in all analyses. The average age of patients with pneumonia was 73 years, they had a high burden of cardiovascular risk factors, and most were functional (92% had either 1 or no ADL impairments) and had normal or near-normal cognition (93% had an average modified mini-mental status examination score of 75) before pneumonia occurred (Table and eTable 3 in the Supplement). Of the 591 pneumonia cases, 211 cases (35.7%) had severe pneumonia. Mortality was high following pneumonia hospitalization. The cumulative mortality in pneumonia cases at 30 days after pneumonia was 111 deaths (18.8%); at 90 days, 146 deaths (24.7%); at 1 year, 212 deaths (35.9%); at 5 years, 348 deaths (58.9%); and at 10 years, 419 deaths (70.9%). Among controls, the corresponding mortality was lower (at 30 days, 8 deaths [0.68%]; at 90 days, 26 deaths [2.2%]; at 1 year, 92 deaths [7.8%]; at 5 years, 400 deaths [33.8%]; and at 10 years, 759 deaths [64.2%]).
Figure 1. Selection of the Nested Analysis Cohorts From the Cardiovascular Health Study and the Atherosclerosis Risk in Communities Study.
ARIC indicates Atherosclerosis Risk in Communities study; CHS, Cardiovascular Health Study; CVD, cardiovascular disease. We used an incidence density approach to match pneumonia cases to controls. Controls were not hospitalized with pneumonia when matched or prior to matching but could develop pneumonia at a later time during follow-up; thus, 54 controls in CHS and 22 controls in ARIC developed pneumonia after matching.
Table.
Characteristics of the Nested Analysis Cohorts in the Cardiovascular Health Study and the Atherosclerosis Risk in Communities Study
| CHS, No. (%) (n = 1773)a |
ARIC, No. (%) (n = 2040)b |
|||||
|---|---|---|---|---|---|---|
| Characteristics at Enrollment | Pneumonia Cases (n = 591) |
Controls (n = 1182) |
P Value | Pneumonia Cases (n = 680) |
Controls (n = 1360) |
P Value |
| Age, mean (SD), y | 73.9 (5.8) | 72.6 (5.6) | <.001 | 55.8 (5.8) | 55.4 (5.9) | .12 |
|
| ||||||
| Women | 342 (57.8) | 778 (65.8) | .001 | 366 (53.8) | 773 (56.8) | .20 |
|
| ||||||
| African Americans | 85 (14.4) | 157 (13.3) | .22 | 215 (31.7) | 333 (24.5) | <.001 |
|
| ||||||
| Atrial fibrillation | 38 (6.4) | 68 (5.8) | .57 | 0 (0.0) | 2 (0.15) | c |
|
| ||||||
| Diabetes | 109 (18.7) | 160 (13.6) | <.005 | 106 (15.8) | 133 (9.9) | <.01 |
|
| ||||||
| Hypertension | 423 (71.6) | 911 (77.1) | .011 | 256 (37.8) | 430 (31.8) | <.001 |
|
| ||||||
| Chronic kidney diseased | 175 (29.9) | 319 (27.0) | .21 | 158 (23.7) | 293 (21.8) | .35 |
|
| ||||||
| BMI, mean (SD) | 26.2 (4.9) | 26.9 (5.0) | <.002 | 27.6 (6.2) | 27.3 (5.3) | .29 |
|
| ||||||
| Ever smokers | 381 (64.5) | 664 (56.2) | <.001 | 492 (72.4) | 750 (55.2) | <.001 |
|
| ||||||
| Pack-years, median (IQR) | 6.5 (0.0-9.0) | 0.0 (0.0-22.0) | <.001 | 20.1 (0.0-40.0) | 1.95 (0.0-24.2) | <.001 |
|
| ||||||
| Alcohol abuse | 33 (5.6) | 56 (4.7) | .44 | 79 (11.8) | 136 (10.1) | .24 |
|
| ||||||
| LDL, mg/dL | ||||||
| <100 | 158 (27.3) | 235 (20.0) | <.001 | 125 (19.2) | 224 (17.0) | .47 |
|
|
|
|||||
| 100-129 | 196 (33.9) | 390 (33.2) | 176 (27.1) | 366 (27.7) | ||
|
|
|
|||||
| ≥130 | 224 (38.8) | 549 (46.8) | 349 (53.7) | 730 (55.3) | ||
|
| ||||||
| HDL, mg/dL | ||||||
| <35 | 30 (5.1) | 43 (3.7) | .32 | 108 (16.2) | 153 (11.4) | .008 |
|
|
|
|||||
| 35-59 | 365 (60.9) | 738 (62.6) | 388 (58.4) | 803 (60.1) | ||
|
|
|
|||||
| ≥60 | 199 (34.0) | 398 (33.8) | 169 (24.4) | 381 (28.5) | ||
|
| ||||||
| Total cholesterol, mg/dL | ||||||
| <200 | 289 (49.3) | 540 (45.8) | .32 | 258 (38.8) | 503 (37.6) | .86 |
|
|
|
|||||
| 200-239 | 201 (34.3) | 431 (35.7) | 248 (37.3) | 503 (37.6) | ||
|
|
|
|||||
| ≥240 | 96 (16.4) | 219 (18.6) | 159 (23.9) | 331 (24.8) | ||
|
| ||||||
| Serum CRP, median (IQR), mg/L | 2.67 (1.29-4.98) | 2.27 (1.19-4.07) | .002 | |||
|
| ||||||
| Percentage of predicted FEV1, mean (SD) | 84.7 (23.9) | 94.7 (20.4) | <.001 | 81.9 (22.9) | 95.3 (16.3) | <.001 |
|
| ||||||
| Subclinical CVDe | 408 (69.0) | 719 (60.8) | <.001 | |||
|
| ||||||
| Diagnostic Q waves on electrocardiogram | 3 (0.4) | 2 (0.2) | .33 | |||
|
| ||||||
| Ankle brachial index <0.9 | 47 (6.9) | 38 (2.8) | <.001 | |||
|
| ||||||
| Carotid artery wall thickness, mm | 0.77 (0.21) | 0.72 (0.18) | <.001 | |||
|
| ||||||
| Carotid atherosclerotic plaque on ultrasound | 283 (41.6) | 711 (52.3) | <.001 | |||
|
| ||||||
|
Longitudinal Trajectories
| ||||||
| Activities of daily livingf | ||||||
| No decline | 430 (72.8) | 893 (75.6) | .001 | |||
|
|
|
|||||
| Minimal decline | 115 (19.5) | 242 (20.5) | ||||
|
|
|
|||||
| Rapid decline | 46 (7.8) | 47 (4.0) | ||||
|
| ||||||
| Independent activities of daily livingf | ||||||
| No decline | 404 (68.4) | 900 (76.1) | <.001 | |||
|
|
|
|||||
| Rapid decline | 187 (31.6) | 282 (23.9) | ||||
|
|
|
|||||
| Cognition based on mini-mental state scoref | ||||||
| No decline | 420 (71.1) | 949 (80.3) | <.001 | |||
|
|
|
|||||
| Minimal decline | 129 (21.8) | 190 (16.1) | ||||
|
|
|
|||||
| Rapid decline | 42 (7.1) | 43 (3.6) | ||||
Abbreviations: ARIC, Atherosclerosis Risk in Communities; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CHS, Cardiovascular Health Study; CRP, C-reactive protein; CVD, cardiovascular disease; FEV1, forced expiratory volume in first second; HDL, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein.
SI conversion factors: To convert HDL, LDL, and total cholesterol to mmol/L, multiply by 0.0259.
Values represent the most recent available measurement before inclusion into the analysis cohort.
Values represent measurements at enrollment in ARIC.
Estimation of a P value is not valid due to the low number of events.
Defined as glomerular filtration rate <60 mL/min/1.73 m2 estimated by the Modification of Diet in Renal Disease study equation.27
Subclinical disease was defined as having any 1 of the following: (1) major electrocardiogram abnormalities based on the Minnesota Code, (2) ankle-brachial index of ≤0.9, (3) increased carotid or internal carotid artery wall thickness (>80th percentile of the CHS distribution) or stenosis of >25% (based on ultrasonographic findings), (4) echocardiogram wall motion abnormality or low ejection fraction, or (5) positive responses to the Rose Questionnaire for angina or intermittent claudication (without clinical history of these diagnoses).29
Participants on trajectories of no and minimal decline had either normal or borderline scores (score <79 suggests dementia) throughout the study. Participants on trajectories of no and minimal decline for activities of daily living (ADL) and independent ADL (IADL) required assistance for ≤1 ADLs or IADLs. Additional information on the calculation of these trajectories is provided in eMethods 2 of the Supplement.30
Based on all patients hospitalized with pneumonia, 206 participants (34.85%) had CVD events over 10 years after pneumonia hospitalization. Of these, 104 cases (50.5%) were myocardial infarction, 35 cases (17.0%) were stroke, and 67 (32.5%) were fatal coronary heart disease events. The cumulative number of pneumonia cases that experienced CVD events by 30 days after pneumonia were 68 (11.5%); at 90 days, 79 (13.4%); at 1 year, 106 (17.9%); and at 5 years, 177 (30.0%).
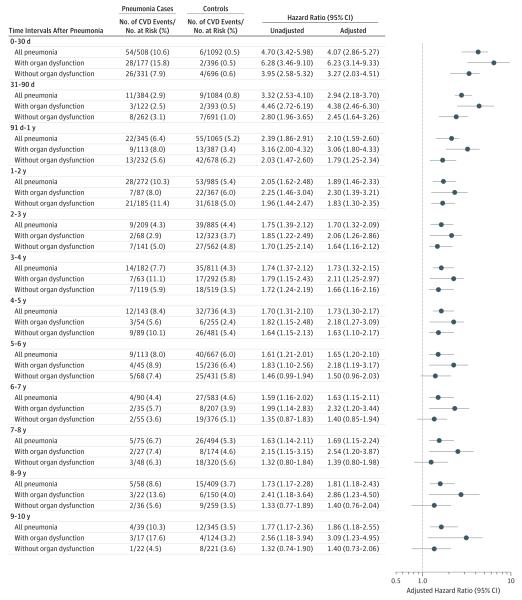
Compared with controls, the 10-year risk of CVD was higher among participants with pneumonia (P < .001) (Figure 2). The risk was highest during the first year. During this period, CVD occurred in 54 cases (10.6%) and 6 controls (0.5%) in the first 30 days (HR, 4.07 [95% CI, 2.86-5.27]); 11 cases (2.9%) and 9 controls (0.8%) between 31 and 90 days (HR, 2.94 [95% CI, 2.18-3.70]); and 22 cases (6.4%) and 55 controls (5.2%) between 91 days and 1 year (HR, 2.10 [95% CI, 1.59-2.60]). After the first year, the risk of CVD was highest in the second year, when 28 cases (10.3%) and 53 controls (5.4%) developed CVD (HR, 1.89 [95% CI, 1.46-2.33]); and lowest in the seventh year, when 4 cases (4.4%) and 27 controls (4.6%) developed CVD (HR, 1.63 [95% CI, 1.15-2.11]). For reference, the adjusted HRs for CVD over 10 years associated with smoking were 1.18; with hypertension, 1.84; and with diabetes, 1.19.
Figure 2. Risk of Cardiovascular Disease Events After Hospitalization for Pneumonia in the Cardiovascular Health Study.
CVD indicates cardiovascular disease. The analysis included 1773 participants (591 pneumonia cases and 1182 controls). Stratified analyses by severity of pneumonia included 633 participants (211 pneumonia cases and 422 controls) for pneumonia with organ dysfunction and 1140 participants (380 pneumonia cases and 422 controls) for pneumonia without organ dysfunction. The number of participants at risk and those who developed an event over each time interval were estimated using a complete case approach and participants with missing data for covariates were excluded. The estimates were adjusted for age, sex, race, hypertension, diabetes mellitus, serum total, high-density lipoprotein and low-density lipoprotein cholesterol, smoking, alcohol abuse,26 atrial fibrillation, chronic kidney disease,27 serum C-reactive protein,28 presence of subclinical cardiovascular disease,29 percentage of predicted forced expiratory volume in first second of expiration (FEV1) measured by spirometry, trajectories of activities of daily living and independent activities of daily living over time,30 and trajectories of modified mini-mental status examination scores over time.30 Adjusted hazard ratios were calculated using the most recently available measurements before inclusion in the nested analysis cohort.
The association between pneumonia and increased long-term risk of CVD was observed for both severe and nonsevere pneumonia cases (Figure 2). In general, the adjusted HRs for severe pneumonia were higher and remained above 1 for longer duration compared with the cases of nonsevere pneumonia, but the 95% CIs of these estimates overlapped between the 2 groups.
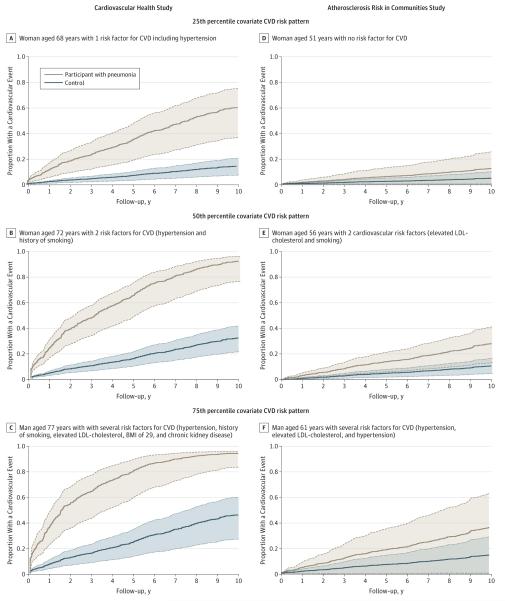
Figure 3 shows that the increased hazard for CVD following pneumonia was seen across all participants. For instance, the 10-year risk of CVD for a woman aged 72 years who had 2 cardiovascular risk factors, including hypertension and smoking, increased from 31% without pneumonia to 90% with pneumonia (panel B), and was approximately 2-fold higher compared with a man aged 77 years with several risk factors including hypertension, smoking, elevated low-density lipoprotein cholesterol, high BMI, and chronic kidney disease (panel C). Thus, pneumonia hospitalization was associated with a large increase in the risk of CVD.
Figure 3. Adjusted Failure Plots to Show the Magnitude of Risk Increase for Cardiovascular Disease That Was Associated With Hospitalization for Pneumonia in CHS and ARIC.
ARIC indicates Atherosclerosis Risk in Communities study; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CHS, Cardiovascular Health Study; CVD, cardiovascular disease; LDL, low-density lipoprotein. The failure plots are adjusted for demographics and cardiovascular risk factors (see Methods for additional details). The plots in brown represent participants with pneumonia and the plots in blue represent controls with a covariate pattern in the 25th, 50th, and 75th percentiles in CHS and ARIC. The shaded areas around each curve represent 95% CIs.
In sensitivity analyses, the HRs for the association between pneumonia and CVD remained similar and significant throughout the 10-year follow-up period when controls included only participants who required hospitalization for other causes, when analyses were restricted to cases in which pneumonia was recorded in the primary discharge diagnosis field, and when participants who were diagnosed with pneumonia and heart failure in the same admission were excluded (eTable 4, eTable 5, and eTable 6 in the Supplement).
Atherosclerosis Risk in Communities Study
There were 13 810 eligible participants. After matching, the analysis cohort nested within ARIC consisted of 2040 participants (680 pneumonia cases and 1360 controls, Figure 1B). After their selection as controls, 22 participants (1.6%) subsequently developed pneumonia. The average age of participants with pneumonia was 55 years. Similar to CHS, these participants had a high burden of cardiovascular risk factors (Table and eTable 3 in the Supplement) and high mortality after pneumonia. The cumulative mortality among pneumonia cases at 30 days after pneumonia was 80 deaths (11.8%); at 90 days, 107 deaths (15.7%); at 1 year, 168 deaths (24.7%); at 5 years, 300 deaths (44.1%); and at 10 years, 518 deaths (76.2%). Among controls, the corresponding mortality was also lower (at 30 days, 4 deaths [0.29%]; at 90 days, 7 deaths [0.51%]; at 1 year, 24 deaths [1.8%]; at 5 years, 116 deaths [8.5%]; and at 10 years, 286 deaths [21.0%]).
Based on all patients hospitalized with pneumonia, 112 cases (16.5%) had CVD over 10 years after pneumonia hospitalization (33 cases [29.5%] with myocardial infarction, 36 cases [32.1%] with stroke, and 43 cases [38.4%] with fatal coronary heart disease). The cumulative number of pneumonia cases that developed CVD at 30 days after pneumonia were 6 (0.9%); at 90 days, 12 (1.8%); at 1 year, 31 (4.6%); and at 5 years, 70 (10.3%).
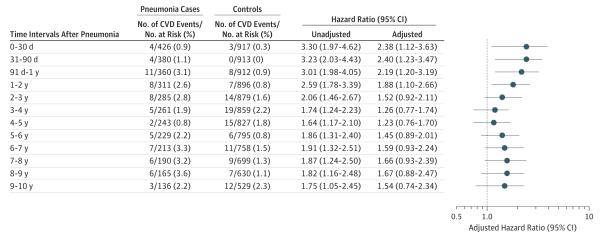
Similar to CHS, participants hospitalized with pneumonia had a higher risk of CVD after the infection compared with controls (P < .001). The risk was highest during the first year and persisted through the second year. During these periods, CVD developed in 4 cases (0.9%) and 3 controls (0.3%) in the first 30 days (HR, 2.38 [95% CI, 1.12-3.63]); 4 cases (1.1%) and 0 controls (0.0%) between 31 and 90 days (HR, 2.40 [95% CI, 1.23-3.47]); 11 cases (3.1%) and 8 controls (0.9%) between 91 days and 1 year (HR, 2.19 [95% CI, 1.20-3.19]); and 8 cases (2.6%) and 7 controls (0.8%) during the second year (HR, 1.88 [95% CI, 1.10-2.66]) (Figure 4). After the second year, the HRs remained elevated but the results were not statistically significant. Similar to CHS, pneumonia was associated with a large increase in the risk of CVD in younger adults across different risk groups (Figure 3).
Figure 4. Risk of Cardiovascular Disease (CVD) Events After Hospitalization for All Pneumonia in the Atherosclerosis Risk in Communities Study.
The analysis included 2040 participants (680 pneumonia cases and 1360 controls). The number of participants at risk and those who developed an event over each time interval were estimated using a complete case approach and participants with missing data for covariates were excluded. The estimates were adjusted for age, sex, race, hypertension, diabetes mellitus, plasma total, high-density lipoprotein and low-density lipoprotein cholesterol, smoking, alcohol abuse,26 atrial fibrillation, chronic kidney disease,27 presence of diagnostic Q waves in electrocardiogram, peripheral arterial disease (defined by ankle brachial index <0.9), carotid artery wall thickness, presence of carotid atherosclerotic plaque by ultrasound, and percentage of predicted forced expiratory volume in first second of expiration (FEV1) measured by spirometry. Adjusted hazard ratios were calculated using baseline (at study entry) covariates measurements.
Discussion
In this study, hospitalization with pneumonia in older adults was associated with subsequent increase in the risk of CVD. The risk was highest (4-fold) in the first 30 days after pneumonia and although it progressively declined during the first year, it remained approximately 1.5-fold higher in subsequent years. This association persisted after adjusting for demographics, the burden of cardiovascular risk factors, and crude measures of frailty, and it was observed across a range of infection severity, was validated in a cohort of younger adults, and it was robust to sensitivity analyses using different control groups and stringent case definitions for pneumonia hospitalization. Moreover, in our analyses, the magnitude of risk for CVD associated with pneumonia was similar or higher compared with the risk of CVD associated with traditional risk factors, such as smoking, diabetes, and hypertension. Thus, our results suggest that pneumonia is an important risk factor for CVD.
The high short-term risk of CVD within 30 days after infections, mainly of the respiratory tract, is well established.3 Nonetheless, results of studies addressing more lasting effects of infections on CVD risk have been conflicting. Using case-only analyses, Smeeth and colleagues7 and Elkind and colleagues8 found that the association between infection and CVD was detected only up to 3 months after infection. However, case-only analyses (self-controlled cases series and case-crossover designs) are not designed to assess long-term effects of acute exposures.33,34 In CHS, we9 previously showed that hospitalization for pneumonia was associated with a 2-fold increased risk of death from coronary heart disease over 5 years after infection, but we did not adjust for potential confounders, particularly cardiovascular risk factors and subclinical CVD before pneumonia. Using medical claims data, Foley and colleagues10 reported that septicemia is associated with increased rates of CVD up to 5 years after infection. However, they analyzed only patients on dialysis, and their results cannot be generalized to the broader population. Thus, this study is the first to document the temporal variation in the long-term risk of CVD in adults using rigorous methods to adjust for many potential confounders.
The mechanisms by which infections might increase CVD risk in the short-term are discussed elsewhere.3,4 However, the mechanism by which infections could affect long-term risk of CVD are poorly understood. Experimental models of infection in mice prone to atherosclerosis and autopsy studies in humans suggest that infections can induce pro-inflammatory changes in the cellular composition of the atherosclerotic lesions and make them more vulnerable to cause coronary and cerebrovascular events.35,36 Persistent systemic inflammatory activity is a known risk factor for CVD.37 Although more than 80% of patients hospitalized for pneumonia recover by 1 week, half of these patients continue exhibiting high circulating inflammatory markers.38,39 Furthermore, higher interleukin-6 levels at hospital discharge are associated with increased cardiovascular mortality at 1 year after pneumonia.5 Thus, persistent inflammation after pneumonia can contribute to subsequent progression to CVD. Similarly, survivors of pneumonia hospitalization may also have a persistent procoagulant state, and higher levels of coagulation markers at hospital discharge have been associated with increased risk of cardiovascular deaths.6 Finally, organ dysfunction during acute infection may persist after recovery and increase risk of CVD. For example, acute kidney injury is common during pneumonia and its progression to chronic kidney disease could increase cardiovascular risk.40
Our study has several implications. First, because we included only participants without CVD prior to pneumonia, our findings suggest that hospitalization for pneumonia should be considered an independent cardiovascular risk factor in future strategies for screening and primary prevention of CVD. This is particularly important in the elderly because their risk of pneumonia and subsequent CVD was high. Second, because previous studies demonstrate that patients hospitalized with pneumonia have increased long-term mortality that is not explained by their high burden of comorbidities before the infection,11,12 and that CVD is a leading cause of death in these cases,5 our findings should prompt clinical trials to test targeted strategies in this population. However, because mortality differences between participants with and without pneumonia were attenuated in CHS at 10 years after the occurrence of pneumonia in our study, such strategies may not be beneficial in very old individuals. Finally, the association of pneumonia with CVD risk should also be considered when estimating the cost and benefit of interventions to prevent pneumonia.
Strengths of our study are inclusion of representative community samples of middle-aged and elderly adults, large sample size, a validated approach to identify pneumonia events, rigorous methodology to adjudicate cardiovascular events, replication of results in another cohort, and adjustment for a large number of potential confounders.
Our study also has limitations. First, although we included fatal and nonfatal cardiovascular events, we did not account for competing risk of death due to noncardiovascular reasons. However, this limitation would bias our estimates toward the null. Second, we did not include patients with pneumonia that did not require hospitalization, and thus our results cannot be generalized to that population. Third, we ascertained only first hospitalizations for pneumonia and thus, our analyses did not account for the potential effect of recurrent pneumonia episodes. Fourth, we adjusted for a large number of confounders but could not assess the role of pneumococcal vaccination. However, pneumococcal vaccine use is likely to be low in ARIC and the magnitude of the association beyond 90 days was similar in both cohorts. Thus, pneumococcal vaccination is unlikely to confound this association. Fifth, we did not adjust for interventions that could affect the course of pneumonia, such as type of antibiotic therapy. This is important because the long-term risk of CVD may be due to a combination of the disease itself and accompanying therapies. Finally, because of our reliance on administrative data for identification of pneumonia, we cannot rule out misclassification bias. Nevertheless, our risk estimates did not meaningfully change when we restricted our analyses to cases in which pneumonia was recorded in the primary discharge diagnosis field and excluded cases where both pneumonia and heart failure were recorded.
Conclusions
Hospitalization for pneumonia is associated with increased short-term and long-term risk of CVD, suggesting that pneumonia may be an important risk factor for CVD.
Supplementary Material
Acknowledgments
Funding/Support: The Cardiovascular Health Study (CHS) was supported by contracts HHSN268201200036C, HHSN268200800007C, N01 HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grant HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by grant AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. The Atherosclerosis Risk in Communities study was supported by contracts HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C from the NHLBI. This article is also supported by a Junior Investigator Award from the Department of Medicine of The Ottawa Hospital and the Ottawa Hospital Research Institute in Ottawa, Ontario, Canada (Dr Corrales-Medina) and by K23GM083215 and R01GM097471 from the National Institute of General Medical Sciences (Dr Yende).
Footnotes
Author Contributions: Drs Corrales-Medina and Yende had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Corrales-Medina, Angus, Chirinos, Newman, Loehr, Yende.
Acquisition, analysis, or interpretation of data: Alvarez, Weissfeld, Chirinos, Chang, Newman, Loehr, Folsom, Elkind, Lyles, Kronmal, Yende.
Drafting of the manuscript: Corrales-Medina, Alvarez, Yende.
Critical revision of the manuscript for important intellectual content: Weissfeld, Angus, Chirinos, Chang, Newman, Loehr, Folsom, Elkind, Lyles, Kronmal, Yende.
Statistical analysis: Alvarez, Weissfeld, Chang, Loehr, Yende.
Obtained funding: Newman, Yende.
Administrative, technical, or material support: Kronmal, Yende.
Study supervision: Corrales-Medina, Angus, Chirinos, Yende.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Angus reports grant funding from the National Institutes of Health. Dr Elkind reports consulting for Biogen Idec, Bristol-Myers Squibb/Pfizer, Boehringer Ingelheim, Daiichi Sankyo, and Janssen; giving expert testimony for Merck/Organon; serving on the advisory board for CardioNet; and receiving grant funding from Bristol-Myers Squibb/ Sanofi and diaDexus. No other disclosures were reported.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr Angus, a JAMA associate editor, had no role in the evaluation of or decision to publish this article.
REFERENCES
- 1.NHLBI [Accessed June 13, 2013];Morbidity and mortality: 2012 chartbook on cardiovascular lung and blood diseases. http://www.nhlbi.nih.gov/files/docs/research/2012_ChartBook_508.pdf.
- 2.Go AS, Mozaffarian D, Roger VL, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corrales-Medina VF, Madjid M, Musher DM. Role of acute infection in triggering acute coronary syndromes. Lancet Infect Dis. 2010;10(2):83–92. doi: 10.1016/S1473-3099(09)70331-7. [DOI] [PubMed] [Google Scholar]
- 4.Corrales-Medina VF, Musher DM, Shachkina S, Chirinos JA. Acute pneumonia and the cardiovascular system. Lancet. 2013;381(9865):496–505. doi: 10.1016/S0140-6736(12)61266-5. [DOI] [PubMed] [Google Scholar]
- 5.Yende S, D’Angelo G, Kellum JA, et al. GenIMS Investigators. Inflammatory markers at hospital discharge predict subsequent mortality after pneumonia and sepsis. Am J Respir Crit Care Med. 2008;177(11):1242–1247. doi: 10.1164/rccm.200712-1777OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yende S, D’Angelo G, Mayr F, et al. GenIMS Investigators Elevated hemostasis markers after pneumonia increases 1-year risk of all-cause and cardiovascular deaths. PLoS One. 2011;6(8):e22847. doi: 10.1371/journal.pone.0022847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351(25):2611–2618. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 8.Elkind MS, Carty CL, O’Meara ES, et al. Hospitalization for infection and risk of acute ischemic stroke: the Cardiovascular Health Study. Stroke. 2011;42(7):1851–1856. doi: 10.1161/STROKEAHA.110.608588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Meara ES, White M, Siscovick DS, Lyles MF, Kuller LH. Hospitalization for pneumonia in the Cardiovascular Health Study: incidence, mortality, and influence on longer-term survival. J Am Geriatr Soc. 2005;53(7):1108–1116. doi: 10.1111/j.1532-5415.2005.53352.x. [DOI] [PubMed] [Google Scholar]
- 10.Foley RN, Guo H, Snyder JJ, Gilbertson DT, Collins AJ. Septicemia in the United States dialysis population, 1991 to 1999. J Am Soc Nephrol. 2004;15(4):1038–1045. doi: 10.1097/01.asn.0000119144.95922.c4. [DOI] [PubMed] [Google Scholar]
- 11.Quartin AA, Schein RM, Kett DH, Peduzzi PN, Department of Veterans Affairs Systemic Sepsis Cooperative Studies Group Magnitude and duration of the effect of sepsis on survival. JAMA. 1997;277(13):1058–1063. [PubMed] [Google Scholar]
- 12.Yende S, Angus DC, Ali IS, et al. Influence of comorbid conditions on long-term mortality after pneumonia in older people. J Am Geriatr Soc. 2007;55(4):518–525. doi: 10.1111/j.1532-5415.2007.01100.x. [DOI] [PubMed] [Google Scholar]
- 13.Loeb M. Community-acquired pneumonia [published online August 18, 2010] Clin Evid. (Online) [Google Scholar]
- 14.Hall MJ, DeFrances CJ, Williams SN, Golosinskiy A, Schwartzman A. National Hospital Discharge Survey: 2007 summary. Natl Health Stat Report. 2010;(29):1–20. 24. [PubMed] [Google Scholar]
- 15.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1(3):263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 16.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives: the ARIC investigators. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 17.Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events: the Cardiovascular Health Study. Ann Epidemiol. 1995;5(4):278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 18.Price TR, Psaty B, O’Leary D, Burke G, Gardin J. Assessment of cerebrovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1993;3(5):504–507. doi: 10.1016/1047-2797(93)90105-d. [DOI] [PubMed] [Google Scholar]
- 19.White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial 2 years’ experience. J Clin Epidemiol. 1996;49(2):223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 20.Rosamond WD, Folsom AR, Chambless LE, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30(4):736–743. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- 21.Guevara RE, Butler JC, Marston BJ, Plouffe JF, File TM, Jr, Breiman RF. Accuracy of ICD-9-CM codes in detecting community-acquired pneumococcal pneumonia for incidence and vaccine efficacy studies. Am J Epidemiol. 1999;149(3):282–289. doi: 10.1093/oxfordjournals.aje.a009804. [DOI] [PubMed] [Google Scholar]
- 22.Lindenauer PK, Lagu T, Shieh MS, Pekow PS, Rothberg MB. Association of diagnostic coding with trends in hospitalizations and mortality of patients with pneumonia, 2003-2009. JAMA. 2012;307(13):1405–1413. doi: 10.1001/jama.2012.384. [DOI] [PubMed] [Google Scholar]
- 23.Yende S, Alvarez K, Loehr L, et al. Atherosclerosis Risk in Communities Study. Cardiovascular Health Study. Health, Aging, and Body Composition Study Epidemiology and long-term clinical and biologic risk factors for pneumonia in community-dwelling older Americans: analysis of 3 cohorts. Chest. 2013;144(3):1008–1017. doi: 10.1378/chest.12-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwashyna TJ, Cooke CR, Wunsch H, Kahn JM. Population burden of long-term survivorship after severe sepsis in older Americans. J Am Geriatr Soc. 2012;60(6):1070–1077. doi: 10.1111/j.1532-5415.2012.03989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang MH, Shugart YY, Cole SR, Platz EA. A simulation study of control sampling methods for nested case-control studies of genetic and molecular biomarkers and prostate cancer progression. Cancer Epidemiol Biomarkers Prev. 2009;18(3):706–711. doi: 10.1158/1055-9965.EPI-08-0839. [DOI] [PubMed] [Google Scholar]
- 26.Town M, Naimi TS, Mokdad AH, Brewer RD. Health care access among US adults who drink alcohol excessively: missed opportunities for prevention. Prev Chronic Dis. 2006;3(2):A53. [PMC free article] [PubMed] [Google Scholar]
- 27.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function—measured and estimated glomerular filtration rate. N Engl J Med. 2006;354(23):2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 28.Tracy RP, Lemaitre RN, Psaty BM, et al. Relationship of C-reactive protein to risk of cardiovascular disease in the elderly: results from the Cardiovascular Health Study and the Rural Health Promotion Project. Arterioscler Thromb Vasc Biol. 1997;17(6):1121–1127. doi: 10.1161/01.atv.17.6.1121. [DOI] [PubMed] [Google Scholar]
- 29.Kuller L, Borhani N, Furberg C, et al. Prevalence of subclinical atherosclerosis and cardiovascular disease and association with risk factors in the Cardiovascular Health Study. Am J Epidemiol. 1994;139(12):1164–1179. doi: 10.1093/oxfordjournals.aje.a116963. [DOI] [PubMed] [Google Scholar]
- 30.Shah FA, Pike F, Alvarez K, et al. Bidirectional relationship between cognitive function and pneumonia. Am J Respir Crit Care Med. 2013;188(5):586–592. doi: 10.1164/rccm.201212-2154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kasal J, Jovanovic Z, Clermont G, et al. Comparison of Cox and Gray’s survival models in severe sepsis. Crit Care Med. 2004;32(3):700–707. doi: 10.1097/01.ccm.0000114819.37569.4b. [DOI] [PubMed] [Google Scholar]
- 32.Corrales-Medina VF, Serpa J, Rueda AM, et al. Acute bacterial pneumonia is associated with the occurrence of acute coronary syndromes. Medicine (Baltimore) 2009;88(3):154–159. doi: 10.1097/MD.0b013e3181a692f0. [DOI] [PubMed] [Google Scholar]
- 33.Whitaker HJ, Hocine MN, Farrington CP. The methodology of self-controlled case series studies. Stat Methods Med Res. 2009;18(1):7–26. doi: 10.1177/0962280208092342. [DOI] [PubMed] [Google Scholar]
- 34.Maclure M, Mittleman MA. Should we use a case-crossover design? Annu Rev Public Health. 2000;21:193–221. doi: 10.1146/annurev.publhealth.21.1.193. [DOI] [PubMed] [Google Scholar]
- 35.Naghavi M, Wyde P, Litovsky S, et al. Influenza infection exerts prominent inflammatory and thrombotic effects on the atherosclerotic plaques of apolipoprotein E-deficient mice. Circulation. 2003;107(5):762–768. doi: 10.1161/01.cir.0000048190.68071.2b. [DOI] [PubMed] [Google Scholar]
- 36.Madjid M, Vela D, Khalili-Tabrizi H, Casscells SW, Litovsky S. Systemic infections cause exaggerated local inflammation in atherosclerotic coronary arteries: clues to the triggering effect of acute infections on acute coronary syndromes. Tex Heart Inst J. 2007;34(1):11–18. [PMC free article] [PubMed] [Google Scholar]
- 37.Kaptoge S, Seshasai SR, Gao P, et al. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur Heart J. 2014;35(9):578–589. doi: 10.1093/eurheartj/eht367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kellum JA, Kong L, Fink MP, et al. GenIMS Investigators Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med. 2007;167(15):1655–1663. doi: 10.1001/archinte.167.15.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Halm EA, Fine MJ, Marrie TJ, et al. Time to clinical stability in patients hospitalized with community-acquired pneumonia: implications for practice guidelines. JAMA. 1998;279(18):1452–1457. doi: 10.1001/jama.279.18.1452. [DOI] [PubMed] [Google Scholar]
- 40.Murugan R, Karajala-Subramanyam V, Lee M, et al. Genetic and Inflammatory Markers of Sepsis (GenIMS) Investigators Acute kidney injury in nonsevere pneumonia is associated with an increased immune response and lower survival. Kidney Int. 2010;77(6):527–535. doi: 10.1038/ki.2009.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.