Abstract
Purpose:
To evaluate whether medication counseling with emphasis on auxiliary labels improves recall of auxiliary label information and adherence to medication schedules.
Methods:
A prospective, randomized study of an educational intervention in community pharmacies near Baltimore, Maryland. Fifty literate, English-speaking adults receiving one of the 18 commonly dispensed antibiotics were randomized to receive a counseling session or no counseling. Five to seven days after medication pickup, a structured phone interview was conducted to capture data on recall of auxiliary labels and adherence.
Results:
A total of 39 subjects completed the phone interview (78%). The rate of correct recall was high: 77% correct recall for all three labels. Among those with incorrect recall, 7 out of 9 subjects received no counseling (p = 0.11). The auxiliary labels incorrectly recalled were all related to dietary restrictions.
Conclusion:
The findings from this study suggest that medication counseling emphasizing auxiliary label information may lead to improved recall and adherence to antibiotics. Additional studies are required to confirm the preliminary findings and determine whether they correspond to improved adherence. Information most commonly misunderstood were related to dietary restrictions. Additional research focusing on counseling related to dietary restrictions is recommended.
Keywords: Medication, counseling, auxiliary label, adherence, pharmacy
Purpose
The objective of this study was to evaluate whether medication counseling with emphasis on auxiliary labels leads to enhanced recall of auxiliary label information and improved adherence to the medication schedules compared with providing written medication information to patients alone.
A patient’s ability to adhere to a medication regimen may be compromised if he or she cannot understand how to take the prescribed medication.1 In the 2006 Institute of Medicine report, Preventing Medication Errors, the importance of medication education to improve adherence was emphasized.2,3 An estimated 1.5 million preventable adverse effects occur in the United States annually; many were related to patient misunderstanding of drug information and medication nonadherence.2
Pharmacists are well positioned to improve patients’ comprehension and adherence.4 The two primary means of communicating drug information are through written instructions and verbal consultation. Unfortunately, research has found that many patients do not understand the written instructions provided with their prescriptions.2,5–7 In addition, most primary prescription labels do not contain all the necessary information for patients to fully comply with a specific medication’s conditions for use, such as “do not crush or chew” or “do not drink alcoholic beverages when taking this medication.”5,8
In an attempt to improve adherence, pharmacists provide preprinted auxiliary labels with additional instructions on warnings, side effects, drug interactions, and proper medication use to patients. Recall and comprehension of auxiliary labels have been assessed, but results between studies have been conflicting.9,10 Auxiliary medication labels are intended to highlight key precautions, but due to the small size of the prescription container, the details contained on the auxiliary label must be brief and use small print.11 Several studies have concluded that small print, complex instructions, and low literacy were prime causes of failure to adequately recall instructions.6,9,12,13 Studies conducted by the US Public Health Service found that 60% of patients were unaware of their medication precautions and that 63% failed to notice the auxiliary labels affixed to their medication bottles.14,15 Other studies determined that less than 10% of patients read or examined their auxiliary labels.13,15 Similarly, printed consumer medication information has not proven to be effective in improving patient comprehension or adherence.16 According to the US Food and Drug Administration’s December 2008 news release, consumer medication information provided with new prescriptions did “not consistently provide easy-to-read, understandable information about the use and risks of medications.”17
Verbal consultation, also known as patient or medication counseling, has been shown to increase patient understanding and adherence.18 Medication counseling is endorsed by the American Pharmacists Association (APhA) and the American Association of Colleges of Pharmacy (AACP), and was emphasized by Congress with the enactment of the Omnibus Budget Reconciliation Act of 1990 (OBRA ‘90). OBRA ‘90 codified the importance of pharmacists counseling patients on the correct use, schedule, and duration in addition to side effects and other warnings.19
With the limitations of written medication information, counseling by pharmacists is the most established way to consistently improve patient’s comprehension and adherence to medications. In addition, simple pharmacist interventions, such as targeted patient counseling methods, have resulted in improvements in medication adherence.20 Since auxiliary labels contain key drug information required to optimize medication use, performing optimal patient counseling and emphasizing the information on auxiliary labels could result in significant improvements in patient comprehension and adherence.
Methods
Study design
This was a randomized educational intervention study enrolling 50 subjects from two community pharmacies in Maryland. Enrollment for the study began in September of 2009 and was completed in March of 2011. The study’s protocol, design, and informed consent were Institutional Review Board (IRB) approved and conducted in accordance with the ethical principles in the Declaration of Helsinki and Good Clinical Practices. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. The authors declare no conflict of interest in preparing this article.
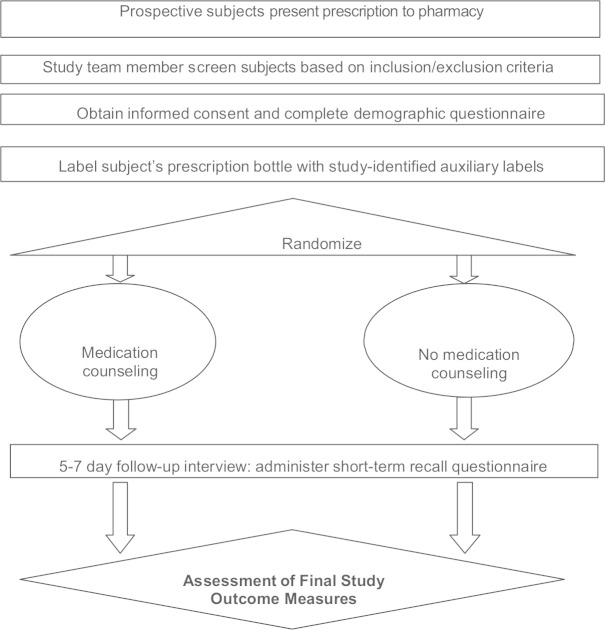
Community pharmacy patients dropping off a prescription for one of the study-identified medications were informed by the pharmacist that they may be eligible for the study and if interested, could talk with a study team member. Patients who met study criteria were enrolled after signing an IRB-approved informed consent. Patient enrollment in the study was voluntary; patients did not receive any compensation for study participation. Figure 1 describes the basic design of the study.
Figure 1.
Schematic of study design.
Eligibility criteria
Subjects included into the study were literate, English-speaking adult patients receiving one of the 18 medications, including amoxicillin, amoxicillin/clavalunate, penicillin v potassium, cephalexin, cefuroxime, cefdinir, doxycycline, minocycline, tetracycline, ciprofloxacin, moxifloxacin, levofloxacin, azithromycin, clarithromycin, erythromycin, trimethoprim/sulfamethoxazole, nitrofurantoin, and clindamycin.
Subjects were excluded from the study if they were obtaining or had obtained a degree in medicine, nursing, or pharmacy; were receiving chronic antibiotic therapy; or received the same antibiotic within the last 3 months. At screening, all subjects were offered counseling in accordance with OBRA ’90. Subjects who were initially seeking counseling or who had specific questions regarding their antibiotics were directed to the pharmacist to provide the required counseling and then were discontinued from the study. In addition, individuals who had severely impaired vision or hearing, or were self-described as having dementia were not enrolled into the study.
Selection of medications and corresponding auxiliary labels
The medications chosen for this study were 18 antibiotics commonly dispensed from community pharmacies. The study intended to evaluate recall and adherence upon receiving a new prescription. Antibiotics were chosen for this study due to the likelihood that a prescription for an antibiotic would be a new prescription, and adherence to prescribing directions is particularly important for these medications to be effective.
Each medication bottle included the primary prescription label in accordance with the information prescribed by their doctor and legal requirements for state and federal pharmacy practice. In addition to the primary label, three auxiliary labels providing instructions on drug administration were affixed to the bottle. The label, “It is very important that you take or use this exactly as directed. Do not skip doses or discontinue unless directed by your doctor,” was affixed to every prescription bottle. The two additional labels affixed to the bottle were dependent upon the prescribed drug. The nine different auxiliary labels used in this study are listed in Table 1.
Table 1.
Auxiliary labels.
| Label number | Content of label | Medications with label |
|---|---|---|
| 1 | It is very important that you take this or use this exactly as directed. | All |
| 2 | Do not drink alcoholic beverages when taking this medication. | Amoxicillin, amoxicillin/clavalunate, penicillin v potassium, cephalexin, cefuroxime, cefdinir, doxycycline, ciprofloxacin, azithromycin, clarithromycin, erythromycin, nitrofurantoin, and clindamycin |
| 3 | Do not take dairy products, antacids, or iron preparations within 1 h of these medications. | Cefdinir, doxycycline, minocycline, tetracycline, ciprofloxacin, moxifloxacin, levofloxacin, and azithromycin |
| 4 | May take with food to lessen chance of upset stomach. | Amoxicillin, amoxicillin/clavalunate, cephalexin, cefuroxime, and nitrofurantoin |
| 5 | Take medication on an empty stomach 1 h before or 2–3 h after a meal unless otherwise directed by your doctor. | Penicillin v potassium |
| 6 | Medication should be taken with plenty of water. | Minocycline, tetracycline, trimethoprim/sulfamethoxazole, and clindamycin |
| 7 | Do not chew or crush. Swallow whole. | Moxifloxacin and levofloxacin |
| 8 | Do not eat grapefruit or drink grapefruit juice, while taking this medication. | Clarithromycin and erythromycin |
| 9 | You should avoid prolonged or excessive exposure to direct and/or artificial sunlight, while taking this medication. | Trimethoprim/sulfamethoxazole |
Study intervention
Upon medication pickup, subjects were randomly assigned to Arm A, medication counseling, or Arm B, no medication counseling. If an individual was randomized to receive no medication counseling but had questions about their prescription upon pickup, they were discontinued from the study and referred to their staff pharmacist to provide the required counseling.
Counseling took approximately 10–15 min and was delivered by the same pharmacist for all patients in this study. A prescription-specific counseling form was prepared in advance for each subject randomized to Arm A to ensure that the medication counseling included the most pertinent information from the medication labeling and the information included in the auxiliary labels, and that the content of the counseling was delivered as consistently as possible. The structure of the counseling session was based on the Indian Health Service’s Prime Counseling Method21 with an emphasis on the auxiliary label information affixed to the prescription bottle.
The “Indian Health Services Method” is a common and well-established counseling technique taught by pharmacy schools.2 According to this method, three open-ended questions are used to guide the counseling session prior to explanation by the pharmacist. The first question, “What did your doctor tell you the medication was for?” was used to explain uses for the prescribed antibiotic. The second question, “How did your doctor tell you to take this medication?” was used to describe the antibiotic schedule, duration, and instructions for use. Most of the counseling session was dedicated to this question in order to explain the importance of adhering to the antibiotic schedule and duration prescribed by their doctor and to explain the instructions described on the auxiliary labels for correct use of the antibiotic. The third question, “What did your doctor tell you to expect about your medication?” was used to describe any side effects that should be expected while taking their antibiotic.
Five to seven days after medication pickup, a follow-up phone-call was conducted by a study investigator to collect data on the subject’s short-term recall of medication instructions. At least three telephone attempts were made for each subject and, if possible, a message was left requesting that the subject return the phone-call. Three failed attempts without any call-back were considered a failure.
The study investigator responsible for conducting the follow-up phone-call was a licensed pharmacist who was trained to administer the questionnaire and blinded to whether the subject received counseling. Since a standardized psychometrically validated questionnaire to measure the endpoints in this study did not exist, a scripted questionnaire was developed based on a literature search for consistency. All data during the follow-up interview were collected on the follow-up questionnaire form. The questionnaire content and patient answers were assessed by a blinded study investigator after the first 10 patients were enrolled; no revisions were required based on the experience from these patients.
Outcome measures
Auxiliary label recall was the primary outcome measure for this study. For auxiliary label recall, subjects were asked a series of nine true or false questions. Three questions pertained to the auxiliary label information associated with the subject’s antibiotic prescription, while the other six acted as distracters. Table 2 lists the true or false questions and the auxiliary label associated with each question. If a subject answered the question associated with an auxiliary label correctly, then he or she was recorded as having correct recall for that auxiliary label. If the subject answered the question incorrectly, then the subject was recorded as having incorrect recall for that auxiliary label.
Table 2.
Auxiliary label recall questions.
| Label number | True or false (T or F) auxiliary label recall questions |
|---|---|
| 1 | “T or F: You may stop taking this medication once you start feeling better.” |
| 2 | “T or F: You may drink alcohol in small amounts while taking this medication.” |
| 3 | “T or F: You can take this medication with antacids.” |
| 4 | “T or F: You may take food with this medication if it upsets your stomach.” |
| 5 | “T or F: You must take this medication on an empty stomach.” |
| 6 | “T or F: You need to drink plenty of water with this medication.” |
| 7 | “T or F: You cannot chew this medication.” |
| 8 | “T or F: You should avoid grapefruit juice while taking this medication.” |
| 9 | “T or F: You should use sunscreen while taking this medication.” |
The secondary outcome measures for the study included patient-reported assessments of adherence to the antibiotic schedule and duration of use. The secondary outcome measures were captured on the follow-up questionnaire form as verbatim answers.
For adherence to schedule and duration, subjects were asked when they started taking their prescription, whether they had or had not completed their prescription, and reasons for continuing or stopping their prescription. If subjects were still taking their prescription, they were later asked to retrieve their prescription bottles and count the number of remaining tablets/capsules. The remaining tablets/capsules indicated by the subject was later compared with the expected number of remaining tablets/capsules by calculating the antibiotic schedule and time the subject started their prescription. If they had already completed their prescription, they were asked to recall when they completed their prescription. The date and time in which the subject completed their medication was also compared with the expected date of medication completion. Subjects were asked how often and at what times they took their prescription versus how often and at what times they were supposed to take their prescription. Subjects were specifically asked whether they had missed any doses, and if so, how many times had they missed a dose.
All verbatim responses were later provided to a reviewing committee consisting of three registered pharmacists. This committee was only involved in determining adherence based on the answers provided on the questionnaire form and was blinded to randomization assignment to reduce potential bias. Members were trained to review the verbatim responses provided by each subject and then rate whether the subject was adherent with their prescription schedule and duration. The first two committee members independently provided their assessments. The third committee member acted as an adjudicator only if there was a disagreement in assessment between the first two committee members.
Statistical analyses
The rate of correct and incorrect recall of auxiliary label content was evaluated as the primary outcome measure. The study had an 80% power (two-sided alpha = 0.05) to detect a difference of 0.5 in the mean correct answers (scale of 0–3; standard deviation (SD) = 0.5) using a two-sample t-test. This required a total sample size of 17 per group (34 total). Fifty patients were enrolled to account for dropouts. These assumptions were based on a previous study that evaluated medication information recall in patients.12 Adherence to antibiotic schedule and duration was evaluated as a secondary outcome measure. Demographic information was presented using simple summary statistics. The group analysis was done using summary statistics, proportions, measures of proportional analysis, and t-tests to detect differences between means for continuous data. The t-tests were also used to assess any associations between continuous demographic data and correct recall of auxiliary labels. Chi-square analyses were used to evaluate differences between arms for the primary and secondary analyses. Kappa statistic was used to evaluate interrater reliability between reviewing committee members assessing verbatim answers. All statistical analyses were performed using SAS software, version 9.2 (SAS Institute, Inc., Cary, North Carolina).
Results
Fifty subjects were enrolled into the study. In all, 24 subjects were randomly assigned to Arm A (counseling), and 26 subjects were assigned to Arm B (no counseling). A total of 39 subjects completed the follow-up interview 5–7 days following prescription pickup. Eighteen subjects completing the study were in Arm A, and 21 were in Arm B.
Eleven subjects (22%) were lost to follow-up, 6 subjects in Arm A and 5 in Arm B. These subjects were screened and randomized to Arm A or Arm B, but investigators were unable to reach these subjects to complete the follow-up study questionnaire. A sensitivity analysis performed on the missing data confirmed that intervention arms remained balanced despite lost to follow-up, and that the missing data did not alter the results of the study.
The demographic characteristics are presented in Table 3 and were relatively comparable (±5%) when comparing age, race, education level completed, prior antibiotic history, and payment method. A total of 50 subjects were enrolled in the study with a median age of 40 years and mean age of 42.5 years (SD = 15.4; range = 19–100 years). Approximately three quarters of the subjects in the study were women (76%) and were African American (76%); 14% were Caucasian, and 10% were other races. Nearly, all the subjects had completed a high school level of education, and 60% had completed some level of college education after high school. Although a large number of the subjects in the study were female and African American, the number in each of these demographic groups was also balanced between the two arms.
Table 3.
Subject demographics (n = 50).
| Demographics | Arm A/counseling (%) | Arm B/no counseling (%) | Overall (%) |
|---|---|---|---|
| Agea | 39.4 (13.6) | 45.3 (16.7) | 42.5 (15.4) |
| Female | 19 (79) | 19 (73) | 38 (76) |
| Race | |||
| African American | 16 (67) | 22 (85) | 38 (76) |
| Caucasian | 6 (25) | 1 (4) | 7 (14) |
| Other | 2 (8) | 3 (12) | 5 (10) |
| Highest grade completed | |||
| Less than high school | 1 (4) | 3 (12) | 4 (8) |
| High school or GED | 8 (33) | 8 (31) | 16 (32) |
| At least some college | 15 (63) | 15 (58) | 30 (60) |
| Have received antibiotic before | 14 (58) | 15 (58) | 29 (58) |
| Source of payment | |||
| Private insurance | 16 (67) | 19 (73) | 35 (70) |
| Medicaid | 3 (11) | 5 (19) | 8 (16) |
| Self-pay | 5 (21) | 1 (4) | 6 (12) |
| Other | 0 (0) | 1 (4) | 1 (2) |
GED: general educational development.
Values are in years and so are means and standard deviations.
Subjects were taking an average of three prescription medications, and approximately two-thirds (58%) of the subjects reported receiving the same antibiotic before. The most commonly prescribed antibiotic in this study was azithromycin, followed by amoxicillin and ciprofloxacin. Table 4 lists the number of subjects receiving each antibiotic in each intervention arm.
Table 4.
Distribution of antibiotics (n = 50).
| Antibiotic name | Arm A/counseling | Arm B/no counseling | Overall |
|---|---|---|---|
| Azithromycin | 8 | 5 | 13 |
| Amoxicillin | 3 | 4 | 7 |
| Ciprofloxacin | 3 | 3 | 6 |
| Penicillin v potassium | 2 | 2 | 4 |
| Cephalexin | 1 | 2 | 3 |
| Clarithromycin | 1 | 2 | 3 |
| Doxycycline | 0 | 3 | 3 |
| Levofloxacin | 1 | 2 | 3 |
| Clindamycin | 1 | 1 | 2 |
| Nitrofurantoin | 2 | 0 | 2 |
| Amoxicillin/clavalunate | 1 | 0 | 1 |
| Cefuroxime | 0 | 1 | 1 |
| Minocycline | 0 | 1 | 1 |
| Trimethoprim/sulfamethoxazole | 1 | 0 | 1 |
| Cefdinir | 0 | 0 | 0 |
| Erythromycin | 0 | 0 | 0 |
| Moxifloxacin | 0 | 0 | 0 |
| Tetracycline | 0 | 0 | 0 |
Table 5 lists the nine different auxiliary labels and the number of times each auxiliary label was used in Arms A and B. The auxiliary label used most often in this study was Label 1, followed by Labels 2, 3, and 4.
Table 5.
Distribution of auxiliary warning labels (n = 50).
| Label number | Arm A/counseling | Arm B/no counseling | Overall |
|---|---|---|---|
| 1 | 24 | 26 | 50 |
| 2 | 22 | 23 | 45 |
| 3 | 12 | 14 | 26 |
| 4 | 7 | 7 | 14 |
| 5 | 2 | 2 | 4 |
| 6 | 2 | 2 | 4 |
| 7 | 1 | 2 | 3 |
| 8 | 1 | 2 | 3 |
| 9 | 1 | 0 | 1 |
Primary outcome
The results for the primary outcome measure are provided in Table 6. There was an unanticipated high rate of recall (76.9%) for the 39 subjects who completed the study. Only 9 of the 39 (23.1%) subjects answered at least one auxiliary label incorrectly: 2 of the subjects were randomized to Arm A (counseling) (22.2%) and 7 were randomized to Arm B (no counseling) (77.7%). A numerical difference favoring better patient recall in Arm A (counseling) was observed when comparing Arm A (counseling) to Arm B (no counseling). However, the difference between the study arms did not reach statistical significance (p = 0.11). No demographic characteristic was associated with significantly higher levels of recall; however, analyses of these subpopulations were limited by the size of this study.
Table 6.
Patient recall of auxiliary labeling information (primary outcome measure).
| Arm A/counseling | Arm B/no counseling | p-value | |
|---|---|---|---|
| Subjects completed | 18 | 21 | 0.11a |
| One label recalled incorrectly | 2 | 7 | |
| All labels recalled correctly | 16 | 14 |
SD: standard deviation.
Two-sample t-test (scale of 0–3, SD = 0.5).
A review of the auxiliary labels showed that the labels most incorrectly recalled were all related to dietary restrictions required for specific medications. The most incorrectly recalled label was Label 3, “Do not take dairy products, antacids or iron preparations within one hour of these medications” (Table 1). Of the nine subjects who answered at least one auxiliary label incorrectly, Label 3 was answered incorrectly five out of the nine times. All five of these subjects were in Arm B (no counseling). Label 4, “May take with food to lessen chance of upset stomach” was answered incorrectly three times, and Label 8, “Do not eat grapefruit or drink grapefruit juice while taking this medication” was answered incorrectly once.
Secondary outcome
Subjects who missed one or more doses were classified as being nonadherent to the schedule and duration of their prescribed antibiotic. These criteria for nonadherence have been used for other studies investigating adherence20,22 and were deemed appropriate, given the short-term duration of the regimens for the prescribed medications in this study. Twelve subjects (30.8%) were classified in this category compared with 27 subjects (69.2%) classified as being adherent. In Arm A (counseling), 5 of 18 (27.8%) subjects were classified as being nonadherent. In Arm B (no counseling), 7 of 21 (33.3%) subjects were classified as being nonadherent. There was no statistically significant difference in adherence between the two arms (p = 0.7). The kappa statistic calculated for the reviewing committee members scoring adherence to schedule and duration was 0.45.
Discussion
This was a small study conducted in community pharmacies in Maryland. The recall of auxiliary labeling information for this study sample was 77%. This high level of recall would require an increased sample size to see the absolute difference that this study was powered to detect. There were only two recall errors in the intervention compared to seven in the control group. While not statistically significant, this represents a 78% reduction in recall errors for patients counseled on auxiliary labeling information compared to those who did not receive medication counseling. In addition, the number of nonadherent patients identified in this study was higher for patients who did not receive counseling compared to patients who received counseling (p = NS).
While not always directly correlated, the unanticipated high recall rate observed in this study may have been due to the high education levels of the patients enrolled in this study and a corresponding high level of health literacy. Other explanations include the possibility of familiarity with auxiliary label content for antibiotics or a high degree of medical sophistication among the patients in this study. The health literacy level and degree of medical sophistication were not directly evaluated in this study. The possibility of other factors, such as friends or neighbors with medical knowledge, was also not explored.
Focused counseling on dietary restrictions appeared to be the most critical auxiliary labeling information for patient medication counseling based on the subjects’ rate of recall. These auxiliary labels were answered incorrectly more often than other labels, especially in the arm that received no counseling, which is likely due to patient’s not understanding the dietary restrictions associated with antibiotics.
In contrast to the dietary restriction labels, auxiliary labels used to stress the importance of adherence to antibiotic schedule and duration, such as Label 1 did not appear to be effective. This auxiliary label was affixed to every subject’s medication bottle and was answered correctly by every retained subject during the follow-up interview. Despite the ability to correctly recall this auxiliary label, 30% of subjects in both intervention arms were still nonadherent to their antibiotic medication. This finding shows that correctly recalling medication information for these types of labels does not translate to improved patient adherence.
Despite having patients perform pill counts to assess compliance, the study was limited by the patient-reported methodology when assessing medication adherence since these pill counts were self-reported and not all of the patients performed the requested pill counts due to completion of therapy or the unavailability of the medication bottle at the time of the follow-up interview.
Issues with generalizability were a concern since the study was conducted in a small suburban region near Baltimore, Maryland, and the subject population consisted of primarily African American females with a high level of education. Subjects were aware that they were enrolled into a counseling study and that there would be a follow-up interview with a pharmacist, which could result in a Hawthorne effect. Additionally, improvement in the content and format of auxiliary labels using patient feedback could also result in improved recall and adherence of medication information.23
The data related to adherence yielded interesting results about the association between recall of auxiliary labels and whether recall impacts adherence. Further investigation is needed to derive definitive results in these two areas, perhaps in a larger, adequately powered study using a more definitive methodology to assess medication adherence. A larger study that enrolls patients with lower levels of health literacy may also be of interest since these patients may have more marked responses to patient counseling with an emphasis on auxiliary label information since it is less likely that they understand and can apply written medication information.
Conclusion
The recall of general auxiliary labeling information was high (77%) for patients who did and did not receive counseling on general auxiliary labeling information. However, the nonstatistical findings from this study suggest that medication counseling with an emphasis on auxiliary label information may lead to improved patient recall and medication adherence for antibiotic treatment regimens. The medication information that was most commonly misunderstood by patients who did not receive counseling on auxiliary labels was related to dietary restrictions required to achieve optimal therapeutic outcomes. Additional adequately powered studies are required to confirm the preliminary findings from this study and to determine whether these findings correspond to an improvement in medication adherence. Additional research focusing on counseling interventions related to medication dietary restrictions is also recommended.
Acknowledgments
The authors thank Richard Gorman, MD; Mary Roth McClurg, PharmD; Paras Patel, RPh; Jeen Min, RPh; and Hamet Toure, PharmD, MPh.
Footnotes
Declaration of conflicting interests: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Ngoh LN. Health literacy: a barrier to pharmacist-patient communication and medication adherence. J Am Pharm Assoc 2009; 49: 132–149. [DOI] [PubMed] [Google Scholar]
- 2. Shrank WH, Avorn J. Educating patients about their medications: the potential and limitations of written drug information. Health Aff (Millwood) 2007; 26: 731–740. [DOI] [PubMed] [Google Scholar]
- 3. Aspden P, et al. Preventing medication errors. Washington, DC: National Academies Press, 2006. [Google Scholar]
- 4. Lau R, Stewart K, Mcnamara KP, et al. Evaluation of a community pharmacy-based intervention for improving patient adherence to antihypertensives: a randomised controlled trial. BMC Health Serv Res 2010; 10: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Holt GA, Dorcheus L, Hall EL. Patient interpretation of label instructions. Am Pharm 1992; NS32(3): 58–62. [DOI] [PubMed] [Google Scholar]
- 6. Wolf MS, Davis TC, Tilson HH. Misunderstanding of prescription drug warning labels among patients with low literacy. Am J Health Syst Pharm 2006; 63: 1048–1055. [DOI] [PubMed] [Google Scholar]
- 7. Moisan J, Gaudet M, Gregoire JP, et al. Non-adherence with drug treatment and reading difficulties with regard to prescription labeling among seniors. Gerontology 2002; 48: 44–51. [DOI] [PubMed] [Google Scholar]
- 8. Shrank WH, Agnew-Blais J, Choudhry NK. The variability and quality of medication container labels. Arch Intern Med 2007; 167: 1760–1765. [DOI] [PubMed] [Google Scholar]
- 9. Davis TC, Wolf MS, Bass PF. Low literacy impairs comprehension of prescription drug warning labels. J Gen Intern Med 2006; 21: 847–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hassan Y, Aziz NA, Sariff A. Comprehension of antibiotic instructions in an outpatient Malaysian practice. Hosp Pharm 1994; 29: 48–53. [PubMed] [Google Scholar]
- 11. Sharpe TR, Mikeal RL. Patient adherence with antibiotic regimens. Am J Hosp Pharm 1974; 31: 479–484. [PubMed] [Google Scholar]
- 12. McKnight PT, Schneider PJ, Brier KL. Effect of label format on information recall in patients receiving prescription medication. Contemp Pharm Pract 1981; 4: 150–154. [PubMed] [Google Scholar]
- 13. Davis TC, Wolf MS, Bass PF. Literacy and misunderstanding prescription labels. Ann Intern Med 2006; 145: 887–894. [DOI] [PubMed] [Google Scholar]
- 14. Brown CS, Solovitz BL. Short and long-term effects of auxiliary labels on patient knowledge of precautionary drug information. Drug Intell Clin Pharm 1988; 22: 470–474. [DOI] [PubMed] [Google Scholar]
- 15. Wolf MS, Davis TC, Shrank W. To err is human: patient misinterpretations of prescription drug label instructions. Patient Educ Couns 2007; 67: 293–300. [DOI] [PubMed] [Google Scholar]
- 16. Shiffman S, Gerlach KK, Sembower MA, et al. Consumer understanding of prescription drug information: an illustration using an antidepressant medication. Ann Pharmacother 2011; 45(4): 452–458. [DOI] [PubMed] [Google Scholar]
- 17. Food and Drug Administration. Study finds much of private-sector consumer medication information not consistently useful (News release, 16 December 2008), http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2008/ucm116992.htm (accessed 14 September 2012).
- 18. Lee S, Cheung P, Chow MS. Benefits of individualized counseling by the pharmacist on the treatment outcomes of hyperlipidemia in Hong Kong. J Clin Pharmacol 2004; 44: 632–639. [DOI] [PubMed] [Google Scholar]
- 19. McGivney MS, Meyer SM. Medication therapy management: it’s relationship to patient counseling, disease management, and pharmaceutical care. J Am Pharm Assoc 2007; 47: 620–628. [DOI] [PubMed] [Google Scholar]
- 20. Haynes RB, Yao X, Degani A, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev 2005; 4: CD000011. [DOI] [PubMed] [Google Scholar]
- 21. Foster SL, Smith EB, Seybold MR. Advanced counseling techniques: integrating assessment and intervention. Am Pharm 1995; NS35(10): 40–50. [DOI] [PubMed] [Google Scholar]
- 22. Sherr L, Lampe FC, Clucas C, et al. Self-reported non-adherence to ART and virological outcome in a multiclinic UK study. AIDS Care 2010; 22(8): 939–945. [DOI] [PubMed] [Google Scholar]
- 23. Wolf M, Davis T, Parker R. Improving prescription drug warnings to promote patient comprehension. Arch Intern Med 2010; 170(1): 50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]