Abstract
We employed next-generation, transcriptome-wide RNA sequencing (RNA-Seq) technology to assess the effects of two different exercise training protocols on transcriptional profiles in diaphragm second-order arterioles (D2a) and in the diaphragm feed artery (DFA) from Otsuka Long Evans Tokushima Fatty (OLETF) rats. Arterioles were isolated from the diaphragm of OLETF rats that underwent an endurance exercise training program (EX; n = 13), interval sprint training program (SPRINT; n = 14), or remained sedentary (Sed; n = 12). Our hypothesis was that exercise training would have similar effects on gene expression in the diaphragm and soleus muscle arterioles because diaphragm blood flow increases during exercise to a similar extent as in soleus. Results reveal that several canonical pathways that were significantly altered by exercise in limb skeletal muscles were not among the pathways significantly changed in the diaphragm arterioles including actin cytoskeleton signaling, role of NFAT in regulation of immune response, protein kinase A signaling, and protein ubiquitination pathway. EX training altered the expression of a smaller number of genes than did SPRINT in the DFA but induced a larger number of genes with altered expression in the D2a than did SPRINT. In fact, FDR differential expression analysis (FDR, 10%) indicated that only two genes exhibited altered expression in D2a of SPRINT rats. Very few of the genes that exhibited altered expression in the DFA or D2a were also altered in limb muscle arterioles. Finally, results indicate that the 2a arterioles of soleus muscle (S2a) from endurance-trained animals and the DFA of SPRINT animals exhibited the largest number of genes with altered expression.
Keywords: interval sprint training, endurance exercise, blood flow, next generation sequencing, gene expression, resistance arteries
our laboratory has a longstanding interest in the effects of muscle fiber type composition and muscle fiber recruitment patterns on characteristics of skeletal muscle vascular trees. The soleus muscle (slow-twitch, oxidative muscle in rats) has blood flow at rest that is two- to fourfold greater than blood flow to fast-twitch, glycolytic gastrocnemius muscle (9, 35). During exercise muscle fibers in the gastrocnemius muscle are recruited and blood flow increases with increasing running speed so that soleus and gastrocnemius muscles have similar blood flow during intense exercise (8). The diaphragm has been shown to have a blood flow capacity and blood flows during exercise similar to those of high oxidative limb skeletal muscle (38). Previous work demonstrated that second branch order arterioles of diaphragm muscle (D2a) exhibit endothelium-dependent dilator (EDD) responses, dilator responses to isoproterenol, dilator responses to NO, and vasoconstrictor responses to endothelin-1 that are similar to those of red and white gastrocnemius 2a arterioles (RG2a and WG2a) (1, 2). In contrast, D2a arterioles are less sensitive to α1-adrenergic constriction than RG2a and WG2a arterioles (1) but more sensitive to dilator responses to adenosine than RG2a (2).
We recently observed that the number of genes differentially expressed with obesity tended to decrease with increasing branch order arteriole number (i.e., decreasing size of the artery) in the diaphragm whereas in the soleus and gastrocnemius muscles, the opposite pattern of obesity-induced alterations in gene expression was observed (49). Given these dramatic differences between the effects of obesity/Type II diabetes (T2D) on vascular gene expression in diaphragm and limb muscles, we wondered whether the effects of exercise training on gene expression in diaphragm arteries/arterioles may also be different from that seen in limb muscles.
T2D is associated with decreased endothelium-dependent dilation (EDD) and vascular rarefaction in skeletal muscle microcirculation (29, 32, 60). Also, T2D-induced blunting of EDD differs with muscle fiber type composition, and exercise training reverses this dysfunction in a fiber type-dependent manner (11, 44, 47). This observation adds importantly to the growing body of evidence for differences among vasomotor properties of arteries of different tissues (1, 2, 43) and among/along arteries in the same tissue (3, 4, 6, 7, 18–20, 23, 31, 33, 34, 37, 38, 41–43, 45). Furthermore, we have previously demonstrated that exercise training improves EDD nonuniformly in the arterial tree of limb skeletal muscle of a rodent model of obesity/T2D (11, 26, 44, 47, 48). It is not known whether similar phenomena are seen in diaphragm.
In the study reported herein, transcriptional profiles in diaphragm arterioles and feed arteries from Otsuka Long Evans Tokushima Fatty (OLETF) rats were assessed using transcriptome-wide RNA sequencing (RNA-Seq) analysis as described previously (27, 51) to gain insights into the molecular events underpinning exercise-induced vascular adaptations in obesity and T2D. We contrasted the changes present in diaphragm arterioles to those seen in soleus and gastrocnemius muscle reported in the companion paper (40). One group of OLETF rats underwent an endurance exercise training program (EX), a second group underwent an interval sprint training program (SPRINT), and a third group were restricted to cage activity (Sed). Our hypothesis was that exercise training would have similar effects on gene expression in the diaphragm and soleus muscle arterioles because diaphragm blood flow increases during exercise to a similar extent as in soleus muscle. Thus, if shear stress is a primary signal for the effects of exercise on gene expression in arterioles of both types of skeletal muscle, exercise training should have similar effects across muscle.
METHODS
Animals and experimental design.
Male OLETF rats (n = 39) were obtained at age 4 wk (Japan SLC, 3371-8, Kotoh-Cho, Hamamatsu, Shizuoka, Japan). The OLETF rat has a mutated cholecystokinin-1 receptor that results in a hyperphagic phenotype and has become an established model of obesity, insulin resistance, and T2D (28). Each rat was individually housed in a temperature-controlled (21°C) environment with 0600–1800 light and 1800-0600 dark cycles. All animals were given ad libitum access to standard chow with a macronutrient composition of 56% carbohydrate, 17% fat, and 27% protein (Formulab 5008, Purina Mills, St. Louis, MO). At 20 wk of age, rats were randomly assigned to one of three groups: 1) Sed (n = 12), 2) EX (n = 13), and 3) SPRINT (n = 14). We used the EX program we have used extensively previously (8, 9, 35, 42, 45, 46) in which treadmill running duration and intensity were increased progressively over the first 4 wk to reach 60 min of treadmill running at 20 m/min at a 15% incline for the remaining 8 wk. We also used an SPRINT exercise training program we have used extensively (8, 9, 35, 42, 45, 46) which consists of six bouts of treadmill running, with 4.5-min rest periods that progressively increase in duration and intensity over the first 5 wk to reach running speeds of 40 m/min at a 15% incline for 2.5 min/bout for the remaining 7 wk (0.6 km/day). Both EX and SPRINT groups exercised 5 days/wk. The rats used in this study are the same as those used in our previous reports on soleus and gastrocnemius feed arteries (27, 51) and vasomotor function of four arterioles of soleus and gastrocnemius muscles (44).
Rats were anesthetized at 30–32 wk of age with an intraperitoneal injection of pentobarbital sodium (50 mg/kg) between 0800 and 0930. Tissues were then harvested, and the animals were killed by exsanguination. The last exercise bout for EX and SPRINT animals was performed ∼18 h prior to death. Food was removed from the cages 12 h prior to death. All protocols were approved by the University of Missouri Animal Care and Use Committee.
Body weight, body composition, food intake, and citrate synthase.
Body weights and food intakes were monitored and recorded on a weekly basis. Weekly food intakes were averaged across the period of the intervention (age 20–30 wk). Body composition was assessed by dual-energy X-ray absorptiometry (DXA; Hologic QDR-1000, calibrated for rodents) on the day of death. Omental, retroperitoneal, and epididymal adipose tissue depots were then removed and weighed to the nearest 0.01 g. Citrate synthase activity was measured from whole muscle homogenate of the red and white portions of the vastus lateralis muscle using the spectrophotometric method of Srere (64).
Blood parameters.
Whole blood was collected on the day of euthanasia for analysis of glycosylated hemoglobin (HbA1c) by the boronate-affinity high-performance liquid chromatography method (Primus Diagnostics, Kansas City, MO) in the Diabetes Diagnostics Laboratory at the University of Missouri. Serum samples were prepared by centrifugation and stored at −80°C until analysis. Glucose, triglyceride (TG), and nonesterified fatty acid (NEFA) assays were performed by a commercial laboratory (Comparative Clinical Pathology Services, Columbia, MO) on an Olympus AU680 automated chemistry analyzer (Beckman-Coulter, Brea, CA) using commercially available assays according to manufacturer's guidelines. Plasma insulin concentrations were determined using a commercially available, rat-specific enzyme-linked immunosorbent assay (Alpco Diagnostics, Salem, NH). Samples were run in duplicate and manufacturer's controls and calibrators were used according to assay instructions.
Isolation of skeletal muscle arterioles.
Immediately after harvesting the diaphragm and the gastrocnemius/soleus muscle complex, the muscles were pinned down in a Petri dish containing a cold RNA-stabilizing agent (RNAlater; Ambion, Austin, TX). Under the microscope, diaphragm, soleus, and gastrocnemius feed arteries and second (2a)-order branch arterioles were dissected clean of perivascular adipose tissue and excess adventitia. For the diaphragm we followed dissection procedures outlined previously (1, 2). Results presented herein are for the diaphragm feed artery (DFA) (which is the terminal portion of the phrenic artery where it enters the muscle) and diaphragm 2a arterioles (D2a). Thus from each rat we isolated the following: the soleus feed artery (SFA) and 2a arteriole (S2a); and the feed artery (GFA), 2a arterioles from red (RG2a) and white (WG2a) portions of the gastrocnemius muscle and DFA and D2a. Samples were kept in RNAlater for 48 h at 4°C, and then removed from the RNAlater solution and stored at −80°C until analysis. Results from the soleus and gastrocnemius muscle have been reported previously and are included in our analysis of the DFA and D2a data so that it was appropriate to compare results with those of limb muscles (27, 51, 52). Because of the substantial number of differences in the effects of exercise training on gene expression in the diaphragm arteries and the skeletal muscle arteries we decided to publish these data in two papers, this one and the companion paper focused on soleus and gastrocnemius feed arteries and second-order arterioles (40).
RNA extraction and quality control.
Total RNA isolations were performed as described previously using the NucleoMag 96 RNA kit procedure (Clontech part no. 744350.1) (27, 51). For assessing total RNA yield and integrity, tandem Agilent Bioanalyzer 2100 instruments were used in combination with the Total RNA 6000 Pico Assay as described (27, 51). At the time of this study, the RNA Pico LabChip Kit was the only platform to give unbiased assessment of RNA integrity (via RIN) and accurate results with small amounts of RNA. Typical yields for these samples were ∼500-1,000 pg/μl.
Illumina library preparation (SMARTer amplification and RNA-seq).
Total RNA could not directly be used in traditional Illumina gene expression profiling methods (RNA-Seq) due to the low concentration of RNA in some samples (standard RNA-Seq kits during this project required 0.1–1 μg of total RNA). Thus the SMARTer Ultra Low RNA Kit for Illumina Sequencing (Clontech cat. no. 634935) was utilized for generating full-length cDNA transcripts prior to Illumina RNA-Seq library preparation as described previously (27, 51, 52) using SMARTer first-strand cDNA synthesis and purification, utilizing the SMARTer anchor sequence and poly(A) sequence that serve as universal priming sites for end-to-end generation of single-stranded cDNA, followed by cDNA amplification with LongDistance PCR (LD-PCR) using the manufacturer's recommended Advantage 2 PCR system (Clontech cat. no. PT3281-1) as designed for Illumina sequencing. We used 100-1,000 pg of total RNA as input to the SMARTer 1st cDNA reaction.
Following cDNA generation, validation was performed using the Bioanalyzer 2100 High Sensitivity DNA Assay (Agilent, cat. no. 5067-4626) to accurately size and quantitate DNA up to 12 kb in length, using minimal sample volumes (1 μl). After 14 cycles of LD-PCR amplification the final cDNA yields were estimated at ∼1–10 ng for each sample, a suitable input amount for library preparation for cDNA/ChIP Seq library preparation. To generate Illumina Paired-End RNAseq libraries, cDNA was fragmented to ∼200 bp using the Q700 DNA fragmentation system (QSonica) and then used directly with the NextFlex DNA preparation kit (Bioo Scientific, cat. no. 5140-02). Fragment cDNA was end repaired and purified with 1.8 × SPRI beads to remove reaction components (Agencourt) and A-tailed in preparation for cohesive ligation to the Illumina specific sequencing adapters (NextFlex DNA Adapters, Bioo Scientific, cat. 514104). Ligated DNA was purified (2×) with 1.0 × SPRI to remove adapter dimers and perform gel-free size selection, and then amplified through 14 cycles of PCR. The final sequencing construct was purified with a 1.0 × SPRI to remove low-molecular-weight adapter dimer artifacts (if any), and libraries were validated to contain ∼330-bp fragments using the Bioanalyzer 2100 High Sensitivity DNA Assay. Library quantitation was performed using the Qubit 2.0 fluorometer and the High Sensitivity DNA assay (Life Tech, cat. no. Q32851).
RNA sequencing.
As previously described (27, 51, 52), large-scale multiplexing (48 bar codes) was used to form many library pools that were later sequenced. Samples were randomized to the pools in a stratified manner with dynamic allocation used to maximize the representation and balance of key covariates (e.g., vessel, treatment, and date of death). The final pools were each loaded on a single lane of single read 50-base sequencing on the Illumina HiSeq2000 yielding ∼175–200 million useable reads per lane (14–17 million reads per RNAseq sample). Because the harvesting and sequencing could not all be done at one time, the pools were sequenced in five batches over a 4-mo period.
Statistical analysis.
The analysis of the RNA-Seq data was carried out for the DFA, and D2a, and for SFA, S2a, GFA, RG2a, WG2a, samples as described previously (27, 51). Nonspecific filtering of genes prior to statistical testing was carried out to increase detection power (15), based on the requirement that a gene have mean expression level greater than 2 counts per million reads mapped (CPM) after averaging across all samples. This CPM cutoff was established empirically based on the point at which the External RNA Controls Consortium (ERCC) Spike-ins at different concentrations were no longer distinguishable. ERCC Spike-ins are used to control for variability by using a common set of external RNA controls developed by the External RNA Controls Consortium (ERCC), an ad hoc group of academic, private, and public organizations hosted by the National Institute of Standards and Technology (NIST). Adjustment to the P values was made to account for multiple testing using the false discovery rate (FDR) method of Benjamini and Hochberg (12). For all comparisons we chose 10% as our FDR threshold for statistical significance. As an empirical measure of the false discovery rate, we evaluated what proportion of the identical ERCC probe/concentration combinations (Set B) appeared in our list of differentially expressed genes. Similarly, we looked at a set of 13 putative housekeeping genes derived from a study of more than 13,000 rat samples (17) to have another estimate of our false discovery rate. The set of genes was Actb, B2m, Gapdh, Gusb, Hprt1, Hmbs, Hsp90b1, Rpl13a, Rps29, Rplp0, Ppia, Tbp, and Tuba1. For both of these sets of controls, we also estimated the fold change of each of the genes as a measure of the accuracy of the fold change estimates.
To investigate enriched canonical pathways, a broader cutoff was used (P < 0.01) to determine differentially expressed gene lists with the rationale being that Type I error would be controlled by comparing similarity of pathways enriched across multiple vessel/treatment combination. Canonical pathways were assessed for enrichment for each gene list produced (i.e., each gene list was separately analyzed for enriched pathways). We used Fisher's exact test for enrichment with a P < 0.01 for significance. Many pathways appeared in multiple gene lists so the significance of such pathways was computed by combining P values (across each gene list) using the method of Fisher. Novel networks were generated through the use of Ingenuity Pathways Analysis (Ingenuity Systems, http://www.ingenuity.com), henceforth IPA, as previously described (27, 51, 52). Finally, for the remaining data (i.e., non RNA-Seq) a one-way ANOVA with LSD post hoc was performed to examine the between-group differences for all descriptive variables, and statistical significance was declared at P ≤ 0.05.
RESULTS
Results characterizing the animals from which the arterioles were isolated are only summarized here because they have been reported previously (40, 51). Both exercise training programs produced decreased body weight (Sed 687 ± 12 g, EX = 592 ± 11 g, SPRINT = 589 ± 13 g) and % body fat (Sed 35 ± 1, EX = 25 ± 1, SPRINT = 27 ± 1). In addition, food intake per day relative to body weight was similar in all three groups, but both EX and IST produced small decreases in absolute food intake. Retroperitoneal, omental, and epididymal adipose tissue masses were also significantly less in both SPRINT and EX groups compared with Sed. Heart weight-to-body weight ratios were significantly greater in EX and SPRINT groups due to differences in body weight as heart weights were similar across all three groups. Both SPRINT and EX trained rats exhibited significantly higher citrate synthase activity in the red and white portions of the vastus lateralis muscle (red: Sed 28.3 ± 1.6 μmol·min−1·g−1, EX = 35.9 ± 1.3, SPRINT = 35.6 ± 1.5. white: Sed 8.0 ± 0.3 μmol·min−1·g−1, EX = 11.0 ± 0.8, SPRINT = 16.8 ± 0.9) establishing the effectiveness of the training programs. Total and HDL-cholesterol levels were lower in both trained groups while LDL-cholesterol was not different among the groups. Triglycerides and nonesterified fatty acid levels were lower in EX and SPRINT than in sedentary rats. Insulin (Sed 8.1 ± 1.4 ng/ml, EX = 4.1 ± 0.5, SPRINT = 3.9 ± 0.6), HOMA-IR index, and % glycosylated hemoglobin (HbA1c) (Sed 7.2 ± 0.3%, EX = 5.4 ± 0.1%, SPRINT = 5.5 ± 0.1%) were lower in both trained groups while glucose levels were only significantly lower in the SPRINT vs. sedentary animals (51).
For group comparisons reported in diaphragm vessels, the average ERCC Spike-in (Set B) empirical FDR was 9.2% at the FDR cutoff of 10% (mean fold = 1.14), while for the putative housekeeping genes the average was 2.1% (fold = 1.09). For group comparisons across limb skeletal muscles (40) and diaphragm vessels the average ERCC Spike-in (Set B) empirical FDR was 2.6% at the FDR cutoff of 10% (mean fold = 1.12), while for the putative housekeeping genes the average was 2.4% (fold = 0.999). In their entirety these findings strongly support the methodology used because, on average, the fold changes for these controls are approximately equal to 1 and the empirical FDR is less than the target FDR (10%).
Effects of EX and SPRINT on gene expression FDR < 10%.
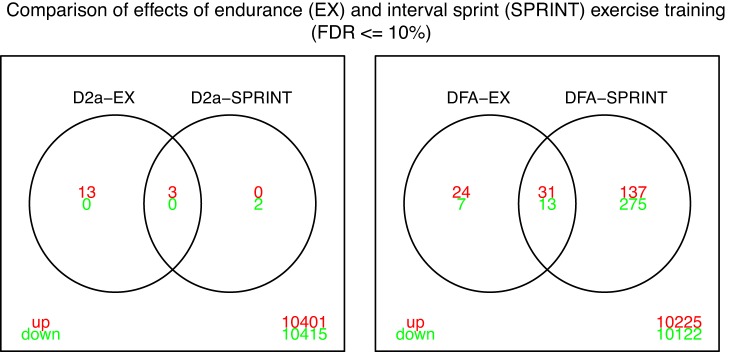
Figure 1 presents results showing the number of genes in the D2a and DFA whose expression was changed by EX or SPRINT. The intersections of the Venn diagrams represent genes whose expression was changed in both EX and SPRINT. These results show that both EX and SPRINT caused the largest number of changes in gene expression in the DFA with fewer changes in the D2a. These results also show that EX and SPRINT produced the same change in expression of only three genes in the D2a while SPRINT and EX produced increased expression of 31 common genes and decreased expression of 13 common genes in the DFA.
Fig. 1.
Venn diagrams illustrating the number of genes whose expression was altered by endurance exercise (EX) and by interval sprint training (SPRINT) in diaphragm 2a arterioles (D2a) and diaphragm feed artery (DFA). Red font indicates upregulated genes and green font downregulated genes relative to expression in sedentary rats. Overlapping area represents genes whose expression were changed by both EX and SPRINT. Thus 16 genes exhibited increased expression in the D2a after EX and 3 after SPRINT. Also, SPRINT caused decreased expression of 2 genes in D2a. The three genes with increased expression in D2a after EX and IST are Cyp2c13, Fkbp5, and Cys1.
Results of Ingenuity canonical pathway analysis of effects of EX and SPRINT.
IPA analysis was used to evaluate the effects of EX and SPRINT on expression of genes in established canonical pathways (301 metabolic pathways and 341 signaling pathways) which revealed that 23 pathways had significant changes in gene expression when analyzed across vessels, and 16 of 23 were signaling pathways. Several of these pathways had a large number of genes with altered expression in diaphragm vessels (primarily DFA) including fatty acid oxidation 1, triacylglycerol degradation, retinol biosynthesis, PEDF signaling, 14-3-3 mediated signaling, acute phase response signaling, estrogen receptor signaling, AMPK signaling, B cell receptor signaling, epithelial adherens junction signaling, ERK/MAPK signaling, Type II diabetes mellitus signaling, insulin receptor signaling, gap junction signaling, glucocorticoid receptor, Myc mediated apoptosis signaling, NRF2-mediated oxidative stress responses, PI3K/AKT signaling, PPAR/RXR activation, remodeling of epithelial adherens junctions, germ cell-sertoli cell junction signaling, sertoli cell-sertoli cell junction signaling, STAT3 pathway, and tight junction signaling. Also, based on past experience concerning known vascular cell signaling pathways that are altered by exercise training we selected the following canonical pathways for further analysis: endothelin-1, eNOS, production of NO and ROS, and insulin receptor signaling pathways. It is important that several pathways that were significantly altered by exercise in limb skeletal muscles (40) were not among the list of pathways significantly changed in the diaphragm arterioles including actin cytoskeleton signaling, role of NFAT in regulation of immune response, protein kinase A signaling, and protein ubiquitination pathway.
Some pathways contained substantial numbers of genes with changes in expression across both DFA and D2a but showed much different patterns of altered gene expression than previously reported for skeletal muscle arterioles. For example, in the gastrocnemius feed artery (GFA) actin cytoskeleton signaling pathway contained a number of genes that exhibited altered expression levels in EX and/or IST relative to sedentary OLETF rats. Some genes coding for contractile and/or structural proteins were decreased or unchanged by EX and SPRINT in the DFA but exhibited increased expression in the GFA as reported previously and thus the actin cytoskeleton signaling pathway was not among those changed significantly in the DFA or D2a. Tubulin, beta 2A class IIa (Tubb2a) was increased by EX and SPRINT in D2a, and tubulin, beta 2B class IIb (Tubb2b) was increased in D2a and DFA by both EX and SPRINT. Products of tubulin genes are believed to be important in gap junction signaling and in formation of cytoarchitecture.
SPRINT DFA exhibited increased mRNA levels for 5 genes in the aldosterone signaling pathway. Expression of three genes (Dnajb2, Dnaja1, and Hspa5) was increased in DFA by both EX and SPRINT.
EX and SPRINT increased expression of five genes of DFA in the AMPK signaling pathway (Lipe, Gys2, Ak3, Adrb3, Eif4ebp1) and caused decreased expression of seven other genes in the DFA (Ppp2cb, Akt1, Cpt1a, Ppm1b, Stk11, Prkaca, Hlth). EX increased expression of Adrb3 in D2a, and IST decreased expression of Lipe in D2a. In the eicosanoid pathway Tbxas1 expression was increased in D2a and DFA by both EX and SPRINT.
The ERK-MAPK IPA signaling pathway had a large number of genes whose expression was changed by EX and/or SPRINT. As can be seen in Table 1, EX and SPRINT had similar effects on expression of genes in this pathway in the DFA in that both EX and SPRINT increased expression of 5 genes (Pparg, Ywhab, Eif4ebp1, Pla2g5 and Ppp2rla) while in DFA expression of 10 genes was decreased by EX and SPRINT (Table 1). EX and SPRINT had little effect on expression of genes in the ERK-MAPK pathway gene in the D2a's.
Table 1.
ERK-MAPK signaling IPA canonical pathway
| Symbol | GeneID | DFA.OE | DFA.OI | D2a.OE | D2a.OI |
|---|---|---|---|---|---|
| Pparg | 25664 | 3.505* | 2.565* | 0.874 | 0.668 |
| Fyn | 25150 | 0.669* | 0.55* | 0.915 | 0.84 |
| Ywhag | 56010 | 0.751 | 0.512* | 0.674 | 0.792 |
| Ywhab | 56011 | 1.42* | 1.396* | 1.012 | 0.906 |
| Crebbp | 54244 | 0.927 | 0.662* | 0.964 | 1.049 |
| Sos2 | 85384 | 0.838* | 0.842* | 1.19 | 0.908 |
| Rac1 | 363875 | 0.59* | 0.61* | 1.207 | 1.275 |
| Eif4ebp1 | 116636 | 2.177* | 2.337* | 0.936 | 0.736 |
| Ppp2cb | 24673 | 0.682* | 0.598* | 0.779 | 0.916 |
| Sh1 | 85385 | 0.813 | 0.608* | 0.808 | 0.869 |
| Fos | 314322 | 0.346* | 0.325* | 0.976 | 0.983 |
| Ets2 | 304063 | 0.577* | 0.464* | 0.843 | 0.965 |
| Pla2 g5 | 29354 | 1.32 | 1.62* | 0.895 | 1.163 |
| Prkaca | 25636 | 0.589* | 0.467* | 0.896 | 0.67 |
| Ptk2 | 25614 | 0.876 | 0.798 | 0.895 | 1.047 |
| Myc | 24577 | 1.162 | 0.957 | 1.506* | 0.962 |
| Ppp2rla | 117281 | 1.191 | 1.357* | 0.969 | 1.012 |
| Dusp6 | 116663 | 1.183 | 1.081 | 1.249 | 1.009 |
| Pik3r6 | 497932 | 1.032 | 1.036 | 1.28 | 0.848 |
| Stat1 | 25124 | 1.052 | 0.98 | 0.767 | 0.86 |
| Ppplr14b | 259225 | 0.697 | 0.845 | 0.76 | 0.9 |
| Stat3 | 25125 | 1.009 | 0.843 | 0.856 | 0.937 |
| Prkar1a | 25725 | 1.067 | 1.127 | 0.842 | 1.002 |
Results of IPA analysis to evaluate the effects of EX and SPRINT on expression of genes in this signaling IPA canonical pathway. Results are expressed as fold change for the EX (OE) or SPRINT (OI) values relative to that of the sedentary OLETF value for that sample. Thus any entry that is >1.00 (italic font) reflects a gene with increased expression and any entry that is <1.00 (nonitalic font) reflects a gene with decreased expression. The * indicate a value that is significantly changed relative to sedentary OLETF rats (P < 0.01). The Symbol and Gene IDs are from the National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov/) gene bank and defined therein.
The protein kinase A signaling pathway is another canonical signaling pathway exhibiting a large number of genes whose expression was changed by EX and/or SPRINT (Table 2). The expression of some genes in the protein kinase A signaling pathway was increased in both arteries by EX and/or SPRINT, and overall, both EX and SPRINT had greater effects on gene expression of this pathway in the DFA than in the D2a. Examples of genes in this pathway whose expression was increased by EX and SPRINT in both DFA and D2a include an inhibitor of NF-kappa-B (Nfkbia), and glycogen synthase 2 (Gys2). An example of a gene whose expression was decreased by EX and SPRINT in DFA but increased in D2a is protein kinase C, zeta (Prkcz) (Table 2). Desert Hedgehog (Dhh) expression was decreased by EX and SPRINT in DFA not D2a as was true for protein kinase, cAMP-dependent, catalytic, alpha (Prkaca). Adenylate cyclase 4 (Adcy4) was not significantly changed in DFA or D2a by EX or SPRINT. Finally, hormone sensitive lipase (Lipe) exhibited increased expression to both EX and SPRINT in the DFA while both EX and SPRINT decreased expression of this gene in the D2a.
Table 2.
Protein kinase A signaling IPA canonical pathway
| Symbol | GeneID | DFA.OE | DFA.OI | D2a.OE | D2a.OI |
|---|---|---|---|---|---|
| Nfkbia | 25493 | 1.853* | 1.919* | 1.366 | 1.306 |
| Lipe | 25330 | 2.524* | 2.511* | 0.69 | 0.578* |
| Gys2 | 25623 | 6.874* | 4.68* | 2.925* | 0.425* |
| Ptprc | 24699 | 0.754 | 0.587* | 0.936 | 0.925 |
| Myh10 | 79433 | 0.618* | 0.595* | 1.348 | 1.053 |
| Ywhag | 56010 | 0.751 | 0.512* | 0.674 | 0.792 |
| Ptpn2 | 117063 | 1.272 | 1.474* | 1.298 | 1.271 |
| Ywhab | 56011 | 1.426* | 1.396* | 1.012 | 0.906 |
| Cdc14b | 361195 | 0.628* | 0.404* | 0.862 | 0.873 |
| Crebbp | 54244 | 0.927 | 0.662* | 0.964 | 1.049 |
| Ptch1 | 89830 | 0.673 | 0.675 | 1.121 | 0.83 |
| Prkaca | 25636 | 0.589* | 0.467* | 0.896 | 0.67 |
| Gsk3b | 84027 | 0.711 | 0.504* | 0.808 | 1.24 |
| Prkcz | 25522 | 0.684 | 0.352* | 2.042* | 1.381 |
| Crem | 25620 | 0.864 | 0.826 | 0.97 | 0.798 |
| Myh2 | 691644 | 1.048 | 1.017 | 0.89 | 1.093 |
| Tnni2 | 29389 | 0.626 | 1.965 | 0.787 | 1.159 |
| Mylpf | 24584 | 0.55 | 1.825 | 0.638 | 1.15 |
| Myl1 | 56781 | 0.724 | 1.659 | 0.682 | 1.262 |
| Myh4 | 360543 | 0.301 | 0.416 | 0.678 | 1.161 |
| Gys1 | 690987 | 0.787 | 0.792 | 0.69 | 0.86 |
| Pygm | 24701 | 0.662 | 1.092 | 0.758 | 1.051 |
| Ptk2 | 25614 | 0.876 | 0.798 | 0.895 | 1.047 |
| Plcd1 | 24655 | 0.907 | 1.011 | 0.936 | 0.95 |
| Dhh | 84380 | 0.664* | 0.67* | 0.892 | 1.083 |
| Gnb4 | 294962 | 0.875 | 1.106 | 1.286 | 1.167 |
| Finb | 306204 | 0.857 | 0.941 | 1.105 | 1.211 |
| Ptprj | 29645 | 0.721 | 0.708 | 1.293 | 1.68$ |
| Dusp6 | 116663 | 1.183 | 1.081 | 1.249 | 1.009 |
| Adcy4 | 54223 | 0.859 | 0.845 | 1.069 | 1.081 |
| Eya3 | 313027 | 0.882 | 0.683* | 0.989 | 1.079 |
| Akap10 | 360540 | 0.8 | 0.6* | 0.766 | 1.119 |
| Pygb | 25739 | 0.915 | 0.941 | 0.965 | 1.406* |
| Gng5 | 79218 | 1.167 | 1.277* | 1.004 | 1.077 |
| Ppp1r14b | 259225 | 0.697 | 0.845 | 0.76 | 0.9 |
| Nfatc3 | 361400 | 1.038 | 0.978 | 1.052 | 0.949 |
| Pdia3 | 29468 | 1.304 | 1.568* | 0.775 | 0.906 |
| Tgfbr1 | 29591 | 0.875 | 0.881 | 1.07 | 1.338 |
| Rhoa | 117273 | 1.022 | 1.089 | 0.974 | 1.031 |
| Tcf7l1 | 312451 | 0.962 | 0.998 | 1.18 | 1.007 |
| Prkar1a | 25725 | 1.067 | 1.127 | 0.842 | 1.002 |
Results of IPA analysis to evaluate the effects of EX and SPRINT on expression of genes in this IPA canonical signaling pathway. Results are expressed as fold change for the EX (OE) or SPRINT (OI) values relative to that of the sedentary OLETF value for that sample. Thus any entry that is >1.00 (italic font) reflects a gene with increased expression and any entry that is <1.00 (nonitalic font) reflects a gene with decreased expression. The * indicate a value that is significantly changed relative to sedentary OLETF rats (P < 0.01);
P < 0.05. The Symbol and Gene IDs are from the National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov/) gene bank and defined therein.
The endothelin-1 signaling pathway (Table 3) contained a number of genes showing altered expression due to EX and/or SPRINT in some cases in both arterioles examined and in others only in one. EX and/or SPRINT decreased expression of endothelin converting enzyme (Ece1) in DFA. Of interest, MAPK6 (Mapk6), which is activated following insulin stimulation, showed decreased expression or no change in expression in both DFA and D2a with both EX and SPRINT. Myelocytomatosis oncogene (Myc) expression is not altered by EX or SPRINT in DFA but was increased in D2a by EX only. Also, phospholipase A2, group V (Pla2g5) expression was increased by EX and SPRINT in DFA, and protein kinase C, zeta (Prkcz) was decreased in DFA and increased in D2a.
Table 3.
ET-1 signaling IPA canonical pathway
| Symbol | GeneID | DFA.OE | DFA.OI | D2a.OE | D2a.OI |
|---|---|---|---|---|---|
| Shc1 | 85385 | 0.813 | 0.608* | 0.808 | 0.869 |
| Fos | 314322 | 0.346* | 0.325* | 0.976 | 0.983 |
| Pla2 g5 | 29354 | 1.352* | 1.626* | 0.895 | 1.163 |
| Prkcz | 25522 | 0.684 | 0.352* | 2.042* | 1.381 |
| Prdx6 | 94167 | 1.517* | 1.476* | 1.188 | 1.082 |
| Casq1 | 686019 | 0.535 | 1.112 | 0.664 | 1.221 |
| Plcd1 | 24655 | 0.907 | 1.011 | 0.935 | 0.95 |
| Pla2 g16 | 24913 | 1.513* | 1.12 | 1.014 | 1.084 |
| Myc | 24577 | 1.162 | 0.957 | 1.506* | 0.962 |
| Adcy4 | 54223 | 0.859 | 0.845 | 1.069 | 1.081 |
| Pik3r6 | 497932 | 1.032 | 1.036 | 1.28 | 0.848 |
| Pld4 | 362792 | 0.999 | 0.766* | 1.32 | 0.903 |
| Pdia3 | 29468 | 1.304 | 1.568* | 0.775 | 0.906 |
| Mapk6 | 58840 | 0.633* | 0.698* | 0.645* | 0.997 |
| Gnat1 | 363143 | 1.113 | 0.796 | 1.226 | 1.002 |
| Ece1 | 94204 | 0.674 | 0.67 | 0.903 | 0.941 |
Results of IPA analysis to evaluate the effects of EX and SPRINT on expression of genes in this IPA Canonical pathway. Results are expressed as fold change for the EX (OE) or SPRINT (OI) values relative to that of the sedentary OLETF value for that sample. Thus any entry that is >1.00 (italic font) reflects a gene with increased expression and any entry that is <1.00 (nonitalic font) reflects a gene with decreased expression. The * indicate a value that is significantly changed relative to sedentary OLETF rats (P < 0.01). The Symbol and Gene IDs are from the National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov/) gene bank and defined therein.
NOS3 mRNA expression was not altered by EX or SPRINT in DFA or D2a, but Akt1 expression was decreased by EX and SPRINT in the DFA (Table 4). Neither EX nor SPRINT increased expression of heat shock protein Hspa5 or Hsp90b1 in DFA. Also, PKG1 (Prkg1) showed decreased expression in DFA and D2a with EX but was not altered by SPRINT.
Table 4.
eNOS signaling IPA canonical pathway
| Symbol | GeneID | DFA.OE | DFA.OI | D2a.OE | D2a.OI |
|---|---|---|---|---|---|
| Lpar4 | 302378 | 0.874 | 0.35* | 1.072 | 1.084 |
| Akt1 | 24185 | 0.673* | 0.549* | 0.979 | 1.146 |
| Prkaca | 25636 | 0.589* | 0.467* | 0.896 | 0.67 |
| Hspa5 | 25617 | 1.257* | 1.525 | 0.959 | 1.312* |
| Prkcz | 25522 | 0.684 | 0.352* | 2.042* | 1.381 |
| Aqp4 | 25293 | 0.319 | 0.432 | 1.024 | 1.297 |
| Hsp90b1 | 362862 | 1.009 | 0.978 | 1.096 | 1.192* |
| Prkg1 | 54286 | 0.735 | 0.995 | 0.708 | 1.015 |
| Adcy4 | 54223 | 0.859 | 0.845 | 1.069 | 1.081 |
| Pik3r6 | 497932 | 1.032 | 1.036 | 1.28 | 0.848 |
| Prkar1a | 25725 | 1.067 | 1.127 | 0.842 | 1.002 |
Results of IPA analysis to evaluate the effects of EX and SPRINT on expression of genes in this IPA Canonical pathway. Results are expressed as fold change for the EX (OE) or SPRINT (OI) values relative to that of the sedentary OLETF value for that sample. Thus any entry that is >1.00 (italic font) reflects a gene with increased expression and any entry that is <1.00 (nonitalic font) reflects a gene with decreased expression. The * indicate a value that is significantly changed relative to sedentary OLETF rats (P < 0.01). The Symbol and Gene IDs are from the National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov/) gene bank and defined therein.
Table 5 shows results of IPA analysis to evaluate the effects of EX and SPRINT on expression of genes in the Production of NO and ROS canonical pathway.
Table 5.
Production of NO and ROS IPA canonical pathway
| Symbol | GeneID | DFA.OE | DFA.OI | D2a.OE | D2a.OI |
|---|---|---|---|---|---|
| Nfkbia | 25493 | 1.853* | 1.919* | 1.366 | 1.306 |
| Rbp4 | 25703 | 2.96* | 2.555* | 1.275 | 0.436* |
| Ppp2cb | 24673 | 0.682* | 0.598* | 0.779 | 0.916 |
| Map3k12 | 25579 | 0.809 | 0.49* | 0.798 | 0.836 |
| Fos | 314322 | 0.346* | 0.325* | 0.976 | 0.983 |
| Akt1 | 24185 | 0.673* | 0.549* | 0.979 | 1.146 |
| Crebbp | 54244 | 0.927 | 0.662* | 0.964 | 1.049 |
| Rac1 | 363875 | 0.59* | 0.61* | 1.207 | 1.275 |
| Map3k7 | 313121 | 0.762* | 0.663* | 1.119 | 1.056 |
| Prkcz | 25522 | 0.684 | 0.352* | 2.042* | 1.381 |
| Ppp2r1a | 117281 | 1.191 | 1.357* | 0.969 | 1.012 |
| Pik3r6 | 497932 | 1.032 | 1.036 | 1.28 | 0.848 |
| Arg2 | 29215 | 1.214 | 0.841 | 1.127 | 1.329 |
| Stat1 | 25124 | 1.052 | 0.98 | 0.767 | 0.86 |
| Ppp1r14b | 259225 | 0.697 | 0.845 | 0.76 | 0.9 |
| Map3k6 | 313022 | 1.262 | 1.151 | 1.37 | 1.263 |
| Rhoa | 117273 | 1.022 | 1.089 | 0.974 | 1.031 |
| Rhot2 | 287156 | 1.345$ | 1.207 | 0.807 | 1.129 |
Results of IPA analysis to evaluate the effects of EX and SPRINT on expression of genes in this IPA canonical pathway. Results are expressed as fold change for the EX (OE) or SPRINT (OI) values relative to that of the sedentary OLETF value for that sample. Thus any entry that is >1.00 (italic font) reflects a gene with increased expression and any entry that is <1.00 (nonitalic font) reflects a gene with decreased expression. The * indicate a value that is significantly changed relative to sedentary OLETF rats (P < 0.01);
P < 0.05. The Symbol and Gene IDs are from the National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov/) gene bank and defined therein.
Because the Nrf2-mediated oxidative stress response canonical pathway was significantly affected by EX and SPRINT and because Nrf2 (a nuclear transcription factor that enhances antioxidant defenses and counteracts oxidative stress) is considered to attenuate cardiovascular disease (59) we analyzed the effects of EX and SPRINT on this canonical pathway. As shown in Table 6, SPRINT increased expression of 11 Nrf2 pathway genes and EX increased expression of 5 Nrf2 pathway genes in the DFA. EX caused decreased expression of 5 Nrf2 pathway genes and SPRINT caused decreased expression of 9 Nrf2 pathway genes in DFA. Only FK506 binding protein (works with glucocorticoid receptors) (Fkbp5) and flavin containing monooxygenase 1 (Fmo1) were increased by EX and SPRINT in both DFA and D2a. Superoxide dismutase 3 (Sod3) was increased by EX and SPRINT in the DFA but not significantly altered in D2as.
Table 6.
NRF2-mediated oxidative stress responses IPA canonical pathway
| Symbol | GeneID | DFA.OE | DFA.OI | D2a.OE | D2a.OI |
|---|---|---|---|---|---|
| Fkbp5 | 361810 | 2.098* | 1.753* | 3.106* | 3.031* |
| Fmo1 | 25256 | 2.691* | 1.699* | 1.56* | 1.058 |
| Map2k6 | 114495 | 0.856 | 0.456* | 1.091 | 1.02 |
| Actb | 81822 | 1.344 | 1.656* | 0.942 | 0.975 |
| Crebbp | 54244 | 0.927 | 0.662* | 0.964 | 1.049 |
| Herpud1 | 85430 | 1.3 | 1.629* | 1.257 | 1.184 |
| Dnajb2 | 689593 | 1.332 | 1.518* | 0.906 | 1.194 |
| Dnaja1 | 65028 | 1.475 | 1.974* | 1.171 | 1.177 |
| Prkcz | 25522 | 0.684 | 0.352* | 2.042* | 1.881 |
| Sod3 | 25352 | 1.889* | 2.354* | 1.24 | 0.978 |
| Fos | 314322 | 0.346* | 0.329* | 0.976 | 0.983 |
| Akt1 | 24185 | 0.673* | 0.549* | 0.979 | 1.146 |
| Map3k7 | 313121 | 0.762* | 0.663* | 0.966 | 0.934 |
| Jund | 24518 | 0.629* | 0.471* | 0.98 | 0.9 |
| Gsk3b | 84027 | 0.711* | 0.504* | 0.808 | 1.24 |
| Actg2 | 25365 | 1.332 | 2.435* | 1.044 | 1.287 |
| Actg1 | 287876 | 1.201 | 1.4* | 0.976 | 1 |
| Fth1 | 25319 | 1.891* | 2.128* | 1.271 | 1.033 |
| Enc1 | 294674 | 1.021 | 0.825 | 1.264 | 1.172 |
| Prdx1 | 117254 | 1.392* | 1.419* | 0.859 | 1.079 |
| Pik3r6 | 497932 | 1.032 | 1.036 | 1.28 | 0.848 |
| Dnajc15 | 290370 | 1.306 | 1.64* | 0.85 | 1.021 |
| Actc1 | 29275 | 0.782 | 1.189 | 0.752 | 1.255 |
| Gstp1 | 24426 | 1.064 | 1.16 | 1.224 | 1.191 |
| Mgst3 | 289197 | 0.868 | 0.949 | 0.945 | 1.171 |
| Ephx1 | 25315 | 1.263 | 1.265 | 0.956 | 1.001 |
| Mgst2 | 295037 | 1.165 | 0.742* | 1.213 | 0.892 |
| Gsta2 | 24422 | 0.987 | 0.898 | 1.203 | 0.987 |
| Stip1 | 192277 | 1.071 | 1.183 | 0.992 | 1.183 |
Results of IPA analysis to evaluate the effects of EX and SPRINT on expression of genes in this IPA canonical pathway. Results are expressed as fold change for the EX (OE) or SPRINT (OI) values relative to that of the sedentary OLETF value for that sample. Thus any entry that is >1.00 (italic font) reflects a gene with increased expression and any entry that is <1.00 (nonitalic font) reflects a gene with decreased expression. Value = 1 (boldface) reflects no change in expression. The * indicate a value that is significantly changed relative to sedentary OLETF rats (P < 0.01). The Symbol and Gene IDs are from the National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov/) gene bank and defined therein.
Both EX and SPRINT altered the expression of a number of genes in the protein ubiquitination pathway in the DFA (Table 7). It is of interest that the DFA (13 genes for SPRINT and 8 genes for EX) showed greater numbers of ubiquitination pathway genes with altered expression in EX and SPRINT groups than did the D2as (3 genes for SPRINT and 2 for EX) (Table 7). EX resulted in decreased expression of 5 genes in the ubiquitination pathway in DFA and decreased expression of 1 gene in this pathway in D2a. SPRINT resulted in decreased expression of 8 genes in the ubiquitination pathway in DFA and decreased expression of 3 genes in this pathway in D2a. Ubd expression was not changed by EX or SPRINT in either vessel. The WntI2-catenin signaling pathway analysis revealed decreased expression of Ubd, a gene coding for a protein involved in antigen processing and presentation (FAT10) for activating NF-kB. Ubd is decreased in DFA and D2a by both EX and SPRINT. Myc expression was not altered by EX or SPRINT in either vessel.
Table 7.
Protein ubiquitination canonical pathway
| Symbol | GeneID | DFA.OE | DFA.OI | D2a.OE | D2a.OI |
|---|---|---|---|---|---|
| Birc6 | 313876 | 0.647* | 0.589* | 0.829 | 0.837 |
| Usp28 | 315639 | 0.734 | 0.544* | 0.865 | 1.018 |
| Ube4b | 298652 | 0.823 | 0.632* | 0.861 | 0.992 |
| Dnajb2 | 689593 | 1.332* | 1.518* | 0.906 | 1.194 |
| Usp1 | 313387 | 0.697$ | 0.534* | 1.014 | 1.113 |
| Dnaja1 | 65028 | 1.475$ | 1.974* | 1.171 | 1.177 |
| Hspa5 | 25617 | 1.257 | 1.525* | 0.959 | 1.312$ |
| Ube4a | 315608 | 0.835 | 0.695* | 1.094 | 1.002 |
| Usp7 | 360471 | 0.69 | 0.62* | 0.866 | 0.832 |
| Psme1 | 29630 | 1.402* | 1.592* | 1.112 | 1.091 |
| Usp42 | 288482 | 0.606* | 0.603* | 1.444 | 1.272 |
| Usp47 | 308896 | 0.708* | 0.681* | 0.891 | 0.989 |
| Psma3 | 29670 | 1.138 | 1.288* | 0.893 | 1.08 |
| Ube2j2 | 298689 | 1.314 | 1.428* | 0.846 | 0.851 |
| Cul1 | 362356 | 0.902 | 0.948 | 0.88 | 0.954 |
| Psmb3 | 29676 | 0.896 | 1.023 | 1.061 | 1.057 |
| Cryab | 25420 | 0.919 | 1.279 | 1.068 | 1.424 |
| Usp18 | 312688 | 1.398 | 0.859 | 0.618 | 0.661 |
| Dnajc15 | 290370 | 1.306 | 1.64* | 0.85 | 1.021 |
| Skp1 | 287280 | 1.24 | 1.344$ | 0.936 | 1.22 |
| Tceb1 | 64525 | 0.931 | 1.199 | 0.922 | 1.164 |
| Ubd | 29168 | 0.448 | 0.563 | 0.459 | 0.416 |
| Dnajc24 | 362184 | 0.777 | 0.942 | 0.842 | 0.93 |
| Hsp90b1 | 362862 | 1.009 | 0.978 | 1.096 | 1.192 |
| Psmb2 | 29675 | 1.036 | 1.209 | 0.927 | 1.048 |
| Psmd12 | 287772 | 1.061 | 1.107 | 0.82 | 1.048 |
| Psma5 | 29672 | 0.932 | 0.952 | 0.919 | 1.026 |
| Psmb1 | 94198 | 0.915 | 1.078 | 1.02 | 1.112 |
| Usp46 | 289584 | 1.11 | 0.757 | 0.901 | 0.983 |
| Psma2 | 29669 | 1.013 | 1.094 | 0.943 | 1.171 |
| Birc2 | 60371 | 0.936 | 0.857 | 0.871 | 0.907 |
| Psmd5 | 296651 | 1.177 | 1.092 | 0.816 | 0.944 |
| Uchl1 | 29545 | 0.747 | 0.743 | 1.339 | 1.72 |
| Dnajc12 | 619393 | 0.878 | 0.894 | 1.496$ | 1.273 |
| Psma4 | 29671 | 1.041 | 1.137 | 0.948 | 1.015 |
| Psmd14 | 311078 | 1.055 | 1.182 | 0.76 | 0.895 |
| Psmc2 | 25581 | 0.946 | 0.967 | 0.882 | 0.961 |
Results of IPA analysis to evaluate the effects of EX and SPRINT on expression of genes in this IPA canonical pathway. Results are expressed as fold change for the EX (OE) or SPRINT (OI) values relative to that of the sedentary OLETF value for that sample. Thus any entry that is >1.00 (italic font) reflects a gene with increased expression and any entry that is <1.00 (nonitalic font) reflects a gene with decreased expression. The * indicate a value that is significantly changed relative to sedentary OLETF rats (P < 0.01);
P < 0.05. The Symbol and Gene IDs are from the National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov/) gene bank and defined therein.
Fzd6, fizzled family receptor 6, is decreased in DFA by SPRINT, and fizzled family receptor 8 expression was decreased in D2a by SPRINT. SHC transforming protein 1 (Shc1), a growth factor, exhibited decreased expression in the IST-DFA and tended to be decreased in the EX-DFA and D2a. Shc1 is also a component of other IPA canonical pathways examined, including endothelin-1 signaling, insulin receptor signaling, integrin signaling, PLC signaling, PPAR-signaling, renin-angiotensin and the VEGF signaling pathways. Thus it is not possible at this time to determine for which pathway decreased Shc1 expression is most important. SPRINT also produced decreased expression of 8 genes in the insulin receptor signaling pathway (Shc1, Fyn, Akt1, Asic1, Prkaca, Gsk3b, Prkcz, and Sgk1) of DFA, and EX tended to decrease expression of these same genes in the DFA. Two genes listed above (Lipe, Gys2) and Eif4ebp1 showed increased expression in the DFA of both SPRINT and EX groups. There were minimal EX and SPRINT effects on gene expression in the insulin receptor pathway of D2a.
Analysis of results also revealed that the solute carrier group of membrane transport proteins (Slc) family of genes, had a number of genes whose expression was altered differentially by EX and SPRINT. Results indicate that expression of 30 Slc genes was altered by EX and/or SPRINT (Table 8). As can be appreciated from Table 8, EX and SPRINT induced a number of changes in Slc gene expression (EX: 8 increased and 6 decreased; and SPRINT: 3 increased and 15 decreased), but the patterns of changes were not the same for EX and SPRINT. EX and SPRINT produced the greatest changes in Slc gene expression in the DFA relative to the D2a. In most cases, Slc genes exhibited altered expression in only one vessel and with only one type of EX. Exceptions to this are Slc25a25 (decreased by SPRINT in both DFA and D2a) and Slc27a2, which had increased expression in the DFA with EX but decreased expression in the D2a of SPRINT. Another interesting observation is that several of the mitochondrial transport genes were changed in either DAF or D2a.
Table 8.
Effects of exercise on expression of genes coding for solute carrier transporter proteins in diaphragm arterioles/arteries
| Symbol | GeneID | DFA.OE | DFA.OI | D2a.OE | D2a.OI |
|---|---|---|---|---|---|
| Slc1a5 | 292657 | ↓ | |||
| Slc2a1 | 24546 | ||||
| Slc2a4 | 25139 | ↓ | |||
| Slc2a12 | 308028 | ↓ | |||
| Slc3a1 | 29484 | ↓ | |||
| Slc4alap | 298805 | ||||
| Slc4a1 | 24779 | ↓ | |||
| Slc6a4 | 25553 | ||||
| Slc6a6 | 29464 | ↑ | |||
| Slc6a8 | 50690 | ||||
| Slc7a1 | 25648 | ||||
| Slc7a3 | 29485 | ||||
| Slc7a15 | 307811 | ↑ | |||
| Slc9a3r2 | 116501 | ↑ | |||
| Slc9b1 | 365946 | ↓ | |||
| Slc10a6 | 289459 | ||||
| Slc10a7 | 291942 | ↓ | |||
| Slc12a7 | 308069 | ↓ | |||
| Slc15a3 | 246239 | ↓ | |||
| Slc16a4 | 295356 | ↑ | |||
| Slc20a1 | 81826 | ↓ | |||
| Slc22a18 | 309131 | ↑ | |||
| Slc24a6 | 498185 | ↓ | |||
| Slc25a5 | 25176 | ↑ | |||
| Slc25a19 | 303676 | ↑ | |||
| Slc25a21 | 171151 | ↓ | |||
| Slc25a25 | 310791 | ↓ | ↓ | ||
| Slc25a35 | 497933 | ↑ | |||
| Slc25a28 | 688811 | ↓ | |||
| Slc25a44 | 365841 | ↓ | |||
| Slc27a2 | 65192 | ↑ | ↓ | ||
| Slc28a2 | 60423 | ↓ | |||
| Slc30a9 | 498358 | ||||
| Slc35b1 | 287642 | ||||
| Slc35c1 | 311204 | ||||
| Slc35e3 | 362883 | ||||
| Slc35e4 | 266687 | ||||
| Slc35f1 | 502421 | ||||
| Slc37a1 | 294321 | ||||
| Slc37a4 | 29573 | ||||
| Slc38a5 | 92745 | ||||
| Slc38a14 | 306009 | ↑ | |||
| Slc38a5 | 192208 | ↓ | |||
| Slc39a13 | 295928 | ||||
| Slc40a1 | 170840 | ||||
| Slc41a1 | 363985 | ↓ | |||
| Slc43a2 | 287532 | ↓ | |||
| Slc43a3 | 311170 | ↑ | |||
| Slc46a3 | 288454 | ||||
| Slco2a1 | 24546 | ||||
| Ucp3 | 25708 |
Results are reflected as increased expression (upward arrow) or decreased expression (downward arrow). Only significant differences are shown (P < 0.01) for the EX (OE) or SPRINT (OI) values relative to that of the sedentary OLETF value for that sample. The Symbol and Gene IDs are from the National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov/) gene bank and defined therein.
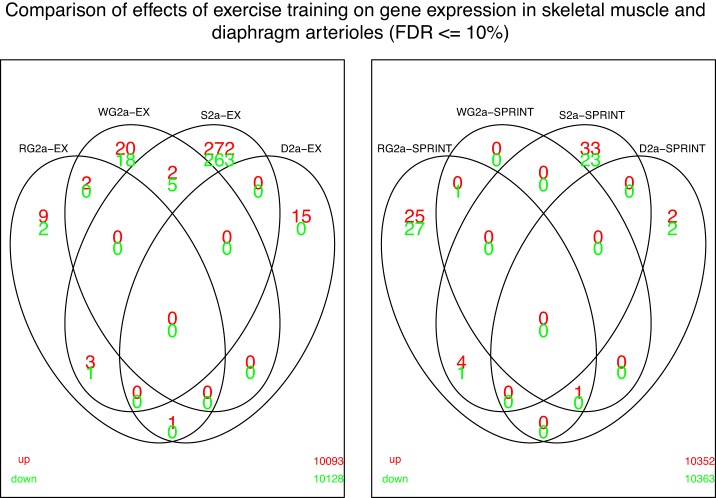
Figure 2 uses Venn diagrams to present results showing the number of genes in the D2a whose expression was changed by EX and SPRINT, compared with similar arterioles from red gastrocnemius (RG2a), white gastrocnemius (WG2a) and soleus 2a arterioles (S2a), and at the intersections of the circles, genes changed in all four types of arterioles (FDR of 10%). These results show that there were no intersections where EX or SPRINT altered expression of the same genes in all four arterioles. While soleus and diaphragm 2as exhibited the largest number of genes with altered expression following EX, none of these genes were common to both S2a and D2a. RG2a shared some genes with altered expression following EX with WG2a, S2a, and D2a. There was only one gene whose expression was altered by EX in the RG2a and D2a. The D2a only had three upregulated genes and 2 downregulated genes following SPRINT. The D2a only had one gene with altered expression shared with another arteriole and that was the WG2a (Fig. 2).
Fig. 2.
Venn diagrams illustrating the number of genes whose expression was altered by endurance exercise (EX) and by interval sprint training (SPRINT) in diaphragm 2a arterioles (D2a) contrasted with results in the various arterioles from red gastrocnemius and white portion of the gastrocnemius (RG2a and WG2a) and soleus (S2a) muscles. Red font indicates upregulated genes and green font downregulated genes relative to expression in sedentary rats. Overlapping areas represent genes whose expression were changed in the different arterioles by either EX or SPRINT. These intersections are complex. In the center left of the figure note that there are no genes up- or downregulated in all 4 arterioles by EX and on the right by SPRINT. A striking finding is that there were no genes with altered expression in RG2a and S2a that were also changed in D2a for either EX or SPRINT. The figure also illustrates in the lower left portion that both the D2a and RG2a exhibited increased expression of one gene (Mup5) after EX. In contrast, in the lower right is illustrated that D2a and RG2a both exhibited increased expression of a gene (Fkbp5) after SPRINT. Fkbp5 was also increased in WG2a.
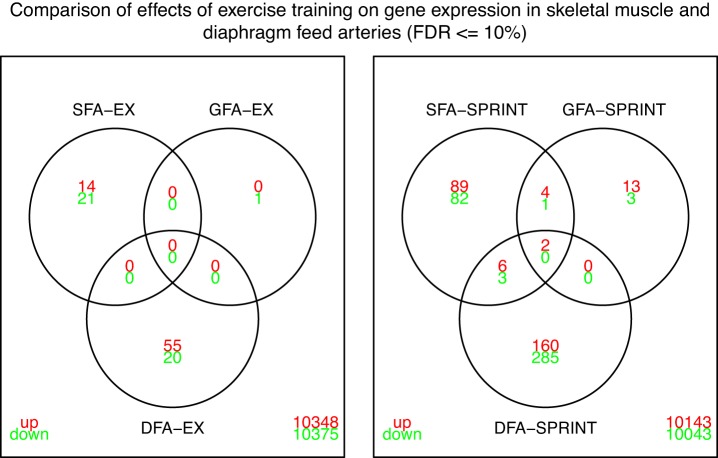
Figure 3 uses Venn diagrams to present results showing the number of genes in the DFA, gastrocnemius feed artery (GFA), and soleus feed artery (SFA) whose expression was changed by EX and SPRINT. Results show that DFA had no changes in gene expression shared by SFA or GFA after EX. With SPRINT, the DFA shared 2 genes whose expression was increased by SPRINT in all three feed arteries. The SFA also shared 6 upregulated and 3 downregulated genes with DFA, but the GFA only shared the 2 genes changed in all three feed arteries. Overall, the results in Figs. 2 and 3 indicate that while there were 456 genes whose expression was changed in the DFA of SPRINT rats and 16 whose expression was increased in D2a by EX, there are few similarities between the alterations in gene expression induced by EX and SPRINT in diaphragm arteries/arterioles and the arteries/arterioles of limb skeletal muscles.
Fig. 3.
Venn diagrams illustrating the number of genes whose expression was altered by endurance exercise (EX) and by interval sprint training (SPRINT) in in diaphragm feed artery (DFA) contrasted with results in the gastrocnemius feed artery (GFA), soleus feed artery (SFA). Red font indicates upregulated genes and green font downregulated genes relative to expression in sedentary rats. Overlapping area represents genes whose expression were changed by EX or SPRINT in two or more arteries. A striking finding is that there were no genes with altered expression after EX in SFA and GFA that were also changed in DFA. In contrast, there were 2 genes (Wisp2 and Tubb2b) that had increased expression after SPRINT in SFA and GFA that were also increased in DFA. Finally, the data in this figure demonstrate that SFA and DFA shared 6 genes that were upregulated by SPRINT and 3 that were downregulated. Thus SFA showed more similar changes in transcriptome to that of DFA than did GFA.
DISCUSSION
The purpose of this study was to determine the effects of EX and SPRINT on the transcriptome of DFA and D2a of OLETF rats, a model of T2D, and to compare and contrast these effects with those observed in limb skeletal muscle (40). Our hypothesis was that the effects of exercise training on the transcriptome of diaphragm arteries/arterioles would be similar to those we reported for the soleus muscle (40) because the soleus and diaphragm have similar resting and exercise-induced blood flows (8–10, 35, 36, 54, 62). This hypothesis is based on the concept that shear stress on the vessel wall caused by increased blood flow during exercise training bouts is a major signal for changes in vascular gene expression in both soleus and diaphragm. In addition, given that understanding exercise-induced alterations in the phenotype of arterioles and/or arteriolar vascular cells is one key to establishing mechanisms responsible for the beneficial effects of exercise in prevention and treatment of cardiovascular effects of T2D we explored the differential effects of two exercise training protocols. We tested our hypothesis by evaluating the impact of EX and SPRINT on vascular gene expression profiles in the DFA and D2a using transcriptome-wide RNA-Seq analysis to comprehensively characterize vascular adaptations and allow comparison to limb skeletal muscle arterioles. A significant finding of the study is that treadmill exercise in rats, both EX and SPRINT, alters gene expression patterns differently in the D2a than in DFA and that these changes in gene expression are also not the same as in limb skeletal muscle arterioles (40). These results do not support our hypothesis that the effects of both types of exercise training would be similar in soleus and diaphragm arterioles. This finding is puzzling given the knowledge that treadmill bouts of exercise appear to produce similar changes in blood flow to soleus and diaphragm of rats.
The hypothesis that hemodynamic forces, including shear stress and wall stretch, that are increased in arteries providing blood flow to active striated muscle are among the key signals for altered gene expression in the arteriolar network of skeletal muscle drove this series of experiments. Available literature indicates that diaphragm blood flow in rats prior to treadmill (8–10, 35, 36, 54, 62) or swimming (39) exercise is in the range of 50–150 ml·min−1·100 g−1 and can increase to over 300 ml·min−1·100 g−1 during maximal exercise (8, 10). So it is clear that diaphragm and soleus muscle have relatively high resting blood flows which increase during exercise. Although some results from this study are consistent with this concept of exercise-induced adaptation, most of the genes whose expression was altered by SPRINT and EX are not considered shear stress-responsive genes (14, 16). Overall the number of genes with altered expression and the variety of biological processes controlled by these genes suggests that this conception of the signal for adaptation is oversimplified. Clearly the adaptions of diaphragm muscle vasculature are complex and the biological processes involved in signaling the altered gene expression patterns are not fully apparent at this time.
Because the diaphragm has been reported to not undergo adaptations to exercise training, perhaps because the respiratory system is “built for exercise” (53, 55, 56, 58), it would not have been surprising to find little to no effect of exercise training on diaphragm arterioles. Indeed we expected that both diaphragm and soleus arterioles would be less impacted by exercise training than are those of fast-twitch skeletal muscle because the relative effects of exercise on metabolism and blood flow are much greater in fast-twitch skeletal muscle (40). Although there were reports of no effects of exercise training on the diaphragm in the 1980's, it now seems clear that metabolic pathways and contractile proteins in the diaphragm undergo adaptive changes in response to exercise training (i.e., small but significant increases in oxidative capacity and antioxidant capacity) (55, 56, 58, 63). Contrary to our hypothesis that exercise training would have greater effects in arterioles of fast-twitch muscle, our results indicate that the 2a arterioles of soleus muscle (S2a) from endurance-trained animals and the DFA of IST animals exhibited the largest number of genes with altered expression (Figs. 2 and 3). Thus arterioles perfusing these two muscles, soleus and diaphragm, exhibit the largest number of alterations in gene expression, not the arterioles of fast-twitch gastrocnemius muscle as we proposed.
The diaphragm of mammals is a unique skeletal muscle in that it is chronically active in life, even during sleep. Thus, while slow-twitch postural muscles are somewhat similar in that they are active when the animal maintains posture, the diaphragm is perhaps more similar to cardiac muscle in that it is always active. Also, the diaphragm is among the most aerobically adapted skeletal muscles with oxidative capacity and capillary density similar to that of cardiac muscle. Another interesting difference between the diaphragm and limb skeletal muscle is the effects of obesity. It appears that severe obesity increases the load on respiratory muscles inducing adaptations in the diaphragm (21, 57). For example, Powers et al. (57) and Farkas et al. (21) reported that oxidative capacity was increased in the diaphragm muscles of obese Zucker rats. This effect of obesity on diaphragm muscle may help explain the different effects of obesity on the transcriptome of limb and diaphragm muscle arteries (49). That is, we previously reported that, in limb skeletal muscle, the number of genes whose expression was significantly altered by obesity increased going from feed artery down the arteriolar tree to the small arterioles (50). In contrast, in the diaphragm the number of genes whose expression was significantly altered by obesity decreased going from the feed artery down the arteriolar tree (50). Thus obesity appeared to have differential effects on the transcriptome of diaphragm and limb skeletal muscle arterioles. These obesity-induced differential changes in transcriptome between limb muscle arterioles and diaphragm may be related to the adaptations of the muscle fibers induced in the diaphragm by obesity.
Present results indicate that both EX and SPRINT induced significant changes in the expression of more genes in the DFA than in the D2a (Fig. 1), and these effects are different from what was observed in similar branch orders of limb skeletal muscle arterioles (Figs. 2 and 3). Also, in our previous work we reported that in the soleus muscle, EX had greater effects on gene expression in the 2a arteriole than did SPRINT. Here, in a similar fashion, EX had greater effects on gene expression in the D2a than did SPRINT (Fig. 1), but in contrast, SPRINT had greater effects on gene expression in the DFA than did EX (Fig. 1). We previously reported that while SPRINT altered expression of 368 genes in RG2a, 247 genes in the GFA, and 147 genes in the WG2a, the phenotypic effects of SPRINT on these three vessels was quite different. Also we reported that EX had effects on fewer genes in the gastrocnemius circulation than did SPRINT (40). As shown in Fig. 2, EX had greater effects on gene expression in the D2a than did SPRINT. This was also true of S2a but the number of genes with altered expression was much greater in S2a for both SPRINT and EX than for D2a (Fig. 2). Thus, based on these patterns of gene expression change, both types of exercise training had dramatically different effects on arteries/arterioles of diaphragm and gastrocnemius. Further, while effects of exercise on gene expression in diaphragm arteries/arterioles is more similar to those of soleus muscle, there were many more changes in S2a than in D2a whereas there were many more genes with altered expression in the DFA than in the SFA of these animals (Figs. 2 and 3) (40).
IPA pathway analysis of results indicates that the top canonical pathways influenced by EX in DFA were fatty acid β-oxidation 1, triacylglycerol degradation, retinol biosynthesis, LXR/RXR activation, and PEDF signaling. In contrast, SPRINT had the greatest effects on NRF2-mediated oxidative stress response, 14-3-3-mediated signaling, B cell receptor signaling, sertoli cell-sertoli cell junction signaling, and glucocorticoid receptor signaling in the DFA. In the D2a, EX had the greatest effect on the following pathways: bupropion degradation, acetone degradation 1, PPARα/RXRα activation, estrogen biosynthesis, and nicotine degradation III. The top canonical pathways of D2a of SPRINT were fatty acid activation, mitochondrial l-carnitine pathway, linoleate biosynthesis II, sonic hedgehog signaling, and phenylalanine degradation IV. Thus effects of EX and SPRINT on genes coding for canonical pathways were different in D2a vs. DFA and generally EX and SPRINT altered different pathways in these two vessels. Contrary to our hypothesis, the top canonical pathways altered by EX and SPRINT in DFA and D2a were different than in soleus feed arteries and 2a arterioles as well as being different from the pathways changed in gastrocnemius arteries/arterioles (Figs. 2 and 3).
One important feature of the 14-3-3 proteins is their ability to bind a number of different signaling proteins, including kinases, phosphatases, and transmembrane receptors. This plethora of interacting proteins allows 14-3-3 to play important roles in a wide range of vital regulatory processes, such as mitogenic signal transduction, apoptotic cell death, and cell cycle control. The role of this family of highly divergent proteins plays in the vascular adaptation to SPRINT in the DFA is not clear at this time. These proteins are known to modulate function of their targets at various levels, such as subcellular localization, stability, phosphorylation, biological activity, and/or dynamic interactions.
Previous results indicate that SPRINT training had the greatest effects on expression of GFA genes coding for structural/contractile proteins with additional effects on signaling pathways/genes in the GFA and that, in general, genes whose expression was changed by EX and SPRINT in the GFA were largely different from those in other limb muscle arterioles (40). Importantly several pathways that were significantly altered by exercise in limb skeletal muscles were not among the list of pathways significantly changed in the diaphragm vessels (40).
Although SPRINT had different effects on phenotype of soleus arterioles than those in the gastrocnemius muscle it appears that soleus and diaphragm show some similarities in the effects of EX and SPRINT on the transcriptome of the arterioles/feed arteries. Glucocorticoid receptor signaling and NRF2-mediated oxidative stress response pathways were two of the top canonical pathways altered by SPRINT in the SFA and DFA but not the GFA, consistent with our hypothesis that soleus and diaphragm arterioles would exhibit similar adaptations. The only canonical pathway altered by SPRINT in DFA, SFA, and GFA was the epithelial adherens junction signaling pathway.
The top canonical pathways for the S2a of SPRINT rats were completely different from those of the D2a of SPRINT rats (Fig. 2). Thus most genes with altered expression in SPRINT-S2as are not altered in D2a. As shown in Fig. 2, none of the 61 genes whose expression was changed by SPRINT in the S2a were also changed by SPRINT in the D2a. Also, EX induced changes in expression of 538 genes in the S2a, but none of these genes exhibited altered expression in the D2a of EX.
Effects of SPRINT are different from effects of EX in diaphragm arteries/arterioles.
EX training altered the expression of a smaller number of genes than did SPRINT in the DFA but induced a larger number of genes with altered expression in the D2a than did SPRINT. In fact, FDR 10% analysis indicated that only 2 genes exhibited altered expression in D2a of SPRINT rats. As shown in Figs. 2 and 3, SPRINT and EX altered expression of different genes in the arteries/arterioles of the diaphragm than in either soleus or gastrocnemius muscle.
Amaral and Michelini (5) previously examined exercise training-induced structural vascular adaptation in diaphragm of normal and SHR rats and found that exercise training increased capillary density in diaphragm of normal and SHR rats and decreased wall to lumen ratio in SHR diaphragm arterioles. Amaral and Michelini (5) also reported that microvascular rarefaction (decreased numbers of microvessels) was decreased in limb skeletal muscles by exercise in hypertensive rats (SHR), suggesting that the beneficial effects of exercise occur in limb skeletal muscle as well as diaphragm (5). It is not known whether similar responses occur in T2D so we examined our results for evidence of changes in expression of genes linked to structural vascular adaptation. In contrast to what was reported in the GFA of these rats (40), there were few changes in expression of genes coding for structural proteins in the DFA or D2a suggesting less vascular remodeling in the diaphragm arteriolar tree than in the gastrocnemius muscle.
EX and/or SPRINT altered expression of 30 genes coding for the solute carrier group of membrane transport proteins (Slc) involved in transporting solutes across membranes and transport proteins in DFA and D2a (Table 8). Some Slc proteins are PDZ scaffolding proteins that mediate cellular processes by binding to and regulating the membrane expression and protein-protein interactions of membrane receptors. The Slc transport proteins include over 386 protein-coding genes from 52 gene families (13, 22, 24, 61). EX induced increased expression of 8 genes and decreased expression of 6 genes. SPRINT induced increased expression of 3 genes and decreased expression of 15 genes (Table 8). The patterns of changes were not the same for EX and SPRINT. EX and SPRINT produced the greatest changes in Slc gene expression in the DFA relative to the D2a arterioles. In most cases, Slc genes exhibited altered expression in only one arteriole and with only one type of EX. Exceptions to this are Slc25a25 (decreased by SPRINT in both DFA and D2a) and Slc27a2 which had increased expression in the DFA with EX but decreased expression in the D2a of SPRINT. Overall these results support our conclusion that changes in vascular gene expression patterns differ along the arteriolar tree in a given muscle.
Because Vassilakopoulos et al. (65) reported that exercise training resulted in increased NOS activity in the rat diaphragm as well as increased NOS3 protein, we expected to find that EX and/or SPRINT increased NOS3 message in the DFA and D2a. However, results indicated that NOS3 transcript was not altered by either form of exercise training. If NOS3 expression was increased by EX or SPRINT in the OLETF diaphragms it would suggest that this increase is more localized in the diaphragm muscle fibers than in the blood vessels as eNOS expression has been demonstrated associated with mitochondria in diaphragm muscle fibers (25, 30). Future research is required to address the question of whether EX or SPRINT increases NOS3 in the muscle fibers of OLETF rat diaphragm.
Caveats.
Results were obtained from whole arterioles so we cannot at this time establish which cell type in the feed arteries/arterioles is responsible for the altered gene expression. In each case the changes could be in endothelial, vascular smooth muscle, fibroblast and/or nerve cells. Future research is required to address this important question. Also, because we only examined the treatment effects of EX and SPRINT in OLETF rats the effects of these exercise training programs on vascular gene expression patterns may be different in nonobese rats.
We analyzed and interpreted the results of this study from our perspective of a search for mechanisms responsible for vascular adaptation in skeletal muscle arterioles that may contribute to the vascular health benefits in T2D. We recognize that this is a limited view of the large amount of data obtained in this study. We anticipate that others who will examine the results provided in the Supplemental Data, available with the online version of this article, will make equally important interpretations. There is no doubt that the effects of exercise training on vascular cell gene expression in the arteriolar network of diaphragm and limb skeletal muscle are complex. Present results combined with previous results (40) stimulate the conclusion that these effects differ with skeletal muscle fiber type and with location of the vascular cells in the vascular tree. Given the complex nature of changes in vascular gene expression reported herein, we consider that hemodynamic forces are but one of the exercise-induced signals mediating the regulation of vascular gene expression in skeletal muscle arteriolar trees.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants NIH-RO1-HL-036088 (M. H. Laughlin and J. W. Davis), T32-AR-048523 (N. T. Jenkins and J. S. Martin), and VHA-CDA2 1299-02 (R. S. Rector). J. Padilla is currently supported by National Heart, Lung, and Blood Institute Grant K01-HL-125503. This work was also supported in part with resources and the use of facilities at the Harry S Truman Memorial Veterans Hospital in Columbia, MO.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.H.L., J.S.M., and J.W.D. conception and design of research; M.H.L., J.S.M., and R.S.R. performed experiments; M.H.L., S.A., and J.W.D. analyzed data; M.H.L., J.S.M., R.S.R., and J.W.D. interpreted results of experiments; M.H.L., S.A., and J.W.D. prepared figures; M.H.L. drafted manuscript; M.H.L., J.S.M., R.S.R., S.A., and J.W.D. edited and revised manuscript; M.H.L. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank N. Fleming, E. Gibson, K. Tacchi, and M. Brielmaier for assisting in the care of the rats and exercise training. S. Blake (Global Biologics) performed the RNA extractions, and RNA libraries were submitted to the University of Missouri DNA Core Facility for high-throughput sequencing services.
REFERENCES
- 1.Aaker A, Laughlin MH. Diaphragm arterioles are less responsive to alpha-1-adrenergic constriction than gastrocnemius arterioles. J Appl Physiol 92: 1808–1816, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Aaker A, Laughlin MH. Differential adenosine sensitivity of diaphragm and skeletal muscle arterioles. J Appl Physiol 93: 848–856, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Aird WC. Phenotypic heterogeneity of the endothelium. I. Structure, function, and mechanisms. Circ Res 100: 158–173, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Aird WC. Phenotypic heterogeneity of the endothelium. II. Representative vascular beds. Circ Res 100: 174–190, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Amaral SL, Michelini LC. Effect of gender on training-induced vascular remodeling in SHR. Braz J Med Biol Res 44: 814–965, 2011. [DOI] [PubMed] [Google Scholar]
- 6.Anderson KM, Faber JE. Differential sensitivity of arteriolar α1- and α2-adrenoceptor constriction to metabolic inhibition during rat skeletal muscle contraction. Circ Res 69: 174–184, 1991. [DOI] [PubMed] [Google Scholar]
- 7.Ando H, Kubin T, Schaper W, Schaper J. Cardiac microvascular endothelial cells express alpha-smooth muscle actin and show low NOS III activity. Am J Physiol Heart Circ Physiol 276: H1755–H1768, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong RB, Laughlin MH. Blood flows within and among rat muscles as a function of time during high speed treadmill exercise. J Physiol 344: 187–208, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armstrong RB, Laughlin MH. Exercise blood flow patterns within and among rat muscles after training. Am J Physiol Heart Circ Physiol 246: H59–H68, 1984. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong RB, Laughlin MH. Rat muscle blood flows during high-speed locomotion. J Appl Physiol 59: 1322–1328, 1985. [DOI] [PubMed] [Google Scholar]
- 11.Bender SB, Newcomer SC, Harold Laughlin M. Differential vulnerability of skeletal muscle feed arteries to dysfunction in insulin resistance: impact of fiber type and daily activity. Am J Physiol Heart Circ Physiol 300: H1434–H1441, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Statistical Soc 57: 289–300, 1995. [Google Scholar]
- 13.Bhattacharya R, Wang E, Dutta SK, Vohra PK, EG, Prakash YS, Mukhopadhyay D. NHERF-2 maintains endothelial homeostasis. Blood 119: 4798–4806, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Björck HM, Renner J, Maleki S, Nilsson SFE, Kihlberg J, Folkersen L, Karlsson M, Ebbers T, Eriksson P, Länne T. Characterization of shear-sensitive genes in the normal rat aorta identifies Hand2 as a major flow-responsive transcription factor. PloS one 7: e52227, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bourgon R, Gentleman R, Huber W. Independent filtering increases detection power for high-throughput experiments. Proc Natl Acad Sci USA 107: 9546–9551, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu TJ, Peters DG. Serial analysis of the vascular endothelial transcriptome under static and shear stress conditions. Physiol Genomics 34: 185–192, 2008. [DOI] [PubMed] [Google Scholar]
- 17.de Jonge HJM, Fehrmann RSN, de Bont ESJM, Hofstra RMW, Gerbens F, Kamps WA, de Vries EGE, van der Zee AGJ, te Meerman GJ, ter Elst A. Evidence based selection of housekeeping genes. PloS one 2: e898, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ekelund U, Mellander S. Role of endothelium-derived nitric oxide in the regulation of tonus in large-bore arterial resistance vessels, arterioles and veins in cat skeletal muscle. Acta Physiol Scand 140: 301–309, 1990. [DOI] [PubMed] [Google Scholar]
- 19.Faber JE. In situ analysis of alpha-adrenoceptors on arteriolar and venular smooth muscle in rat skeletal muscle microcirculation. Circ Res 62: 37–50, 1988. [DOI] [PubMed] [Google Scholar]
- 20.Faber JE, Meininger GA. Selective interaction of alpha-adrenoceptors with myogenic regulation of microvascular smooth muscle. Am J Physiol Heart Circ Physiol 259: H1126–H1133, 1990. [DOI] [PubMed] [Google Scholar]
- 21.Farkas GA, Gosselin LE, Zhan W, Schlenker EH, Siek GC. Histochemical and metabolic properties of diaphragm muscle in morbidly obese Zucker rats. J Appl Physiol 77: 2250–2259, 1994. [DOI] [PubMed] [Google Scholar]
- 22.He L, Vasiliou K, Nebert DW. Analysis and update of the human solute carrier (SLC) gene superfamily. Hum Genomics 3: 195–206, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hester RL, Eraslan A, Saito Y. Differences in EDNO contribution to arteriolar diameters at rest and during functional dilation in striated muscle. Am J Physiol Heart Circ Physiol 265: H146–H151, 1993. [DOI] [PubMed] [Google Scholar]
- 24.Höglund PJ, Nordström KJ, Schiöth HB, Fredriksson R. The solute carrier families have a remarkably long evolutionary history with the majority of the human families present before divergence of Bilaterian species. Mol Biol Evol 28: 1531–1541, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hussain SN, El-Dwairi Q, Abdul-Hussain MN, Sakkal D. Expression of nitric oxide synthase isoforms in normal ventilatory and limb muscles. J Appl Physiol 83: 348–353, 1997. [DOI] [PubMed] [Google Scholar]
- 26.Jenkins NT, Padilla J, Martin JS, Crissey JM, Thyfault JP, Rector RS, Laughlin MH. Differential vasomotor effects of insulin on gastrocnemius and soleus feed arteries in the OLETF rat model: role of endothelin-1. Exp Physiol 99: 262–271, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jenkins NT, Padilla J, Thorne PK, Martin JS, Rector RS, Davis JW, Laughlin MH. Transcriptome-wide RNA sequencing analysis of rat skeletal muscle feed arteries. I. Impact of obesity. J Appl Physiol (1985) 116: 1017–1032, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes 41: 1422–1428, 1992. [DOI] [PubMed] [Google Scholar]
- 29.Kingwell BA, Formosa M, Muhlmann M, Bradley SJ, McConell GK. Type 2 diabetic individuals have impaired leg blood flow responses to exercise: role of endothelium-dependent vasodilation. Diabetes Care 26: 899–904, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Kobzik L, Stringer B, Balligand JL, Reid MB, Stamler JS. Endothelial type nitric oxide synthase in skeletal muscle fibers: mitochondrial relationships. Biochem Biophys Res Commun 211: 375–381, 1995. [DOI] [PubMed] [Google Scholar]
- 31.Kuo L, Davis MJ, Chilian WM. Longitudinal gradients for endothelium-dependent and -independent vascular responses in the coronary microcirculation. Circulation 92: 518–525, 1995. [DOI] [PubMed] [Google Scholar]
- 32.Lalande S, Gusso S, Hofman PL, Baldi JC. Reduced leg blood flow during submaximal exercise in type 2 diabetes. Med Sci Sports Exerc 40: 612–617, 2008. [DOI] [PubMed] [Google Scholar]
- 33.Lash JM. Contribution of arterial feed vessels to skeletal muscle functional hyperemia. J Appl Physiol 76: 1512–1519, 1994. [DOI] [PubMed] [Google Scholar]
- 34.Lash JM, Bohlen HG. Time- and order-dependent changes in functional and NO-mediated dilation during exercise training. J Appl Physiol 82: 460–468, 1997. [DOI] [PubMed] [Google Scholar]
- 35.Laughlin MH, Armstrong RB. Muscular blood flow distribution patterns as a function of running speed in rats. Am J Physiol Heart Circ Physiol 243: H296–H306, 1982. [DOI] [PubMed] [Google Scholar]
- 36.Laughlin MH, Armstrong RB. Rat muscle blood flow as a function of time during prolonged slow treadmill exercise. Am J Physiol Heart Circ Physiol 244: H814–H824, 1983. [DOI] [PubMed] [Google Scholar]
- 37.Laughlin MH, Davis MJ, Secher NH, van Lieshout JJ, Arce-Esquivel AA, Simmons GH, Bender SB, Padilla J, Bache RJ, Merkus D, Duncker DJ. Peripheral circulation. Compr Physiol 2: 321–447, 2012. [DOI] [PubMed] [Google Scholar]
- 38.Laughlin MH, Korthuis RJ, Duncker DJ, Bache RJ. Control of blood flow to cardiac and skeletal muscle during exercise. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD: Am. Physiol. Soc, 1996, sect. 12, chapt. 16, p. 705–769. [Google Scholar]
- 39.Laughlin MH, Mohrman SJ, Armstrong RB. Muscular blood flow distribution patterns in the hindlimb of swimming rats. Am J Physiol Heart Circ Physiol 246: H398–H403, 1984. [DOI] [PubMed] [Google Scholar]
- 40.Laughlin MH, Padilla J, Jenkins NT, Thorne PK, Martin JS, Rector RS, Akter S, Davis JW. Exercise-induced differential changes in gene expression among arterioles of skeletal muscles of obese rats. J Appl Physiol. doi: 10.1152/japplphysiol.00316.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laughlin MH, Pollock JS, Amann JF, Hollis ML, Woodman CR, Price EM. Training induces nonuniform increases in eNOS content along the coronary arterial tree. J Appl Physiol 90: 501–510, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Laughlin MH, Woodman CR, Schrage WG, Gute D, Price EM. Interval sprint training enhances endothelial function and eNOS content in some arteries that perfuse white gastrocnemius muscle. J Appl Physiol 96: 233–244, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Li S, Fan YS, Chow LH, Van Den Diepstraten C, van Der Veer E, Sims SM, Pickering JG. Innate diversity of adult human arterial smooth muscle cells: cloning of distinct subtypes from the internal thoracic artery. Circ Res 89: 517–525, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Martin JS, Padilla J, Jenkins NT, Crissey JM, Bender SB, Rector RS, Thyfault JP, Laughlin MH. Functional adaptations in the skeletal muscle microvasculature to endurance and interval sprint training in the type 2 diabetic OLETF rat. J Appl Physiol 113: 1223–1232, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McAllister RM, Jasperse JL, Laughlin MH. Nonuniform effects of endurance exercise training on vasodilation in rat skeletal muscle. J Appl Physiol 98: 753–761, 2005. [DOI] [PubMed] [Google Scholar]
- 46.McAllister RM, Newcomer SC, Laughlin MH. Vascular nitric oxide: effects of exercise training in animals. Appl Physiol Nutr Metab 33: 173–178, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mikus CR, Rector RS, Arce-Esquivel AA, Libla JL, Booth FW, Ibdah JA, Laughlin MH, Thyfault JP. Daily physical activity enhances reactivity to insulin in skeletal muscle arterioles of hyperphagic Otsuka Long-Evans Tokushima Fatty rats. J Appl Physiol 109: 1203–1210, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mikus CR, Roseguini BT, Uptergrove GM, Matthew Morris E, Scott Rector R, Libla JL, Oberlin DJ, Borengasser SJ, Taylor AM, Ibdah JA, Harold Laughlin M, Thyfault JP. Voluntary wheel running selectively augments insulin-stimulated vasodilation in arterioles from white skeletal muscle of insulin resistant rats. Microcirculation 19: 729–738, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Padilla J, Jenkins NT, Thorne PK, Martin JS, Rector RS, Davis JW, Laughlin MH. Identification of genes whose expression is altered by obesity throughout the arterial tree. Physiol Genomics 46: 821–832, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Padilla J, Jenkins NT, Thorne PK, Martin JS, Rector RS, Davis JW, Laughlin MH. Identification of genes whose expression is altered by obesity throughout the arterial tree. Physiol Genomics 46: 821–832, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Padilla J, Jenkins NT, Thorne PK, Martin JS, Rector RS, Davis JW, Laughlin MH. Transcriptome-wide RNA sequencing analysis of rat skeletal muscle feed arteries. II. Impact of exercise training in obesity. J Appl Physiol (1985) 116: 1033–1047, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Padilla J, Simmons GH, Davis JW, Whyte JJ, Zderic TW, Hamilton MT, Bowles DK, Laughlin MH. Impact of exercise training on endothelial transcriptional profiles in healthy swine: a genome-wide microarray analysis. Am J Physiol Heart Circ Physiol 301: H555–H564, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Polla B, D'Antona G, Boltinelle R, Reggiani C. Respiratory muscle fibres: specialisation and plasticity. Thorax 59: 808–817, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Poole DC, Sexton WL, Behnke BJ, Ferguson CS, Hageman KS, Musch TI. Respiratory muscle blood flows during physiological and chemical hyperpnea in the rat. J Appl Physiol 88: 186–194, 2000. [DOI] [PubMed] [Google Scholar]
- 55.Poole DC, Sexton WL, Farkas GA, Powers SK, Reid MB. Diaphragm structure and function in health and disease. Med Sci Sports Exerc 29: 738–754, 1997. [DOI] [PubMed] [Google Scholar]
- 56.Powers SK, Criswell DS. Adaptive strategies of respiratory muscles in response to endurance exercise. Med Sci Sports Exerc 28: 1115–1122, 1996. [DOI] [PubMed] [Google Scholar]
- 57.Powers SK, Farkas GA, Demirel H, Coombes JS, Fletcher L, Hughes MG, Hodge k Dodd SL, Schlenker EH. Effects of aging and obesity on respiratory muscle phenotype in Zucker rats. J Appl Physiol 81: 1347–1354, 1996. [DOI] [PubMed] [Google Scholar]
- 58.Powers SK, Shanely RA. Exercise-induced changes in diaphragmatic bioenergetic and antioxidant capacity. Exerc Sport Sci Rev 30: 69–74, 2002. [DOI] [PubMed] [Google Scholar]
- 59.Reuland DJ, McCord JM, Hamilton KL. The role of Nrf2 in the attenuation of cardiovascular disease. Exerc Sport Sci Rev 41: 162–168, 2013. [DOI] [PubMed] [Google Scholar]
- 60.Reusch JE, Bridenstine M, Regensteiner JG. Type 2 diabetes mellitus and exercise impairment. Rev Endocr Metab Disord 14: 77–86, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schlessinger A, Khuri N, Giacomini KM, Sali A. Molecular modeling and ligand docking for solute carrier (SLC) transporters. Curr Top Med Chem 13: 843–856, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sexton WL, Poole DC. Costal diaphragm blood flow heterogeneity at rest and during exercise. Respir Physiol 101: 171–182, 1995. [DOI] [PubMed] [Google Scholar]
- 63.Smuder AJ, Min K, Hudson MB, Kavazis AN, Kwon O, Nelson WB, Powers SK. Endurance exercise attenuates ventilator-induced diaphragm dysfunction. J Appl Physiol 112: 501–510, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Srere PA. Citrate synthase. Methods Enzymol 13: 3–5, 1969. [Google Scholar]
- 65.Vassilakopoulos T, Deckman G, Kebbewar M, Rallis G, Harfouche R, Hussain SN. Regulation of nitric oxide production in limb and ventilatory muscles during chronic exercise training. Am J Physiol Lung Cell Mol Physiol 284: L452–L457, 2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.