Abstract
Intravenous (IV) iron therapy is widely used in iron deficiency anaemias when oral iron is not tolerated or ineffective. Administration of IV‐iron is considered a safe procedure, but severe hypersensitivity reactions (HSRs) can occur at a very low frequency. Recently, new guidelines have been published by the European Medicines Agency with the intention of making IV‐iron therapy safer; however, the current protocols are still non‐specific, non‐evidence‐based empirical measures which neglect the fact that the majority of IV‐iron reactions are not IgE‐mediated anaphylactic reactions. The field would benefit from new specific and effective methods for the prevention and treatment of these HSRs, and the main goal of this review was to highlight a possible new approach based on the assumption that IV‐iron reactions represent complement activation‐related pseudo‐allergy (CARPA), at least in part. The review compares the features of IV‐iron reactions to those of immune and non‐immune HSRs caused by a variety of other infused drugs and thus make indirect inferences on IV‐iron reactions. The process of comparison highlights many unresolved issues in allergy research, such as the unsettled terminology, multiple redundant classifications and a lack of validated animal models and lege artis clinical studies. Facts and arguments are listed in support of the involvement of CARPA in IV‐iron reactions, and the review addresses the mechanism of low reactogenic administration protocols (LRPs) based on slow infusion. It is suggested that consideration of CARPA and the use of LRPs might lead to useful new additions to the management of high‐risk IV‐iron patients.
Abbreviations
- ADRs
adverse drug reactions
- API
active pharmaceutical ingredient
- CARPA
complement activation‐related pseudo‐allergy
- CHMP
Committee for Medicinal Products for Human Use
- EMA
European Medicinal Agency
- HMW‐ID
high molecular weight iron dextran
- HSRs
hypersensitivity reactions
- ID
intravenous dextran
- IV‐iron
intravenous iron
- LMW‐ID
low molecular weight iron dextran
- LRP
low reactogenic administration protocol
- Mab
monoclonal antibody
- MBL
mannose binding lectin
- MRI
magnetic resonance imaging
- SPIO
superparamagnetic iron oxide
- WAO
World Allergy Organisation
Tables of Links
| TARGETS |
|---|
| GPCRs |
| ATR, anaphylatoxin receptor |
| C3aR |
| C5aR |
| LIGANDS |
|---|
| C3a |
| C5a |
| Doxorubicin |
| Paracetamol (acetaminophen) |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013).
Introduction
Roughly 25% of all adverse drug reactions (ADRs) are hypersensitivity reactions (HSRs), affecting up to some half‐million patients in the USA every year (Lazarou et al., 1998). Such reactions can be caused by almost all types of drugs, irrespective of their chemical composition, complexity or route of administration. Because of recent public concern about the HSRs caused by one of such reactogenic drugs, namely intravenous iron (IV‐iron) compounds (European Medicines Agency– Committee for Medicinal Products for Human Use (EMA‐CHMP), 2013), this review focuses on the HSRs caused by these preparations.
Parenteral iron (IV‐iron) has become an important treatment for iron deficiency anaemia in a wide range of therapeutic areas, when oral iron is inappropriate, ineffective or not tolerated. A recent safety review performed by the EMA‐CHMP concluded that all available IV‐iron preparations on the European market have a positive risk‐benefit ratio and low risk of causing HSRs. Nevertheless, in order to minimize the risk of life‐threatening reactions, new recommendations were introduced for all health care professionals who provide treatment with IV‐iron. The document (EMA‐CHMP, 2013) calls for changes in the practice of IV‐iron infusions (Table 1) and for uniform reporting of HSRs (Ring and Messmer, 1977; Ring et al., 2010).
Table 1.
New recommendations by the EMA Committee for Medicinal Products for Human Use (CHMP) to manage risk of allergic reactions with IV‐iron (EMA‐CHMP, 2013)
| 1. All prescribers should inform patients of the risk and seriousness of a hypersensitivity reaction and the importance of seeking medical attention if a reaction occurs. |
| 2. Patients need to be closely observed for any allergic reactions during and for at least 30 min after IV‐iron injection. |
| 3. IV‐iron preparations should only be administered |
| A. by staff trained to evaluate and manage anaphylactic and anaphylactoid reactions, |
| B. in an environment where resuscitation facilities are available so that the patient can be treated immediately. |
| 4. The current practice of first giving the patient a small test dose is not a reliable way to predict how the patient will respond when the full dose is given. A test dose is therefore no longer recommended, but instead, caution is warranted with every dose of IV‐iron that is given, even if previous administrations have been well tolerated. |
| 5. In case of hypersensitivity reactions, healthcare professionals should immediately stop the iron administration and consider appropriate treatment for the hypersensitivity reaction. |
| 6. IV‐iron medicines should not be used during pregnancy unless clearly necessary. Treatment should be confined to the second or third trimester, provided the benefits of treatment outweigh the risk to the unborn baby. |
However, the field would also benefit from scientific evidence‐based introduction of more specific and effective measures than the currently applied common empirical treatments. To achieve this, the mechanisms of IV‐iron reactions should be better understood, and this challenge represents the main thrust of the present review. Our approach is to highlight the similarities and differences among iron‐induced and other IV drug‐induced HSRs that might give clues regarding the mechanisms and hence make possible new methods of prevention and treatment of reactions to IV‐iron. The comparison focuses on the symptoms of reactions categorized by their various properties. The process of comparison entails addressing some critical unsolved issues in the field, such as the incoherent terminology and lack of credible clinical data. A summary of current premedication and treatment options alludes to the inappropriateness of using antihistamines, because they mimic some of the features of HSRs, and provides rationale for the testing of low reactogenic administration protocols (LRPs), such as low‐dose slow priming followed by slow dose escalation. One particular new perspective outlined for the first time in this review is the possible causal role of complement activation in IV‐iron reactions, a proposal which has both conceptual and therapeutic implications.
Features of HSRs to IV‐iron compared with those to other IV drugs
Figure 1 lists the symptoms and various features of HSRs caused by IV drugs, including IV‐iron, by which the symptoms have been categorized: organ systems affected, severity, kinetics, prevalence, duration, mechanism and tolerance induction. Changes of the variables in these categories depend on the reactogenicity of the drugs involved, the method of their administration and the patient's sensitivity. A closer analysis of these variables reveals both similarities and major differences between the symptoms of reactions to IV‐iron and to other IV drugs, as detailed below.
Figure 1.
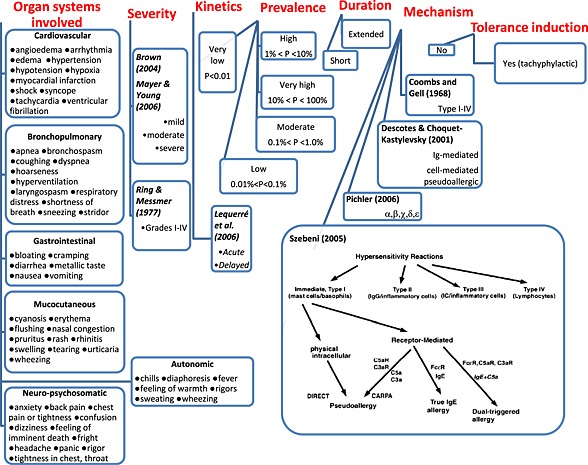
Hypersensitivity reaction parameters and their different classifications.
The symptoms and their organ expression do not seem to differ between IV‐iron and other drugs, at least on the basis that almost all listed symptoms and all mentioned organ systems have been cited for HSRs to both IV‐iron and non‐iron drugs. Nevertheless, because the active pharmaceutical ingredients usually have an effect on the physicochemical features of the drug and hence on its immune reactivity, it is not impossible that a head‐to‐head comparison would reveal specific effects of iron on HSR symptoms. At this time, however, we are not aware of such quantitative data in the literature.
For the description of the severity of symptoms, the best known classification of HSRs was described by Ring and Messmer (Ring and Messmer, 1977; Ring et al., 2010), who graded the symptoms in categories I–IV. This classification, which should not be confused with Gell and Coombs' types I–IV HSRs, which was recommended by the EMA (EMA, 2012) for ADR reporting, despite the existence of a simpler (Brown, 2004) and a more recent symptom‐based grading systems (Mayer and Young, 2006). Irrespective of how HSRs are quantified, from mild to severe, life‐threatening reactions can occur with either IV‐iron formulation or other IV drugs. Severity does not differentiate between iron and non‐iron reactions. Likewise, iron and non‐iron HSRs can be acute, short, delayed and extended. Thus, the kinetics and duration of symptoms do not provide distinguishing features.
Prevalence is the feature of HSRs that does radically differ between reactogenic drugs. When expressed as the percentage of patients displaying HSRs among all those treated, the range spans 5 orders of magnitude (0.001–70%). To enable the comparison of prevalence statistics within such wide limits, we propose clustering the % values into five groups (very high, high, moderate, low and very low) with numerical ranges coinciding with the orders of magnitude in the 10−3–102 range (third column in Figure 2).
Figure 2.

Scheme illustrating the anaphylatoxin concept of HSRs. Iron and other nanoparticles activate the complement system (upper red arrow) that leads to the formation of anaphylatoxins. Their blood level is determined by generation via this activation process, and by consumption, due partly to cellular uptake and partly to metabolism by carboxypeptidases (green). The level in blood determines whether or not anaphylatoxins trigger mast cells for release reaction (lower red arrow). A scheme of complement activation (left insert) and the main vasoactive mediators released from mast cells are also shown.
Table 2 categorizes the main drug types according to HSR prevalence. It turns out from the compilation that the highest rate of HSRs occur with certain monoclonal antibodies (mAbs), liposomal drugs and anticancer agents (taxanes), which are delivered in micellar solvents, such as Cremophor EL. The frequency of HSRs to penicillin, the textbook example of drug‐induced allergic reactions, falls at the moderate/high borderline (1%). On the other hand, the prevalence of HSRs to different IV‐iron preparations is very low, a feature shared only with the safest radiocontrast agents (Table 2). In fact, the vast majority of IV‐iron administrations in clinical practice occur with no or minor adverse events. The heightened public and regulatory concern about these reactions may therefore be considered to be controversial.
Table 2.
Frequency of HSRsa in patients treated IV with different reactogenic drugs
| Reaction rate | Drugs | Drug Type |
|---|---|---|
| Very high P > 10% | Rituximab, infliximab | mAb |
| Doxil (Caelyx), AmB (AmBisome) | Liposome‐encapsulated | |
| Taxanes (paclitaxel, docetaxel), platinum | Micellarized anticancer | |
| High 1% < P <10% | Natalizumab, cetuximab, trastuzumab, panitumumab, gentuzumab | mAb |
| Amphotec, Myocet, Amphocyl, DaunoXome, Abelcet, Visudyne | Liposome | |
| Penicillin | Antibiotic | |
| Platinum compounds (cisplatin, carboplatin), | Anticancer drugs | |
| Moderate 0.1 < P % <1 | Omalizumab | Monoclonal antibodies (mAbs) |
| Alemtuzumab | ||
| Trastuzumab | ||
| Cephalosporins/carbapenems, aztreonam, imipenem | Antibiotics | |
| Iodinated contrast agents (Ioxaglate, Iohexol, Iopamidol, Ioversol, Iopromide, Ioxilan) | Radiocontrast agents | |
| low 0.01 < P % <0.1 | Bevacizumab | mAb |
| Epipodophyllotoxins (teniposide, etoposide), asparaginase, procarbazine, doxorubicin, 6‐mercaptopurine | Anticancer drugs | |
| Acetaminophen (paracetamol), aspirin, ibuprofen | Anaesthetics, analgesics antalgics, antipyretics and non‐steroidal anti‐inflammatory drugs | |
| Phenytoin, carbamazepine phenobarbital sodium Lamotrigine, primidone diphenylhydantoin, sulfonamides (procainamide), sulfonylureas | Anticonvulsants (antiepileptics) | |
| Iodinated contrast agents (ioxaglate, iohexol, iopamidol, ioversol, iopromide, ioxilan, iodixanol, Gd‐GTPA | Contrast agents | |
| Venofer, Cosmofer, Ferinject, Monofer, Ferrlecit, Ferumoxytol | IV‐ironb | |
| Very low P < 0.01 | SonoVue | Contrast agents |
| Venofer, Cosmofer, Ferinject, Monofer, Ferrlecit, Ferumoxytol | IV‐ironb |
All types of reactions regardless of severity. Rates were obtained from individual box labels, public (internet) information or Summaries of Product Characteristics.
Data uncertain to select the exact category, P = prevalence.
Despite the very low prevalence of IV‐iron reactions, minor but significant differences between the risk rates might have major clinical implications. Analyses of commercial and public databases (e.g. post‐marketing surveillance data and voluntary submission of ADR reports) have led some authors to claim differences in the safety profiles of currently available IV‐iron products (Chertow et al., 2004; Bailie et al., 2005; Chertow et al., 2006; Bailie et al., 2011; Bailie, 2012; Bailie and Verhoef, 2012). These reports were recently challenged by the EMA document (EMA‐CHMP, 2013) and a recent review by Bircher and Auerbach (Bircher and Auerbach, 2014) that concluded that insufficient reliable data are available to support this conclusion. There are many reasons for this uncertainty, such as the confusing terminology and classification of HSRs, under‐reporting or differential reporting of IV‐iron reactions, absence of brand names and a lack of accurate or uniform denominator information (i.e. whether the HSR rate is given per infusion, per patient, per time or per all ADRs) (Wysowski et al., 2010). Moreover, randomized clinical trials are not powered to compare very rare clinical events (Black, 1996). Critchly and Dunbar (2007) concluded that a study to compare the adverse event rate for two IV‐iron compounds would need about 6600 patients to achieve the necessary statistical power. Nonetheless, a relatively higher rate and generally more severe HSRs were observed with high molecular weight iron‐dextran compared with the low molecular weight formulation (Fletes et al., 2001; Chertow et al., 2004). Since that time, both high molecular weight‐dextrans (Imferon® and Dexferrum®) have been removed from the market by the marketing authorisation holders in USA. The problems with the terminology and classification of HSRs will be discussed in more detail later.
Mechanisms of HSRs to IV‐iron versus those to other IV drugs
The mechanisms of HSRs are categorized in many different ways, four of which are shown in Figure 1. The oldest and best known is the classical scheme of Gell and Coombs, which distinguishes four types of HSRs, Types I–IV (Coombs and Gell, 1968). The system is based on pathophysiological principles and has been criticized (Descotes and Choquet‐Kastylevsky, 2001; Rajan, 2003) on the basis that the adverse immune effects of drugs occur mostly via complex mechanisms, which cannot be cleanly fitted into Gell and Coomb's categories. One important example is direct complement activation causing type I reactions, because these reactions are defined by Gell and Coombs as being solely IgE‐mediated.
Among the alternative classifications of HSR mechanisms, Descotes proposed three categories: immunoglobulin‐mediated, cell‐mediated and pseudoallergic (Descotes and Choquet‐Kastylevsky, 2001). Pichler labelled his five categories with Greek letters: α‐type reactions involve cytokine release, β‐type reactions are immune reactions against biological agents, γ‐type reactions are immune or cytokine imbalance syndromes, δ‐type reactions arise because of cross‐reactivity, and ε‐type reactions do not directly involve the immune system (Pichler, 2006). The last mechanistic scheme was proposed by one of us (Szebeni, 2005), in which the Gell and Coombs' type I reactions were subdivided according to the mechanism of mast cell (and basophil) activation. The scheme suggests a distinction between direct and receptor‐mediated stimulation of mast cells and basophils, with the latter arm subdivided to (1) FcεR, (2) anaphylatoxin receptor (ATR, C5aR and C3aR) and (3) both FcεR and ATR‐mediated mixed responses. The IgE‐R‐mediated HSRs are the classical type I reactions, while the ATR‐mediated release reactions underlie complement activation‐related pseudoallergy (CARPA) (Szebeni, 2005), which can occur without the presence of any specific antibodies or immune competent cells.
As for mechanistic distinction between IV‐iron reactions and other IV‐drug‐induced reactions, the weight of evidence suggests that IV‐iron reactions are not IgE‐mediated (Fleming et al., 1992; Novey et al., 1994). However, other than this conclusion, there is insufficient information to prove any other ‘immune’ mechanism. Among the non‐immune mechanisms, there are several lines of indirect evidence suggesting that CARPA may play a causal role in IV‐iron reactions. This hypothesis will be described in substantial detail later in this review.
Risk factors of HSRs to IV‐iron versus other IV drugs
The risk factors for IV‐iron reactions include genetic predisposition, general nonspecific factors and unique temporary conditions. Among the genetic predispositions, atopic constitution is the best recognized, in which the patients have an innate proneness for asthma or allergy to drugs, pollens and other allergens (Enright et al., 1989; Goss et al., 1995; Brannagan, 2002; Laman et al., 2005; Hong et al., 2012; Kelsall et al., 2012; Auerbach et al., 1998, 2011; Fletes et al., 2001; Fishbane, 2003). The acquired lasting risk factors include, among others, old age, concurrent or past cardiovascular disease, autoimmune diseases and mastocytosis (Ansell et al., 1980; Shehadi, 1982; Goss et al., 1995; Simons et al., 2011), while the acquired temporary risk factors are exemplified by infectious diseases, certain medications [e.g. β‐adrenoceptor blockers, ACE inhibitors (Goss et al., 1995; Simons et al., 2011) and even psychological distress (Lalli, 1974; Misbah and Chapel, 1993]. We are not aware of significant differences between IV‐iron and other IV drugs in relation to the risk factors mentioned above.
Unsolved issues in clinical research on HSRs to IV‐iron and other IV drugs
As pointed out by Auerbach et al., there is an unmet medical need for a uniform and commonly accepted definition of adverse events to iron compounds, the absence of which at present is a major reason for the statement that ‘reliable comparative safety data do not exist’ (Auerbach and Ballard, 2010). In fact, one major problem in clinical HSR research is its complex and sometimes confusing terminology.
There is a long list of different descriptive, qualitative and quantitative terms for essentially the same acute illness (abnormal immune stimulation), leading the World Allergy Organization to a Nomenclature Review conference in 2004 (Johansson et al., 2004). Subsequently, the list of terms has expanded (Table 3), among which the use of ‘immunological’ and ‘non‐immunological’ HSRs to differentiate between IgE‐mediated reactions from all others is particularly questionable, because the involvement of IgE or other specific antibodies in HSRs often cannot be proved or has not even been tested (Johansson et al., 2004; Kemp et al., 2008). This problem applies to all HSRs, including IV‐iron reactions.
Table 3.
Terms used for different hypersensitivity reactions
| Term | Type | Definition | Mechanism | Severity |
|---|---|---|---|---|
| Drug allergy | Descriptive | Hypersensitivity to drugs | Any | Mild‐to‐severe |
| Infusion reaction | Descriptive | HSR arising as a consequence of infusion | Any | Mild‐to‐severe |
| Idiosyncratic reaction | Descriptive | HSR without known reason | Any | Mild‐to‐severe |
| Anaphylaxis | Quantitative | Severe, life‐threatening generalized or systemic HSR | Any | Severe |
| Pseudoallergy | Qualitative | Systemic and/or local signs of HSR | Any mechanism with no role specific IgE | Mild‐to‐severe |
| Non‐allergic hypersensitivity | Qualitative | Systemic and/or local signs of HSR | Any mechanism with no role specific IgE | Mild‐to‐severe |
| Non‐immune hypersensitivity | Qualitative | Systemic and/or local signs of HSR | Any mechanism with no role specific IgE | Mild‐to‐severe |
| Complement‐activation‐related pseudoallergy (CARPA) | Qualitative | Systemic and/or local signs of HSR | Complement activation is involved directly or indirectly | Mild‐to‐severe |
| Immunological anaphylaxis | Qualitative and quantitative | mediated by specific IgE | Severe | |
| Non‐immunological anaphylaxis | Qualitative and quantitative | Not mediated by specific IgE | Severe | |
| Anaphylactoid reaction | Qualitative and quantitative | Not mediated by specific IgE | Mild‐to‐severe | |
| Type B adverse drug reaction | Qualitative and quantitative | Drug dose‐independent systemic and/or local signs of HSRa | Any | Severe |
Scott and Thompson (2014).
In addition to the ambiguous terminology, anaphylaxis research is hindered by two other factors: (1) the rarity of severe reactions, making it difficult, if not impossible, to set up comparative trials according to the principles of evidence‐based medicine, and (2) the lack of animal models, which mimic the symptoms of human HSRs. As for the first problem, instead of Phase I toxicity/safety‐evaluation followed by randomized, placebo‐controlled clinical trials, clinical evidence in the field of anaphylaxis is mostly based on retrospective analyses of large databases and medical records, which can seldom answer specific, prospective questions regarding safety or efficacy.
The problem of animal models of anaphylaxis lies in their divergence from humans in their sensitivity to different reactogenic drugs, and in the frequency of occurrence and symptoms of reactions. For these reasons there is no validated animal model of human HSRs provoked either by drugs, or other causes, for example foods, insect stings, surgery and exercise. Nevertheless, animal models are useful for the study of some common steps in anaphylaxis at the cellular and subcellular level, for example mast cell degranulation (Nauta et al., 2008).
One aspect of progress in this field that awaits professional and regulatory recognition is the use of pig models of CARPA, because pigs, unlike rats and mice, resemble humans in their haemodynamical, haematological, biochemical and skin responses to many nanomedicines (liposomes, micellar drugs, radiocontrast agents, polymers, antibodies, protein drugs and enzymes) with unique sensitivity and reproducibility (2007, 2006, 2000, 2012a, 2012b, 2012c, 1999; Szebeni, 2014; Merkel et al., 2011; Dézsi et al., 2014). It is this model that presents both tachyphylactic and non‐tachyphylactic reactions and therefore allows prediction of the efficacy of slow infusion and prophylactic desensitization with drug carriers (such as empty liposomes) (2012a, 2012b, 2012c, 1999, 2011; Dézsi et al., 2014). Whether IV‐iron nanoparticles will trigger CARPA in pigs awaits experimental exploration.
Current prevention and treatment of HSRs to IV‐iron and other IV drugs
The prevention and treatment options for HSRs to IV‐iron and other IV drugs differ according to the severity and prevalence of HSRs. For reaction prevention, there are two options: premedication and the use of LRPs.
The premedication regimes usually include corticosteroids and antihistamines, along with a variety of additional agents, including paracetamol (acetaminophen). However, the efficacy of premedication to prevent mild and moderate HSRs has been queried, and many authors believe that premedication for all patients are not justified and that premedication should be reserved only for patients at increased risk (Tramer et al., 2006). Among other causes of scepticism, the benefit of antihistamines has been questioned on the basis that they increase the frequency of ADRs (Lorenz and Doenicke, 1985; Baller and Huchzermeyer, 1989; Wasserman et al., 2004) and that the symptoms they produce can mimic those of a mild HSR (Auerbach and Ballard, 2010). Paracetamol is as effective alone as it is given together with antihistamines (Keshavarzian et al., 2007). Taking this evidence together, we conclude that there is now no place for the use of antihistamines for the prevention or treatment of HSRs to IV‐iron.
The use of LRPs is the other potential safety measure to prevent HSRs to IV drugs. Two groups of IV medicines were shown to benefit most from this approach: therapeutic mAbs and liposomal drugs. LRP for mAbs was introduced by Puchner et al. (Puchner et al., 2001) to prevent HSRs to infliximab. Their protocol involved an 11‐step progressive dose escalation over 4 h, a technique which was later applied for other antineoplastic mAbs including rituximab, cetuximab and trastuzumab (Duburque et al., 2006; Castells, 2008; Castells et al., 2008; Brennan et al., 2009). The infusion rates and timing of escalation steps were different in the various protocols, and they were often combined with anti‐allergic pre‐medications. What is common to all LRPs is that the infusion is started at a very low rate carrying 0.001 to 0.01% of the full drug dose in 5–15 min. This priming may serve two functions: (1) desensitization of the patient to the drug and (2) alarming for the presence of hypersensitivity.
The use of LRP to prevent liposome reactions was first applied for the infusion of doxorubicin‐HCl liposomes (Doxil); Gabizon and Muggia, 1998). The liposome infusion was initially administered at a rate of 1 mg doxorubicin min−1, and if no reactions occurred, the rate was increased to complete the IV therapy over 1 h. This method remains the recommended protocol for Doxil administration today (Doxil prescribing information, 2014).
LRP has also been applied successfully for the prevention of IV‐iron reaction in high‐risk patients, with a history of life‐threatening reactions (Altman and Petersen, 1988; Monaghan et al., 1994; Hickman et al., 2000). However, because HSRs to IV‐iron occur at a low, or very low rate, such an approach may not be needed in the average, risk factor‐free patient.
The treatment options for HSRs to IV drugs and iron, because they are therapeutic rather than preventive, are also referred to as ‘reactive’ (Lequerre et al., 2006; Mayer and Young, 2006).
As summarized in Table 4, these options also depend on the severity of reactions and are identical regardless of the cause of HSR.
Table 4.
Reactive treatment options to manage HSRs to intravenous drugs including IV‐iron (Rampton et al., 2014)
| Symptoms | Treatment options | |
|---|---|---|
| Mild HSRs | Itching, urticaria, flushing, sensation of heat, slight chest tightness, hypertension and back/joint pains | Stop infusion temporarily and watch symptoms and signs. If symptoms improve the infusion can be restarted cautiously. |
| Moderate HSRs | As in mild reaction + cough, chest tightness, nausea, shortness of breath, tachycardia and hypotension | Stop infusion and consider IV‐fluids and IV‐corticosteroids. |
| Severe HSRs = life‐threatening anaphylaxis | As in moderate + sudden onset and rapid aggravation of symptoms + wheezing, stridor, periorbital oedema, cyanosis, loss of consciousness and cardiac/respiratory arrest | As for moderate HSRs + IM or IV adrenaline (epinephrine) + consider β2‐adrenoceptor agonist inhaler, O2 by facemask, act according to local standard anaphylaxis guidelines. |
The treatment of very severe reactions, that is anaphylaxis, is special in that these events are very rare, and the medications applied are rarely ‘evidence‐based’. As stated earlier, it is impracticable to perform randomized controlled trials on clinical conditions that are very rare (Black, 1996), a conclusion which also applies to clinical investigations into the most effective treatment of severe anaphylaxis (Ring et al., 2010; Working Group of the Resuscitation Council, UK, 2013). In this context, Ranft and Kochs (2004) concluded that ‘None of the traditionally applied remedies against anaphylaxis – epinephrine and intravascular volume replacement, histamine receptor blockade, inhaled beta‐mimetics and steroids – have been proved efficacious by means of evidence‐based medicine – there is a lack of consensus as to the substantial elements of therapy’. Despite the methodological challenges, Ring et al. (2010) concluded that ‘Adrenaline (epinephrine) is the essential anti‐anaphylactic drug. Glucocorticoids are given in order to prevent a protracted or biphasic course of anaphylaxis; they are of little help in the acute treatment. H1 antagonists are valuable in mild anaphylactic reactions; they should be given intravenously if possible. The replacement of volume is crucial in anti‐anaphylactic treatment’. These recommendations are largely in agreement with most major guidelines for the treatment of life‐threatening anaphylaxis (Simons et al., 2011; Working Group of the Resuscitation Committee, 2013).
The anaphylatoxin concept of HSRs to IV‐iron and efficacy of LRPs
Perhaps the most important conclusion from the comparison of HSRs to IV‐iron and other IV drugs is that, other than prevalence, there are no major qualitative or quantitative differences between these reactions. Considering that the overwhelming majority (77%) of all HSRs are not IgE‐mediated (Demoly et al., 1999), it seems reasonable to assume that IV‐iron reactions could have the same ‘non‐immune’ underlying mechanism as the majority of other IV‐drugs. One of the non‐immune mechanisms that have received much attention recently is CARPA, which has been claimed to be a common cause for, or contributing factor to all acute HSRs provoked by any infusion that contains complement‐activating antibodies or nanoparticles.
That complement activation plays a causal role in the HSRs to rituximab, was shown by van der Kolk et al. (van der Kolk et al., 2001), while for liposomal preparations, including Doxil, several lines of evidence have accumulated over the past two decades (2014, 1994). For example, a clinical investigation showed that strong complement activation in patients with cancer infused with Doxil correlated with the severity and frequency of HSRs and that the rate of drug infusion was critical both in the risk of HSR and complement activation (Chanan‐Khan et al., 2003). The study could even accurately predict the upper threshold of safe initial infusion rate (0.38 mg doxorubicin min−1) on the basis of a significant correlation between initial infusion rate and in vivo production of SC5b‐9, an index of complement activation. Such measurements and calculations may be useful for the development of LRPs for other reactogenic drugs.
Earlier preclinical evidence for complement activation underlying the infusion rate‐dependence of HSRs was provided in a porcine model of liposome‐induced CARPA, wherein the speed of liposome infusion showed remarkable correlation with the rise of pulmonary arterial pressure (Szebeni et al., 2000), which, in turn, was shown to arise as a consequence of complement activation‐related anaphylatoxin production (Szebeni et al., 1999, 2000). These facts taken together suggest that the slow‐infusion‐based LRPs may be effective when complement activation is a major pathogenic factor for HSRs.
Among further in vivo evidence for CARPA as a common underlying mechanism of HSRs, it has been shown that HSRs, not only to liposomes, but also to polymers, dendrimers, carbon nanotubes and a wide range of other nanoparticles, are also complement activation‐related, and the symptoms are similar and closely mimic human HSRs and anaphylaxis (Szebeni, 2012). Studies on this phenomenon have established that the reactogenic complement‐activating nanoparticles are usually highly charged and/or coated by repetitive surface projections from polymers, carbohydrate, peptides, etc., that bind antigen or pattern recognition molecules (IgM, IgG, C1q, mannose binding lectin (MBL) and ficolin). Crystalline surfaces (shown for silica and paclitaxel; Szebeni et al., 2003) as well as 71% cholesterol‐containing liposomes, in which the cholesterol is partially crystallized (Szebeni et al., 2001; Baranyi et al., 2003), are also effective complement activators and inducers of HSR. Because all existing IV‐iron medicines consist of crystalline iron oxide/hydroxide nanoparticle cores (up to 10–20 nm) and a carbohydrate shell (from mannitol, dextran, gluconate sucrose, carboxymaltose, isomaltoside, etc.) (Fütterer et al., 2013), preconditions for complement activation via crystal and carbohydrate recognition molecules exist with all IV‐iron medicines. In addition, some of the iron–carbohydrate complexes can also agglomerate to form large clusters (up to 200 nm) (Fütterer et al., 2013), providing further surface for complement activation.
Although we are not aware of studies showing complement activation by reactogenic IV‐iron medicines, a recent study provided evidence for such activation by dextran‐coated superparamagnetic iron oxide (SPIO) nanoparticles used as a magnetic resonance imaging (MRI) contrast agent (Banda et al., 2014). The latter study also investigated the mechanism of complement activation in human and mouse serum and found evidence for prominent involvement of the lectin pathway (via MBL or L‐ficolin) and triggering of alternative amplification loop (Banda et al., 2014). Because the SPIO nanoparticles can also cause major HSRs in patients, and because the basic structure of all IV‐iron medicines and iron‐oxide core/dextran shell containing MRI contrast agents are very similar, it is highly likely that complement activation by IV‐iron can occur in man via the same or similar mechanism and may cause or contribute to the rare occurrence of non‐IgE‐mediated HSRs.
These facts and considerations, taken together with the reported success of slow infusion protocols in preventing life‐threatening reactions to IV‐iron (Altman and Petersen, 1988; Monaghan et al., 1994; Hickman et al., 2000) suggest that CARPA, the proposed mechanism of very frequent liposome and antibody and taxane reactions, also represents the most likely mechanism underlying the reactions to IV‐iron. The difference in prevalence might arise from differences in the extent of complement activation and/or the general sensitivity of patients for activation by these agents.
As for the chain of events leading from complement activation to HSRs, the anaphylatoxins C3a and C5a bind to mast cells (and basophil leukocytes and macrophages) via specific receptors on these cells and trigger the release of a great number of vasoactive mediators that cause the symptoms of HSRs listed in Figure 1.
Figure 2 illustrates the anaphylatoxin concept of HSRs caused by drug particles. In the rare patients developing these reactions, iron, or other nanoparticle‐based drugs, activate the complement system (upper red arrow) that leads to the formation of anaphylatoxins. Their blood level is determined by generation via this activation process, and by consumption, due partly to cellular uptake and partly to metabolism by carboxypeptidases (green) (Campbell et al., 2002). The level in blood determines whether or not anaphylatoxins trigger mast cells for release reaction (lower red arrow). A scheme of complement activation (left insert) and the main vasoactive mediators released from mast cells are also shown.
Figure 2 may also explain why a low speed of infusion is an effective way of preventing HSRs to all reactogenic drugs, including IV‐iron; it is hypothesized that the changes in anaphylatoxin equilibrium are fast, so that a slow infusion may keep the concentration of anaphylatoxins below the HSR threshold of allergy‐mediating cells (mast cells, basophils and certain macrophages), while massive and rapid exposure to a complement activator may tip the balance towards anaphylatoxin build‐up, and hence exceed the HSR threshold (Figure 2).
Conclusions and outlook
This paper represents an extension of an earlier review of IV‐iron reactions, which focused on risk minimization and management (Rampton et al., 2014). Here we compare the various features of IV‐iron and other IV drug‐induced HSRs in order to uncover possible clues regarding their mechanism and how to improve therapy. Our particular scientific question was as follows: ‘What is the available direct or indirect evidence for shared molecular mechanisms that have implications for the prevention and treatment of HSRs, in general, and of IV‐iron reactions, in particular?’ The comparison focused on the symptoms, prevalence, kinetics, duration, tolerance and molecular mechanisms of HSRs and came to the conclusion that complement activation and subsequent anaphylatoxin production may be a common underlying cause for many HSRs to IV drugs, including IV‐iron. The review addresses the difficulties of nomenclature and clinical studies in allergy research and points out that the treatment of IV‐iron reactions is based on empirical traditions rather than clinical research. It is also emphasized that as further evidence becomes available, the recent EMA guidance on risk minimization for very rare IV‐iron reactions should be extended or revised with recommendations for specific and effective new interventions against HSRs to iron, for example by using slow infusion‐based LRPs that are known to attenuate CARPA. It is clear that future research needs for the prevention and management of immune‐mediated drug hypersensitivity reactions (Adkinson et al., 2002), and the anaphylatoxin concept may spur many more complement‐focused desensitization or treatment ideas.
Conflict of interest
J. S. and S. F. have nothing to disclose. M. H. reports minor speaker honoraria from Vifor, Takeda and Pharmacosmos. S. H. reports personal fees from Pharmacosmos and Vifor. F. L. reports memberships of advisory boards and/or speaker invitations at symposia supported by Abbvie, Amgen, Pharmacosmos, Keryx, Rockwell, Sanofi, Takeda, Vifor‐Fresenius Pharma. S. P. reports personal fees from Pharmacosmos, outside the submitted work. D. S. R. reports personal fees from Pharmacosmos and Vifor outside the submitted work. G. W. reports personal fees from Pharmacosmos and Vifor outside the submitted work. J. F. reports personal fees from Pharmacosmos outside the submitted work.
Acknowledgements
Financial support from the following sources is acknowledged: J. S., TÁMOP‐4.2.1.B to the ‘Applied Materials and Nanotechnology Center of Excellence’, Miskolc University; EU project FP7‐NMP‐2012‐LARGE‐6‐309820 (NanoAthero); and Gedeon Richter NyRT, to the Nanomedicine Research and Education Center at Semmelweis University.
Szebeni, J. , Fishbane, S. , Hedenus, M. , Howaldt, S. , Locatelli, F. , Patni, S. , Rampton, D. , Weiss, G. , and Folkersen, J. (2015) Hypersensitivity to intravenous iron: classification, terminology, mechanisms and management. British Journal of Pharmacology, 172: 5025–5036. doi: 10.1111/bph.13268.
References
- Adkinson NFJ, Essayan D, Gruchalla R, Haggerty H, Kawabata T, Sandler JD et al. (2002). Task force report: future research needs for the prevention and management of immune‐mediated drug hypersensitivity reactions. J Allergy Clin Immunol 109: S461–478. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M et al. (2013). The Concise Guide to PHARMACOLOGY 2013/14: G protein‐coupled receptors. Br J Pharmacol 170: 1459–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman LC, Petersen PE (1988). Successful prevention of an anaphylactoid reaction to iron dextran. Ann Intern Med 109: 346–347. [DOI] [PubMed] [Google Scholar]
- Ansell G, Tweedie MC, West CR, Evans P, Couch L (1980). The current status of reactions to intravenous contrast media. Invest Radiol 15: S32–S39. [DOI] [PubMed] [Google Scholar]
- Auerbach M, Ballard H (2010). Clinical use of intravenous iron: administration, efficacy, and safety. Hematology. Am Soc Hematol Educ Program 2010: 338–347. [DOI] [PubMed] [Google Scholar]
- Auerbach M, Chaudhry M, Goldman H, Ballard H (1998). Value of methylprednisolone in prevention of the arthralgia‐myalgia syndrome associated with the total dose infusion of iron dextran: a double blind randomized trial. J Lab Clin Med 131: 257–260. [DOI] [PubMed] [Google Scholar]
- Auerbach M, Pappadakis JA, Bahrain H, Auerbach SA, Ballard H, Dahl NV (2011). Safety and efficacy of rapidly administered (one hour) one gram of low molecular weight iron dextran (INFeD) for the treatment of iron deficient anemia. Am J Hematol 86: 860–862. [DOI] [PubMed] [Google Scholar]
- Bailie GR (2012). Comparison of rates of reported adverse events associated with i.v. iron products in the United States. Am J Health Syst Pharm 69: 310–320. [DOI] [PubMed] [Google Scholar]
- Bailie GR, Clark JA, Lane CE, Lane PL (2005). Hypersensitivity reactions and deaths associated with intravenous iron preparations. Nephrol Dial Transplant 20: 1443–1449. [DOI] [PubMed] [Google Scholar]
- Bailie GR, Horl WH, Verhoef JJ (2011). Differences in spontaneously reported hypersensitivity and serious adverse events for intravenous iron preparations: comparison of Europe and North America. Arzneimittelforschung 61: 267–275. [DOI] [PubMed] [Google Scholar]
- Bailie GR, Verhoef JJ (2012). Differences in the reporting rates of serious allergic adverse events from intravenous iron by country and population. Clin Adv Hematol Oncol 10: 101–108. [PubMed] [Google Scholar]
- Baller D, Huchzermeyer H (1989). Histamine effects on the heart with special reference to cardiac side effects of H2 receptor antagonists. Klin Wochenschr 67: 743–755. [DOI] [PubMed] [Google Scholar]
- Banda NK, Mehta G, Chao Y, Wang G, Inturi S, Fossati‐Jimack L et al. (2014). Mechanisms of complement activation by dextran‐coated superparamagnetic iron oxide (SPIO) nanoworms in mouse versus human serum. Part Fibre Toxicol 11: 64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranyi L, Szebeni J, Savay S, Bodo M, Basta M, Bentley TB et al. (2003). Complement‐dependent shock and tissue damage induced by intravenous injection of cholesterol‐enriched liposomes in rats. J Applied Res 3: 221–231. [Google Scholar]
- Bircher AJ, Auerbach M (2014). Hypersensitivity from intravenous iron products. Immunol Allergy Clin North Am 34: 707–723. [DOI] [PubMed] [Google Scholar]
- Black N (1996). Why we need observational studies to evaluate the effectiveness of health care. BMJ 312: 1215–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannagan TH (2002). Intravenous gammaglobulin (IVIg) for treatment of CIDP and related immune‐mediated neuropathies. Neurology 59: S33–S40. [DOI] [PubMed] [Google Scholar]
- Brennan PJ, Rodriguez BT, Hsu FI, Sloane DE, Castells MC (2009). Hypersensitivity reactions to mAbs: 105 desensitizations in 23 patients, from evaluation to treatment. J Allergy Clin Immunol 124: 1259–1266. [DOI] [PubMed] [Google Scholar]
- Brown SG (2004). Clinical features and severity grading of anaphylaxis. J Allergy Clin Immunol 114: 37–1376. [DOI] [PubMed] [Google Scholar]
- Campbell WD, Lazoura E et al. (2002). Inactivation of C3a and C5a octapeptides by carboxypeptidase R and carboxypeptidase N. Microbiol Immunol 46: 131–134. [DOI] [PubMed] [Google Scholar]
- Castells MC (2008). Hypersensitivity to antineoplastic agents. Curr Pharm Des 14: 2892–2901. [DOI] [PubMed] [Google Scholar]
- Castells MC, Tennant NM, Sloane DE, Hsu FI, Barrett NA, Hong DI et al. (2008). Hypersensitivity reactions to chemotherapy: outcomes and safety of rapid desensitization in 413 cases. J Allergy Clin Immunol 122: 574–580. [DOI] [PubMed] [Google Scholar]
- Chanan‐Khan A, Szebeni J, Savay S, Liebes L, Rafique NM, Alving CR et al. (2003). Complement activation following first exposure to pegylated liposomal doxorubicin (Doxil): possible role in hypersensitivity reactions. Ann Oncol 14: 1430–1437. [DOI] [PubMed] [Google Scholar]
- Chertow GM, Mason PD, Vaage‐Nilsen O, Ahlmen J (2004). On the relative safety of parenteral iron formulations. Nephrol Dial Transplant 19: 1571–1575. [DOI] [PubMed] [Google Scholar]
- Chertow GM, Mason PD, Vaage‐Nilsen O, Ahlmen J (2006). Update on adverse drug events associated with parenteral iron. Nephrol Dial Transplant 21: 378–382. [DOI] [PubMed] [Google Scholar]
- Coombs RRA, Gell PGH (1968). Classification of allergic reactions responsible for drug hypersensitivity reactions In: Coombs RRA, Gell PGH. (eds). Clinical Aspects of Immunology, 2nd edn. Davis: Philadelphia, PA: pp 575–596. [Google Scholar]
- Critchly JU, Dunbar YE (2007). Adverse events associated with intravenous iron infusion (low‐molecular‐weight iron dextran and iron sucrose): a systematic review. TATM 9: 8–36. [Google Scholar]
- Demoly P, Lebel B, Messaad D, Sahla H, Rongier M, Daures JP et al. (1999). Predictive capacity of histamine release for the diagnosis of drug allergy. Allergy 54: 500–506. [DOI] [PubMed] [Google Scholar]
- Descotes J, Choquet‐Kastylevsky G (2001). Gell and Coombs's classification: is it still valid? Toxicology 158: 3–49. [DOI] [PubMed] [Google Scholar]
- Dézsi L, Fülöp T, Mészáros T, Szénási G, Urbanics R, Vázsonyi C et al. (2014). Features of complement activation‐related pseudoallergy to liposomes with different surface charge and PEGylation: Comparison of the porcine and rat responses. J Contr Release 195: 2–10. [DOI] [PubMed] [Google Scholar]
- Doxil prescribing information, Doxil.com (2014) https://www.doxil.com/shared/product/doxil/prescribing‐information.pdf (accessed 10/11/2015).
- Duburque C, Lelong J, Iacob R, Seddik M, Desreumaux P, Fournier C et al. (2006). Successful induction of tolerance to infliximab in patients with Crohn's disease and prior severe infusion reactions. Aliment Pharmacol Ther 24: 851–858. [DOI] [PubMed] [Google Scholar]
- EMA‐CHMP (2013). New recommendations to manage risk of allergic reactions with intravenous iron‐containing medicines Vol. EMA/579491/2013, pp 1–3: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/IV_iron_31/WC500151308.pdf (accessed 10/11/2015).
- Enright T, Chua‐Lim A, Duda E, Lim DT (1989). The role of a documented allergic profile as a risk factor for radiographic contrast media reaction. Ann Allergy 62: 302–305. [PubMed] [Google Scholar]
- Fishbane S (2003). Safety in iron management. Am J Kidney Dis 41: 18–26. [DOI] [PubMed] [Google Scholar]
- Fleming LW, Stewart WK, Parratt D (1992). Dextran antibodies, complement conversion and circulating immune complexes after intravenous iron dextran therapy in dialysed patients. Nephrol Dial Transplant 7: 35–39. [PubMed] [Google Scholar]
- Fletes R, Lazarus JM, Gage J, Chertow GM (2001). Suspected iron dextran‐related adverse drug events in hemodialysis patients. Am J Kidney Dis 37: 743–749. [DOI] [PubMed] [Google Scholar]
- Fütterer S, Andrusenko I, Kolb U, Hofmeister W, Langguth P (2013). Structural characterization of iron oxide/hydroxide nanoparticles in nine different parenteral drugs for the treatment of iron deficiency anaemia by electron diffraction (ED) and X‐ray powder diffraction (XRPD). J Pharm Biomed Anal 86: 151–60. [DOI] [PubMed] [Google Scholar]
- Gabizon AA, Muggia FM (1998). Initial clinical evaluation of pegylated liposomal doxorubicin in solid tumors In: Woodle MC, Storm G. (eds). Long‐Circulating Liposomes: Old Drugs, New Therapeutics, edn. Landes Bioscience: Austin, TX: pp 155–174. [Google Scholar]
- Goss JE, Chambers CE, Heupler FA Jr (1995). Systemic anaphylactoid reactions to iodinated contrast media during cardiac catheterization procedures: guidelines for prevention, diagnosis, and treatment. Laboratory Performance Standards Committee of the Society for Cardiac Angiography and Interventions. Cathet Cardiovasc Diagn 34: 99–104. [DOI] [PubMed] [Google Scholar]
- Hickman MA, Bern stein IL, Palascak JE (2000). Successful administration of iron dextran in a patient who experienced a life threatening reaction to intravenous iron dextran. Ann Allergy Asthma Immunol 84: 262–263. [DOI] [PubMed] [Google Scholar]
- Hong DI, Bankova L, Cahill KN, Kyin T, Castells MC (2012). Allergy to monoclonal antibodies: cutting‐edge desensitization methods for cutting‐edge therapies. Expert Rev Clin Immunol 8: 43–52. [DOI] [PubMed] [Google Scholar]
- Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF et al. (2004). Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol 113: 832–836. [DOI] [PubMed] [Google Scholar]
- Kelsall J, Rogers P, Galindo G, De Vera MA (2012). Safety of infliximab treatment in patients with rheumatoid arthritis in a real‐world clinical setting: description and evaluation of infusion reactions. J Rheumatol 39: 1539–1545. [DOI] [PubMed] [Google Scholar]
- Kemp SF, Lockey RF, Simons FE (2008). Epinephrine: the drug of choice for anaphylaxis. A statement of the World Allergy Organization. Allergy 63: 1061–1070. [DOI] [PubMed] [Google Scholar]
- Keshavarzian A, Mayer L, Salzberg B, Garone M, Finkelstein W, Cappa J et al. (2007). A multicenter retrospective experience of infliximab in Crohn's disease patients: infusion reaction rates and treatment persistency. Gastroenterol Hepatol(NY) 3: 381–390. [PMC free article] [PubMed] [Google Scholar]
- Lalli AF (1974). Urographic contrast media reactions and anxiety. Radiology 112: 267–271. [DOI] [PubMed] [Google Scholar]
- Laman CA, Silverstein SB, Rodgers GM (2005). Parenteral iron therapy: a single institution's experience over a 5‐year period. J Natl Compr Canc Netw 3: 791–795. [DOI] [PubMed] [Google Scholar]
- Lazarou J, Pomeranz BH, Corey PN (1998). Incidence of adverse drug reactions in hospitalized patients. A meta‐analysis of prospective studies. JAMA 279: 1200–1205. [DOI] [PubMed] [Google Scholar]
- Lequerre T, Vittecoq O, Klemmer N, Goeb V, Pouplin S, Menard JF et al. (2006). Management of infusion reactions to infliximab in patients with rheumatoid arthritis or spondyloarthritis: experience from an immunotherapy unit of rheumatology. J Rheumatol 33: 1307–1314. [PubMed] [Google Scholar]
- Lorenz W, Doenicke A (1985). H1 and H2 blockade: a prophylactic principle in anesthesia and surgery against histamine‐release responses of any degree of severity: Part 1. N Engl Reg Allergy Proc 6: 37–57. [DOI] [PubMed] [Google Scholar]
- Mayer L, Young Y (2006). Infusion reactions and their management. Gastroenterol Clin North Am 35: 857–866. [DOI] [PubMed] [Google Scholar]
- Merkel OM, Urbanics R, Bedőcs P, Rozsnyay Z, Rosivall L, Toth M et al. (2011). In vitro and in vivo complement activation and related anaphylactic effects associated with polyethylenimine and polyethylenimine‐grafted‐poly (ethylene glycol) block copolymers. Biomaterials 32: 4936–4942. [DOI] [PubMed] [Google Scholar]
- Misbah SA, Chapel HM (1993). Adverse effects of intravenous immunoglobulin. Drug Saf 9: 254–262. [DOI] [PubMed] [Google Scholar]
- Monaghan MS, Glasco G, St John G, Bradsher RW, Olsen KM (1994). Safe administration of iron dextran to a patient who reacted to the test dose. South Med J 87: 1010–2. [DOI] [PubMed] [Google Scholar]
- Nauta AJ, Engels F, Knippels LM, Garssen J, Nijkamp FP, Redegeld FA (2008). Mechanisms of allergy and asthma. Eur J Pharmacol 585: 354–360. [DOI] [PubMed] [Google Scholar]
- Novey HS, Pahl M, Haydik I, Vaziri ND (1994). Immunologic studies of anaphylaxis to iron dextran in patients on renal dialysis. Ann Allergy 72: 224–228. [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP et al. (2014). The IUPHAR/BPS Guide to PHARMACOLOGY: an expert‐driven knowledge base of drug targets and their ligands. Nucl Acids Res 42: D1098–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichler WJ (2006). Adverse side‐effects to biological agents. Allergy 61: 912–920. [DOI] [PubMed] [Google Scholar]
- Puchner TC, Kugathasan S, Kelly KJ, Binion DG (2001). Successful desensitization and therapeutic use of infliximab in adult and pediatric Crohn's disease patients with prior anaphylactic reaction. Inflamm Bowel Dis 7: 34–37. [DOI] [PubMed] [Google Scholar]
- Rajan TV (2003). The Gell–Coombs classification of hypersensitivity reactions: a re‐interpretation. Trends in Immunol 24: 376–379. [DOI] [PubMed] [Google Scholar]
- Rampton G, Folkersen J, Fishbane S, Hedenus M, Howaldt S, Locatelli F et al. (2014). Hypersensitivity reactions to intravenous iron: guidance for risk minimisation and management. Haematologica 99: 1671–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranft A, Kochs EF (2004). Treatment of anaphylactic reactions: a review of guidelines and recommendations. AnasthesiolIntensivmedNotfallmed Schmerzther 39: 2–9. [DOI] [PubMed] [Google Scholar]
- Ring J, Grosber M, Mohrenschlager M, Brockow K (2010). Anaphylaxis: acute treatment and management. Chem Immunol Allergy 95: 201–10. doi:10.1159/000315953 Epub;%2010 Jun 1.: 201–210.. [DOI] [PubMed] [Google Scholar]
- Ring J, Messmer K (1977). Incidence and severity of anaphylactoid reactions to colloid volume substitutes. Lancet 1: 466–469. [DOI] [PubMed] [Google Scholar]
- Scott S, Thompson J (2014). Adverse drug reactions. Anest Intens Care Med 15: 245–249. [Google Scholar]
- Shehadi WH (1982). Contrast media adverse reactions: occurrence, recurrence, and distribution patterns. Radiology 143: 11–17. [DOI] [PubMed] [Google Scholar]
- Simons FE, Ardusso LR, Bilo MB, El‐Gamal YM, Ledford DK, Ring J et al. (2011). World allergy organization guidelines for the assessment and management of anaphylaxis. World Allergy Organ J 4: 13–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szebeni J, Alving CR, Savay S, Barenholz Y, Priev A, Danino D et al. (2001). Formation of complement‐activating particles in aqueous solutions of Taxol: possible role in hypersensitivity reactions. Int Immunopharmacol 1: 721–735. [DOI] [PubMed] [Google Scholar]
- Szebeni J (2005). Complement activation‐related pseudoallergy: a new class of drug‐induced immune toxicity. Toxicology 216: 106–121. [DOI] [PubMed] [Google Scholar]
- Szebeni J (2012). Hemocompatibility testing for nanomedicines and biologicals: predictive assays for complement mediated infusion reactions. Eur J Nanomed 5: 33–53. [Google Scholar]
- Szebeni J (2014). Complement activation‐related pseudoallergy: a stress reaction in blood triggered by nanomedicines and biologicals. Mol Immunol 61: 163–173. [DOI] [PubMed] [Google Scholar]
- Szebeni J, Alving CR, Rosivall L, Bünger R, Baranyi L, Bedöcs P et al. (2007). Animal models of complement‐mediated hypersensitivity reactions to liposomes and other lipid‐based nanoparticles. J Liposome Res 17: 107–117. [DOI] [PubMed] [Google Scholar]
- Szebeni J, Baranyi L, Sávay S, Bodó M, Milosevits J, Alving CR et al. (2006). Complement activation‐related cardiac anaphylaxis in pigs: role of C5a anaphylatoxin and adenosine in liposome‐induced abnormalities in ECG and heart function. Am J Physiol 290: H1050–1058. [DOI] [PubMed] [Google Scholar]
- Szebeni J, Baranyi L, Savay S, Bodo M, Morse DS, Basta M et al. (2000). Liposome‐induced pulmonary hypertension: properties and mechanism of a complement‐mediated pseudoallergic reaction. Am J Physiol Heart Circ Physiol 279: H1319–H1328. [DOI] [PubMed] [Google Scholar]
- Szebeni J, Bedőcs P, Csukás D, Rosivall L, Bunger R, Urbanics R (2012a). A porcine model of complement‐mediated infusion reactions to drug carrier nanosystems and other medicines. Adv Drug Deliv Rev 64: 1706–1716. [DOI] [PubMed] [Google Scholar]
- Szebeni J, Bedőcs P, Rozsnyay Z, Weiszhár Z, Urbanics R, Rosivall L et al. (2012b). Liposome‐induced complement activation and related cardiopulmonary distress in pigs: factors promoting reactogenicity of Doxil and AmBisome. Nanomedicine NBM 8: 176–184. [DOI] [PubMed] [Google Scholar]
- Szebeni J, Bedőcs P, Urbanics R, Bunger R, Rosivall L, Tóth M et al. (2012c). Prevention of infusion reactions to PEGylated liposomal doxorubicin via tachyphylaxis induction by placebo vesicles: a porcine model. J Contr Rel 160: 382–387. [DOI] [PubMed] [Google Scholar]
- Szebeni J, Fontana JL, Wassef NM, Mongan PD, Morse DS, Dobbins DE et al. (1999). Hemodynamic changes induced by liposomes and liposome‐encapsulated hemoglobin in pigs: a model for pseudoallergic cardiopulmonary reactions to liposomes. Role of complement and inhibition by soluble CR1 and anti‐C5a antibody. Circulation 99: 2302–2309. [DOI] [PubMed] [Google Scholar]
- Szebeni J, Muggia F, Gabizon A, Barenholz Y (2011). Activation of complement by therapeutic liposomes and other lipid excipient‐based therapeutic products: prediction and prevention. Adv Drug Deliv Rev 63: 1020–1030. [DOI] [PubMed] [Google Scholar]
- Szebeni J, Wassef NM, Spielberg H, Rudolph AS, Alving CR (1994). Complement activation in rats by liposomes and liposome‐encapsulated hemoglobin: evidence for anti‐lipid antibodies and alternative pathway activation. Biochem Biophys Res Comm 205: 255–263. [DOI] [PubMed] [Google Scholar]
- Tramer MR, von Elm E, Loubeyre P, Hauser C (2006). Pharmacological prevention of serious anaphylactic reactions due to iodinated contrast media: systematic review. BMJ 333: 675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kolk LE, Grillo‐López AJ, Baars JW, Hack CE, van Oers MH (2001). Complement activation plays a key role in the side‐effects of rituximab treatment. Br J Haematol 115: 807–811. [DOI] [PubMed] [Google Scholar]
- Wasserman MJ, Weber DA, Guthrie JA, Bykerk VP, Lee P, Keystone EC (2004). Infusion‐related reactions to infliximab in patients with rheumatoid arthritis in a clinical practice setting: relationship to dose, antihistamine pretreatment, and infusion number. J Rheumatol 31: 1912–1917. [PubMed] [Google Scholar]
- Working Group of the Resuscitation Committee (2013). Emergency treatment of anaphylactic reactions ‐ guideline for health care providers. Resuscitation Council (UK) http://www.resus.org.uk/pages/reaction.pdf (accessed 10/11/2015).
- Wysowski DK, Swartz L, Borders‐Hemphill BV, Goulding MR, Dormitzer C (2010). Use of parenteral iron products and serious anaphylactic‐type reactions. Am J Hematol 85: 650–654. [DOI] [PubMed] [Google Scholar]


