Abstract
Platinum‐based drugs such as cisplatin and carboplatin are on the WHO model list of essential medicines, as highly effective chemotherapeutic drugs for the treatment of various solid tumors. These drugs react with purine residues in DNA, thereby causing DNA damage, inhibition of cell division, and eventually cell death. However, the mechanisms whereby platinum‐based drugs enter cancer cells remained poorly understood. In this issue, Planells‐Cases et al (2015) provide evidence that cells take up cisplatin and carboplatin via volume‐regulated anion channels (VRACs), more specifically VRACs composed of LRRC8A and LRRC8D subunits.
Subject Categories: Autophagy & Cell Death, Cancer, Membrane & Intracellular Transport
Fifty years ago, while testing the effect of electrical fields on the growth of bacteria, researchers discovered that metal complexes of platinum released from the electrodes had a strong inhibitory effect on bacterial cell division (Rosenberg et al, 1965). This serendipitous discovery fueled further successful research into the antiproliferative effects of different platinum‐containing molecules in mammalian cancer cells. By the end of the 1970s, a first platinum‐based drug, cisplatin (Fig 1A), was approved for the treatment of testicular and ovarian cancers. Currently, cisplatin and second‐ and third‐generation platinum‐based drugs such as carboplatin and oxaliplatin are amongst the most widely used and effective anticancer drugs (Kelland, 2007).
Figure 1. Permeability of VRACs for platinum‐based drugs.
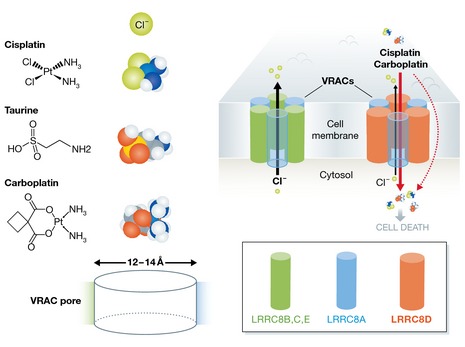
Left: structure and dimension of platinum‐based drugs in comparison with chloride ions and the VRAC‐permeant osmolyte taurine. Right: VRACs are composed of the obligatory LRRC8A subunit complemented with additional LRRC8B‐E subunits. VRACs containing LRRC8D exhibit high permeability for the platinum‐based anticancer drugs cisplatin and carboplatin, allowing drug entry into cells to cause cell death. The hexameric stoichiometry of VRACs is an educated guess based on limited homology with pannexin/connexin channels.
In the last decades, the biochemical mechanisms whereby cisplatin and related compounds inhibit cell proliferation have been largely unraveled. Briefly, once inside cells, platinum‐based drugs undergo aquation (i.e. the incorporation of water molecules) and enter the nucleus where they react covalently with the N7 nitrogen in guanines (Galluzzi et al, 2012). This causes distortions in the DNA, which can provoke cell cycle arrest and ultimately programmed cell death (Kelland, 2007; Galluzzi et al, 2012). However, before cisplatin and related drugs can exert their antiproliferative effects, they first need to be taken up by the targeted cells (Galluzzi et al, 2012). Cisplatin and carboplatin are relatively polar, compared to other classes of small‐molecule (anticancer) drugs. Consequently, their passive diffusion through the plasma membrane is slow, and other transport pathways, including transporters and channels, have been postulated. In particular, the copper transporter CTR1 has been proposed to mediate cisplatin uptake (Ishida et al, 2002), although this has been disputed (Ivy & Kaplan, 2013). Planells‐Cases et al (2015) now propose that VRACs act as entry channels for cisplatin and carboplatin. VRACs are ubiquitous channels that typically open in response to cell swelling or any other maneuver that dilutes the cellular ionic content (Nilius et al, 1997; Pedersen et al, 2015). VRACs are best known for their key role in the homeostasis of cell volume, but they have also been implicated in cellular processes including regulation of the membrane potential, release of transmitter and cell proliferation (Nilius et al, 1997).
Earlier work had already suggested a functional role for VRACs in cisplatin‐induced cell death. However, since the molecular nature of the functionally well‐characterized VRACs had remained elusive for decades (Pedersen et al, 2015), the exact role of these channels in platinum drug‐induced effects remained murky. Recently, the chloride channel field breathed a sigh of relief when two groups independently identified leucine‐rich repeat‐containing protein 8A (LRRC8A; also dubbed SWELL1) as an essential structural component of VRACs (Qiu et al, 2014; Voss et al, 2014). LRRC8 proteins form a small family of integral membrane proteins with four transmembrane helices and include five homologues (LRRC8A‐E) in mammals and most other chordates (Abascal & Zardoya, 2012). Current evidence indicates that functional VRAC channels are heteromultimers that contain the obligatory LRRC8A subunit supplemented with at least one of the other four isoforms (Voss et al, 2014). Based on limited homology with pannexins, a hexameric architecture has been proposed, but this stoichiometry still demands experimental verification (Abascal & Zardoya, 2012).
In a genomewide screen for genes that mediate cisplatin and carboplatin resistance, Planells‐Cases et al (2015) discovered that gene‐inactivating insertions in the LRRC8A and LRRC8D genes resulted in significantly increased survival of cancer cells when grown in the presence of the two platinum‐based drugs. Moreover, cells deficient in either LRRC8A or LRRC8D exhibited strongly reduced cellular platinum levels, suggesting that resistance to cisplatin and carboplatin is due to impaired cellular uptake of these drugs rather than to effects on downstream processes. Interestingly, hypotonic cell swelling caused a striking increase in cisplatin/carboplatin uptake. Overall, the results support the idea that the platinum‐based drugs can permeate VRACs composed of LRRC8A and LRRC8D subunits.
It may seem dubious to assume that the pore of an anion‐selective channel could accommodate a large, non‐charged, platinum complex. Since cisplatin and oxaliplatin do not carry charge, it is technically impossible to directly measure their flux through VRACs using electrophysiological techniques. Nevertheless, earlier work has provided ample indications that VRACs may be permeable to larger (and not necessarily negatively charged) molecules, including amino acids, sugars, ATP, and the sulfonic acid taurine, and estimates of the diameter of the VRAC pore are in the range of 12–14 Å (Pedersen et al, 2015). As such, the molecular dimensions of cisplatin and carboplatin would be at least compatible with pore permeation (Fig 1). Planells‐Cases et al (2015) provide evidence that permeability to such larger molecules is dependent on the subunit composition of VRACs, in that channels containing the LRRC8D subunit conduct more cisplatin and taurine (Fig 1). This suggests that LRRC8D differs from the other LRRC8 isoforms in regions that contribute to the VRAC pore. At present, the localization and structure of the VRAC pore is unknown. In fact, it cannot yet be excluded that other non‐LRRC8 proteins contribute to the VRAC pore. Mutating a threonine residue at the top of the first transmembrane domain (TM1) of LRRC8A caused mild changes in anion selectivity, suggesting that this residue may be in the vicinity of the permeation pathway (Qiu et al, 2014). Sequence alignments of all LRRC8 proteins indicate that the first extracellular loop connecting TM1 and TM2 in LRRC8D is about 40 amino acids longer than in the other isoforms (Abascal & Zardoya, 2012). It is therefore tempting to speculate that this first extracellular domain of LRRC8 proteins forms a reentrant pore loop in VRACs and that the extended loop in LRRC8D results in an enlarged pore that facilitates permeation of platinum‐based drugs and organic osmolytes.
In addition to providing important insights into the VRAC pore, the new results may have significant clinical impact. Reduced cellular uptake is one key mechanism, whereby cancer cells can become resistant to platinum‐based drugs (Galluzzi et al, 2012). In this respect, Planells‐Cases et al (2015) provide a retrospective analysis of two small cohorts of ovarian cancer patients that received treatment with platinum‐based drugs, which reveals that lower expression levels of LRRC8D correlate with lower survival rates. If these results are further confirmed, assessment of LRRC8D expression may be tested in cancer patients to predict the efficacy of platinum‐based drugs and to adjust the treatment strategy accordingly. It might even be considered to develop agonists that selectively activate LRRC8D‐containing VRACs, irrespective of cell volume changes, which could then be used to increase the effectiveness of platinum‐based drugs. Some caution is required here, as several earlier studies have linked VRAC activity to cell cycle progression, and pharmacological agents that inhibit VRACs were generally found to act as potent inhibitors of cell proliferation (Voets et al, 1995; Shen et al, 2000; Pedersen et al, 2015). Therefore, pharmacological upregulation of VRAC function in vivo may have unwanted stimulatory effects on cell growth. It is also known that various widely used drugs, including for instance the anti‐estrogen tamoxifen and the antidepressant fluoxetine (Prozac), potently inhibit VRACs (Pedersen et al, 2015) and may thus potentially influence the uptake of platinum drugs by cancer cells in patients. Finally, knowledge about the entry mechanisms of platinum‐based drugs might also be exploited to prevent their entry into non‐cancer cells. For instance, one of the most common and burdensome side effects of platinum‐based drugs is the development of neuropathic pain, which is due to an effect of the drugs on peripheral nerves and the surround tissue (Avan et al, 2015). Peripheral inhibition of LRRC8D‐containing VRACs might be a strategy to prevent these adverse effects.
See also: R Planells‐Cases et al (December 2015)
References
- Abascal F, Zardoya R (2012) LRRC8 proteins share a common ancestor with pannexins, and may form hexameric channels involved in cell‐cell communication. BioEssays 34: 551–560 [DOI] [PubMed] [Google Scholar]
- Avan A, Postma TJ, Ceresa C, Cavaletti G, Giovannetti E, Peters GJ (2015) Platinum‐induced neurotoxicity and preventive strategies: past, present, and future. Oncologist 20: 411–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, Castedo M, Kroemer G (2012) Molecular mechanisms of cisplatin resistance. Oncogene 31: 1869–1883 [DOI] [PubMed] [Google Scholar]
- Ishida S, Lee J, Thiele DJ, Herskowitz I (2002) Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc Natl Acad Sci USA 99: 14298–14302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy KD, Kaplan JH (2013) A re‐evaluation of the role of hCTR1, the human high‐affinity copper transporter, in platinum‐drug entry into human cells. Mol Pharmacol 83: 1237–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelland L (2007) The resurgence of platinum‐based cancer chemotherapy. Nat Rev Cancer 7: 573–584 [DOI] [PubMed] [Google Scholar]
- Nilius B, Eggermont J, Voets T, Buyse G, Manolopoulos V, Droogmans G (1997) Properties of volume‐regulated anion channels in mammalian cells. Prog Biophys Mol Biol 68: 69–119 [DOI] [PubMed] [Google Scholar]
- Planells‐Cases R, Lutter D, Guyader C, Gerhards NM, Ullrich F, Elger DA, Kucukosmanoglu A, Xu G, Voss FK, Reincke SM, Stauber T, Blomen VA, Vis DJ, Wessels LF, Brummelkamp TR, Borst P, Rottenberg S, Jentsch TJ (2015) Subunit composition of VRAC channels determines substrate specificity and cellular resistance to Pt‐based anti‐cancer drugs. EMBO J 34: 2993–3008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen SF, Klausen TK, Nilius B (2015) The identification of a volume‐regulated anion channel: an amazing Odyssey. Acta Physiol 213: 868–881 [DOI] [PubMed] [Google Scholar]
- Qiu Z, Dubin AE, Mathur J, Tu B, Reddy K, Miraglia LJ, Reinhardt J, Orth AP, Patapoutian A (2014) SWELL1, a plasma membrane protein, is an essential component of volume‐regulated anion channel. Cell 157: 447–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg B, Vancamp L, Krigas T (1965) Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature 205: 698–699 [DOI] [PubMed] [Google Scholar]
- Shen MR, Droogmans G, Eggermont J, Voets T, Ellory JC, Nilius B (2000) Differential expression of volume‐regulated anion channels during cell cycle progression of human cervical cancer cells. J Physiol 529(Pt 2): 385–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets T, Szucs G, Droogmans G, Nilius B (1995) Blockers of volume‐activated Cl− currents inhibit endothelial cell proliferation. Pflugers Arch 431: 132–134 [DOI] [PubMed] [Google Scholar]
- Voss FK, Ullrich F, Munch J, Lazarow K, Lutter D, Mah N, Andrade‐Navarro MA, von Kries JP, Stauber T, Jentsch TJ (2014) Identification of LRRC8 heteromers as an essential component of the volume‐regulated anion channel VRAC. Science 344: 634–638 [DOI] [PubMed] [Google Scholar]


