Abstract
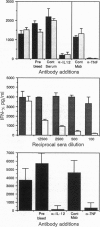
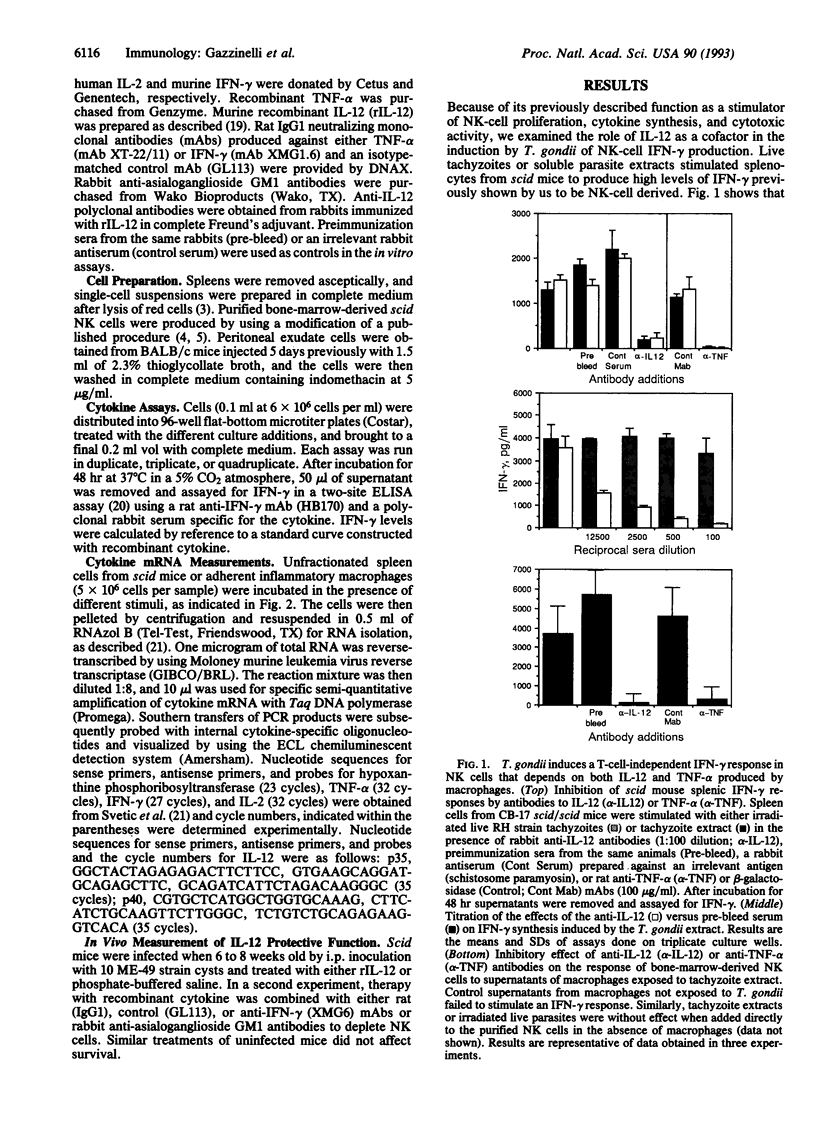
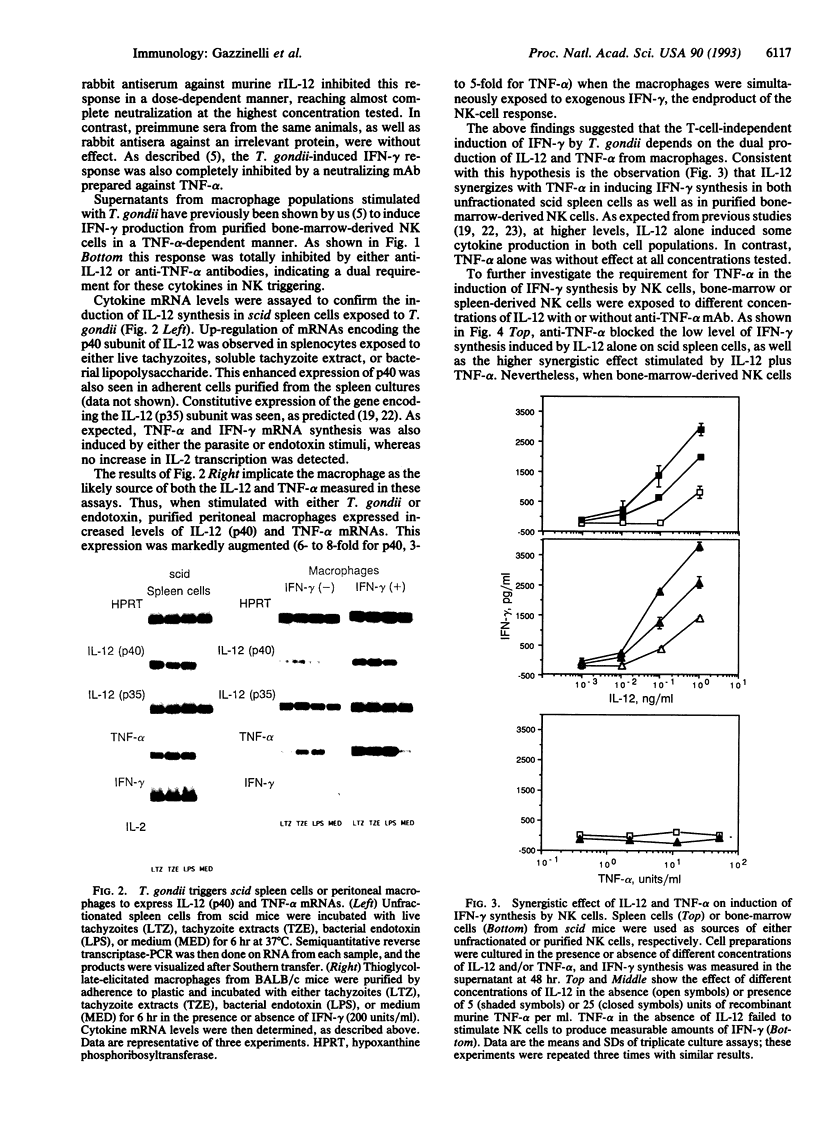
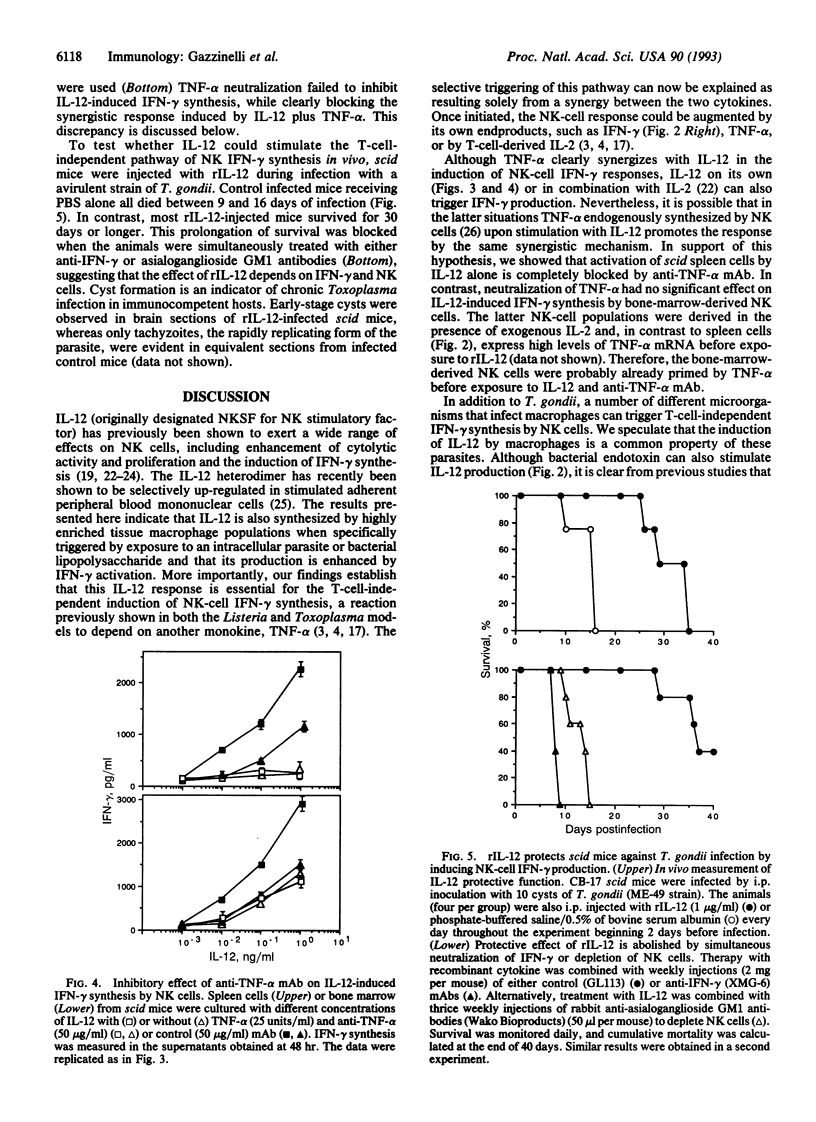
Immunity against the intracellular protozoan Toxoplasma gondii is highly dependent on interferon gamma (IFN-gamma). We have previously shown that, in addition to T lymphocytes, natural killer (NK) cells can be stimulated by the parasite to produce this cytokine by a reaction requiring adherent accessory cells and tumor necrosis factor alpha. We now demonstrate that a recently characterized cytokine, interleukin 12 (IL-12), is also necessary for parasite-induced IFN-gamma synthesis by NK cells. Anti-IL-12 antibodies completely inhibited T. gondii or bacterial endotoxin-stimulated IFN-gamma production by NK-enriched spleen cells from severe combined immunodeficient mice. Moreover, potent NK cytokine responses were induced by the combination of IL-12 and tumor necrosis factor alpha. In addition, adherent spleen cells from scid/scid mice or thyoglycollate-elicited macrophages from BALB/c animals produced high levels of both IL-12 (p40) and tumor necrosis factor alpha mRNAs when exposed to either live tachyzoites, parasite extracts, or endotoxin, confirming that these cytokines are produced by accessory cells. Finally, in vivo studies showed that treatment with recombinant IL-12 results in prolonged survival of scid mice after infection with T. gondii by means of a response dependent on both IFN-gamma and NK cells. Together the data argue that IL-12 is required for the T-cell-independent triggering of NK cells by intracellular parasites and that the cytokine may be useful for inducing this protective pathway in immunodeficient hosts.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bancroft G. J., Schreiber R. D., Unanue E. R. Natural immunity: a T-cell-independent pathway of macrophage activation, defined in the scid mouse. Immunol Rev. 1991 Dec;124:5–24. doi: 10.1111/j.1600-065x.1991.tb00613.x. [DOI] [PubMed] [Google Scholar]
- Bancroft G. J., Sheehan K. C., Schreiber R. D., Unanue E. R. Tumor necrosis factor is involved in the T cell-independent pathway of macrophage activation in scid mice. J Immunol. 1989 Jul 1;143(1):127–130. [PubMed] [Google Scholar]
- Chan S. H., Kobayashi M., Santoli D., Perussia B., Trinchieri G. Mechanisms of IFN-gamma induction by natural killer cell stimulatory factor (NKSF/IL-12). Role of transcription and mRNA stability in the synergistic interaction between NKSF and IL-2. J Immunol. 1992 Jan 1;148(1):92–98. [PubMed] [Google Scholar]
- Chan S. H., Perussia B., Gupta J. W., Kobayashi M., Pospísil M., Young H. A., Wolf S. F., Young D., Clark S. C., Trinchieri G. Induction of interferon gamma production by natural killer cell stimulatory factor: characterization of the responder cells and synergy with other inducers. J Exp Med. 1991 Apr 1;173(4):869–879. doi: 10.1084/jem.173.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chehimi J., Starr S. E., Frank I., Rengaraju M., Jackson S. J., Llanes C., Kobayashi M., Perussia B., Young D., Nickbarg E. Natural killer (NK) cell stimulatory factor increases the cytotoxic activity of NK cells from both healthy donors and human immunodeficiency virus-infected patients. J Exp Med. 1992 Mar 1;175(3):789–796. doi: 10.1084/jem.175.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen A. W., Overdulve J. P., Hoenderboom J. M. Separation of Isospora (Toxoplasma) gondii cysts and cystozoites from mouse brain tissue by continuous density-gradient centrifugation. Parasitology. 1981 Aug;83(Pt 1):103–108. doi: 10.1017/s0031182000050071. [DOI] [PubMed] [Google Scholar]
- D'Andrea A., Rengaraju M., Valiante N. M., Chehimi J., Kubin M., Aste M., Chan S. H., Kobayashi M., Young D., Nickbarg E. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J Exp Med. 1992 Nov 1;176(5):1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel J. K. Pathophysiology of toxoplasmosis. Parasitol Today. 1988 Oct;4(10):273–278. doi: 10.1016/0169-4758(88)90018-x. [DOI] [PubMed] [Google Scholar]
- Gajewski T. F., Joyce J., Fitch F. W. Antiproliferative effect of IFN-gamma in immune regulation. III. Differential selection of TH1 and TH2 murine helper T lymphocyte clones using recombinant IL-2 and recombinant IFN-gamma. J Immunol. 1989 Jul 1;143(1):15–22. [PubMed] [Google Scholar]
- Gazzinelli R. T., Hakim F. T., Hieny S., Shearer G. M., Sher A. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J Immunol. 1991 Jan 1;146(1):286–292. [PubMed] [Google Scholar]
- Gazzinelli R., Xu Y., Hieny S., Cheever A., Sher A. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J Immunol. 1992 Jul 1;149(1):175–180. [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Lambert L. H., Jr, Remington J. S. Resistance to murine tumors conferred by chronic infection with intracellular protozoa, Toxoplasma gondii and Besnoitia jellisoni. J Infect Dis. 1971 Dec;124(6):587–592. doi: 10.1093/infdis/124.6.587. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Fitz L., Ryan M., Hewick R. M., Clark S. C., Chan S., Loudon R., Sherman F., Perussia B., Trinchieri G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989 Sep 1;170(3):827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft B. J., Brooks R. G., Conley F. K., McCabe R. E., Remington J. S. Toxoplasmic encephalitis in patients with acquired immune deficiency syndrome. JAMA. 1984 Aug 17;252(7):913–917. [PubMed] [Google Scholar]
- Mahmoud A. A., Warren K. S., Strickland G. T. Acquired resistance to infection with Schistosoma mansoni induced by Toxoplasma gondii. Nature. 1976 Sep 2;263(5572):56–57. doi: 10.1038/263056a0. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Fong T. A. Specific assays for cytokine production by T cells. J Immunol Methods. 1989 Jan 17;116(2):151–158. doi: 10.1016/0022-1759(89)90198-1. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Hillman J. K., Rubin B. Y., Kelly C. D., Jacobs J. L., Tyler L. W., Donelly D. M., Carriero S. M., Godbold J. H., Roberts R. B. Patients at risk for AIDS-related opportunistic infections. Clinical manifestations and impaired gamma interferon production. N Engl J Med. 1985 Dec 12;313(24):1504–1510. doi: 10.1056/NEJM198512123132403. [DOI] [PubMed] [Google Scholar]
- Romagnani S. Induction of TH1 and TH2 responses: a key role for the 'natural' immune response? Immunol Today. 1992 Oct;13(10):379–381. doi: 10.1016/0167-5699(92)90083-J. [DOI] [PubMed] [Google Scholar]
- Ruskin J., Remington J. S. Immunity and intracellular infection: resistance to bacteria in mice infected with a protozoan. Science. 1968 Apr 5;160(3823):72–74. doi: 10.1126/science.160.3823.72. [DOI] [PubMed] [Google Scholar]
- Schoenhaut D. S., Chua A. O., Wolitzky A. G., Quinn P. M., Dwyer C. M., McComas W., Familletti P. C., Gately M. K., Gubler U. Cloning and expression of murine IL-12. J Immunol. 1992 Jun 1;148(11):3433–3440. [PubMed] [Google Scholar]
- Sher A., Oswald I. P., Hieny S., Gazzinelli R. T. Toxoplasma gondii induces a T-independent IFN-gamma response in natural killer cells that requires both adherent accessory cells and tumor necrosis factor-alpha. J Immunol. 1993 May 1;150(9):3982–3989. [PubMed] [Google Scholar]
- Suzuki Y., Orellana M. A., Schreiber R. D., Remington J. S. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988 Apr 22;240(4851):516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Remington J. S. The effect of anti-IFN-gamma antibody on the protective effect of Lyt-2+ immune T cells against toxoplasmosis in mice. J Immunol. 1990 Mar 1;144(5):1954–1956. [PubMed] [Google Scholar]
- Svetić A., Finkelman F. D., Jian Y. C., Dieffenbach C. W., Scott D. E., McCarthy K. F., Steinberg A. D., Gause W. C. Cytokine gene expression after in vivo primary immunization with goat antibody to mouse IgD antibody. J Immunol. 1991 Oct 1;147(7):2391–2397. [PubMed] [Google Scholar]
- Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry J. C., Schreiber R. D., Unanue E. R. Regulation of gamma interferon production by natural killer cells in scid mice: roles of tumor necrosis factor and bacterial stimuli. Infect Immun. 1991 May;59(5):1709–1715. doi: 10.1128/iai.59.5.1709-1715.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]