Abstract
Background and Purpose
Substance P and its preferred neurokinin receptor NK1 have been implicated in stress and anxiety and have been proposed as possible therapeutic targets for the treatment of anxiety/depression. Attention is also being focused on the role this neuropeptide system may play in drug addiction, because stress‐related mechanisms promote drug abuse.
Experimental Approach
The effects of the rat‐specific NK1 receptor antagonist, L822429, on alcohol intake and seeking behaviour was investigated in genetically selected Marchigian Sardinian alcohol preferring rats. These rats demonstrate an anxious phenotype and are highly sensitive to stress and stress‐induced drinking.
Key Results
Systemic administration of L822429 significantly reduced operant alcohol self‐administration in Marchigian Sardinian alcohol preferring rats, but did not reduce alcohol self‐administration in stock Wistar rats. NK1 receptor antagonism also attenuated yohimbine‐induced reinstatement of alcohol seeking at all doses tested but had no effect on cue‐induced reinstatement of alcohol seeking. L822429 reduced operant alcohol self‐administration when injected into the lateral cerebroventricles or the medial amygdala. L822429 injected into the medial amygdala also significantly reduced anxiety‐like behaviour in the elevated plus maze test. No effects on alcohol intake were observed following injection of L822429 into the dorsal or the ventral hippocampus.
Conclusions and Implications
Our results suggest that NK1 receptor antagonists may be useful for the treatment of alcohol addiction associated with stress or comorbid anxiety disorders. The medial amygdala appears to be an important brain site of action of NK1 receptor antagonism.
Abbreviations
- AP
antero‐posterior
- CeA
central amygdala
- CRF
corticotropin releasing factor
- DH
dorsal hippocampus
- DV
dorsal‐ventral
- EPM
elevated plus maze
- ML
medial‐lateral
- msP rats
Marchigian Sardinian (alcohol) preferring rats
- SP
substance P
- TO
time out
- VH
ventral hippocampus
Tables of Links
| TARGETS |
|---|
| GPCRs |
| NK1 receptor |
| CRF1 receptor |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013).
Introduction
Substance P (SP) and its preferred neurokinin NK1 receptor are involved in stress and anxiety‐like behaviour (Santarelli et al., 2001; McLean, 2005; Ebner and Singewald, 2006; Ebner et al., 2008). Neuropeptidergic systems involved in stress and anxiety have frequently attracted the interest of the researchers as targets for the pharmacotherapy of alcoholism (Ubaldi et al., 2013). SP and NK1 receptors are highly expressed in areas involved in the modulation of stress, anxiety and mood responses, such as the cingulate cortex, caudate putamen, nucleus accumbens, septum, hippocampus, amygdala, dorsal raphe and locus coeruleus (McLean, 2005; Ebner et al., 2008). Emotional and physical stressors modulate SP tissue levels or SP immunoreactivity in brain areas that are involved in fear and anxiety (Siegel et al., 1987; Nakamura et al., 1990). Blockade of NK1 receptors has consistently been shown to exert anxiolytic and anti‐depressant effects (see Mclean, 2005).
The NK1 receptor system may additionally play a role in excessive alcohol drinking and relapse (see Schank et al., 2012; Schank, 2014). For instance, systemic administration of an NK1 receptor antagonist attenuated stress‐induced reinstatement of alcohol seeking in non‐selected Wistar rats (Schank et al., 2011). At the same doses, the NK1 receptor antagonist did not have effects on self‐administration of alcohol under baseline conditions, nor did it affect cue‐induced reinstatement of alcohol seeking or novel environment‐induced locomotion (Schank et al., 2011). This suggests that NK1 receptors affect alcohol drinking and seeking that are motivated by the need to attenuate stress and anxiety in vulnerable individuals. To investigate this hypothesis, we evaluated the effect of NK1 receptor blockade on alcohol‐related behaviours in genetically selected Marchigian Sardinian alcohol preferring (msP) rats.
This strain of msP rats is highly sensitive to stress and stress‐induced excessive alcohol seeking (Ciccocioppo et al., 2006), show an anxious phenotype (Hansson et al., 2006), morphological and functional brain alterations, similar to those in depressed patients (Gozzi et al., 2013), and depressive‐like symptoms that recover following alcohol consumption (Ciccocioppo et al., 1999). The msP rats also display a high alcohol preference and intake, with an excessive daily alcohol drinking (6–8 g·kg−1 body weight) in a binge‐type pattern, leading to significant blood alcohol levels (Ciccocioppo, 2013). Altogether, these data suggest that rats of this strain may be useful as a model of a sub‐population of alcoholic patients in whom excessive drinking and relapse vulnerability is associated with co‐morbid anxiety and is motivated by negative reinforcement, or ‘tension relief’ mechanisms.
It is also relevant to note that msP rats have an innate up‐regulation of the corticotropin releasing factor CRF1 receptor transcript and receptor density (Hansson et al., 2006). This strain of rats show a reduction in alcohol drinking in response to CRF1 receptor antagonists, at doses that have no effect in non‐dependent, non‐selected rats (Hansson et al., 2006), whereas CRF1 receptor gene expression is reduced following voluntary drinking in these animals (Hansson et al., 2007). There could be some similarity between the actions of CRF1 receptor antagonists and NK1 receptor antagonists (Schank et al., 2011). For instance, NK1 receptor antagonists can reduce alcohol consumption in conditions of high or escalated intake similar to CRF1 receptor antagonists (Cippitelli et al., 2012). Furthermore, as with CRF1 receptor antagonists, NK1 receptor antagonists attenuated stress‐induced reinstatement to alcohol seeking in non‐selected Wistar lines, an effect not observed in the cue‐induced model (Marinelli et al., 2007, Liu and Weiss, 2002, Schank et al., 2011). It was therefore of great interest to investigate the effect of NK1 receptor antagonism on alcohol drinking in the msP strain of rats.
Based on these findings, we predicted that NK1 receptor blockade, contrary to what has been observed in heterogeneous, non‐selected Wistar rats, should reduce drinking in msP rats and that this effect should occur concomitantly with an anxiolytic action. To explore this hypothesis, we used L822429, an NK1 receptor antagonist developed to possess high affinity for the rat NK1 receptor (Ebner et al., 2004; Ebner et al., 2008; Singewald et al., 2008; Schank et al., 2011), and that exhibits anxiolytic properties (Ebner et al., 2004). The effect of L822429 was assessed on alcohol self‐administration in msP rats and for comparison in Wistar rats. Moreover, in the msP rats, we evaluated the effect of NK1 receptor blockade on stress and cue‐induced reinstatement of alcohol seeking. In the attempt to determine the brain sites of action of the NK1 receptor antagonist on alcohol intake, intracranial administrations of L822429 were also carried out in areas rich in SP fibres and NK1 receptors, which include the hippocampus and medial amygdala (Roberts et al., 1982; Nakaya et al., 1994; Borhegyi and Leranth, 1997; Ribeiro‐da‐Silva and Hokfelt, 2000; Ogier and Raggenbass, 2003). Finally, to link the reduction of alcohol drinking by the NK1 receptor antagonist to its anxiolytic action, we explored the consequences of injecting L822429 directly into the medial amygdala, on the results from the elevated plus maze (EPM) test, an established measure of anxiety in rodents.
Methods
Animals
All animal care and experimental procedures followed the EU directive for Care and Use of Laboratory Animals and were approved by the Ethical Committee of the University of Camerino. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2011; McGrath and Lilley, 2015). A total of 59 animals were used in the experiments described here.
Male msP and Wistar rats (Charles River, Calco, Italy) were used for the study. They were housed two per cage on a reverse 12 h light/dark cycle (lights off at 0900) at a constant temperature of 20 ± 2 °C and relative humidity of 45–55%. Food and water were available ad libitum. At the time of drug treatments, their body weights ranged between 450 and 550 g. Animals were handled daily for 1 week prior the start of experimentation.
Preparation of drugs used
Alcohol solution (10% v/v) was prepared by diluting 95% alcohol (F.L. Carsetti s.n.c., Italy) in tap water. The NK1 receptor antagonist, L822429 [2‐cyclopropoxy‐5‐(5‐(trifluoromethyl) tetrazol‐1‐yl)ben‐zyl]‐(2‐phenylpiperidin‐3‐yl)amine, was synthesized in the laboratory of Dr K. Rice at the NIDA Intramural Research Program. L822429 was dissolved in 45% w/v 2‐hydroxypropyl β‐cyclodextrin and the pH adjusted. The compound was injected i.p. in a volume of 1–2 mL·kg−1. Yohimbine (Sigma‐Aldrich, Milano, Italy) was dissolved in distilled water and injected i.p. in a volume of 1 mL·kg−1.
Operant self‐administration apparatus and training
Training and testing were conducted in standard operant conditioning chambers housed in sound‐attenuating cubicles (Med Associates Inc., USA). Each operant chamber was equipped with a drinking reservoir (volume capacity: 0.3 mL) positioned 4 cm above the grid floor in the centre of the front panel of the chamber and two retractable levers positioned laterally (3 cm) to either side of the drinking receptacle. Auditory and visual stimuli were presented via a speaker located on the front panel and light located on the back panel respectively. A microcomputer controlled the delivery of the fluids, presentation of auditory and visual stimuli and recording of the behavioural data. Each response on the active lever resulted in delivery of 0.1 mL of fluid, while responses on the second lever had no consequences. For 30 min·each day, rats were trained to self‐administer 10% (v/v) alcohol solution on a fixed ratio 1 schedule of reinforcement with a time‐out (TO) of 5 s. The TO period (during which lever responses are recorded but not reinforced) was signalled by illumination of a white house light located at the back panel for 5 s. Experiments were commenced when a stable baseline of responding was achieved.
Stereotaxic surgery
The msP rats were anaesthetised by i.m. injection of 250–300 μL of a solution (Zoletil®) containing 58.17 mg·mL tiletamine chlorhydrate and 57.5 mg·ml−1 zolazepam chlorhydrate (VIRBAC, Carros, France). Atropine sulphate (80 µg in 80 μL; Fattro S.P.A., Ozzano Emilia (BO), Italy) was also administered to the rats. For intracerebroventricular (i.c.v.) injections, a stainless steel guide cannula (7 mm length) aimed at the lateral ventricle was stereotaxically implanted. Taking bregma as reference point, the following coordinates were used: AP = −1.0 mm, ML = 1.8 mm and DV = −2.0 mm. For the dorsal hippocampus (DH), ventral hippocampus (VH) and medial amygdala injections, each animal was stereotaxically implanted with a unilateral guide stainless steel cannula (12 mm length) in each hemisphere over the DH, VH or MeA. Taking bregma as reference point, the following coordinates were used: DH: AP = −4.2 mm, ML = 2.6 mm and DV = −2.9 mm; VH: AP = −4.8 mm, ML = 5.1 mm and DV = −5.0 mm; MeA: AP = −2.7 mm, ML = 3.4 mm and DV= −7.0 mm (Paxinos and Watson, 1988). Injector lengths used for the injection were 1.5 mm longer than the cannula for DH and VH and 2.0 mm longer for MeA. At the end of the surgery, each guide cannula was sealed with a stainless steel wire (0.3 mm in diameter), and rats were given a single i.m. injection (500 μL) of rubrocillin (Intervet, Schering‐Plough Animal Health, Segrate (MI), Italy) as a prophylactic antibiotic. Rats were allowed to recover from surgery for 1 week during which they were monitored daily for signs of pain or distress. Correct cannula placement of the ICV guide cannulae was confirmed with angiotensin II (Gosnell et al., 1990; Choi et al., 2003) (Supporting Information).
At completion of the experiment, the animals were killed by CO2 inhalation and then the brains were quickly removed and snap‐frozen at −20 °C in isopentane. The brains were then stored at −80 °C. The correct placement of the cannulae was further verified by histological examination (Supporting Information). Only behavioural data obtained from rats that had the cannulae correctly placed in both hemispheres of the brain were analysed (N = 7 for DH, N = 9 for VH, N = 8 and N = 14 for MeA, self‐administration and EPM experiments, respectively).
Effect of L822429 on alcohol and saccharin self‐administration
One group of msP rats (N = 7) and another of non‐selected Wistar rats (N = 10) were trained to self‐administer 10% (v/v) alcohol solution as described previously. In a within‐subject Latin square counterbalanced order, 1 h before the beginning of the session, rats were treated with different doses of L822429 (0, 5, 15 and 30 mg·kg−1) i.p. Drug treatment was performed every fourth day. Thus, four rounds of drug treatment were performed, with each rat receiving all four doses of L822429. The first day after drug test, rats remained in their home cage, whereas on days 2 and 3, the baseline alcohol self‐administration was re‐established. Under identical experimental conditions, the study was replicated in another group of msP rats (N = 7) with saccharin solution (0.2%) as the reinforcer.
Effect of L822429 on yohimbine‐induced reinstatement of alcohol seeking in msP rats
A group of msP rats (N = 9) were trained to self‐administer 10% (v/v) alcohol solution as described previously. The rats were then subjected to 30 min extinction sessions for 16 consecutive days. Sessions were identical to those of the self‐administration training, with the exception that lever responses were not associated with alcohol delivery. Following the extinction phase, the rats were treated i.p. with L822429 (0, 5, 15 and 30 mg·kg−1) according to a counterbalanced Latin square (within‐subject) design 1 h before the reinstatement session. Yohimbine (1.25 mg·kg−1) was given i.p. to all the rats 30 min after the L822429 administration. A 4‐day interval, during which animals were subjected to extinction sessions, was allowed between drug tests (Cippitelli et al., 2008). Thus, four rounds of drug treatment were performed, with each rat receiving all four doses of L822429.
Effect of L822429 on cue‐induced reinstatement of alcohol seeking in msP rats
These msP rats (N = 9) were trained to discriminate between 10% alcohol and water in 30 min daily sessions. The discriminative stimulus for alcohol was the odour of an orange extract (S+), whereas water availability was signalled by an anise extract (S−). In addition, each lever press resulting in alcohol delivery was paired with illumination of the chamber's house light for 5 s. The corresponding cue during water sessions was a 5 s tone (70 dB). Concurrently with the presentation of these stimuli, a 5 s TO period was in effect during which responses were recorded but not reinforced. Following the conditioning phase, rats were subjected to 30 min extinction sessions for six consecutive days. During this phase, sessions began by extension of the levers without presentation of the discriminative stimuli, associated delivery of liquids or response‐contingent cues. After the extinction phase, reinstatement tests began under conditions identical to those during the conditioning phase, except that alcohol and water were not made available. The msP rats were tested under the S− condition on day 1. On day 2, the rats were treated i.p. with L822429 (0, 5, 15 and 30 mg·kg−1) 1 h before the beginning of the reinstatement test under the S+ condition according to a counterbalanced Latin square (within‐subject) design. A 4‐day interval, during which animals remained in their home cage, was allowed between drug tests (Ciccocioppo et al., 2002; Cippitelli et al., 2008). Thus, four rounds of drug treatment were performed, with each rat receiving all four doses of L822429.
Effect of L822429 administered intracranially on alcohol self‐administration in msP rats
A group of msP rats (N = 8) was trained to self‐administer 10% (v/v) alcohol solution as described previously. The rats then underwent surgery to implant a cannula into the lateral cerebroventricle and, after recovery, were subjected to alcohol self‐administration again. Following acquisition of a stable baseline of alcohol self‐administration, L822429 treatment began. In a within‐subject Latin square counterbalanced design, rats were treated with L822429 (30 µg in·2 μL per rat) or its vehicle 10 min before the beginning of the session. Drug treatment was performed every third day. The first day after drug test, rats remained in their home cage, whereas on day 2, the baseline alcohol self‐administration was re‐established. This experiment was also performed using lower doses (0, 7.5 and 15 µg·µl−1 per rat) of the NK1 receptor antagonist (see Supporting Information for results).
In a similar experiment, three groups of msP rats (N = 7–9 per group) trained to self‐administer 10% (v/v) alcohol were implanted bilaterally with cannulae into the DH, VH and medial amygdala respectively. The doses of L822429 evaluated here were 0, 7.5 and 15 µg per rat (0.3 μL per side). Treatment was carried out in a within‐subject Latin square counterbalanced design.
Effect of injection of L822429 into medial amygdala on anxiety‐like behaviour in msP rats
To measure anxiety‐like responses, the elevated plus maze (EPM) test was used. The maze, elevated 50 cm above the floor, was located in a sound attenuated room illuminated by a red dim light (~30 lx). Two groups of rats (N = 7 per group) were injected with L822429 (15 µg per rat; 0.3 μL per side) or its vehicle into the MeA. Ten minutes later, animals were tested in the EPM arena. The 5 min test procedure began when the animals were individually placed in the centre of the maze, facing a closed arm. A rat was considered to be on the central platform when at least two of its paws were on it. An entry was defined as the presence of all four paws in the arms. The percent of time spent in the open arm, the number of open arm entries and the number of total arm entries were recorded by an automated videotracking system (Noldus Ethovision XT 7.0). Data were then analysed by an experimenter unaware of the experimental treatments.
Data analysis
Self‐administration, reinstatement and intracranial experiments were evaluated by one‐way ANOVA with repeated measures using drug dose as a within‐subject factor. Analysis of variance was followed by the Newman–Keuls test when found to be significant. Statistical significance was set at P < 0.05. Data obtained from the EPM experiment were analysed using Student's t‐test. Statistical significance was set at P < 0.05. Statistica version 8 (StatSoft, Inc. Tulsa, OK, USA) was used for statistical analysis of the data.
Results
L822429 reduces alcohol self‐administration in msP but not Wistar rats
Rats rapidly acquired stable alcohol self‐administration. As shown in Figure 1A, treatment with L822429 significantly reduced operant responding for alcohol in msP rats [F(3,6) = 24.5; P < 0.001]. Post hoc analysis revealed a significant reduction of alcohol self‐administration at the doses of 5 mg·kg−1 (P < 0.01), 15 mg·kg−1 (P < 0.01) and 30 mg·kg−1 (P < 0.001), demonstrating a dose‐dependent effect. There was no significant effect of L822429 treatment on alcohol self‐administration in Wistar rats (Figure 1B). ANOVA did not show any significant main effect of treatment [F(3,9)=1.34, NS]. In msP rats, L822429 did not significantly modify operant responding for saccharin [[F(3,6)=0.07, NS] (Figure 2)].
Figure 1.
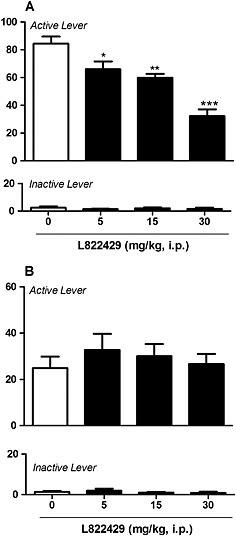
Effect of L822429 (0, 5, 15 and 30 mg·kg−1) on alcohol self‐administration in msP (A) and Wistar (B) rats under fixed ratio 1 schedule of reinforcement. Panel A shows that alcohol self‐administration is dose‐dependently reduced by L822429 in msP rats (N = 7). Panel B shows that there is no significant effect of L822429 treatment on alcohol self‐administration in Wistar rats (N = 10). Data are the mean (±SEM) number of rewards in 30 min. L822429 was given i.p. ***P < 0.001; **P < 0.01; *P < 0.05, significant differences from vehicle‐treated (0 mg·kg−1) rats.
Figure 2.
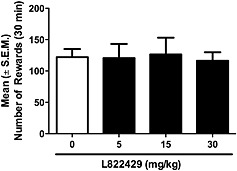
Effect of L822429 (5, 10, 15 and 30 mg·kg−1) on operant saccharin self‐administration in msP rats under fixed ratio 1 schedule of reinforcement. Data are the mean ±SEM of total number of rewards earned in 30 min. L822429 was given i.p. No significant differences were found between vehicle‐treated (0 mg·kg−1) and drug treated groups (N = 7).
In the experiments, responses at the inactive lever were extremely low and were not affected by L822429 treatment (data not shown).
L822429 attenuates yohimbine‐induced reinstatement of alcohol seeking in msP rats
After a stable baseline of responding to 10% alcohol was established during the self‐administration phase (mean ± SEM of the last 3 days: 52 ± 4.6), extinction was initiated and the responses to alcohol progressively decreased. As shown in Figure 3A, administration of yohimbine (1.25 mg·kg−1) significantly reinstated the operant responding for alcohol [F(1,8) = 9.75, P < 0.05]. ANOVA showed that pre‐treatment with L822429 significantly reduced the effect of yohimbine [F(3,8) = 4.25, P < 0.05]. Post hoc analysis revealed a significant dose‐related inhibition of reinstatement following administration of the NK1 receptor antagonist (5, 15 and 30 mg·kg−1). Statistical analysis of the inactive lever responses showed that there was no effect of yohimbine treatment, indicating its selectivity to elicit reinstatement of alcohol seeking (data not shown).
Figure 3.
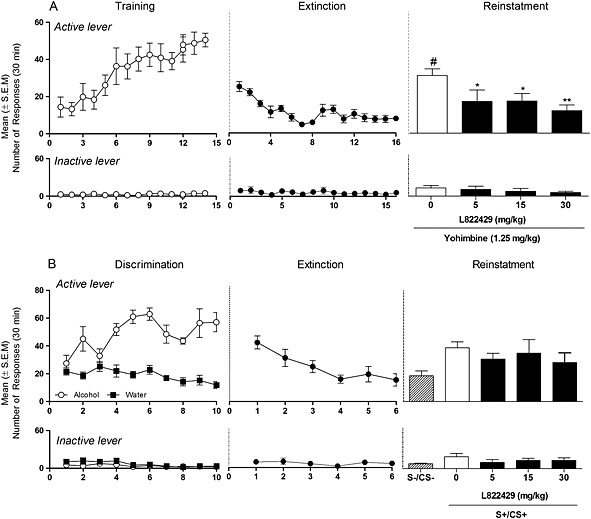
Effect of L822429 (0, 5, 15 and 30 mg·kg−1) on (A) yohimbine‐induced reinstatement of alcohol seeking and (B) cue‐induced reinstatement of alcohol seeking. S−, S+ refer to the discriminative stimuli indicating water and alcohol availability respectively. Data are the mean (±SEM) number of responses on the active lever in 30 min (N = 9 for each experiment). L822429 was administered via the intraperitoneal route. **P < 0.01; *P < 0.05, significant differences from vehicle‐treated group (0 mg·kg−1). # P < 0.01, significant difference from extinction. Data from the last day of extinction with water‐paired cues are shown as S‐/CS‐; the alcohol‐paired cues are shown as S+/CS+.
L822429 does not affect cue‐induced reinstatement of alcohol seeking in msP rats
ANOVA showed a significant overall effect of conditioning [F(1,16)= 27.82, P < 0.001] during the discrimination phase. Animals responded at a significantly higher level for alcohol (mean ± SEM of the last 3 days for alcohol: 53 ± 4.5) than for water (mean ± SEM of the last 3 days for water: 14 ± 2.1). During extinction, lever pressing progressively decreased. In the reinstatement test, ANOVA showed that cues had a significant overall effect on alcohol seeking [F(2,8) = 11.46; P < 0.001]. A more detailed analysis showed a robust reinstatement of responding under the S+ (P < 0.01) but not under the S− (P > 0.05, NS) compared with the last day of extinction. As shown in Figure 3B, pre‐treatment with L822429 did not affect conditioned reinstatement of alcohol seeking [F(3,8) = 16.59; NS]. Responses at the inactive lever were not influenced by the treatment.
Intracerebroventricular administration of L822429 reduces alcohol self‐administration in msP rats
ANOVA revealed a significant effect of the treatment [F(1,7) = 24.0, P ≤ 0.01] on alcohol self‐administration. As shown in Figure 4, the i.c.v administration of L822429 at the dose of 30 µg per rat significantly reduced alcohol self‐administration in the msP rats. Inactive lever responses were not influenced by the L822429 treatment. No appreciable effect of L822429 was found at the lower doses of 7.5 and 15 µg per rat (Figure S2).
Figure 4.
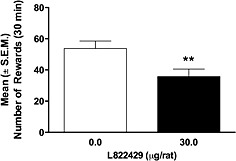
Effect of i.c.v. injection of L822429 (0.0, 30.0 µg per rat) on alcohol self‐administration in msP rats under fixed ratio 1 schedule of reinforcement. Data are the mean ± SEM of number of rewards earned in 30 min (N = 8). **P < 0.01, significant difference from vehicle‐treated group (0 µg per rat).
L822429 attenuates alcohol self‐administration in msP rats when administered into the medial amygdala but not the dorsal hippocampus or ventral hippocampus
ANOVA showed that the administration of L822429 into the dorsal hippocampus (Figure 5A) did not significantly modify alcohol self‐administration [F(2,6) = 2.23, NS]. As shown in Figure 5B, there was also no significant effect of L822429 administration into the ventral hippocampus [F(2,8) = 0.26, NS]. However, there was a significant reduction of alcohol self‐administration when the drug was injected into the medial amygdala [F(2,7) = 6.3, P < 0.05]. As shown in Figure 5C, post hoc analysis revealed that alcohol self‐administration was significantly reduced at the dose of 15 µg per rat.
Figure 5.
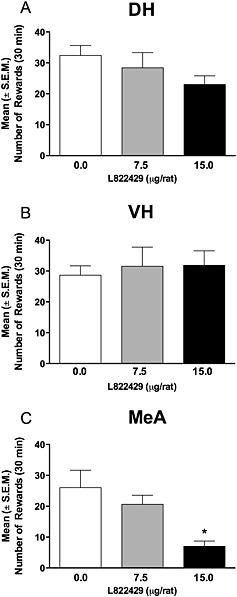
Effect of L822429 injection (0, 7.5 and 15 µg per rat) into the dorsal hippocampus (A), ventral hippocampus (B) and medial amygdala (C) on alcohol self‐administration in msP rats under fixed ratio 1 schedule of reinforcement. Data are the mean ± SEM of total number of rewards earned in 30 min (N = 7–9 per group). *P < 0.05, significant difference from vehicle‐treated group (0 mg·kg−1).
Micro‐injection of L822429 into the medial amygdala attenuates anxiety‐like behaviour of msP rats in elevated plus maze test
As shown in Figure 6, L822429 treatment (15 µg per rat) significantly increased the percentage of time spent in the open arms [t(12) = 5.69, P < 0.0001] and the percentage of open arm entries [t(12) = 4.97, P < 0.0001]. Conversely, drug exposure reduced the total number of closed arm entries [t(12) = 4.97, P < 0.0001]. Total arm entries were not affected by treatment [t(12) = 1.19, P > 0.05].
Figure 6.
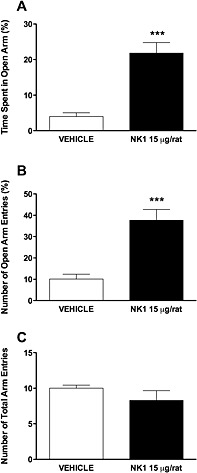
Effect of L822429 injection (0, 15 µg per rat) into the medial amygdala on the time spent on the open arms (A), the percentage of entries into the open arms (B) and the total number of entries (C) in the elevated plus maze test. Data are expressed as the mean ±SEM (N = 7 per group). ***P < 0.001, significant difference from vehicle‐treated group.
Discussion
In agreement with previous reports (Schank et al., 2011; Schank et al., 2013), we found that the NK1 receptor antagonist L822429 did not modify basal alcohol self‐administration in non‐selected Wistar rats. In contrast, in genetically selected msP rats, L822429 did attenuate alcohol self‐administration at doses that did not modify saccharin‐reinforced operant responding. This indicates that the action of the NK1 receptor antagonist is selective for alcohol in msP rats, consistent with previous findings with another selected line, the alcohol preferring (P) rat (Schank et al., 2013). Furthermore, and also in agreement with previous work (Schank et al., 2011; Schank et al., 2014), NK1 receptor blockade suppressed reinstatement of alcohol seeking elicited by the pharmacological stressor yohimbine, but not by cues previously associated with alcohol availability. We also confirmed that L82242 exerted its effects via central mechanisms and that the medial amygdala is an important site of action for the NK1 receptor antagonist on alcohol intake in msP rats. Finally, we demonstrated that the injection of L822429 into the medial amygdala of msP rats decreased anxiety‐like behaviour in the EPM test under baseline conditions, suggesting that the reduction of alcohol intake elicited by the NK1 receptor antagonist may be driven by inhibition of innate anxiety and negative emotionality in these rats.
The NK1 receptor mediates anxiety and stress (McLean, 2005; Ebner and Singewald, 2006), and it is known that these negative emotional states promote the compulsivity of alcohol drinking (Koob and Le Moal, 2006). Also, NK1 receptor antagonists are effective in the reduction of anxiety, depression and stress‐related states (Ebner and Singewald, 2006). Our findings therefore suggest that the likely mechanism by which the NK1 receptor antagonist was able to reduce alcohol drinking was by attenuating these negative emotional states that drive drinking behaviour in vulnerable individuals. This may explain why NK1 receptor antagonists reduced alcohol self‐administration in the msP rats, which are postulated to drink to alleviate an innate high anxiety state, but not in their Wistar counterparts. This is in agreement with the study by Schank et al. (2011) that showed no effects of the NK1 receptor antagonist on alcohol drinking in non‐selected Wistar rats (Schank et al., 2011). To our knowledge in fact, only one study has reported inhibition of alcohol drinking in heterogeneous rats following NK1 receptor antagonism, but the effect was observed at doses that also suppressed sucrose consumption (Steensland et al., 2010). This indicates that high doses of a NK1 receptor antagonist could elicit a general inhibitory effect on appetitive behaviour.
Interestingly, inhibition of the NK1 receptor by pharmacological antagonism or genetic deletion reduced alcohol intake in mice of the C57/BL6 strain (George et al., 2008; Thorsell et al., 2010), which have relatively high alcohol preference (Belknap et al., 1993). This would be compatible with our observations in alcohol preferring rat strains and suggests that NK1 receptor antagonists can reduce alcohol consumption in conditions of high or escalated intake similar to CRF1 receptor antagonists (Cippitelli et al., 2012).
The NK1 receptor antagonist attenuated stress‐induced reinstatement to alcohol seeking in this study. The stressor employed was yohimbine, an α2‐adrenoceptor antagonist that increases noradrenergic cell firing (Aghajanian and VanderMaelen, 1982) and enhances noradrenaline release in terminal areas (Abercrombie et al., 1988; Pacak et al., 1992). Yohimbine induces anxiety‐like responses in both humans (Holmberg and Gershon, 1961; Bremner et al., 1996b) and laboratory animals (Bremner et al., 1996a) and triggers craving in alcohol‐dependent patients (Umhau et al., 2011). The ability of the NK1 receptor antagonist to attenuate stress‐induced reinstatement to alcohol seeking suggests the possible involvement of the SP/NK1 receptor system in stress‐induced craving in alcohol dependence. Indeed, a recent clinical trial using an NK1 receptor antagonist found beneficial effects on baseline and stress‐elicited alcohol craving (George et al., 2008). The ability of the NK1 receptor antagonist to attenuate stress‐induced reinstatement to alcohol seeking and not affect cue‐induced reinstatement to alcohol seeking parallels the study of Schank and colleagues, where similar effects were observed in non‐selected Wistar rats (Schank et al., 2011). This further supports the notion that stress may be a necessary precondition for NK1 receptor antagonists to elicit their alcohol‐seeking suppressing effects. These findings also underscore another important similarity between the antagonists of SP/NK1 receptor and CRF/CRF1 receptor systems in the modulation of alcohol intake. CRF1 receptor antagonists suppress stress‐induced reinstatement to alcohol seeking in msP lines (Hansson et al., 2006) and non‐selected Wistar lines (Marinelli et al., 2007) while being ineffective on cue‐reinstatement models (Liu and Weiss, 2002). It is tempting to speculate that both the SP/NK1 receptor and the CRF/CRF1 receptor systems might share common neurobiological pathways. For instance, like CRF, SP and the NK1 receptor might be involved in the neuroadaptations induced by history of alcohol dependence or escalated intake. To support this hypothesis, NK1 receptor knock‐out mice failed to escalate their alcohol consumption after repeated cycles of deprivation (Thorsell et al., 2010), suggesting that the SP/NK1 receptor system may mediate neuroadaptive changes that contribute to the escalation of alcohol intake, a hallmark of addiction.
The ability of the NK1 receptor antagonist to reduce alcohol drinking in the up‐regulated CRF state of msP rats suggests that there could be an interaction between the actions of SP and CRF in the brain. In a study that investigated the differential gene expression profile induced by SP in human astronoma and rat astroglial cells using microarray techniques, SP was shown to induce the expression of CRF1 receptors, an effect that was inhibited by a selective NK1 receptor antagonist (Hamke et al., 2006). In addition, SP has also been shown to increase CRF1 receptor mRNA and induce the expression of the CRF1 receptor protein in human mast cells (Asadi et al., 2012). This indicates that SP could induce CRF1 receptor expression in brain regions that mediate anxiety and stress‐induced alcohol intake. Taken together, these data suggest that a cross‐talk may exist between the two neuropeptide systems in the modulation of stress‐induced drinking in msP rats. However, there is a possibility that NK1 receptor and CRF1 receptor systems use parallel pathways to mediate stress‐induced behaviours, or that these systems converge on a common downstream target. Future research should focus on exploring these possibilities.
The hippocampus is a brain structure involved in many functional processes such as learning and memory as well as regulation of emotions (Eichenbaum, 2000; Engin and Treit, 2007) and has a high density of SP‐containing fibres, which derive from intrinsic and extrinsic origins (Borhegyi and Leranth, 1997). In the hippocampus, SP effects are mediated by NK1 receptors (Ogier and Raggenbass, 2003) even though other neurokinin receptor subtypes are present in this brain structure (Maeno et al., 1993; Quartara and Maggi, 1998). Thus, we were interested in studying the effect of NK1 receptor antagonism in this brain area on alcohol intake. Our results showed that neither NK1 receptor antagonism in the dorsal nor the ventral hippocampus influenced alcohol intake in msP rats. The hippocampus is known to play a role in drug reinforcement, craving and cue‐induced relapse (Volkow, 2004; Martin‐Fardon et al., 2008; Koob and Volkow, 2010b), whereas not much is known about its role in the modulation of negative states associated with addiction. Based on present findings, we would speculate that the NK1 receptor system located in this brain structure was not involved in the modulation of drinking behaviour, nor did it contribute to the regulation of negative affect associated with the innate high alcohol vulnerability of msP rats.
On the other hand, a suppression of alcohol intake was observed when the drug was injected into the MeA. Notably, the effect in the medial amygdala was obtained at a dose of L822429, which was ineffective following i.c.v. administration. This provides strong evidence for a specific role of this area in mediating the effect of NK1 receptor antagonists on alcohol drinking. The amygdala is a critical brain structure for the processing of emotions including fear and anxiety (Aggleton, 1993; LeDoux, 2003) and has been shown to play an important role in negative reinforcement that drives compulsive drug use (Koob and Zorrilla, 2010a). SP‐containing neurons as well as NK1 receptors are highly expressed in this area (Roberts et al., 1982; Nakaya et al., 1994; Ribeiro‐da‐Silva and Hokfelt, 2000). The medial amygdala is reported to contain the highest amounts of SP within the amygdala (Roberts et al., 1982) and immunohistochemical studies have demonstrated a dense plexus of SP‐containing cell bodies and terminals in this area (Ribeiro‐da‐Silva and Hokfelt, 2000; Cippitelli et al., 2012). One study showed that within the amygdala, the medial amygdala is particularly activated by emotional stress more strongly than the central amygdala, as indicated by c‐fos expression (Dayas et al., 1999). Another study demonstrated an immobilization stress‐induced increase of SP release in the medial amygdala but not in the central amygdala (Ebner et al., 2004). Thus, we considered it relevant to investigate the effect of NK1 receptor antagonism in this brain area on anxiety in msP rats.
Results from the EPM confirmed that the administration of L822429 suppressed anxiety‐like responses when micro‐injected into the medial amygdala. This agrees with the study of Ebner et al (2004), which showed that injections of the same NK1 receptor antagonist into the medial amygdala blocked anxiogenic‐like effects in previously stressed (immobilization stress) rats, as tested with the EPM (Ebner et al., 2004). Additionally, Ebner and colleagues found that the NK1 receptor antagonist did not elicit an anxiolytic effect in unstressed rats but reversed the anxiogenic effect of SP microinjected into the medial amygdala (Ebner et al., 2004). Extrapolating these findings to our study, it could be that the msP rats have an innately overfunctioning SP system in the medial amygdala, which may contribute to the typical negative affective state of these animals, in turn facilitating their excessive alcohol consumption. Additionally, because it has also been shown that NK1 receptor activation by SP increases CRF1 receptor expression, it is tempting to speculate that up‐regulation of the extrahypothalamic corticotropin system described in msP rats may result (Hansson et al., 2006; Hansson et al., 2007), at least in part, from over‐activity of the neurokinin system. Together, these data indicate an important role for the SP/NK1 receptor system in the medial amygdala for the modulation of anxiety and stress‐induced excessive alcohol drinking and seeking. However, recent studies in P alcohol preferring rats have identified the central amygdala as the major site of NK1 receptor antagonist activity on excessive alcohol intake in that particular strain (Schank et al., 2013). The underlying mechanism of this divergence is not clear, but it is important to note that these two alcohol preferring lines were independently generated through genetic selection under somewhat different selection conditions.
In conclusion, the findings in this study demonstrate that the SP/NK1 receptor system plays an important role in excessive alcohol intake mediated by stress. Like CRF1 receptor antagonists, NK1 receptor antagonists reduce alcohol drinking in a similar fashion and could represent a viable pharmacological target for the treatment of alcoholism associated with stress or co‐morbid anxiety disorders. The medial amygdala is a probable site of action for the effect of the NK1 receptor antagonist on alcohol intake in genetically selected msP rats.
Author contributions
R. C., J. S. and M. H. planned and coordinated the study. L. A., S. S., M. U., A. C., C. N. and R. D. performed experimental assays. K. C. and K. R. synthesized L822429 and provided critical comments.
Conflicts of interest
The authors declare no conflicts of interest.
Supporting information
Figure S1 Effect of intracerebroventricular injection of L822429 (0.0, 7.5, 15.0 µg/rat) on alcohol self‐administration in msP rats under FR‐1 schedule of reinforcement. Data are the mean ± SEM of total number of rewards earned in 30 min (N = 8). L822429 did not reduce the number of rewards at these doses. No significant differences were found between the treatment groups. Vehicle‐treated group: white bar; drug‐treated groups: black bars.
Figure S2 Microinjections into the Medial Amygdala were performed within the highlighted area. Taking bregma as reference point, the following coordinates were used in accordance with the atlas of Paxinos and Watson, 1998: Antero‐Posterior = 2.7 mm; Medio‐Lateral = 3.4 mm; Dorso‐Ventral = 9.0 mm.
Supporting info item
Acknowledgements
This study was supported by NIH grants nos. AA014351 and AA017447. A portion of this work was supported by the NIH Intramural Research Programs of the National Institute on Drug Abuse (NIDA) and the National Institute of Alcohol Abuse and Alcoholism. We are thankful to Alfredo Fiorelli for technical support as well as Rina Righi and Mariangela Fiorelli for animal care.
Ayanwuyi, L. O. , Stopponi, S. , Ubaldi, M. , Cippitelli, A. , Nasuti, C. , Damadzic, R. , Heilig, M. , Schank, J. , Cheng, K. , Rice, K. C. , and Ciccocioppo, R. (2015) Neurokinin 1 receptor blockade in the medial amygdala attenuates alcohol drinking in rats with innate anxiety but not in Wistar rats. British Journal of Pharmacology, 172: 5136–5146. doi: 10.1111/bph.13280.
References
- Abercrombie ED, Keller RW Jr, Zigmond MJ (1988). Characterization of hippocampal norepinephrine release as measured by microdialysis perfusion: pharmacological and behavioral studies. Neuroscience 27: 897–904. [DOI] [PubMed] [Google Scholar]
- Aggleton JP (1993). The contribution of the amygdala to normal and abnormal emotional states. Trends Neurosci 16: 328–333. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, VanderMaelen CP (1982). Alpha 2‐adrenoceptor‐mediated hyperpolarization of locus coeruleus neurons: intracellular studies in vivo . Science 215: 1394–1396. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M et al. (2013). The concise guide to PHARMACOLOGY 2013/14: G protein‐coupled receptors. Br J Pharmacol 170: 1459–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadi S, Alysandratos KD, Angelidou A, Miniati A, Sismanopoulos N, Vasiadi M et al. (2012). Substance P (SP) induces expression of functional corticotropin‐releasing hormone receptor‐1 (CRHR‐1) in human mast cells. J Invest Dermatol 132: 324–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER (1993). Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology (Berl) 112: 503–510. [DOI] [PubMed] [Google Scholar]
- Borhegyi Z, Leranth C (1997). Substance P innervation of the rat hippocampal formation. J Comp Neurol 384: 41–58. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS (1996a). Noradrenergic mechanisms in stress and anxiety: I. Preclinical studies. Synapse 23: 28–38. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS (1996b). Noradrenergic mechanisms in stress and anxiety: II. Clinical studies. Synapse 23: 39–51. [DOI] [PubMed] [Google Scholar]
- Choi YH, Li C, Page K, Westby A, Della‐Fera MA, Lin J et al. (2003). Melanocortin receptors mediate leptin effects on feeding and body weight but not adipose apoptosis. Physiol Behav 79: 795–801. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R (2013). Genetically selected alcohol preferring rats to model human alcoholism. Curr Top Behav Neurosci 13: 251–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Martin‐Fardon R, Weiss F (2002). Effect of selective blockade of mu(1) or delta opioid receptors on reinstatement of alcohol‐seeking behavior by drug‐associated stimuli in rats. Neuropsychopharmacology 27: 391–399. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Panocka I, Froldi R, Colombo G, Gessa GL, Massi M (1999). Antidepressant‐like effect of ethanol revealed in the forced swimming test in Sardinian alcohol‐preferring rats. Psychopharmacology (Berl) 144: 151–157. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Cippitelli A, Cucculelli M, Ubaldi M, Soverchia L et al. (2006). Genetically selected Marchigian Sardinian alcohol‐preferring (msP) rats: an animal model to study the neurobiology of alcoholism. Addict Biol 11: 339–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Damadzic R, Singley E, Thorsell A, Ciccocioppo R, Eskay RL et al. (2012). Pharmacological blockade of corticotropin‐releasing hormone receptor 1 (CRH1R) reduces voluntary consumption of high alcohol concentrations in non‐dependent Wistar rats. Pharmacol Biochem Behav 100: 522–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Cannella N, Braconi S, Duranti A, Tontini A, Bilbao A et al. (2008). Increase of brain endocannabinoid anandamide levels by FAAH inhibition and alcohol abuse behaviours in the rat. Psychopharmacology (Berl) 198: 449–460. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Day TA (1999). Neuroendocrine responses to an emotional stressor: evidence for involvement of the medial but not the central amygdala. Eur J Neurosci 11: 2312–2322. [DOI] [PubMed] [Google Scholar]
- Ebner K, Singewald N (2006). The role of substance P in stress and anxiety responses. Amino Acids 31: 251–272. [DOI] [PubMed] [Google Scholar]
- Ebner K, Rupniak NM, Saria A, Singewald N (2004). Substance P in the medial amygdala: emotional stress‐sensitive release and modulation of anxiety‐related behavior in rats. Proc Natl Acad Sci U S A 101: 4280–4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner K, Muigg P, Singewald G, Singewald N (2008). Substance P in stress and anxiety: NK‐1 receptor antagonism interacts with key brain areas of the stress circuitry. Ann N Y Acad Sci 1144: 61–73. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H (2000). A cortical‐hippocampal system for declarative memory. Nat Rev Neurosci 1: 41–50. [DOI] [PubMed] [Google Scholar]
- Engin E, Treit D (2007). The role of hippocampus in anxiety: intracerebral infusion studies. Behav Pharmacol 18: 365–374. [DOI] [PubMed] [Google Scholar]
- George DT, Gilman J, Hersh J, Thorsell A, Herion D, Geyer C et al. (2008). Neurokinin 1 receptor antagonism as a possible therapy for alcoholism. Science 319: 1536–1539. [DOI] [PubMed] [Google Scholar]
- Gosnell BA, Majchrzak MJ, Krahn DD (1990). Effects of preferential delta and kappa opioid receptor agonists on the intake of hypotonic saline. Physiol Behav 47: 601–603. [DOI] [PubMed] [Google Scholar]
- Gozzi A, Agosta F, Massi M, Ciccocioppo R, Bifone A (2013). Reduced limbic metabolism and fronto‐cortical volume in rats vulnerable to alcohol addiction. Neuroimage 69: 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamke M, Herpfer I, Lieb K, Wandelt C, Fiebich BL (2006). Substance P induces expression of the corticotropin‐releasing factor receptor 1 by activation of the neurokinin‐1 receptor. Brain Res 1102: 135–144. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Ciccocioppo R, Heilig M (2007). Region‐specific down‐regulation of Crhr1 gene expression in alcohol‐preferring msP rats following ad lib access to alcohol. Addict Biol 12: 30–34. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Fedeli A, Bjork K, Soverchia L et al. (2006). Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc Natl Acad Sci U S A 103: 15236–15241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg G, Gershon S (1961). Autonomic and psychic effects of yohimbine hydrochloride. Psychopharmacologia 2: 93–106. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG, Centre National for the Replacement R, et al. (2011). Animal research: reporting in vivo experiments – the ARRIVE guidelines. J Cereb Blood Flow Metab 31: 991–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G, Le Moal M (2006). Neurobiology of Addiction, Academic Press: London. [Google Scholar]
- Koob GF, Zorrilla EP (2010a). Neurobiological mechanisms of addiction: focus on corticotropin‐releasing factor. Curr Opin Investig Drugs 11: 63–71. [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2010b). Neurocircuitry of addiction. Neuropsychopharmacology 35: 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J (2003). The emotional brain, fear, and the amygdala. Cell Mol Neurobiol 23: 727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Weiss F (2002). Reversal of ethanol‐seeking behavior by D1 and D2 antagonists in an animal model of relapse: differences in antagonist potency in previously ethanol‐dependent versus nondependent rats. J Pharmacol Exp Ther 300: 882–889. [DOI] [PubMed] [Google Scholar]
- Maeno H, Kiyama H, Tohyama M (1993). Distribution of the substance P receptor (NK‐1 receptor) in the central nervous system. Brain Res Mol Brain Res 18: 43–58. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, Harding S, Rice KC, Shaham Y et al. (2007). The CRF1 receptor antagonist antalarmin attenuates yohimbine‐induced increases in operant alcohol self‐administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 195: 345–355. [DOI] [PubMed] [Google Scholar]
- Martin‐Fardon R, Ciccocioppo R, Aujla H, Weiss F (2008). The dorsal subiculum mediates the acquisition of conditioned reinstatement of cocaine‐seeking. Neuropsychopharmacology 33: 1827–1834. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean S (2005). Do substance P and the NK1 receptor have a role in depression and anxiety? Curr Pharm Des 11: 1529–1547. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Moroji T, Nohara S, Nakamura H, Okada A (1990). Effects of whole‐body vibration stress on substance P‐ and neurotensin‐like immunoreactivity in the rat brain. Environ Res 52: 155–163. [DOI] [PubMed] [Google Scholar]
- Nakaya Y, Kaneko T, Shigemoto R, Nakanishi S, Mizuno N (1994). Immunohistochemical localization of substance P receptor in the central nervous system of the adult rat. J Comp Neurol 347: 249–274. [DOI] [PubMed] [Google Scholar]
- Ogier R, Raggenbass M (2003). Action of tachykinins in the rat hippocampus: modulation of inhibitory synaptic transmission. Eur J Neurosci 17: 2639–2647. [DOI] [PubMed] [Google Scholar]
- Pacak K, Armando I, Komoly S, Fukuhara K, Weise VK, Holmes C et al. (1992). Hypercortisolemia inhibits yohimbine‐induced release of norepinephrine in the posterolateral hypothalamus of conscious rats. Endocrinology 131: 1369–1376. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP et al. (2014). The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucleic Acids Res 42: D1098–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1988). The Rat Brain in Stereotaxic Coordinates, 4th edn. San Diego, CA: Academic Press. [Google Scholar]
- Quartara L, Maggi CA (1998). The tachykinin NK1 receptor. Part II: Distribution and pathophysiological roles. Neuropeptides 32: 1–49. [DOI] [PubMed] [Google Scholar]
- Ribeiro‐da‐Silva A, Hokfelt T (2000). Neuroanatomical localisation of Substance P in the CNS and sensory neurons. Neuropeptides 34: 256–271. [DOI] [PubMed] [Google Scholar]
- Roberts GW, Woodhams PL, Polak JM, Crow TJ (1982). Distribution of neuropeptides in the limbic system of the rat: the amygdaloid complex. Neuroscience 7: 99–131. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Gobbi G, Debs PC, Sibille ET, Blier P, Hen R et al. (2001). Genetic and pharmacological disruption of neurokinin 1 receptor function decreases anxiety‐related behaviors and increases serotonergic function. Proc Natl Acad Sci U S A 98: 1912–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank JR (2014). The neurokinin‐1 receptor in addictive processes. J Pharmacol Exp Ther 351: 2–8. [DOI] [PubMed] [Google Scholar]
- Schank JR, Ryabinin AE, Giardino WJ, Ciccocioppo R, Heilig M (2012). Stress‐related neuropeptides and addictive behaviors: beyond the usual suspects. Neuron 76: 192–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank JR, Pickens CL, Rowe KE, Cheng K, Thorsell A, Rice KC et al. (2011). Stress‐induced reinstatement of alcohol‐seeking in rats is selectively suppressed by the neurokinin 1 (NK1) antagonist L822429. Psychopharmacology (Berl) 218: 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank JR, King CE, Sun H, Cheng K, Rice KC, Heilig M et al. (2014). The role of the neurokinin‐1 receptor in stress‐induced reinstatement of alcohol and cocaine seeking. Neuropsychopharmacology 39: 1093–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank JR, Tapocik JD, Barbier E, Damadzic R, Eskay RL, Sun H et al. (2013). Tacr1 gene variation and neurokinin 1 receptor expression is associated with antagonist efficacy in genetically selected alcohol‐preferring rats. Biol Psychiatry 73: 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RA, Duker EM, Pahnke U, Wuttke W (1987). Stress‐induced changes in cholecystokinin and substance P concentrations in discrete regions of the rat hypothalamus. Neuroendocrinology 46: 75–81. [DOI] [PubMed] [Google Scholar]
- Singewald N, Chicchi GG, Thurner CC, Tsao KL, Spetea M, Schmidhammer H et al. (2008). Modulation of basal and stress‐induced amygdaloid substance P release by the potent and selective NK1 receptor antagonist L‐822429. J Neurochem 106: 2476–2488. [DOI] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Nielsen CK, Holgate J, Bito‐Onon JJ, Bartlett SE (2010). The neurokinin 1 receptor antagonist, ezlopitant, reduces appetitive responding for sucrose and ethanol. PLoS One 5: e12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsell A, Schank JR, Singley E, Hunt SP, Heilig M (2010). Neurokinin‐1 receptors (NK1R:s), alcohol consumption, and alcohol reward in mice. Psychopharmacology (Berl) 209: 103–111. [DOI] [PubMed] [Google Scholar]
- Ubaldi M, Bifone A, Ciccocioppo R (2013). Translational approach to develop novel medications on alcohol addiction: focus on neuropeptides. Curr Opin Neurobiol 23: 684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umhau JC, Schwandt ML, Usala J, Geyer C, Singley E, George DT et al. (2011). Pharmacologically induced alcohol craving in treatment seeking alcoholics correlates with alcoholism severity, but is insensitive to acamprosate. Neuropsychopharmacology 36: 1178–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N (2004). Drug dependence and addiction, III: Expectation and brain function in drug abuse. A J Psychiatry 161: 621. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Effect of intracerebroventricular injection of L822429 (0.0, 7.5, 15.0 µg/rat) on alcohol self‐administration in msP rats under FR‐1 schedule of reinforcement. Data are the mean ± SEM of total number of rewards earned in 30 min (N = 8). L822429 did not reduce the number of rewards at these doses. No significant differences were found between the treatment groups. Vehicle‐treated group: white bar; drug‐treated groups: black bars.
Figure S2 Microinjections into the Medial Amygdala were performed within the highlighted area. Taking bregma as reference point, the following coordinates were used in accordance with the atlas of Paxinos and Watson, 1998: Antero‐Posterior = 2.7 mm; Medio‐Lateral = 3.4 mm; Dorso‐Ventral = 9.0 mm.
Supporting info item


