Abstract
Acute limb ischemia is a sudden decrease in limb perfusion that threatens limb viability and requires urgent evaluation and management. Most of the causes of acute limb ischemia are thrombosis of a limb artery or bypass graft, embolism from the heart or a disease artery, dissection, and trauma. Assessment determines whether the limb is viable or irreversibly damaged. Prompt diagnosis and revascularization by means of catheter-based thrombolysis or thrombectomy and by surgery reduce the risk of limb loss and mortality. Amputation is performed in patients with irreversible damage. Despite urgent revascularization, amputation rate is 10%–15% in patients during hospitalization, mostly above the knee, and mortality within 1 year is 10%–15% due to the coexisting conditions.
Keywords: Acute limb ischemia, thrombosis, revascularization, amputation
Introduction
Sudden loss or marked decrease in limb perfusion that threatens limb viability is a vascular emergency. Acute limb ischemia (ALI) may be the first manifestation of arterial disease in a previously asymptomatic patient or may occur as an acute event that causes symptomatic deterioration in a patient with antecedent lower extremity periphery artery disease (PAD) and intermittent claudication. The incidence of this condition is approximately 1.5 cases per 10,000 persons per year.1
The clinical presentation is considered to be acute if it occurs within 2 weeks after symptom onset. Symptoms develop over a period of hours to days and range from new or worsening intermittent claudication to pain in the foot or leg when the patient is at rest, paresthesias, muscle weakness, and paralysis of the affected limb.2 Physical findings may include an absence of pulses distal to the occlusion, cool and pale or mottled skin, reduced sensation, and decreased strength.3 The 30-day mortality and amputation rates are 15% and may be up to 25%.4 Leg symptoms in ALI relate primarily to pain or limb function. The abruptness and time of onset of the pain, its location and intensity, as well as change in severity over time should all be explored. The duration and intensity of the pain and presence of motor or sensory changes are very important in clinical decision making and urgency of revascularization. Outcomes and prognosis of ALI largely depend on the rapid diagnosis and initiation of appropriate and effective therapy. In this review, the diagnosis and treatment of ALI will be discussed.
Description
ALI results from a sudden obstruction in the arterial flow to the extremity due to an embolism or thrombosis.5 Embolic problems result in a greater degree of ischemia than thrombosis. PAD progression is the most thrombotic cause of the ALI. Graft thrombosis or thrombosis of a popliteal aneurysm may also be seen. Cardiac embolization is responsible for 75% of the ALI cases.6 Aortic dissection or embolization, entrapment or cyst, trauma, phlegmasia cerulea, ergotism, hypercoagulable states, and iatrogenic complications related to cardiac catheterization, endovascular procedures, intra-aortic balloon pump, extracorporeal cardiac assistance, as well as vessel closure devices are the potential embolic causes which are related to a sudden decrease in arterial perfusion in the limb (Table 1). The embolus characteristically lodges in vascular bed with no prior collateral development, besides an in situ thrombosis occurs in vessels with prior, gradual atherosclerotic narrowing that has stimulated the formation of collateral channels. The presence of these collaterals lessens the severity and rapidity of symptom development when the atherosclerotic narrowing progresses to occlusion.7
Table 1.
Embolic causes of acute limb ischemia (ALI).
| Periphery artery disease (PAD) progression |
| Cardiac embolization |
| Aortic dissection or embolization |
| Thrombosis of a popliteal aneurysm |
| Graft thrombosis |
| Entrapment or cyst |
| Trauma |
| Phlegmasia cerulea |
| Ergotism |
| Hypercoagulable states |
| Iatrogenic complications related to cardiac catheterization |
| Endovascular procedures |
| Intra-aortic balloon pump |
| Extracorporeal cardiac assistance |
| Vessel closure devices |
Symptoms
Embolic occlusions are usually very sudden and of great intensity, such that patients often present within a few hours of onset. Acute arterial occlusion is associated with intense spasm in the distal arterial tree, and initially, the limb will appear “marble” white. Over the next few hours, the spasm relaxes and the skin fills with deoxygenated blood leading to mottling that is light blue or purple, has a fine reticular pattern, and blanches on pressure.2 At this stage, the limb is still salvageable. The classical description of patients with ALI is represented by the “six Ps”: pain, pallor, paralysis, pulse deficit, paresthesia, and poikilothermia.8 Pallor and the level of coldness (poikilothermia) are important to record to evaluate the progression of ischemia. The pulse deficit is helpful determining the site of occlusion. It should also be remembered that sensory capabilities, such as light touch, two-point tactile discrimination, proprioception, and vibratory perception, are lost early on. Finally, profound paralysis with complete lack of sensation indicates an irreversible state of ischemia, and the patient may be best treated with primary amputation.9
Risk factors
It is often difficult to distinguish an embolus from a thrombosis, but embolic occlusions should be suspected in patients with the following features: (1) acute onset, where the patient is often able to accurately time the moment of the event; (2) a history of embolism; (3) a known embolic source, such as cardiac arrhythmias; (4) no prior history of intermittent claudication; and (5) normal pulse and Doppler examination in the unaffected limb.1 Differential diagnoses of ALI are represented in Table 2.
Table 2.
Differential diagnosis of acute limb ischemia (ALI).
| Conditions mimicking ALI |
|---|
| Systemic shock (especially if associated with chronic occlusive disease) |
| Phlegmasia cerulea dolens |
| Acute compressive neuropathy |
| Differential diagnosis of ALI (other than acute PAD) |
| Trauma |
| Dissection |
| Arteritis |
| Hypercoagulable states |
| Popliteal adventitial cyst |
| Popliteal entrapment |
| Compartment syndrome |
| Acute PAD |
| Atherosclerotic stenosed artery thrombosis |
| Arterial bypass graft thrombosis |
| Embolism from heart, aneurysm, plaque or critical stenosis |
PAD: periphery artery disease.
Source: Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II), http://www.jvascsurg.org (accessed October 2007).
Thrombotic occlusions can occur in any segment of the lower extremities but most commonly involve the superficial femoral artery. With the decline of the incidence of cardiac valvular diseases due to the rheumatic fever, and with the growing use of oral anticoagulant in patients with atrial fibrillation, the proportion of ALI due to cardiac embolism has also declined.10
Thrombosis in situ may occur less commonly with cystic adventitial disease, popliteal entrapment, and popliteal aneurysm in the lower extremities; vasculitis and repetitive trauma in the upper extremities; or trauma and dissection in both. The procoagulative states such as hyperhomocysteinemia and diabetic ketoacidosis can produce thrombosis in situ in the absence of underlying occlusive disease.11,12 In addition, hypercoagulable states; protein S deficiency, protein C deficiency, presence of lupus-like anticoagulant, plasminogen deficiency, and increased platelet reactivity was demonstrated in patients under 51 years of age undergoing lower extremity revascularization for ischemia.13 Also, there are rare causes of ALI that have been reported. Moulakakis et al.14 reported a case with alcohol-paracetamol syndrome who presented with acute left limb ischemia. ALI cases were also reported in patients with hypercoagulability-induced thromboembolism, which is a serious complication of nephrotic syndrome and heparin-induced thrombocytopenia.15,16
Infrainguinal bypass graft occlusion is common, with reported 5-year primary patency of autologous vein and synthetic above-the-knee bypass of 74% and 39%, respectively.17 The most common cause of synthetic arterial bypass graft occlusion is impaired native vessel outflow or poor arterial inflow. Autologous vein grafts develop anastomotic and midportion stenosis due to myointimal hyperplasia. Insufficient valve stripping can result in focal stenosis. Diffuse sclerosis can occur when the vasa vasorum are damaged.
Lipoprotein (a) (Lp(a)) possesses unique antifibrinolytic and prothombotic properties, and may play an important role in vascular thrombosis.18 It has also been shown to be a strong predictor of peripheral vascular disease, independently of cigarette smoking and diabetes in a group of 100 White male patients with mean age of 67 years.19 Two cases with severe PAD due to multiple, thromboembolic arterial occlusions in the absence of advanced arteriosclerosis and traditional cardiovascular risk factors were reported. Markedly elevated Lp(a) above 80 mg/dL was identified as the only significant laboratory abnormality in both unexplained and acute arterial occlusion cases.20 In addition, Lp(a) and other emerging factors such as fibrinogen, C-reactive protein, and homocysteine levels should be considered when assessing the risk of graft occlusion.21
The incidence of peripheral arterial thrombembolization in the setting of transcatheter aortic valve implantation (TAVI) is not clearly known. In the setting of TAVI, debris from the calcified native aortic valve during the implantation of the new valve can embolize and obstruct peripheral arteries.22 Another possible pathophysiological mechanism might be a transient ineffective anticoagulation status. It is conceivable and most likely that the 18-F sheath reducing or obstructing the flow in the femoral artery set the stage for thrombus formation and peripheral embolization of the clots, once the sheath was removed. Identifying factors associated with periprocedural complications and establishing diagnostic and therapeutic algorithms along with technical improvements in devices used for TAVI might reduce the currently high rate of such complications and eventually result in an improved periprocedural survival rate.
Native artery occlusions usually occur in the setting of severe atherosclerotic stenoses; alternatively, an artery may become occluded when an embolus becomes dislodged from a proximal source and is trapped at the site of a peripheral arterial bifurcation.9
Modification of atherosclerotic risk factors plays a crucial role in prevention of limb ischemia. The patients should be treated with aspirin, beta-blockers, and statins, agents that also have been shown to reduce cardiovascular morbidity and mortality.23
Diagnosis
Ankle-brachial index
The value of the clinical findings in patients with ALI can be strongly improved by measuring the ankle-brachial index (ABI). ABI can be measured by dividing the highest ankle systolic pressure to the highest brachial systolic pressure per leg. The ABI is interpreted as follows: noncompressible values > 1.40, normal values of 1.00–1.40, and borderline of 0.91–0.99. Usually an ABI < 0.90 is used to define the decline in limb perfusion.24
Doppler ultrasound
The lesions can be located by two-dimensional (2D) ultrasonography (DUS) and color-Doppler mapping, while the degree of stenosis is estimated mostly by Doppler waveform analysis and peak systolic velocities and ratios. Duplex ultrasound of the extremities is useful to diagnose anatomic location and degree of stenosis of PAD. DUS provides important information on hemodynamics and is also highly useful for the follow-up after angioplasty or to monitor bypass grafts.25,26 The major disadvantage of DUS compared with other imaging techniques (digital subtraction angiography (DSA), computed tomography angiography (CTA), or magnetic resonance angiography (MRA)) is that it does not provide full arterial imaging as a clear roadmap, as do the other techniques. In addition, in some cases, the iliac arteries are more difficult to visualize because of obesity or gas interpositions. Alternative methods should be considered when the imaging is suboptimal.
Angiogram
In most cases, initial diagnostic arteriography may be performed to localize the site of occlusion and to visualize the distal arterial tree. It may also be possible to distinguish an embolic occlusion from an in situ thrombosis. The time required to perform preoperative angiographic evaluation is well worth the information obtained; this information is essential to the operative plan.
DSA
As DSA was considered as the gold standard for decades, it is now reserved for patients undergoing interventions. In current practice, it is not used as a diagnostic tool because of the invasive character and risk of complications.1,27 Indeed, the noninvasive techniques provide satisfying imaging in almost all cases, with less radiation, and complications inherent to the arterial puncture.
CTA and MRA
CTA using multi-detector computed tomography (MDCT) technology allows imaging with high resolution. In a meta-analysis, the sensitivity and specificity of CTA to detect aortoiliac stenoses >50% were were reported as 96% and 98%, respectively.28 The great advantage of CTA remains the visualization of calcifications, clips, stents, and bypasses. In comparison with DSA, MRA has an excellent sensitivity (93%–100%) and specificity (93%–100%).29 Meta-analyses have shown that both CTA28 and MRA30 are highly accurate noninvasive imaging techniques. Some studies even suggest that contrast-enhanced MRA is superior to DSA in visualizing arteries of the lower leg and foot.31,32 According to a recent report by Jens et al.,33 both CTA and contrast-enhanced MRA are accurate techniques in evaluating disease severity of arterial segments from the aorta to the tibial arteries in patients with critical limb ischemia or intermittent claudication. In direct comparison, MRA has the greatest ability to replace diagnostic DSA in symptomatic patients to assist decision making, especially in the case of major allergies. The limitations of the use of MRA are the presence of pacemakers or metal implants, or in patients with claustrophobia. In addition, Gadolinium contrast agents cannot be used in the case of severe renal failure.34
The European Society of Urogenital Radiology (ESUR) defines contrast-induced nephropathy as a state in which nephropathy (increase in blood serum creatinine level of more than 0.5 mg/dL or of more than 25% of the baseline value) occurs within 3 days from the moment of intravascular injection of the contrast medium, assuming that there is no alternative etiology.35 Use of radiological contrast media is the third most common cause of inpatient renal insufficiency, accounting for 11%–12% of all cases.36 It is recommended to discontinue nephrotoxic substances for at least 24 h before contrast medium administration. If the creatinine level is elevated, it is recommended to discontinue metformin for 48 h before contrast administration and for 48 h after contrast administration. It is recommended to administer 0.9% NaCl intravenously, in the dose of 1 mL/kg of body mass/h, for 6 h before contrast agent administration.
Treatment options
The duration of symptoms is of prime importance in the planning of therapy. Percutaneous endovascular options are more effective in patients with ischemia of less than 2 weeks’ duration. On the other hand, symptoms of greater than 2 weeks’ duration are better served with nonthrombotic options.37 Propagation of thrombus after the initial occlusive event may convert a marginally ischemic limb into a severely threatened extremity.
Three general classes are recognized:
Class I: Non-threatened extremity; elective revascularization may or may not be necessary.
Class II: Threatened extremity; revascularization is indicated to prevent tissue loss.
Class III: Ischemia has progressed to infarction and salvage of the extremity is not possible.
The Society for Vascular Surgery and the North American Chapter of the International Society for Cardiovascular Surgery created a classification based on Rutherford classification for acute arterial occlusion (Table 3, Rutherford et al.6).
Table 3.
Classification scheme for acute limb ischemia (ALI).
| Class | Category | Prognosis | Sensory loss | Muscle weakness | DUS—arterial and venous | |
|---|---|---|---|---|---|---|
| I | Viable | No immediate limb threat | None | None | Audible | Audible |
| IIa | Threatened: marginal (salvageable if promptly treated) | Salvageable if treated promptly | Minimal–none | None | Often inaudible | Audible |
| IIb | Threatened: immediate (salvageable with immediate revascularisation) | Salvageable if treated immediately | More than just toes | Mild–moderate | Usually inaudible | Audible |
| III | Major tissue loss or permanent nerve damage inevitable | Limb loss or permanent damage | Profound, anesthetic | Profound, paralysis | None | Inaudible |
DUS: two-dimensional ultrasonography.
It is modified from the classification of Rutherford et al.6
The severity of the ischemia, according to the classification presented above, will dictate the extent of diagnostic tests performed for systemic risk factor assessment. Routine blood studies and coagulation tests should be performed before heparin is administered. Correction of underlying electrolyte imbalances and systemic anticoagulation should proceed concomitantly with the other investigations. A plain chest x-ray and electrocardiogram should be obtained from every patient. In patients with suspected embolism, an echocardiogram should be obtained as soon as time allows.
In 1978, Blaisdell et al.38 first introduced the concept of early heparinization to prevent proximal and distal propagation of thrombus, in combination with delayed intervention. Today, early heparinization remains one of the mainstays in the treatment of ALI. Fortunately, immediate and adequate anticoagulation prevents proximal and/or distal thrombus propagation and preserves the microcirculation.
Endovascular techniques
In the 1960s and the 1970s, balloon-catheter thrombectomy, first introduced by Fogarty et al.,39,40 became the cornerstone of therapy.
Mechanical recanalisation techniques
Percutaneous aspiration thrombectomy
Another method of percutaneous, catheter-guided thrombus removal alternative to open surgery is percutaneous aspiration thrombectomy (PAT). It is an easy, low-cost, rapid technique which is applicable with the use of a large lumen catheter (6–8-F), or even smaller (5-F) for the crural arteries. The catheter is connected to a 60-mL syringe, and the thrombus is forcefully aspirated out of the vessel.40,41 The use of combined mechanical and thrombolytic therapy (pharmaco-mechanical thrombolysis) is used to increase the lytic effect and reduce procedural time, especially in advanced ischemia, when time is crucial for limb salvage. The results of the combined PAT/thrombolysis therapy are very promising. PAT alone has been reported to result in only 31% procedural success rates, but combined with thrombolysis and PAT, the primary success rate reached up to 90%, with a limb salvage rate of 86% and primary patency rates of 58%, in up to 4 years follow-up.42,43 PAT also can be proven highly effective when it comes to the immediate treatment of iatrogenic acute distal atherothrombotic embolization, occurring during percutaneous endovascular therapeutic procedures.
Percutaneous mechanical thrombectomy
Percutaneous mechanical thrombectomy is defined as the endovascular thrombus maceration and removal with the use of Fogarty balloons or dedicated percutaneous thrombectomy devices (PTDs). The use of Fogarty balloons are the cheapest, simplest, and fastest method of thrombectomy compared with the more sophisticated PTDs.40 These balloons are very effective in declotting infrainguinal lesions, through common femoral or popliteal artery puncture, but they are difficult to utilize in chronic stenosis of diseased arteries and well-organized thrombus. PTDs can be categorized in four types, according to their mechanism of action: mechanical clot dissolution catheters, hydrodynamic/rheolytic catheters, mixed type, and ultrasonic catheters.
Although most PTDs have received CE approval for dialysis grafts and native fistulas declotting, special attention should be given when treating ALI. The main difference is that although microembolization does not produce any clinically significant pulmonary events, the same does not apply for the distal vasculature of an ischemic limb, where microembolic events could result in limb loss. It is a requisite that any PTD used for the revascularization of an ischemic limb should not only provide sufficient, immediate, technical success rate but also secure a safe, embolic-free procedure.
For this reason, devices with additional fragment aspiration are preferred for the treatment of ALI. The devices that should be preferred for peripheral applications are the Hydrolyzer, the BSIC Oasis system, the AngioJet, the ThrombCat thrombectomy catheter system, the Bacchus Trellis, the OmniSonics Resolution Wire, and the Ekos Lysus system.44–47
The AngioJet is the only photomultiplier tube (PMT) device that is approved by the Food and Drug Administration (FDA) that can remove thrombus from peripheral arteries using a rapid stream of fluid and hydrodynamic forces to extract the thrombotic material from the lumen.46 The thrombectomy system consists of three major components: the catheter, the pump set, and the drive unit. In patients with very significant ischemia that precludes the obligatory delay associated with pharmacologic thrombolysis, the PMT devices may rapidly clear a channel through the occluded segment. Partial reperfusion of the extremity may provide enough improvement in ischemia to allow complete removal of thrombus, with thrombolytic infusions thereafter. Initial thrombus debulking may also significantly reduce the dose and duration of thrombolytic agents, thereby decreasing the risk of hemorrhagic complications associated with pharmacologic thrombolysis.45,47,48 For instance, for patients who have recently undergone a major surgical procedure, the devices may be employed as sole therapy in patients with contraindications to thrombolytic administration. The advantage of mechanical devices, however, lies in their ability to rapidly debulk the thrombus, significantly reducing the duration of ischemia and probably increasing the exposure of the residual thrombus and distal vessels to pharmacologic thrombolytic agents. According to a clinical report, a significant number of patients required adjunctive thrombolytic therapy for complete thrombus removal.46 Braithwaite et al.49 managed 15 patients with ALI with anticoagulation alone, due to infirmity or surgical and/or thrombolytic contraindications, and obtained dismal 30-day limb salvage and mortality rates of 33% and 60%, respectively.
Due to the lack of well-organized, multicenter, randomized controlled studies that investigate the performance of PTDs, there are no clear indications regarding their application in ALI treatment. Among the great advantages of mechanical thrombectomy is the inferior procedural time, as well as the fact that it can be used as monotherapy when thrombolysis is contraindicated or in combination with a minimal amount of thrombolytic agents in cases of high bleeding diathesis.
The excimer laser has the ability to remove plaque and thrombus by photoacoustic ablation, theoretically reducing the incidence of clinical embolization. The laser-assisted angioplasty for critical limb ischemia (LACI) trial treated patients who were poor surgical candidates with an endovascular strategy consisting of “cool” excimer laser angioplasty followed by adjunctive percutaneous transluminal angioplasty (PTA) and/or stenting. Despite the burden of atherosclerotic lesions in these patients, a remarkably high salvage rate of 93% was achieved at 6 months in surviving patients.50
Ultrasound-based devices, such as the OmniSonics OmniWave Endovascular System and the Ekos Lysus system, seem promising. Local thrombolysis of acute artery occlusion by using the Lysus Peripheral Catheter System may be a safe, effective, and time-saving treatment option. Ultrasound-enhanced lysis achieves more rapid recanalization and a higher rate of early complete thrombus resolution than conventional lysis. In addition, the time of hospitalization was reduced.51
Percutaneous mechanical thrombectomy is recommended in cases of stage Rutherford IIb ischemia and high surgical risk, because thrombolysis is time-consuming and could result in clinical deterioration and/or compartment syndrome. PTDs are not recommended for the treatment of ALI involving the iliac, the common femoral, and the profunda femoral artery.
Acute complications of endovascular procedures and endovascular revascularization techniques, such as angioplasty used for the treatment of peripheral arterial disease, can be the origin of ALI due to arterial thrombosis and/or distal embolization complications in approximately 2%–3%.52 It is reasonable that in the setting of an already accessed artery due to ongoing endovascular procedure, complicated with ALI, the treatment of choice is PAT. This method frequently fails to establish blood flow, because the emboli in the vessels are two small to be reached with a catheter or because the thrombus simply cannot be detached from the vessel wall and retrieved. In that case, thrombolysis, PTDs, and pharmaco-mechanical thrombolysis with the use of PMT devices and lytic agents should be considered.
Pharmacological recanalization techniques
Thrombolytic agents have been successfully employed to dissolve the occluding thrombus, reconstitute blood flow, and improve the status of the tissue bed supplied by the involved vascular segment.48 Intrathrombotic delivery of the thrombolytic agent is more effective than nonselective catheter-directed infusion.
Before revascularization, diagnostic angiography is performed to assess the inflow and arteries and the nature and length of thrombosis. Thereafter, the operator crosses the occlusion with a guidewire and a multi-side-hole catheter, which allows direct delivery of the thrombolytic agent into the thrombus. Once flow is restored, angiography is performed to detect any inciting lesion, such as graft stenosis or retained valve cusps, which can be managed with catheter-based techniques or surgery. Catheters can be successfully positioned across the thrombosed vessel (an essential prerequisite) in 95% of cases. Among patients with ALI caused by an occluded native vessel, stent, or graft, complete or partial thrombus resolution with a satisfactory clinical result occurs after catheter-based thrombolysis in 75%–92% of patients.53 Distal thrombus embolization commonly occurs as the thrombus is lysed, but the embolized thrombus typically clears during the thrombolytic infusion.54 The adjunctive use of glycoprotein IIb/IIIa receptor antagonists may accelerate reperfusion and reduce distal embolization, but the addition of these agents does not improve outcomes.55
The Rochester study randomly assigned 114 patients with acute, limb-threatening ischemia to thrombolysis with urokinase in 57 patients or to immediate operation in 57 patients. After 1-year follow-up, the amputation-free survival rate differences were statistically significant, 75% and 52%, respectively.56 The second large multicenter evaluation was the Surgery versus Thrombolysis for Ischemia of the Lower Extremity (STILE) trial in which 393 patients were randomly assigned to surgery or thrombolysis with either recombinant tissue plasminogen activator (rt-PA) or urokinase. Clinical outcomes for both thrombolytic groups were similar, so their data were combined for an overall comparison of thrombolysis with surgery. Among patients with symptoms of longer duration (>14 days), the surgical group had lower amputation rates than the thrombolysis group at 6 months. In contrast, among patients with symptoms of shorter duration (<14 days), those assigned to thrombolysis had lower rates than did surgical patients.34 The most important multicenter trial to evaluate thrombolytic therapy was the Thrombolysis or Peripheral Arterial Surgery (TOPAS) trial. Recombinant urokinase (r-UK) (4000 IU per min for 4 h followed by 2000 IU per min) was compared with primary operation in 544 patients with lower extremity native artery or bypass graft occlusions of 14 days’ duration or less. The amputation-free survival rates 6 months after randomization were not significantly different: 71.8% in the r-UK group and 74.8% in the operative group. There were also no significant differences in the rates of amputation-free survival or mortality at discharge from the hospital. Among patients assigned to thrombolysis, those with occlusions in bypass grafts had better clinical outcomes and rates of clot dissolution, concurrent with lower rates of major hemorrhagic complications, compared with patients with native artery occlusions. Major hemorrhage complications occurred 12.5% in the r-UK group, as compared with 5.5% in the surgery group among the TOPAS trial patients. Patients’ ages, duration of infusion, and activated partial thromboplastin times at baseline were unrelated to the risk of bleeding. Intracranial hemorrhage occurred in four patients in the r-UK group, one of whom died. In contrast, there were no instances of intracranial hemorrhage in the surgery group. The risk of bleeding was significantly greater when therapeutic heparin was utilized than when it was not. Among patients who received therapeutic heparin, bleeding occurred in 19%. In contrast, in the patients in whom therapeutic heparin was not utilized, bleeding occurred in only 9%.53
Thrombolytic agents work by converting plasminogen to plasmin, which then degrades fibrin. The agents that are currently in use for most peripheral procedures are alteplase, an rt-PA which is present in the Turkey market; reteplase, a genetically engineered mutant of tissue plasminogen activator; and tenecteplase, another genetically engineered mutant of tissue plasminogen activator.57 None of them are approved by the FDA for this indication. Streptokinase, an indirect plasminogen activator, was the first agent used for intra-arterial thrombolysis. Although its use has been largely abandoned in the United States because of lesser efficacy and higher rates of bleeding,48 as compared with other thrombolytic agents, and the potential for allergic reactions, it still can be used in Turkey. The direct plasminogen activator urokinase is no longer available in the United States because of manufacturing issues resulting in a discontinuation of production, and it never existed in the Turkey market (Table 4).
Table 4.
Thrombolytic agents.
| Product | Half-life (min) | Mechanism of action | Technical issues | Complication |
|---|---|---|---|---|
| T-PA | 2–6 | Fibrin-selective. Binds and activates fibrin by cleavage of an arginine–isoleucine bond, after which it activates plasminogen by cleaving Arg560–Val561 | 88.6%–91.8% successful thrombolysis | Major bleeding: 6.1%–6.8% |
| Alteplase | 3–6 | Tissue plasminogen activator produced by recombinant DNA technology. Fibrin-enhanced conversion of plasminogen to plasmin. It produces limited conversion of plasminogen in the absence of fibrin | 88.6%–91.8% successful thrombolysis | Major bleeding: 6.1%–6.8% |
| Reteplase | 14–18 | Similar to Alteplase. Lower fibrin binding and superior penetration ability | Thrombolytic success: 83.8%–86.7% | Major bleeding: 13.3% in 0.5 mg/h regimen, 5.4% in 0.25 mg/h regimen |
| Tenecteplase | 20–24 | Similar to Alteplase. Greater binding affinity for fibrin | Technical success: 91% | Major bleeding: 6.3% |
| Streptokinasea | 12–18 | Irreversible binding and activation of streptokinase to plasminogen. Indirect activation. Vaguely fibrin-specific | ||
| Anistreplase | 70–120 | Similar to streptokinase | ||
| Urokinase | 7–20 | Cleavage of the Arginine–Valine bond in plasminogen leading in active plasmin | Complete clot dissolution: 70% | Major bleeding: 11% |
T-PA: tissue plasminogen activator.
No longer preferred.
Absolute contraindications for catheter-directed thrombolysis include ongoing bleeding, intracranial hemorrhage, compartment syndrome, and severe limb ischemia that require immediate surgical procedure. Relative contraindications include major nonvascular surgery or trauma within past 10 days, intracranial tumor, recent eye surgery or neurosurgery within past 3 months, intracranial trauma within 3 months, recent gastrointestinal bleeding (10 days), an established recent cerebrovascular event, and life expectancy < 1 year. Absolute and relative contraindications are presented in Tables 5 and 6.58
Table 5.
Absolute contraindications to percutaneous catheter-directed thrombolysis.
| Absolute |
|---|
| Active bleeding |
| Intracranial hemorrhage |
| Presence or development of compartment syndrome |
| Severe limb ischemia, requires immediate operative intervention |
Table 6.
Relative contraindications to percutaneous catheter-directed thrombolysis.
| Relative |
|---|
| Major nonvascular surgery or trauma within past 10 days |
| Uncontrolled hypertension: 180 mmHg systolic or 110 mmHg diastolic blood pressure |
| Puncture of noncompressible vessel |
| Intracranial tumor |
| Recent eye surgery |
| Neurosurgery within past 3 months |
| History of severe contrast allergy or hypersensitivity |
| Intracranial trauma within 3 months |
| Recent gastrointestinal bleeding (10 days) |
| Established cerebrovascular event (including transient ischemic attacks within past 2 months) |
| Recent internal or noncompressible hemorrhage |
| Hepatic failure, particularly in cases with coagulopathy |
| Bacterial endocarditis |
| Pregnancy/postpartum status |
| Diabetic hemorrhagic retinopathy |
| Life expectancy < 1 year |
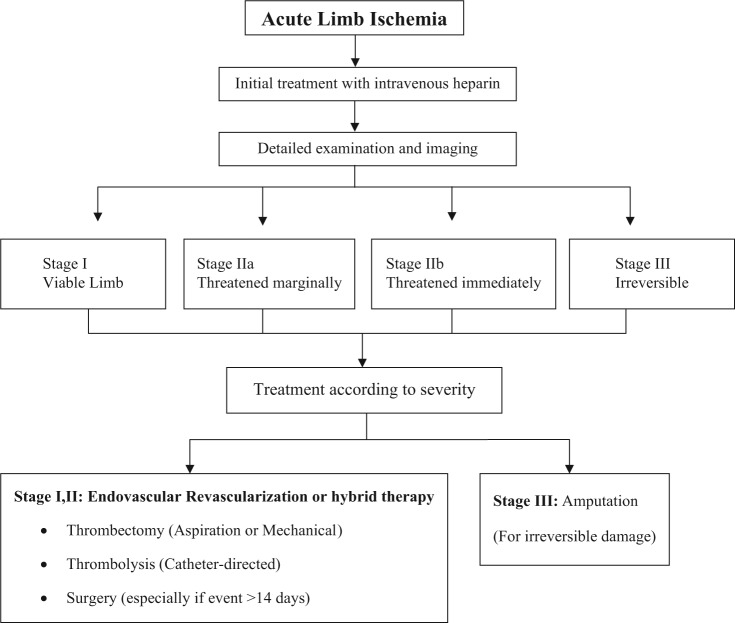
Until today, it has not been clear which therapeutic modality provides the best immediate and long-term results. Evidence-based results provided from randomized clinical trials suggest that thrombolytic therapy is superior to surgery to treat acute (less than 14 days) events regarding bypass graft occlusions and long occlusions without adequate runoff vessel suitable for surgical bypass (Karnabatidis et al.,59 Figure 1).
Figure 1.
The treatment diagram of ALI.
Surgical therapy
Patients in whom ischemia for 12–24 h would not be safe and those with a nonviable limb bypass graft with suspected infection, or contraindication to thrombolysis (e.g. recent intracranial hemorrhage, recent major surgery, vascular brain neoplasm, or active bleeding), should not undergo catheter-directed therapies.
Surgical approaches to the treatment of ALI include thromboembolectomy with a balloon catheter, bypass surgery, and adjuncts such as endarterectomy, patch angioplasty, and intraoperative thrombolysis.59 Frequently, a combination of these techniques is required. The cause of ischemia (embolic vs thrombotic) and anatomical features guide the surgical strategy. Thrombotic occlusion usually occurs in patients with a chronically diseased vascular segment. In such cases, correction of the underlying arterial abnormality is critical. Patients with suspected embolism and an absent femoral pulse ipsilateral to the ischemic limb are best treated by exposure of the common femoral artery bifurcation and balloon-catheter thromboembolectomy.60 A recent refinement for thromboembolectomy is the use of over-the-wire catheters, allowing for selective guidance into distal vessels. After removal of the clot, intraoperative angiography is performed to confirm that the thrombectomy is complete and to guide subsequent treatment if there is persistent inflow or outflow obstruction. The treatment of patients with ALI caused by thrombosis of a popliteal artery aneurysm warrants special mention, because major amputation occurs with high frequency in such patients.61,62 Diffuse thromboembolic occlusion of all major runoff arteries below the knee is frequently seen, and intra-arterial thrombolysis or thrombectomy may be required to restore flow in a runoff artery before aneurysm exclusion and surgical bypass are performed. Restoration of a palpable foot pulse, audible arterial Doppler signals, and visible improvement of foot perfusion suggest treatment success. In some cases, perfusion may be incomplete and close postoperative observation is required to monitor the limb status. Therapeutic anticoagulation with heparin is reinstituted after the procedure. Vasodilators (e.g. nitroglycerin and papaverine) may be useful if there is evidence of vasospasm.
A meta-analysis of five randomized trials comparing catheter-directed thrombolytic therapy with surgery for ALI showed similar rates of limb salvage, but thrombolysis was associated with higher rates of stroke and major hemorrhage within 30 days.56 Individual trial results were inconsistent, however, perhaps because of differences in patients’ characteristics, the duration and severity of ischemia, thrombolytic regimens, and length of follow-up. In one trial, rates of limb salvage were similar with catheter-based thrombolysis and with surgery, but 12-month rates of survival were significantly higher in the thrombolysis group.1 Nevertheless, surgical revascularization is generally preferred for patients with an immediately threatened limb or with symptoms of occlusion for more than 2 weeks.59
Reperfusion injury
Reperfusion may result in injury to the target limb, including profound limb swelling with dramatic increases in compartmental pressures.63 Symptoms and signs include severe pain, hypesthesia, and weakness of the affected limb; myoglobinuria and elevation of the creatine kinase level often occur. Since the anterior compartment of the leg is the most susceptible, assessment of peroneal nerve function (motor function—dorsiflexion of foot; sensory function—dorsum of foot and first web space) should be performed after the revascularization procedure. The diagnosis is made primarily from the clinical findings but can be confirmed if the compartment pressure is more than 30 mmHg or is within 30 mmHg of diastolic pressure.63 If the compartment syndrome occurs, surgical fasciotomy is indicated to prevent irreversible neurologic and soft-tissue damage.
Follow-up care
All postoperative patients should be given warfarin, often for 3–6 months or longer. Patients with thromboembolism will need long-term anticoagulation, possibly lifelong.64 If long-term anticoagulation is contraindicated due to bleeding risk factors, platelet inhibition therapy should be considered. Novel oral anticoagulants that inhibit thrombin or factor Xa, such as dabigatran or rivaroxaban, may be considered in patients with atrial fibrillation, but the efficacy of such drugs in patients with peripheral artery thrombosis is not known.
Adjunctive pharmacotherapy with antithrombotic drugs, statins, and beta-blockers is critical to decrease perioperative cardiovascular complications in patients undergoing surgical vascular reconstruction and to enhance post-revascularization arterial and graft patency.65 According to a study by Tomoi et al.,66 after endovascular therapy for isolated below-the-knee lesions in patients with critical limb ischemia, statin treatment significantly improved overall survival. In addition, statins improve infrainguinal bypass graft patency rates and are associated with reduced graft restenosis and amputation rates.67
Conclusion
Thrombectomy (aspiration or mechanical) is an effective method of treating ALI and could currently be applied combined with lytic infusion in selected cases where rapid recanalization is required or as a stand-alone therapy when the administration of thrombolytic agents is contraindicated. The culprit lesion should then be addressed and treated by surgical techniques if it is necessary. Amputation should be performed in patients with irreversible damage. As a consequence, the morbidity and mortality of acute peripheral arterial occlusion may be reduced by means of the efficient use of the multiplicity of interventional strategies.
Footnotes
Declaration of conflicting interests: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg 2007; 45(Suppl.): S5–S67. [DOI] [PubMed] [Google Scholar]
- 2. Callum K, Bradbury A. ABC of arterial and venous disease: acute limb ischaemia. BMJ 2000; 320(7237): 764–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lyden SP, Shortell CK, Illig KA. Reperfusion and compartment syndromes: strategies for prevention and treatment. Semin Vasc Surg 2001; 14(2): 107–113. [DOI] [PubMed] [Google Scholar]
- 4. Rajan DK, Patel NH, Valji K, et al. ; CIRSE and SIR Standards of Practice Committees. Quality improvement guidelines for percutaneous management of acute limb ischemia. J Vasc Interv Radiol 2005; 16(5): 585–595. [DOI] [PubMed] [Google Scholar]
- 5. Ourel K. Acute limb ischemia. In: Rutherford RB. (ed.) Vascular surgery. 6th ed. Philadelphia, PA: Elsevier, 2005, pp. 959–986. [Google Scholar]
- 6. Rutherford RB, Baker DJ, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg 1997; 26(3): 517–538. [DOI] [PubMed] [Google Scholar]
- 7. Kasirajan K, Marek JM, Longsfeld M. Mechanical thrombectomy as a first-line treatment for arterial occlusion. Semin Vasc Surg 2001; 14(2): 123–131. [DOI] [PubMed] [Google Scholar]
- 8. Mattox KL, Feliciano DV, Burch J, et al. Five thousand seven hundred sixty cardiovascular injuries in 4459 patients. Epidemiologic evolution 1958 to 1987. Ann Surg 1989; 209(6): 698–705, discussion 706–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Costantini V, Lenti M. Treatment of acute occlusion of peripheral arteries. Thromb Res 2002; 106(6): V285–V294. [DOI] [PubMed] [Google Scholar]
- 10. Kasirajan K, Ouriel K. Current options in the diagnosis and management of acute limb ischemia. Prog Cardiovasc Nurs 2002; 17(1): 26–34. [DOI] [PubMed] [Google Scholar]
- 11. Makris M. Hyperhomocysteinemia and thrombosis. Clin Lab Haematol 2000; 22(3): 133–143. [DOI] [PubMed] [Google Scholar]
- 12. Zipser S, Kirsch CM, Lien C, et al. Acute aortoiliac and femoral artery thrombosis complicating diabetic ketoacidosis. J Vasc Interv Radiol 2005; 16(12): 1737–1739. [DOI] [PubMed] [Google Scholar]
- 13. Eldrup-Jorgensen J, Flanigan DP, Brace L, et al. Hypercoagulable states and lower limb ischemia in young adults. J Vasc Surg 1989; 9(2): 334–341. [DOI] [PubMed] [Google Scholar]
- 14. Moulakakis KG, Sfyroeras G, Pavlidis P, et al. Hypercoagulable state due to alcohol-paracetamol syndrome producing acute limb ischemia. Vasc Endovascular Surg 2007; 41(4): 362–365. [DOI] [PubMed] [Google Scholar]
- 15. Lee JK, Baek MS, Mok YM, et al. Successfully treated femoral artery thrombosis in a patient with minimal change nephrotic syndrome. Chonnam Med J 2013; 49(1): 50–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Turba UC, Bozlar U, Simsek S. Catheter-directed thrombolysis of acute lower extremity arterial thrombosis in a patient with heparin-induced thrombocytopenia. Catheter Cardiovasc Interv 2007; 70(7): 1046–1050. [DOI] [PubMed] [Google Scholar]
- 17. Klinkert P, Post PN, Breslau PJ, et al. Saphenous vein versus PTFE for above-knee femoropopliteal bypass: a review of the literature. Eur J Vasc Endovasc Surg 2004; 27(4): 357–362. [DOI] [PubMed] [Google Scholar]
- 18. Koschinsky ML. Lipoprotein(a) and the link between atherosclerosis and thrombosis. Can J Cardiol 2004; 20(Suppl. B): 37B–43B. [PubMed] [Google Scholar]
- 19. Widmann MD, Sumpio BE. Lipoprotein(a): a risk factor for peripheral vascular disease. Ann Vasc Surg 1993; 7(5): 446–451. [DOI] [PubMed] [Google Scholar]
- 20. Bedarida G, Hoffmann U, Tatò F. Acute lower limb ischemia due to thrombo-embolic arterial occlusions in two previously healthy men with markedly elevated Lp(a). Vasc Med 2006; 11(4): 259–262. [DOI] [PubMed] [Google Scholar]
- 21. Fatourou EM, Paraskevas KI, Seifalian AM, et al. The role of established and emerging risk factors in peripheral vascular graft occlusion. Expert Opin Pharmacother 2007; 8(7): 901–911. [DOI] [PubMed] [Google Scholar]
- 22. Malyar NM, Kaleschke G, Reinecke H. Thrombembolic occlusion of crural arteries following transcatheter aortic valve implantation—successful endovascular recanalization using a thrombus aspiration device. Vasa 2012; 41(3): 225–228. [DOI] [PubMed] [Google Scholar]
- 23. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360(9326): 7–22. [DOI] [PubMed] [Google Scholar]
- 24. Aboyans V, Criqui MH, Abraham P, et al. ; American Heart Association Council on Peripheral Vascular Disease; Council on Epidemiology and Prevention; Council on Clinical Cardiology; Council on Cardiovascular Nursing; Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation 2012; 126(24): 2890–2909. [DOI] [PubMed] [Google Scholar]
- 25. Bandyk DF, Chauvapun JP. Duplex ultrasound surveillance can be worthwhile after arterial intervention. Perspect Vasc Surg Endovasc Ther 2007; 19(4): 354–359, discussion 360–361. [DOI] [PubMed] [Google Scholar]
- 26. Ferris BL, Mills JL, Sr, Hughes JD, et al. Is early postoperative duplex scan surveillance of leg bypass grafts clinically important? J Vasc Surg 2003; 37: 495–500. [DOI] [PubMed] [Google Scholar]
- 27. Singh H, Cardella JF, Cole PE, et al. ; Society of Interventional Radiology Standards of Practice Committee. Quality improvement guidelines for diagnostic arteriography. J Vasc Interv Radiol 2003; 14(9 Pt 2): S283–S288. [PubMed] [Google Scholar]
- 28. Met R, Bipat S, Legemate DA, et al. Diagnostic performance of computed tomography angiography in peripheral arterial disease: a systematic review and meta-analysis. JAMA 2009; 301(4): 415–424. [DOI] [PubMed] [Google Scholar]
- 29. Visser K, Hunink MG. Peripheral arterial disease: gadolinium-enhanced MR angiography versus color-guided duplex US—a meta-analysis. Radiology 2000; 216(1): 67–77. [DOI] [PubMed] [Google Scholar]
- 30. Menke J, Larsen J. Meta-analysis: accuracy of contrast-enhanced magnetic resonance angiography for assessing steno-occlusions in peripheral arterial disease. Ann Intern Med 2010; 153(5): 325–334. [DOI] [PubMed] [Google Scholar]
- 31. Leiner T, Kessels AG, Schurink GW, et al. Comparison of contrast-enhanced magnetic resonance angiography and digital subtraction angiography in patients with chronic critical ischemia and tissue loss. Invest Radiol 2004; 39(7): 435–444. [DOI] [PubMed] [Google Scholar]
- 32. Dorweiler B, Neufang A, Kreitner KF, et al. Magnetic resonance angiography unmasks reliable target vessels for pedal bypass grafting in patients with diabetes mellitus. J Vasc Surg 2002; 35: 766–772. [DOI] [PubMed] [Google Scholar]
- 33. Jens S, Koelemay MJW, Reekers JA, et al. Diagnostic performance of computed tomography angiography and contrast-enhanced magnetic resonance angiography in patients with critical limb ischaemia and intermittent claudication: systematic review and meta-analysis. Eur Radiol 2013; 23(11): 3104–3114. [DOI] [PubMed] [Google Scholar]
- 34. Kribben A, Witzke O, Hillen U, et al. Nephrogenic systemic fibrosis: pathogenesis, diagnosis, and therapy. J Am Coll Cardiol 2009; 53(18): 1621–1628. [DOI] [PubMed] [Google Scholar]
- 35. European Society of Urogenital Radiology (ESUR). Renal adverse reactions (ESUR Guidelines 7), http://www.esur.org/guidelines/ (2008).
- 36. Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis 2002; 39(5): 930–936. [DOI] [PubMed] [Google Scholar]
- 37. Results of a prospective randomized trial evaluating surgery versus thrombolysis for ischemia of the lower extremity: the STILE trial. Ann Surg 1994; 220(3): 251–266, discussion 266–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Blaisdell FW, Steele M, Allen RE. Management of acute lower extremity arterial ischemia due to embolism and thrombosis. Surgery 1978; 84(6): 822–834. [PubMed] [Google Scholar]
- 39. Thomsen HS. European Society of Urogenital Radiology (ESUR) guidelines on the safe use of iodinated contrast media. Eur J Radiol 2006; 60(3): 307–313. [DOI] [PubMed] [Google Scholar]
- 40. Fogarty TJ, Cranley JJ, Krause RJ, et al. A method for extraction of arterial emboli and thrombi. Surg Gynecol Obstet 1963; 116: 241–244. [PubMed] [Google Scholar]
- 41. Starck EE, McDermott JC, Crummy AB, et al. Percutaneous aspiration thrombectomy. Radiology 1985; 156(1): 61–66. [DOI] [PubMed] [Google Scholar]
- 42. Sniderman KW, Bodner L, Saddekni S, et al. Percutaneous embolectomy by transcatheter aspiration. Work in progress. Radiology 1984; 150(2): 357–361. [DOI] [PubMed] [Google Scholar]
- 43. Zehnder T, Birrer M, Do DD, et al. Percutaneous catheter thrombus aspiration for acute or subacute arterial occlusion of the legs: how much thrombolysis is needed? Eur J Vasc Endovasc Surg 2000; 20(1): 41–46. [DOI] [PubMed] [Google Scholar]
- 44. Hopfner W, Vicol C, Bohndorf K, et al. Shredding embolectomy thrombectomy catheter for treatment of acute lower-limb ischemia. Ann Vasc Surg 1999; 13(4): 426–435. [DOI] [PubMed] [Google Scholar]
- 45. Wagner H-J, Müler-Hülsbeck S, Pitton MB, et al. Rapid thrombectomy with a hydrodynamic catheter: results from a prospective, multicenter trial. Radiology 1997; 205(3): 675–681. [DOI] [PubMed] [Google Scholar]
- 46. Silva JA, Ramee SR, Collins TJ, et al. Rheolytic thrombectomy in the treatment of acute limb-threatening ischemia: immediate results and six-month follow-up of the multicenter AngioJet registry. Cathet Cardiovasc Diagn 1998; 45(4): 386–393. [DOI] [PubMed] [Google Scholar]
- 47. Henry M, Amor M, Henry I, et al. The Hydrolyser thrombectomy catheter: a single-center experience. J Endovasc Surg 1998; 5(1): 24–31. [DOI] [PubMed] [Google Scholar]
- 48. Kasirajan K, Ouriel K. Management of acute lower extremity ischemia: treatment strategies and outcomes. Curr Interv Cardiol Rep 2000; 2(2): 119–129. [PubMed] [Google Scholar]
- 49. Braithwaite BD, Davies B, Birch PA, et al. Management of acute leg ischemia in the elderly. Br J Surg 1998; 85(2): 217–220. [DOI] [PubMed] [Google Scholar]
- 50. Laird JR, Zeller T, Gray BH, et al. Limb salvage following laser-assisted angioplasty for critical limb ischemia: results of the LACI multicenter trial. J Endovasc Ther 2006; 13(1): 1–11. [DOI] [PubMed] [Google Scholar]
- 51. Wissgott C, Richter A, Kamusella P, et al. Treatment of critical limb ischemia using ultrasound-enhanced thrombolysis (PARES Trial): final results. J Endovasc Ther 2007; 14(4): 438–443. [DOI] [PubMed] [Google Scholar]
- 52. Gardiner GA, Jr, Meyerovitz MF, Stokes KR, et al. Complications of transluminal angioplasty. Radiology 1986; 159(1): 201–208. [DOI] [PubMed] [Google Scholar]
- 53. Ouriel K, Veith FJ, Sasahara AA. A comparison of recombinant urokinase with vascular surgery as initial treatment for acute arterial occlusion of the legs. N Engl J Med 1998; 338: 1105–1111. [DOI] [PubMed] [Google Scholar]
- 54. Earnshaw JJ, Whitman B, Foy C. National Audit of Thrombolysis for Acute Leg Ischemia (NATALI): clinical factors associated with early outcome. J Vasc Surg 2004; 39: 1018–1025. [DOI] [PubMed] [Google Scholar]
- 55. Ouriel K, Castaneda F, McNamara T, et al. Reteplase monotherapy and reteplase/abciximab combination therapy in peripheral arterial occlusive disease: results from the RELAX trial. J Vasc Interv Radiol 2004; 15: 229–238. [DOI] [PubMed] [Google Scholar]
- 56. Ouriel K, Shortell CK, DeWeese JA, et al. A comparison of thrombolytic therapy with operative revascularization in the initial treatment of acute peripheral arterial ischemia. J Vasc Surg 1994; 19: 1021–1030. [DOI] [PubMed] [Google Scholar]
- 57. Morrison HL. Catheter-directed thrombolysis for acute limb ischemia. Semin Intervent Radiol 2006; 23(3): 258–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Comerota A, White JV. Overview of catheter-directed thrombolytic therapy for arterial and graft occlusion. In: Camerta A. (ed.) Thrombolytic therapy for peripheral vascular disease. Philadelphia, PA: Lippincott-Raven, 1995, pp. 249–252. [Google Scholar]
- 59. Karnabatidis D, Spiliopoulos S, Tsetis D, et al. Quality improvement guidelines for percutaneous catheter-directed intra arterial thrombolysis and mechanical thrombectomy for acute lower-limb ischemia. Cardiovasc Intervent Radiol 2011; 34(6): 1123–1136. [DOI] [PubMed] [Google Scholar]
- 60. Kropman RH, Schrijver AM, Kelder JC, et al. Clinical outcome of acute leg ischaemia due to thrombosed popliteal artery aneurysm: systematic review of 895 cases. Eur J Vasc Endovasc Surg 2010; 39: 452–457. [DOI] [PubMed] [Google Scholar]
- 61. Robinson WP, III, Belkin M. Acute limb ischemia due to popliteal artery aneurysm: a continuing surgical challenge. Semin Vasc Surg 2009; 22: 17–24. [DOI] [PubMed] [Google Scholar]
- 62. Berridge DC, Kessel D, Robertson I. Surgery versus thrombolysis for acute limb ischaemia: initial management. Cochrane Database Syst Rev 2002; 3: CD002784. [DOI] [PubMed] [Google Scholar]
- 63. Tiwari A, Haq AI, Myint F, et al. Acute compartment syndromes. Br J Surg 2002; 89: 397–412. [DOI] [PubMed] [Google Scholar]
- 64. Norgren L, Hiatt WR, Dormandy JA, et al. ; TASC II Working Group. Inter-society consensus for the management of peripheral arterial disease. Int Angiol 2007; 26(2): 81–157. [PubMed] [Google Scholar]
- 65. Mangiafico RA, Mangiafico M. Medical treatment of critical limb ischemia: current state and future directions. Curr Vasc Pharmacol 2011; 9(6): 658–676. [DOI] [PubMed] [Google Scholar]
- 66. Tomoi Y, Soga Y, Iida O, et al. Efficacy of statin treatment after endovascular therapy for isolated below-the-knee disease in patients with critical limb ischemia. Cardiovasc Interv Ther 2013; 28(4): 374–382. [DOI] [PubMed] [Google Scholar]
- 67. Paraskevas KI, Giannoukas AD, Mikhailidis DP. Statins and infrainguinal vascular bypass procedures. Curr Vasc Pharmacol 2013; 11(1): 51–57. [PubMed] [Google Scholar]