Abstract
Thyroid hormone (T3) stimulates various metabolic pathways and the hepatic actions of T3 are mediated primarily through the thyroid hormone receptor beta (TRβ). Hypothyroidism has been linked with low grade inflammation, elevated risk of hepatic steatosis and atherosclerosis. Secretory phospholipases (sPLA2) are associated with inflammation, hyperlipidemia and atherosclerosis. Due to potential linkage between thyroid hormone and sPLA2, we investigated the effect of thyroid hormone status on the regulation of secretory phospholipases in mice, rats and human liver. T3 suppressed the expression of the sPLA2 group IIa (PLA2g2a) gene in the liver of BALB/c mice and C57BL/6 transgenic mice expressing the human PLA2g2a. PLA2g2a was elevated with hypothyroidism and high fat diets which may contribute to the low grade inflammation associated with hypothyroidism and diet induced obesity. We also examined the effects of the TRβ agonist eprotirome on hepatic gene regulation. We observed that eprotirome inhibited the expression of selected sPLA2 genes and furthermore the cytokine mediated induction PLA2g2a was suppressed. In addition, eprotirome induced genes involved in fatty acid oxidation and cholesterol clearance while inhibiting lipogenic genes. Our results indicate that in vivo thyroid hormone status regulates the abundance of sPLA2 and the inhibition of PLA2g2a by T3 is conserved across species. By regulating sPLA2 genes, T3 may impact processes associated with atherosclerosis and inflammation and TRβ agonists may ameliorate inflammation and hyperlipidemia.
Keywords: Thyroid hormone, eprotirome, secretory phospholipase A2 and PLA2g2a
Introduction
Thyroid hormone (T3) promotes reverse cholesterol transport, stimulates conversion of cholesterol to bile acids and enhances metabolic rate [1]. There are two isoforms of the thyroid hormone receptor (TR) including TRα and TRβ [2]. The TRα isoform is highly expressed in the heart and is associated with elevated cardiac function [3]. The lipid lowering actions of T3 are mediated primarily through the TRβ isoform which is predominant in liver [4].
Phospholipases A2 are a family of esterase enzymes which hydrolyze the second carbon of membrane phospholipids to release non-esterified free fatty acids and lysophospholipids [5]. The free fatty acid, arachidonic acid, is a precursor for inflammatory mediators such as eicosanoids [5]. PLA2 enzymes have been classified into three major groups: cytosolic phospholipase A2, secretory phospholipase A2 and calcium independent phospholipase A2. Phospholipase group IIa (PLA2g2a) is a secretory phospholipase that is highly expressed in the liver and macrophages [6]. Expression of the PLA2g2a gene is rapidly induced by cytokines, CCAAT enhancer binding protein β (C/EBPβ) and inflammatory signals [7]. High levels of PLA2g2a are associated with the progression of atherosclerosis and arthritis [8,9].
We found that T3 stimulates numerous metabolic genes in the liver including carnitine palmitoyltransferase 1a (CPT1a), phosphoenolpyruvate carboxykinase (PEPCK) and pyruvate dehydrogenase kinase 4 (PDK4) [10,11]. For these genes, the binding of the TRβ is essential for transcriptional activation. However, the stimulation of these genes also involves other transcription factors such as the CCAAT enhancer binding protein (C/EBPβ) as well as several coactivators including peroxisome proliferator activated receptor gamma coactivator (PGC-1α) and the deacetylase Sirtuin 1 (SIRT1) [12,13]. We recently reported that the rat PLA2g2a promoter has a negative thyroid hormone response element (nTRE) and the binding of liganded TRβ to this element recruits nuclear corepressors NCoR1 and SMRT to inhibit PLA2g2a gene transcription [14].
Over the past decade, a number of TRβ agonists have been developed that stimulate TRβ without impacting TRα [15]. These ligands are either selectively cleared by the liver or bind with greater affinity to TRβ. Therapeutically, the goal of these agonists is to enhance the lipid lowering actions of T3 without impacting the cardiovascular system. Several TRβ agonists such as sobetirome (GC-1) and eprotirome (KB2115) decreased circulating cholesterol without elevating heart rate. In a two week clinical trial, KB2115 lowered the LDL cholesterol by 40% [16]. In phase 2 clinical trials, eprotirome was administered to patients already on statin therapy. Following a 100μg or 200μg dose of KB2115 for 12 weeks, there was a 30% decrease in the LDL cholesterol and a significant reduction in triglycerides. There was no change in heart rate or muscle strength [17]. These studies highlight the potential value of TRβ agonists and the need for further study of their hepatic targets.
In present study, we examined effect of thyroid status and the ability of T3 and the TRβ selective agonist KB2115 to regulate sPLA2 the expression. Our data indicate that T3 negatively regulates some secretory phospholipases in mice, rats and human PLA2g2a transgenic mice models. The TRβ agonist KB2115 not only decreased the basal levels of sPLA2s but also the cytokine mediated induction of PLA2g2a. Genes involved in mitochondrial (CPT1a) and peroxisomal fatty acid oxidation (ABCD3, ACAA1) as well as in cholesterol metabolism like CYP7A1 and SRB-I were induced by KB2115. TRβ agonists may be useful in the treatment of low grade inflammation and fatty liver associated hepatic steatosis.
Materials and methods
Primary Rat Hepatocyte Culture and Treatment
Rat hepatocytes were prepared as described previously [11]. Hepatocytes were seeded in 60 mm plates at a density of 3 × 106 cells/plate and maintained for 12 h in RPMI 1640 media with 10% fetal bovine serum. The next day, cells were washed twice with PBS and serum free RPMI 1640 media was added. The hepatocytes were treated with 100 nM T3 or 1μM of KB2115 for 24 hours.
Animals and Treatments
Hypothyroidism was induced in BALB/c mice and male Sprague Dawley rats by feeding an iodine-free diet containing 0.15% propylthiouracil (Teklad 95125) for 5 weeks. The rats and mice were given intraperitoneal injections of T3 or KB2115 (0.33 mg/kg and 0.11 mg/kg of body weight respectively) and after 24 hrs a second dose of T3/KB2115 was given. Animals were sacrificed after 24 hrs and livers were isolated for RNA and protein. For high fat diet experiments, BALB/c mice were divided into three groups 1) chow fed (Teklad Diet 06101), 2) high fat diet (HFD) (TD 95217) and 3) hypothyroid (0.05% PTU) (TD 120714). The energy content of the HFD was 45% fat and 40% carbohydrate while the chow diet has 65% carbohydrate and 17% fat.
C57BL/6 IIA+ mice contained the human PLA2g2a gene under the regulation of its own promoter [9,18]. To induce hypothyroidism, PTU was added at 0.05% in the diet (TD 120714) for 6 weeks. Each group of mice contained seven female mice. C57BL/6 IIA+ were made hyperthyroid by administering two intraperitoneal injections of T3 (0.11 mg/kg of body weight) for two days.
RNA Extraction and Real Time PCR
RNA was extracted from rat liver and primary hepatocytes by RNA-STAT 60 (Tel-Test). The extracted RNA was further purified with the Qiagen RNeasy Mini Kit (74104). cDNA was made by reverse transcribed using Superscript III (Invitrogen). The parameters for RT-PCR were as follows: 95°C for 5 min, 40 cycles of 95°C for 15 s, 60°C for 30 s and 72°C for 10 s. 18S was used as a reference gene and the quantification of the PCR products was carried out using the ΔΔCt method. The relative Ct values for the rat PLA2g2a were 25-27, mouse PLA2g2a 32-34 and human PLA2g2a 12-14. The forward and reverse primers used for real time PCR are as follows: rat PLA2g2a FP catggcctttggctcaattcaggt, rat PLA2g2a RP acagtcatgagtcacacagcacca, rat PLA2g3 FP acagccttgaacttctggtccact, rat PLA2g3 RP gctttgagcaggttgaagcgttg, rat PLA2g5 FP aactgtgtggtcttgaacctccgt, rat PLA2g5 RP acacactctcatgcagcctaccat, and rat PLA2g1b qiagen (QT00179529) mouse PLA2g2a FP agcctcgatcatggccttt, mouse PLA2g2a RP gccgaatcatttccccaaa, human PLA2g2a FP catggcctttggctcaattcaggt, human PLA2g2a RP aggctggaaatctgctggatgtct.
ELISA
HepG2 cells were grown in 24 well plates (0.2×106 cells/well) in DMEM media containing 10% fetal bovine serum (FBS). The following day, the cells were incubated in serum free DMEM media with 1μM KB2115 or 10 ng/mL IL-6. After 24 hours, cell culture media was centrifuged at 1200 rpm to remove cell debris. ELISA was performed for PLA2g2a using EIA Kit from Cayman Chemicals (585000).
Western Blot
Proteins from rat livers were harvested by homogenizing the livers in RIPA buffer (50 mM Tris-HCl, pH 7.4, 100 mM NaCl, 5 mM EDTA pH 8.0, 1% Triton, 1 mM benzamidine, 0.5 mM PMSF). An equal amount of protein was loaded on a 3-8% Tris-acetate acrylamide gel. Proteins were immunoblotted with primary antibodies PLA2g2a (Abcam) and Tubulin (Sigma) [14]. Immunoreactive proteins were detected using Super signal West femto chemiluminescent substrate (Thermo Scientific).
Transient transfections
HepG2 cells were transfected with 2 μg of −448/+58 rPLA2g2a luciferase reporter, 1 μg of an SV40-TRβ and 0.1 μg of TK-renilla by the calcium phosphate method. On the next day, the media was changed to serum free media and KB 2115 was added. Cells were lysed after 24 h and luciferase assays were conducted using the Promega Dual Luciferase. Luciferase values were normalized for renilla luciferase activity.
Lipid Assessments
Plasma cholesterol and lipoproteins were measured in the University of Tennessee Endocrinology/Lipoprotein Laboratory.
Statistical Analysis
All experiments were conducted 3-4 times and the error bar indicates S.E.M. The data was analyzed by Student’s one/two tailed t test or ANOVA. p values < 0.05 were considered to be statistically significant.
Results
Thyroid hormone negatively regulates expression of secretory PLA2 in BALB/c and transgenic C57BL/6 IIA+ mice
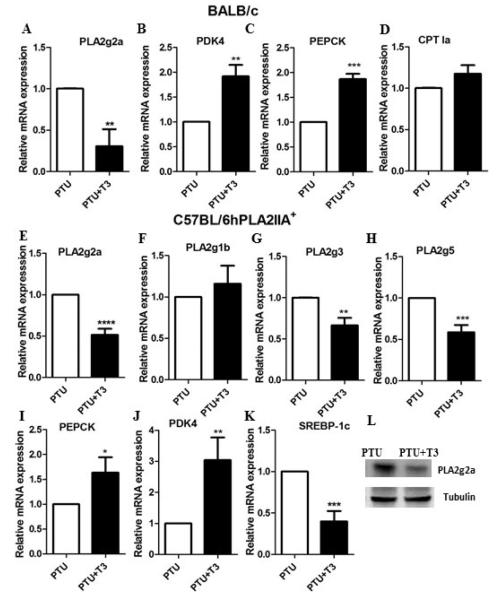
Our first objective was to test whether the thyroid hormone status regulates the PLA2g2a gene in BALB/c mice. BALB/c mice were made hypothyroid by inclusion of 0.05% PTU in the diet. After 5 weeks, mice were given two injections of T3. Administration of T3 reduced the expression of the mouse PLA2g2a gene (Fig 1A). Other genes such as PEPCK and PDK4 were induced by T3 (Fig 1B, 1C), while CPT1a was not elevated by T3 in mice (Fig 1D). The mouse CPT1a has a different pattern of tissue specific expression than the rat or human genes so differential regulation of CPT1a gene by T3 was not unexpected.
Figure 1. Thyroid hormone status regulates expression of secretory PLA2 in BALB/c and C57BL/6 hPLA2 IIA+ mice.
BALB/c mice were made hypothyroid or hyperthyroid as described in the materials and methods. Mice were sacrificed and liver RNA was harvested. The mRNA levels were measured for A) PLA2g2a, B) PDK4, C) PEPCK and D) CPT1a. Values are the average of RNA from 5 mice. C57BL/6 IIA+ mice were fed a PTU supplemented diet for 5 weeks. Mice were injected with T3 and RNA was harvested from the liver. The mRNA levels were measured for E) PLA2g2a, F) PLA2g1b, G) PLA2g3, H) PLA2g5, I) PEPCK, J) PDK4 and K) SREBP-1c. L) PLA2g2a protein abundance was measured by western blotting. Values are the average of RNA from 7 mice. The data are expressed as the mean of the fold induction by PTU+T3 ± S.E.M of mRNA abundance relative to PTU treated mice (* = p value < 0.05; ** = p value <0.01; *** = p value <0.001; ****= p value <0.0001).
To determine if T3 also regulates human PLA2g2a gene in vivo, we tested C57BL/6 transgenic C57BL/6 IIA+ mice expressing the human PLA2g2a gene under the regulation of the human promoter [9]. C57BL/6 mice do not express the PLA2g2a gene due to a frame shift mutation in exon 3 [19]. Following 5 weeks of the 0.05% PTU diet, mice were given two injections of T3. In the liver of the C57BL/6 IIA+ mice, T3 decreased the mRNA abundance of the PLA2g2a gene (Fig 1E) demonstrating that the human PLA2g2a gene is inhibited by T3 in vivo. In addition, the PLA2g3 and PLA2g5 genes were negatively regulated by T3 (Figs 1G and 1H), but PLA2g1b was not reduced (Fig 1F). The PEPCK and PDK4 genes were elevated by T3 (Fig 1I, 1J) while SREBP-1c was inhibited (Fig 1K). We found that T3 reduced the PLA2g2a protein abundance in the transgenic mice (Fig 1L). Overall, these results suggested that in vivo T3 negatively regulates the human and mouse PLA2g2a genes. Furthermore, we measured the effect of T3 on circulating plasma lipids. As shown in Table 1, administration of T3 strongly reduced the circulating levels of cholesterol, triglycerides, HDL and LDL.
Table 1. Administration of T3 to hypothyroid C57BL/6 hPLA2gIIA+ mice alters lipid levels.
C57BL/6 hPLA2gIIA+ mice were fed a PTU supplemented diet for 5 weeks. T3 was administered as described previously. Serum levels of free unbound T3 (FT3), cholesterol, triglycerides, high density lipoproteins (HDL) and low density lipoproteins (LDL) were measured. The averages ± S.E.M. are from seven PTU or seven T3 treated mice (* = p value < 0.05** = p value <0.01; ****= p value <0.0001).
| Mice N=7 |
Cholesterol (mg/dL) |
Triglycerides (mg/dL) |
HDL (mg/dL) |
LDL (mg/dL) |
FT3 (pg/mL) |
|---|---|---|---|---|---|
| PTU Mice | 159±7.1 | 55.2±3.3 | 93.7±2.8 | 54.2±4.6 | 1.5±.30 |
| PTU+T3 Mice | 40.57±4.3**** | 42.86±4.5* | 31.29±3.7**** | 1.5±0.80**** | 18.5±1.1**** |
= p value <0.0001,
= p value < 0.05
Regulation of hepatic gene expression by TRβ agonist KB2115
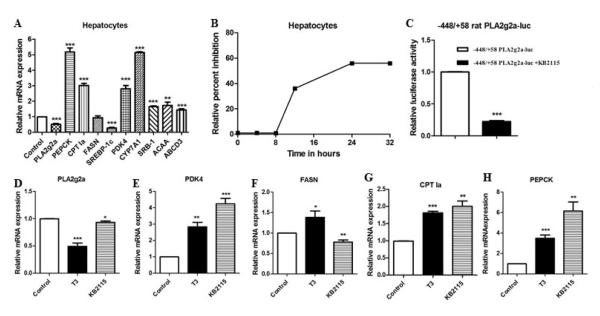
Given the therapeutic advantages of TRβ agonists over T3, we investigated the effect of the TRβ agonist KB2115 on sPLA2 and other hepatic genes in rat hepatocytes and HepG2 cells. In hepatocytes, KB2115 decreased PLA2g2a and inhibited the lipogenic gene SREBP-1c (Fig 2A). The genes associated with cholesterol metabolism including the HDL receptor (SRB-I) and CYP7A1 which is involved in bile acid production were induced by KB2115. Genes involved in mitochondrial and peroxisomal fatty acid oxidation (CPT1a, ABCD3, and ACAA1) were stimulated as were the PEPCK and PDK4 genes (Fig 2A). These results showed that the TRβ agonist regulated gene expression in a similar manner to T3. The time course of PLA2g2a inhibition by KB2115 is shown in figure 2B. To determine if KB2115 directly regulates PLA2g2a gene transcription, the −448/+58 rat PLA2g2a-luc promoter vector was transfected into HepG2 cells along with TRβ and cells were treated with KB2115 for 24 hrs. KB2115 decreased the activity of −448/+58 PLA2g2a-luc indicating that PLA2g2a is inhibited at the promoter level (Fig 2C). We compared the regulation of gene expression in HepG2 cells by T3 and KB2115 (Fig 2D-G). The regulation of several genes was quite similar although KB2115 was not as effective as T3 in reducing the basal level of PLA2g2a gene (Fig 2D).
Figure 2. Effect of the TRβ agonist KB2115 on hepatic gene regulation.
A) Rat hepatocytes were treated with 1μM KB2115 for 24 hrs. RNA was assessed for mRNA abundance. B) Rat hepatocytes were treated with KB2115 for various times and the relative inhibition of PLA2g2a expression was measured. C) HepG2 cells were transfected with 2 μg of −448/+58 rat PLA2g2a luciferase, 1 μg of SV40-TRβ and 0.1 μg of TK-renilla and treated with or without KB2115 for 24 hours. D-H) HepG2 cells were treated with 100 nM T3 or 1μM KB2115 for 24 hrs. RNA was assessed for various genes. All experiments were repeated 4 to 6 times. The data are expressed as the mean of the fold induction ± S.E.M of mRNA abundance relative to treatment control cells (* = p value < 0.05; ** = p value <0.01; *** = p value <0.001).
Hypothyroidism, high fat diets and cytokines induces PLA2g2a gene
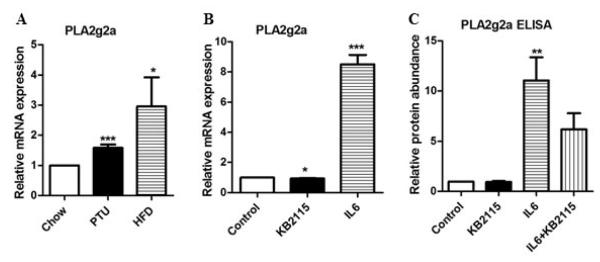
Our next experiments examined the effect of a high fat diet or hypothyroidism on the regulation of the PLA2g2a gene. We found that the PLA2g2a gene was induced 1.8 fold in hypothyroid mice (Fig 3A). Since high fat diets induce low-grade inflammation, we tested whether abundance of PLA2g2a would be altered in mice fed a high fat diet. PLA2g2a was induced 3-4 fold (Fig 3A). We next tested the effect of KB2115 on IL-6 mediated induction of the PLA2g2a gene. The PLA2g2a mRNA and protein levels were increased by interleukin 6 (IL-6) (Fig 3B-C). KB2115 reduced the protein abundance of PLA2g2a following IL-6 treatment suggesting that KB2115 could decrease the cytokine mediated induction of PLA2g2a.
Figure 3. Effect of hypothyroidism and high fat diets on PLA2g2a expression.
A) BALB/c mice were fed a chow diet, PTU diet or a high fat diet for 12 weeks. After 12 weeks, the mRNA levels of PLA2g2a were measured by RT-PCR. The data are expressed as the mean of the fold induction by hypothyroidism (PTU) or high fat diets (HFD) of PLA2g2a mRNA abundance relative to chow fed mice. B) HepG2 cells were treated with 10 ng/mL IL-6 or 1 μM KB2115 or both for 24 hrs. PLA2g2a mRNA abundance was assessed. C) Media was collected from HepG2 cells treated with IL-6 or KB2115 and the PLA2g2a levels were determined by ELISA. All experiments were repeated 3 to 4 times. Data are expressed as relative mRNA or protein abundance (* = p value < 0.05; ** = p value <0.01; *** = p value <0.001).
Comparison of hepatic gene regulation by T3 and KB2115 in rats
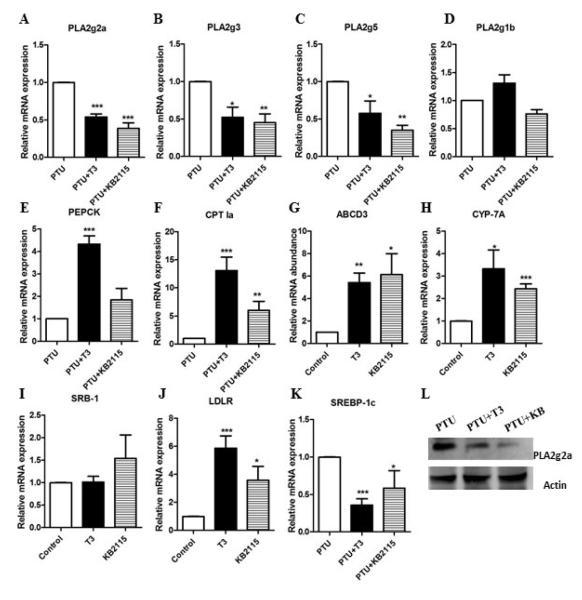
Finally, we evaluated and compared the in vivo regulation of secretory PLA2 genes and other hepatic genes by T3 and KB2115. The secretory PLA2 isoforms including PLA2g2a, PLA2g3, and PLA2g5 were significantly inhibited by KB2115 as well as T3. However, PLA2g1b was not inhibited by either T3 or KB2115 (Fig 4A-D). We also measured PLA2g10 but it was not significantly expressed in the liver of rats or mice (data not shown). Genes involved in fatty acid oxidation were induced by KB2115 (Fig 4F-G). CYP7A1, SRB-I and the LDL receptor (LDLR) were increased, while the lipogenic gene SREBP-1c was significantly inhibited (Fig 4H-K). The protein levels of PLA2g2a were also inhibited by both T3 and KB2115 (Fig 4L). Both T3 and KB2115 reduced the cholesterol, HDL and LDL levels in the rats although the effects of KB2115 were not as strong as T3 (Table 2). Neither T3 nor KB2115 altered the serum triglycerides. The actions of KB2115 are similar but not identical to T3.
Figure 4. Comparison of hepatic gene regulation by T3 and KB2115 in rats.
Rats were made hypothyroid by adding 0.05% PTU in drinking water for three weeks. Hyperthyroidism was induced by i.p. injection of T3 (0.33mg/kg body wt) or KB2115 (0.33mg/kg body wt) for two days. The hepatic mRNA levels were measured for: A) PLA2g2a, B) PLA2g1b, C) PLA2g3, D) PLA2g5, E) PEPCK, F) CPT1a, G) ABCD3, H) CYP-7A, I) SRB-I, J) LDLR, and K) SREBP-1c. L) PLA2g2a protein abundance was measured by western. Values are the average of RNA from 5 mice. The data are expressed as the mean of the fold induction by PTU+T3 or PTU+KB2115 ± S.E.M of mRNA abundance relative to PTU treated mice (* = p value < 0.05; ** = p value <0.01; *** = p value <0.001).
Table 2. Effect of T3 and KB2115 on the serum lipid levels in rats.
Rats were provided PTU supplemented water for 3 weeks. T3 and KB2115 were administered as described in the legend to figure 4. Serum levels of cholesterol, triglycerides, HDL and LDL were measured. The averages ± S.E.M. are from seven PTU or seven T3 or KB2115 treated rats (** = p value <0.01; *** = p value <0.001).
| Rat N=5 |
Cholesterol (mg/dL) |
Triglycerides (mg/dL) |
HDL (mg/dL) |
LDL (mg/dL) |
|---|---|---|---|---|
| PTU | 116±3.8 | 29.4±3.3 | 76.7±2.8 | 34.2±1.6 |
| PTU+T3 | 46.33±4.6*** | 31.6±3.3 | 28.5±2.6*** | 11.3±1.7*** |
| PTU+KB2115 | 84.2 ±8.1** | 43.2.6±3.9 | 48.7±4.7*** | 27.2±4.3 |
= p value <0.001,
= p value < 0.05
Discussion
Hypothyroidism leads to hyperlipidemia and elevated cholesterol in patients [20]. Elevated PLA2g2a is associated with inflammation, hyperlipidemia and cardiovascular disease [21]. PLA2g2a promotes the movement of cholesterol from LDL into arterial plaques and has been associated with the progression of atherosclerosis. In these studies, we examined the T3 regulation of sPLA2 expression in three species. Our data indicate that the PLA2g2a gene is inhibited by T3 in rats, mice and humans. In both rats and mice, other secretory PLA2 isoforms including PLA2g3 and PLA2g5 were suppressed by T3.
Secretory PLA2 enzymes including PLA2g2a, PLA2g3 and PLA2g5 are pro-atherogenic due to their ability to oxidize LDL particles and promote the formation of lipid-laden foam cells from macrophages [6]. Introduction of PLA2g5 bone marrow cells into Ldlr-/- mice increased formation of atherosclerotic lesions. [22]. Likewise, PLA2g3 has been found to enhance the development of atherosclerosis and small LDL particles [23].
T3 has been studied primarily for its effects on metabolism and development. By regulating the expression of sPLA2 genes, T3 may impact genes and processes associated with atherosclerosis and inflammation. It is not known if T3 will inhibit secretory PLA2 genes in non-hepatic tissues, but the ability of T3 to decrease these sPLA2 isoforms suggests a potential avenue for slowing atherogenesis.
There has been considerable interest in the identification of TRβ selective agonists which have beneficial lipid lowering properties without cardiovascular consequences [1]. In primates, the TRβ agonist, GC-1, lowered cholesterol levels and reduced hyperlipidemia [1,17]. A related TRβ agonist, MB07811, induced CPT1a while inhibiting SREBP-1c [24]. MB07811 reversed the hepatic steatosis associated with high fat diets [25]. A recent study showed that GC-1 and KB2115 reduced hepatic steatosis in fat fed rats [26]. In terms of hepatic steatosis, part of the actions of TRβ agonist may include induction of CPT1a. In a limited human trial, administration of KB2115 for 12 weeks reduced cholesterol levels and improved the lipid profile [17]. In present study we found that KB2115 inhibited sPLA2 and the lipogenic gene SREBP-1c. Mitochondrial and peroxisomal fatty acid oxidation genes including CPT1a and ABCD3 were stimulated and similarly CYP7A1 involved in cholesterol metabolism was induced. These actions of KB2115 suggest that TRβ agonists may be useful in the low grade inflammation and hyperlipidemia associated with metabolic diseases and insulin resistance. TRβ ligands could improve the metabolic profile first by decreasing sPLA2 expression and the associated inflammatory response and secondly by reducing lipotoxicity via the stimulation of hepatic fatty acid oxidation and inhibition of lipogenesis. In addition to the effects on lipid and cholesterol metabolism, we postulate that the inhibition of sPLA2 gene by T3 may be another novel beneficial action of TRβ agonists.
Highlights.
Inflammatory and atherosclerotic gene PLA2g2a is inhibited by T3 in vivo
Hypothyroidism and high fat diets induce PLA2g2a
Cytokine mediated induction of the PLA2g2a gene is inhibited by eprotirome
Eprotirome induces genes involved in fatty acid oxidation and cholesterol clearance
TRβ agonists as potential drugs for treatment of hyperlipidemia and meta inflammation
Acknowledgements
This work was supported by grants from the NIH-R01DK059368 (EAP) and the Canadian Institutes of Health Research (EJB). EJB is recipient of a scholarship from the Fonds Québecois de Recherche en Santé (FQRS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Baxter JD, Webb P. Thyroid hormone mimetics: potential applications in atherosclerosis, obesity and type 2 diabetes. Nat Rev Drug Discov. 2009;8:308–320. doi: 10.1038/nrd2830. [DOI] [PubMed] [Google Scholar]
- [2].Zhang J, Lazar MA. The mechanism of action of thyroid hormones. Annu Rev Physiol. 2000;62:439–466. doi: 10.1146/annurev.physiol.62.1.439. [DOI] [PubMed] [Google Scholar]
- [3].Bahouth SW, Cui X, Beauchamp MJ, Park EA. Thyroid hormone induces beta1-adrenergic receptor gene transcription through a direct repeat separated by five nucleotides. J Mol Cell Cardiol. 1997;29:3223–3237. doi: 10.1006/jmcc.1997.0549. [DOI] [PubMed] [Google Scholar]
- [4].Pramfalk C, Pedrelli M, Parini P. Role of thyroid receptor beta in lipid metabolism. Biochim Biophys Acta. 2011;1812:929–937. doi: 10.1016/j.bbadis.2010.12.019. [DOI] [PubMed] [Google Scholar]
- [5].Lambeau G, Gelb MH. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu Rev Biochem. 2008;77:495–520. doi: 10.1146/annurev.biochem.76.062405.154007. [DOI] [PubMed] [Google Scholar]
- [6].Murakami M, Taketomi Y, Sato H, Yamamoto K. Secreted phospholipase A2 revisited. J Biochem. 2011;150:233–255. doi: 10.1093/jb/mvr088. [DOI] [PubMed] [Google Scholar]
- [7].Sullivan CP, Seidl SE, Rich CB, Raymondjean M, Schreiber BM. Secretory phospholipase A2, group IIA is a novel serum amyloid A target gene: activation of smooth muscle cell expression by an interleukin-1 receptor-independent mechanism. J Biol Chem. 2010;285:565–575. doi: 10.1074/jbc.M109.070565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rosenson RS. Phospholipase A2 inhibition and atherosclerotic vascular disease: prospects for targeting secretory and lipoprotein-associated phospholipase A2 enzymes. Curr Opin Lipidol. 2010;21:473–480. doi: 10.1097/MOL.0b013e32833eb581. [DOI] [PubMed] [Google Scholar]
- [9].Boilard E, Lai Y, Larabee K, Balestrieri B, Ghomashchi F, Fujioka D, Gobezie R, Coblyn JS, Weinblatt ME, Massarotti EM, Thornhill TS, Divangahi M, Remold H, Lambeau G, Gelb MH, Arm JP, Lee DM. A novel anti-inflammatory role for secretory phospholipase A2 in immune complex-mediated arthritis. EMBO Molecular Medicine. 2010;2:172–187. doi: 10.1002/emmm.201000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jansen MS, Cook GA, Song S, Park EA. Thyroid hormone regulates carnitine palmitoyltransferase Ialpha gene expression through elements in the promoter and first intron. J Biol Chem. 2000;275:34989–34997. doi: 10.1074/jbc.M001752200. [DOI] [PubMed] [Google Scholar]
- [11].Attia RR, Connnaughton S, Boone LR, Wang F, Elam MB, Ness GC, Cook GA, Park EA. Regulation of pyruvate dehydrogenase kinase 4 (PDK4) by thyroid hormone: role of the peroxisome proliferator-activated receptor gamma coactivator (PGC-1 alpha) J Biol Chem. 2010;285:2375–2385. doi: 10.1074/jbc.M109.039081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Thakran S, Sharma P, Attia RR, Hori RT, Deng X, Elam MB, Park EA. Role of sirtuin 1 in the regulation of hepatic gene expression by thyroid hormone. J Biol Chem. 2013;288:807–818. doi: 10.1074/jbc.M112.437970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhang Y, Ma K, Song S, Elam MB, Cook GA, Park EA. Peroxisomal proliferator-activated receptor-gamma coactivator-1 alpha (PGC-1 alpha) enhances the thyroid hormone induction of carnitine palmitoyltransferase I (CPT-I alpha) J Biol Chem. 2004;279:53963–53971. doi: 10.1074/jbc.M406028200. [DOI] [PubMed] [Google Scholar]
- [14].Sharma P, Thakran S, Deng X, Elam MB, Park EA. Nuclear corepressors mediate the repression of phospholipase A2 group IIa gene transcription by thyroid hormone. J Biol Chem. 2013;288:16321–16333. doi: 10.1074/jbc.M112.445569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Angelin B, Rudling M. Lipid lowering with thyroid hormone and thyromimetics. Curr Opin Lipidol. 2010;21:499–506. doi: 10.1097/MOL.0b013e3283402e9c. [DOI] [PubMed] [Google Scholar]
- [16].Berkenstam A, Kristensen J, Mellstrom K, Carlsson B, Malm J, Rehnmark S, Garg N, Andersson CM, Rudling M, Sjoberg F, Angelin B, Baxter JD. The thyroid hormone mimetic compound KB2115 lowers plasma LDL cholesterol and stimulates bile acid synthesis without cardiac effects in humans. Proc Natl Acad Sci U S A. 2008;105:663–667. doi: 10.1073/pnas.0705286104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ladenson PW, Kristensen JD, Ridgway EC, Olsson AG, Carlsson B, Klein I, Baxter JD, Angelin B. Use of the thyroid hormone analogue eprotirome in statin-treated dyslipidemia. N Engl J Med. 2010;362:906–916. doi: 10.1056/NEJMoa0905633. [DOI] [PubMed] [Google Scholar]
- [18].Grass DS, Felkner RH, Chiang MY, Wallace RE, Nevalainen TJ, Bennett CF, Swanson ME. Expression of human group II PLA2 in transgenic mice results in epidermal hyperplasia in the absence of inflammatory infiltrate. J Clin Invest. 1996;97:2233–2241. doi: 10.1172/JCI118664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kennedy BP, Payette P, Mudgett J, Vadas P, Pruzanski W, Kwan M, Tang C, Rancourt DE, Cromlish WA. A natural disruption of the secretory group II phospholipase A2 gene in inbred mouse strains. J Biol Chem. 1995;270:22378–22385. doi: 10.1074/jbc.270.38.22378. [DOI] [PubMed] [Google Scholar]
- [20].Ichiki T. Thyroid hormone and atherosclerosis. Vascul Pharmacol. 2010;52:151–156. doi: 10.1016/j.vph.2009.09.004. [DOI] [PubMed] [Google Scholar]
- [21].Murakami M, Taketomi Y, Girard C, Yamamoto K, Lambeau G. Emerging roles of secreted phospholipase A2 enzymes: Lessons from transgenic and knockout mice. Biochimie. 2010;92:561–582. doi: 10.1016/j.biochi.2010.03.015. [DOI] [PubMed] [Google Scholar]
- [22].Webb NR, Bostrom MA, Szilvassy SJ, Westhuyzen D.R. van der, Daugherty A, Beer F.C. de. Macrophage-expressed group IIA secretory phospholipase A2 increases atherosclerotic lesion formation in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:263–268. doi: 10.1161/01.atv.0000051701.90972.e5. [DOI] [PubMed] [Google Scholar]
- [23].Sato H, Kato R, Isogai Y, Saka G, Ohtsuki M, Taketomi Y, Yamamoto K, Tsutsumi K, Yamada J, Masuda S, Ishikawa Y, Ishii T, Kobayashi T, Ikeda K, Taguchi R, Hatakeyama S, Hara S, Kudo I, Itabe H, Murakami M. Analyses of group III secreted phospholipase A2 transgenic mice reveal potential participation of this enzyme in plasma lipoprotein modification, macrophage foam cell formation, and atherosclerosis. J Biol Chem. 2008;283:33483–33497. doi: 10.1074/jbc.M804628200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Erion MD, Cable EE, Ito BR, Jiang H, Fujitaki JM, Finn PD, Zhang BH, Hou J, Boyer SH, Poelje P.D. van, Linemeyer DL. Targeting thyroid hormone receptor-beta agonists to the liver reduces cholesterol and triglycerides and improves the therapeutic index. Proc Natl Acad Sci U S A. 2007;104:15490–15495. doi: 10.1073/pnas.0702759104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Moreno M, Lange P. de, Lombardi A, Silvestri E, Lanni A, Goglia F. Metabolic effects of thyroid hormone derivatives. Thyroid. 2008;18:239–253. doi: 10.1089/thy.2007.0248. [DOI] [PubMed] [Google Scholar]
- [26].Vatner DF, Weismann D, Beddow SA, Kumashiro N, Erion DM, Liao XH, Grover GJ, Webb P, Phillips KJ, Weiss RE, Bogan JS, Baxter J, Shulman GI, Samuel VT. Thyroid hormone receptor-beta agonists prevent hepatic steatosis in fat-fed rats but impair insulin sensitivity via discrete pathways. Am J Physiol Endocrinol Metab. 2013;305:E89–E100. doi: 10.1152/ajpendo.00573.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]