Abstract
Irregular participation in HIV medical care hinders HIV RNA suppression and impacts health among people living with HIV. Cluster analysis of clinical data from 1,748 patients attending a large academic medical center yielded three HIV service usage patterns, namely: ‘engaged in care’, ‘sporadic care’, and ‘frequent use’. Patients ‘engaged in care’ exhibited most consistent retention (on average, >88 % of each patient’s observation years had ≥2 visits 90 days apart), annualized visit use (2.9 mean visits/year) and viral suppression (>73 % HIV RNA tests <400 c/mL). Patients in ‘sporadic care’ demonstrated lower retention (46–52 %), visit use (1.7 visits/year) and viral suppression (56 % <400 c/mL). Patients with ‘frequent use’ (5.2 visits/year) had more inpatient and emergency visits. Female, out-of-state residence, low attendance during the first observation year and detectable first-observed HIV RNA were early predictors of subsequent service usage. Patients ‘engaged in care’ were more likely to have HIV RNA <400 than those receiving sporadic care. Results confirm earlier findings that under-utilization of services predicts poorer viral suppression and health out-comes and support recommendations for 2–3 visits/year.
Keywords: HIV care, Health service use, Retention in care, Cluster analysis
Introduction
In the United States, the average survival time for persons living with HIV (PLWH) is now nearly equivalent to that of the general population [1]. This achievement is due in large part to widespread use of antiretroviral therapy (ART), which reduces plasma HIV viral load (VL) and arrests progression to AIDS. Increasingly, the marker of successful primary HIV care is the reduction of HIV RNA copies in blood plasma to below a detectable threshold. Reaching and sustaining a state of viral suppression comes through adopting healthy medical care behaviors, which includes keeping scheduled clinic visits, taking all medications as prescribed, and having regular laboratory assessments. However, many patients find these health behaviors difficult and are challenged to attend medical appointments regularly or are lost-to-follow-up for extended periods, preventing effective management of HIV disease [2]. Estimates vary widely by setting, but recent reports indicate that in the United States only between 40 and 80 % of patients in medical care achieve a suppressed HIV RNA [3–8].
Low or sporadic use of medical care is associated with poor HIV outcomes, including death. The Veterans Administration HIV cohort study found that irregular patterns of medical visits in the year following ART initiation were associated with increased mortality [9]. Similarly, in a study conducted at a medical center in Alabama, missed appointments in the first year of HIV care were associated with increased mortality [10]. To explore the utility of regular medical care beyond 12 months, HIV patients in Kentucky were followed from the initiation of ART until either achieving VL suppression or the end of observation. Only half were optimally retained in care during the observation period, i.e., had at least one visit in each 6-month interval. Time to VL suppression was found to be twice as long for patients with suboptimal retention. Other risk factors for poor retention were having public versus private insurance, and having no AIDS diagnosis versus being diagnosed with AIDS (suggesting a lower level health-related concern). These data suggest that both poverty and complacency about one’s health can affect health outcomes in addition to care-seeking behaviors [11]. AIDS-related mortality has also been found to be associated with geographic region [12]. Nine of the ten states with the highest mortality, North Carolina among them, are located in the southeastern United States, a traditionally impoverished area.
Although there is general consensus about how to treat HIV, the optimal frequency of medical visits needed to achieve and sustain viral suppression and good health of PLWH is a matter of ongoing deliberation. Care delivery research does not lend itself to randomized designs, and understanding health outcomes and medical care is a complex phenomenon, therefore, a variety of observed measures have been analyzed [13–16]. These include: (1) number of missed visits, (2) appointment adherence (percent of visits not missed), (3) gaps in care (time interval without a visit), and (4) visit constancy (e.g., patient was seen at least once in 80 % of all 6-month intervals over a 5-year time period). The US Public Health Service Guidelines state that the current standard of care is one medical evaluation every 3–4 months for at least 1–3 years (until medically stable and virally suppressed), and then every 4–6 months thereafter [17]. The HIV/AIDS Bureau (HAB) defines retention in care as having at least two visits each year, at least 90 days apart [18]. Their measure, hereafter referred to as HAB-Medical Visit (HAB-MV) performance measure, is arguably the most appropriate for program evaluation. However, the HAB guidelines were based on expert opinion, and the optimal measure to use when examining different outcomes has not been empirically demonstrated. In short, there is no ‘gold standard’ measure of medical care for PLWH [19].
To extend prior work and assess the degree to which the use of medical care influences patient outcomes, we examined medical care usage in PLWH attending a large academic medical center in the southeast. Using the HAB-MV performance measure (visit constancy) and annualized attended visits (average number of attended appointments per year), retention in care was categorized. Cluster analysis was undertaken to determine HIV service use patterns in the years subsequent to the first 12 months in a patient’s observation period. The primary research questions we sought to answer were the following: (1) What patterns of HIV care use are prevalent in a clinic based cohort? (2) Are there demographic or early clinical characteristics that are associated with these patterns? (3) Are these patterns of medical care use associated with subsequent VL suppression, immune recovery and hospital or emergency department (ED) admissions? Insights from cluster construction and early cluster membership predictors could inform targeted intervention development for individuals who appear to be at increased risk of falling out of care or having impaired health in later years.
Methods
Data Sources
The study population was comprised of patients receiving care at the University of North Carolina Infectious Diseases (UNC ID) Clinic. Data was provided for all HIV primary care appointments scheduled or completed between 1 Jan 2005 and 1 Feb 2012. Scheduling system data included appointment and demographic information; these were linked to lab test results (HIV RNA and CD4 count tests), medical center ED visits and hospitalizations for the same time period. Study procedures were reviewed and approved by the UNC Institutional Review Board.
Inclusion/Exclusion Criteria
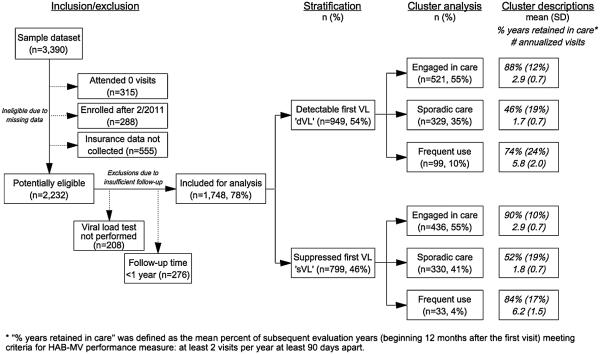
The sample of HIV appointment data included 43,195 unique visits by n = 3,390 patients. Of the scheduled visits, 12,032 (28 %) were cancelled, rescheduled or missed, and 31,163 (72 %) were attended. We excluded patients who either had attended none of their scheduled visits (n = 315); were enrolled after 1 Feb 2011 (i.e., those who did not have an opportunity to be observed for at least one year; n = 288); or whose insurance information was not collected (n = 555). After excluding for missing data, n = 2,232 remaining patients were potentially eligible for analysis. We further excluded patients with insufficient follow-up: those who did not receive a VL test (n = 208); and who dropped out of care at the UNC clinic before one year (n = 276). A final total of n = 1,748 patients (78 % of eligible patients), representing 27,091 attended visits, were retained for cluster analysis (Fig. 1).
Fig. 1.
Analytic procedures based on patient-time contributed to dataset
Cluster Formation
In order to identify patients who were already in care at the onset of the observation period, we stratified patients by their first HIV RNA test into ‘detectable’ or ‘suppressed’ (using a 400 copies/mL cut-point). We chose a threshold of 400 copies/ml to accommodate the varied commercial tests performed over the observation period. A ‘detectable’ first VL test (dVL, defined as C400 copies/mL) was viewed as an indication that more intense medical monitoring was indicated because patients were not yet on anti-retroviral therapy (usually new to care), or were non-adherent to prior ART recommendations. Patients with a ‘suppressed’ first VL (sVL, defined as <400 copies/mL) were considered individuals who were in care prior to the observation period either at the UNC ID clinic or who transitioned from elsewhere. A few of the sVL patients (usually *1 %) are likely elite controllers who have suppressed HIV RNA despite lack of ART [20]. Stratification of the total n = 1,748 patients yielded n = 949 patients with a detectable first VL and n = 799 patients with a suppressed first VL (Fig. 2).
Fig. 2.
Sample selection and cluster analysis flowchart
Following stratification, we performed cluster analysis to create groups of patients with similar HIV service use patterns using two measures: percent years retained in care (using HAB-MV) and number of annualized attended visits. We used these two variables in order to capture both visit constancy and demand for HIV care. Cluster analysis was limited to data following the patient’s first year of care (defined as ‘subsequent years’) to capture long-term service use patterns. These variables are typically available to most providers of HIV care, and the construction of these clusters in other clinical care settings is possible. Evaluation-years were based on the date of their first appointment in the observation time and extended at 365-day intervals ending at the time of the last appointment, rather than by calendar year. Therefore, patients contributed maximal time independent of the month or year in which they first showed up in the data system.
Two analysis sets were constructed from the available data: one for cluster construction and one for cluster validation. A random number generator selected approximately 50 % of the cases (n = 882) for cluster formation and the remainder (n = 866) for validation. We performed cluster analysis using the SPSS two-step cluster analysis procedure, which first assigns cases to pre-clusters (first step) and subsequently performs hierarchical cluster analysis on these pre-clusters (second step). We initially used the SPSS two-step cluster analysis program for its applicability to both categorical and continuous variables and efficiency with large datasets. We used the log-likelihood distance measure, which determines cluster membership by maximizing likelihood of the data given the final cluster solution. Schwarz’s Bayesian information criterion was used initially to determine the number of clusters, which is automatically performed by the two-step cluster algorithm. The initial cluster procedure yielded three clusters, however, we replicated analysis on the construction sample forcing up to six cluster solutions. More than three clusters (per stratum) did not reveal meaningfully different patterns of appointment utilization and some clusters were very small and difficult to interpret, limiting their utility in further analyses. Hence, three clusters per stratum were used for the analysis.
These three clusters described patients with the following HIV service use patterns: ‘engaged in care’, ‘sporadic care’ or ‘frequent use’ (Fig. 2). The ‘engaged in care’ cluster was the largest group, comprising 55 % of each stratum. This cluster exhibited the highest proportion of years in care that met the HAB-MV (dVL: on average, 88 % of each patient’s observation years were retained in care; sVL: 90 % patient-years retained) and annualized attended visits during subsequent years indicating compliance with public health service guidelines recommending one visit every 4 months (mean = 2.9 visits/year in both strata). The ‘sporadic care’ cluster was the second largest group, with patients attending less than 2 visits per year, and meeting HAB-MV during only half of the observation years (dVL: 46 %; sVL: 52 %). The ‘frequent use’ cluster was the smallest group, exhibiting slightly lower HAB-MV measure retention than the ‘engaged in care’ group (dVL: 74 %; sVL: 84 %) but very high annualized attended visits (dVL: 5.8 visits/year; sVL: 6.2 visits/year). This three-cluster solution was confirmed using the validation sample (n = 866), by assessing concordance of the cluster profiles with r correlation coefficients. These clusters were replicated in the validation sample, with each cluster in the construction sample exhibiting a correlation of 0.9 or more with at least one cluster in the validation sample (data not shown). Following cluster confirmation, subsequent analyses were performed using the full sample.
Variables Predicting Cluster Membership
By construction, cluster membership represented multi-year health behaviors contributing to HIV-related health. We sought to identify early HIV-care variables that predict long-term service usage (i.e., in subsequent years). Reasonable candidates for service-use predictors included time-invariant demographics such as patient race/gender group (white male, white female, non-white (black/Hispanic/other) male, and non-white female), age at first visit in the dataset, insurance status (self-reported insurance at first visit: self-pay, public or private), and county of residence (grouped in order of increasing distance by clinic target catchment areas: primary, secondary, outer counties or out of state). We also considered first-year medical care behaviors (number of attended visits, percent visits missed) and clinical HIV indicators (percent of CD4 tests C350 cells/lL, percent of HIV RNA tests suppressed). CD4 counts were dichotomized at a cut-point of <350 or C350 cells/lL also to accommodate the varied commercial tests performed in the observation period. Each of these early clinical predictors was restricted to the patient’s first evaluation-year.
Clinical Outcome Variables
We calculated the percent of CD4 tests C350 and percent of HIV RNA tests suppressed in the final year of observation to represent final clinical outcomes. Patients were considered to have achieved desirable final CD4 counts if 100 % of CD4 tests performed in the final year were C350 or desirable VL suppression if 100 % of VL tests performed in the final year were suppressed. Other outcome variables included hospitalization (‘‘ever been hospitalized at the UNC ID clinic?’’) and ED usage (‘‘ever visited ED at the UNC ID clinic?’’) during the full observation time for each patient.
Data Analysis
We report cluster descriptions (e.g., demographic, lab test and other service use variables) using means and standard deviations for continuous variables and counts and percentages for categorical variables. We performed Chi squared tests of independence and F tests for one-way analysis of variance (ANOVA) to estimate the extent to which predictor variables differed by cluster membership.
The associations between cluster membership (‘engaged in care’, ‘sporadic care’, ‘frequent use’) and the variables used to predict cluster membership (race/gender, insurance status, age, county of residence, first year attended visits, percent of visits missed, percent of CD4 counts C350, and percent of VL tests suppressed) were assessed using multinomial logistic regression models, from which we estimated risk ratios (RRs) and 95 % confidence intervals (CIs) [21]. The ‘engaged in care’ cluster was used as the reference category for the multinomial comparison because it allowed us to determine characteristics associated with either suboptimal or excessive HIV service utilization. Binary logistic regression was used to predict achievement of desirable final VL suppression and CD4 counts, ED use, and hospitalizations from cluster membership, adjusted for demographic covariates (i.e. race/gender, insurance status and age). RRs, risk differences (RDs) and 95 % CIs are reported for the relative and absolute associations for cluster membership and subsequent health outcomes. Risk differences (RDs) provide a measure of public health impact by estimating the difference in likelihood of achieving optimal final outcomes (CD4 and VL) attributable to cluster membership in ‘sporadic care’ and ‘frequent use’ as compared to those in the ‘engaged in care’ cluster (i.e., an RD of -0.01 means that an additional 1 % of individuals could have had a CD4 count [350 cells/lL if they exhibited retention in care behavior characteristic of the ‘engaged in care’ cluster instead). Two-step cluster analyses were conducted using SPSS v20 (SPSS Inc, Chicago, IL, USA) and multinomial and binary logistic regression analyses were conducted using STATA v12 (StataCorp, College Station, TX, USA).
Results
Cluster Description
Demographic distributions across the three clusters were similar in both strata (Table 1). In the dVL stratum only, females comprised a greater proportion of the ‘frequent use’ group regardless of race (F2,946 = 16.1, p = .013). Insurance status was also significantly associated with cluster membership (F2,946 = 12.0, p = .017); in general, patients who reported self-pay were more likely to be in ‘sporadic care,’ and those on public insurance were more likely to be in the ‘frequent use’ group. Lower age at entry was associated with the ‘sporadic care’ cluster in both strata (F2,946 = 7.8, p < .001), though mean differences were within three years of age. County of residence, a proxy for distance to the clinic, was not significantly associated with cluster membership (F2,946 = 9.3, p = .155). First year clinic use differed by cluster membership, where ‘sporadic care’ patients consistently attended fewer appointments and missed twice as many visits as the other clusters in both strata (F2,946 = 20.3, p < .001). ‘Frequent use’ patients exhibited the lowest achievement of CD4 counts C350 and fared no better than ‘sporadic care’ patients in achieving viral suppression in the dVL stratum (F2,940 = 7.1, p < .001). Cluster characteristics in subsequent years also differed in both strata. ‘Engaged in care’ and ‘frequent use’ clusters were associated with longer overall follow-up time (F2,946 = 34.8, p < .001). The number of appointments missed and percent of CD4 counts C350 followed the same patterns as seen in the first year of observation. In addition, the ‘sporadic care’ clusters exhibited the lowest viral suppression (dVL: 56 % of ‘sporadic care’ patients vs. 73 % of ‘engaged in care’ patients were virally suppressed (F2,893 = 22.0, p < .001); sVL: 84 % ‘sporadic’ versus 91 % ‘engaged in care’ were virally suppressed (F2,893 = 22.0, p < .001)). Lastly, ‘frequent use’ patients were almost twice as likely to have ever visited the ED or ever been hospitalized at UNC than the other clusters (ER: dVL: F2,946 = 29.0, p < .001; sVL: F2,796 = 12.4, p < .001. Hospitalizations: dVL: F2,946 = 15.7, p < .001; sVL: F2,796 = 10.8, p < .001).
Table 1.
Demographic and clinical characteristics of patients in UNC cohort by cluster and stratum, n = 1,748
| Stratum | Detectable first viral load (dVL)a |
Suppressed first viral load (sVL)a |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Service use cluster | Engaged in care N (%) |
Sporadic care |
Frequent use |
Engaged in care N (%) |
Sporadic care |
Frequent use |
||||
| Demographic variables |
χ2df | p | χ2df | p | ||||||
| Total | 521 (55 %) | 329 (35 %) | 99 (10 %) | 436 (55 %) | 330 (41 %) | 33 (4 %) | ||||
| Race/gender group | ||||||||||
| White male | 120 (61 %) | 65 (33 %) | 12 (6 %) | 16.16 | .013 | 133 (60 %) | 77 (35 %) | 10 (5 %) | 8.66 | .196 |
| White female | 23 (52 %) | 13 (30 %) | 8 (18 %) | 26 (53 %) | 19 (38 %) | 4 (8 %) | ||||
| Non-white male | 255 (55 %) | 164 (36 %) | 41 (9 %) | 169 (51 %) | 153 (46 %) | 12 (4 %) | ||||
| Non-white female | 123 (50 %) | 87 (35 %) | 38 (15 %) | 108 (55 %) | 81 (41 %) | 7 (4 %) | ||||
| Insurance status | ||||||||||
| Self-pay | 167 (50 %) | 134 (40 %) | 31 (9 %) | 12.04 | .017 | 98 (50 %) | 88 (45 %) | 9 (5 %) | 11.74 | .020 |
| Private | 151 (59 %) | 84 (33 %) | 20 (6 %) | 152 (57 %) | 111 (42 %) | 3 (1 %) | ||||
| Public | 203 (56 %) | 111 (31 %) | 48 (13 %) | 186 (55 %) | 131 (39 %) | 21 (6 %) | ||||
| County of residence | ||||||||||
| Primary | 163 (56 %) | 90 (31 %) | 39 (13 %) | 9.36 | .155 | 122 (58 %) | 78 (37 %) | 11 (5 %) | 6.26 | .408 |
| Secondary | 245 (55 %) | 159 (35 %) | 44 (10 %) | 211 (53 %) | 175 (44 %) | 15 (4 %) | ||||
| Outer | 103 (56 %) | 67 (36 %) | 15 (8 %) | 95 (56 %) | 70 (41 %) | 5 (3 %) | ||||
| Out of state | 10 (42 %) | 13 (54 %) | 1 (4 %) | 8 (47 %) | 7 (41 %) | 2 (12 %) | ||||
| Age at entry (years)b | 41 (11) | 38 (11) | 39 (10) | 7.82,946 | <.001 | 44 (11) | 42 (10) | 45 (10) | 3.42,796 | .036 |
|
| ||||||||||
| Clinical variables | Mean (SD) | Fdf1,df2 | p | mean (SD) | Fdf1,df2 | p | ||||
|
| ||||||||||
| First year | ||||||||||
| Attended visits (n) | 4 9 (2.4) | 4 0 (2.4) | 5.7 (3 7) | 20.32,946 | <.001 | 3.8 (14) | 31 (1 5) | 6 1 (3 3) | 60.72,796 | <.001 |
| Missed visits (%) | 8 (16) | 15 (23) | 10 (18) | 15.622,946 | <.001 | 4 (12) | 8 (18) | 10 (16) | 7.62,796 | <.001 |
| HIV RNA < 400c/ ml (%)c |
28 (31) | 23 (29) | 23 (30) | 3.92,918 | .021 | 93 (17) | 92 (18) | 88 (23) | 2.022,733 | .140 |
| CD4 ≥ 350 cells/ μl (%)c |
48 (43) | 53 (44) | 35 (42) | 7.12,938 | <.001 | 75 (38) | 74 (38) | 68 (42) | 0.72,783 | 542 |
| Subsequent yearsd | ||||||||||
| Missed visits < %) | 26 (14) | 35 (18) | 24 (13) | 37.32,946 | <.001 | 23 (13) | 31 (17) | 18 (9) | 34.62,786 | <.001 |
| HIV RNA < 400c/ ml (%) |
73 (32) | 56 (41) | 58 (35) | 22.022,891 | <.001 | 91 (20) | 84 (28) | 86 (24) | 7.32,789 | <.001 |
| CD4 ≥ 350 cells/ μL (%) |
68 (38) | 63 (43) | 45 (42) | 13.82,934 | <.001 | 82 (32) | 79 (36) | 73 (36) | 1.72,786 | .190 |
| All years | ||||||||||
| Total observation time (years) |
4.6 (1.7) | 3 6(18) | 4.5 (1.8) | 34.82,946 | <.001 | 5.5 (1.5) | 4.5 (1.9) | 4 6 (18) | 39.62,796 | <.001 |
| Ever visited the UNC ER? (%) |
34 (47) | 29 (45) | 69 (47) | 29.02,946 | <.001 | 30 (46) | 24 (43) | 64 (49) | 12.42,796 | <.001 |
| Ever hospitalized at UNC? (%) |
48 (50) | 39 (49) | 71 (46) | 15.72,946 | <.001 | 37 (48) | 27 (45) | 64 (49) | 10.82,796 | <.001 |
Detectable or suppressed first viral load (dVL, sVL) are categorized using a cut-point of ≥400 copies/ mL or <400 copies/mL, respectively
Age at entry is continuous variable, thus we report ANOVA F- statistic
Lab values describe the mean proportion of all VL or CD4 tests above or below their respective cut-point per patient during the specified time period (mean % HIV RNA < 400 or ≥350 CD4 count during the first year or subsequent years)
Subsequent years indicates the observation time starting 12 months after the first visit until the last visit
Early Clinical Predictors of Cluster Membership
The multinomial logistic regression model predicted cluster membership (using ‘engaged in care’ as the reference cluster; see Table 2) from early clinical characteristics. In the dVL stratum, being female was associated with a three-fold increased risk in being ‘frequent use’ versus ‘engaged in care’ as compared to males. Attending more visits in the first year of care was associated with a significantly lower risk of being in the ‘sporadic care’ cluster and higher risk of being in the ‘frequent use’ cluster as compared to the reference cluster [for each additional first-year visit: dVL: RR = 0.85, 95 % CI (0.79, 0.91); sVL: RR = 1.13, 95 % CI (1.04, 1.22)]. First year missed visits were not associated with significant changes in cluster membership. Lastly, in the dVL stratum, a 10 % increase in suppressed first year VL tests or CD4 tests C350 was associated with decreased risk of becoming a ‘frequent use’ patient [RR = 0.88, 95 % CI (0.80, 0.95); CD4: RR = 0.93, 95 % CI (0.88, 0.98)].
Table 2.
Multinomial logistic regression models to predict cluster membership compared with the engaged in care cluster
| Stratum | Detectable first viral load (dVL)a |
Suppressed first viral load (sVL)a |
||
|---|---|---|---|---|
| Service use cluster | Sporadic care | Frequent use | Sporadic care | Frequent use |
| Risk ratio (95 % CI) | Risk ratio (95 % CI) | |||
| Race/gender group | ||||
| White male | (Reference) | (Reference) | (Reference) | (Reference) |
| White female | 0.94 (0.42, 2.08) | 3.32 (1.17, 9.42) | 1.21 (0.59, 2.51) | 2.27 (0.49, 10.57) |
| Non-white male | 1.09 (0.74, 1.61) | 1.34 (0.66, 2.72) | 1.49 (0.99, 2.22) | 0.77 (030, 1.99) |
| Non-white female | 1.18 (0.76, 1.85) | 2.85 (1.36, 5.97) | 1.20 (0.77, 1.88) | 0.52 (0.16, 1.74) |
| Insurance status | ||||
| Self-pay | (Reference) | (Reference) | (Reference) | (Reference) |
| Private | 0.69 (0.47, 1.01) | 0.98 (0.52, 1.84) | 1.00 (0.64, 1.56) | 0.17 (0.03, 0.77) |
| Public | 0.72 (0.50, 1.03) | 1.17 (0.68, 2.02) | 0.91 (0.60, 1.39) | 0.72 (0.28, 1.88) |
| County of residence | ||||
| Primary | (Reference) | (Reference) | (Reference) | (Reference) |
| Secondary | 1.14 (0.80, 1.60) | 0.78 (0.47, 1.29) | 1.13 (0.77, 1.65) | 1.60 (0.57, 4.48) |
| Outer | 1.18 (0.77, 1.81) | 0.67 (0.34, 1.31) | 1.02 (0.65, 1.62) | 1.23 (0.34, 4.45) |
| Out of state | 2.53 (1.03, 6.21) | 0.51 (0.06, 4.18) | 1.27 (0.41, 3.98) | 12.63 (1.85, 86.2) |
| Age at entry (for each additional 10 years) | 1.00 (1.00, 1.00) | 100 (1.00, 1.00) | 1.00 (1.00, 1.00) | 1.00 (1.00, 101) |
| Attended first year visits (for each additional 1 visit) | 0.84 (0.78, 0.91) | 112 (1.04, 1.21) | 0.72 (0.64, 0.81) | 1.61 (1.33, 1.94) |
| Missed visits first year (for each additional 10 % visits missed) | 1.00 (1.00, 1.00) | 1.00 (1.00, 1.00) | 1.00 (1.00, 1.00) | 1.00 (1.00, 1.00) |
| VL suppressed first year (for each additional 10 % suppressed) | 0.98 (0.93, 1.04) | 0.88 (0.81, 0.95) | 0.95 (0.87, 1.04) | 0.92 (0.75, 1.13) |
| CD4 ≥ 350 first year (each additional 10 % above 350) | 1.00 (0.96, 1.03) | 0.93 (0.88, 0.99) | 0.99 (0.94, 1.03) | 1.02 (0.92, 1.14) |
Detectable or suppressed first viral load (dVL, sVL) are categorized using a cut-point of ≥400 copies/mL or <400 copies/mL, respectively
Cluster Membership as a Predictor of Long-Term Health Outcomes
A binary logistic regression model predicted final (last year) CD4 and VL outcomes, as well as ED visits and hospitalizations in subsequent years from cluster membership, adjusting for demographic covariates (Table 3). In the dVL stratum, both ‘sporadic’ and ‘frequent’ use were associated with a significantly lower likelihood of an optimal final CD4, as compared to the ‘engaged in care’ group (RR = 0.82, 95 % CI (0.73,0.92) and RR = 0.67, 95 % CI (0.51,0.83), respectively). Final VL tests were a third less likely to be optimized compared with the engaged in care cluster [‘sporadic care’: RR = 0.66, 95 % CI (0.55,0.76);‘frequentuse’:RR = 0.70,95 %CI (0.55,0.86)]. Associations for sporadic care were similar in the sVL strata. ‘Sporadic care’ was associated with lower final suppressed VL [RR = 0.87, 95 % CI (0.79,0.96)]. ‘Frequent use’ was not associated with final lab outcomes in sVL patients but in both sVL and dVL strata, ‘frequent use’ was associated with an over two-fold increase in risk of ever visiting the ED or being hospitalized, whereas ‘sporadic care’ was associated with a decreased risk of ED use in the sVL stratum.
Table 3.
Risk ratios and risk differences of selected outcomes with the engaged in care cluster
| Stratum | Detectable viral load 1st HIV RNA ≥ 400 c/ml (dVL) |
Suppressed viral load 1st HIV RNA < 400 c/ml (sVL) |
||
|---|---|---|---|---|
| Service use cluster Outcome |
Sporadic care Risk ratio (95 % CI) |
Frequent use | Sporadic care Risk ratio (95 % CI) |
Frequent use |
| 100 % last year HIV tests suppressed | 0.86 (0.55, 0.76) | 0.70 (0.55, 0.86) | 0.87 (0.79, 0.96) | 0.95 (0.82, 1.09) |
| 100 % last year CD4 tests ≥350 | 0.82 (0.73, 0.92) | 0.67 (0.51, 0.83) | 0.94 (0.88, 1.02) | 0.95 (0.80, 1.11) |
| Ever visited ER in subsequent years | 0.72 (0.52, 0.91) | 2.41 (1.75, 3.07) | 0.71 (0.51, 0.92) | 2.54 (1.44, 3.64) |
| Ever been hospitalized in subsequent years | 0.78 (0.56, 1.01) | 2.60 (1.80, 3.41) | 0.63 (0.43, 0.84) | 1.91 (0.80, 3.01) |
| 100 % last year HIV tests suppressed | −0.27 (−0.34, −0.19) | −0.23 (−0.34, −0.11) | −0.11 (−0.18, −0.04) | −0.04 (−0.16, 0.08) |
| 100 % last year CD4 tests ≥350 | −0.13 (−0.19, −0.06) | −0.24 (−0.35, −0.13) | −0.04 (−0.10, 0.01) | −0.04 (−0.17, 0.09) |
| Ever visited ER in subsequent years | −0.07 (−0.12, −0.01) | 0.33 (0.22, 0.44) | −0.06 (−0.11, −0.01) | 0.30 (0.12, 0.49) |
| Ever been hospitalized in subsequent years | −0.04 (−0.09, 0.01) | 0.29 (0.17, 0.41) | −0.04 (−0.08, −0.01) | 0.11 (−0.02, 0.24) |
a Estimated for service use cluster membership (column) on each (row), adjusted for race/gender, insurance status and age at entry into care
In the dVL stratum, 13 per 100 ‘sporadic care’ patients were less likely to have an optimal final CD4 (95 % CI 6–19 %) and 24 % fewer patients had an optimal final VL (95 % CI 13–35 %), as compared to patients ‘engaged in care’. ‘Frequent use’ patients were at even greater risk of failing to achieve optimal final CD4 (RD = -24 %, 95 % CI -13–35 %) and VL (RD = 23 %, 95 % CI 11–34 %). In the sVL stratum, cluster membership was not a statistically significant predictor of CD4 outcomes, and only ‘sporadic care’ is associated with lower likelihood of optimal VLs in the final year. Emergency and inpatient department use was associated with cluster membership. ‘Frequent use’ was associated with over 11–33 % increased absolute risk of ever having an ED visit or hospitalization. The ‘sporadic care’ cluster was associated with a 4–7 % decreased risk of ever having an ED visit or hospitalization.
Discussion
In the cohort described here, long-term HIV medical care behaviors, defined by average number of appointments per year and proportion of follow-up years where visit attendance met the HAB-MV performance measure, were represented by three outpatient medical care use patterns: routine (engaged), sporadic and frequent. Similar patterns were seen regardless of HIV RNA status at entry (dVL/ sVL). The characteristics that were negatively associated with engaged cluster membership in the dVL stratum were being female, and out of state residence. Increased frequency of suppressed VL and CD4 counts [350 cells/lL during the first evaluation-year were positively associated with engaged cluster membership. For each visit attended in the first year, patients were less likely to be in the ‘sporadic care’ cluster but more likely to be in the ‘frequent use’ cluster. The number of missed visits was not associated with any cluster. Similar results were found for patients who entered the observation period with a suppressed VL (sVL). In addition, sVL patients with private insurance were significantly less likely to be in the ‘frequent use’ group. The sporadic level of care predicted a loss of viral control and a fall in CD4 counts in sVL patients. Sporadic cluster members were less likely to have consistently suppressed VLs or CD4 counts [350 cells/lL throughout the final observation year. Frequent use cluster members were also less likely to have consistently suppressed VLs or CD4 counts [350 cells/lL but more likely to have used both emergency and inpatient hospital services. Neither ‘sporadic care’ nor ‘frequent use’ were associated with final CD4 counts in the sVL stratum. However, in the sVL stratum, ‘sporadic care’ members were significantly less likely to have a consistently suppressed final VL compared with those ‘engaged in care’.
The finding that ‘sporadic care’ patients have reduced VL suppression frequencies compared with patients ‘engaged in care’ is particularly important. This observation contributes to prior work that attempts to define the adequacy of medical care for patients living with HIV. Buscher et al. [22] examined 3, 6 and 12-month outcomes in patients who were recommended by their medical provider to return to clinic in 4–6 months versus 3–4 months and found comparable outcomes. They found that in the current ART era, when HIV RNAs are suppressed, visit intervals of 3–4 months do not improve outcomes compared to visit intervals of 4–6 months. Our study examined outcomes at the end of a 4-year observation period. We also found that visit intervals of 4 months maintained viral suppression in 70 or 90 % in patients who have a detectable or suppressed VL, respectively, when first seen. In addition, our work also suggests that patients who are seen an average of every 7 months (1.7 visits/year), and are retained in care for 46–52 % of the observed years, have less than optimal care. This is observed even when patients enter the observation period with a suppressed VL. For those with a detectable VL at entry, the sporadic cluster is also associated with lower CD4 counts.
We identified a small cluster in each stratum with a high demand for care. In the dVL stratum, women were more likely to be in this cluster compared with men. This cluster also had fewer members with completely suppressed VL or 100 % of CD4 counts[350 cells/lL during the final observation year in the dVL stratum only. This group was just as likely to fail to achieve viral suppression as those in sporadic care but less than those in usual care. AIDS-related and non-AIDS-related co-morbid conditions are likely explanations for this pattern of service usage. One possible explanation for the association with gender and this cluster is pregnancy, which is associated with a new diagnosis of HIV, is specific to women, and requires an increased number of visits.
We did not find an association with race/ethnicity and ‘sporadic care’ membership. Nationally, race and ethnicity based differences in HIV care and treatment have been noted [6]. At a clinic level, race and gender differences are reported in some clinical settings but not all. Racial parity was also observed in the private care setting, as reported by the Moore clinic in Baltimore, but disparity in clinic attendance was seen by race and ethnicity at the CORE clinic in Chicago [23–25].
This is one of just a few reports using the HAB-MV performance measure to characterize the quantity of medical care received [26]. This measure has also been compared to visit constancy and gaps in care [16] and found to provide similar estimates of care retention. Established in 2008, it is similar to other measures of care insofar as it identifies patients with suboptimal retention. In an examination of care retention, the proportion of observation years that a patient met the HAB-MV performance measure retention goal was consistent with visit constancy and proportion of time gaps of care that were 6 months or longer [16]. Retention using the HAB-MV performance measure was also associated with VL suppression at 12 months, but when HAB-MV was assessed over a 5.6 year follow-up period and dichotomized to patients who met the criterion in all years of follow-up or not, the measure was not associated with a greater likelihood of VL suppression [13]. More recently this measure was shown to have the highest optimal predictive value compared with VL outcomes [27]. Our work adds to a small but growing body of evidence supporting the usefulness of readily available electronic medical record data and the HAB-MV measure as a performance metric.
Though this dataset was from a high-volume medical center in North Carolina, a notable limitation of our analysis is the potential truncation of a patient’s episode of care at the beginning or end of the dataset. We attempted to account for this uncertainty by stratification into detectable and suppressed first VLs, conceptualizing a suppressed first VL as a proxy for receiving ART and medical care prior to the observation period. We found more statistically significant early predictors in the dVL stratum, which may suggest that interventions to foster retention in care in populations who are new or returning to care may have greater impact. We found that the sporadic care group displayed the lowest ER/hospitalization rates. We do not have any information suggesting why patients are not in care. Existing literature has shown that asymptomatic patients will often feel that they no longer need to continue taking medication. Conversely, we cannot rule out health reasons prompting more scheduled visits and thus higher service usage since the dataset of visit instances did not include information on reason for visit or pregnancy status. We attempted to address this by defining cluster membership using only measures of attended visits so that clusters describe actual visit uptake rather than needs. We ensured temporal precedence of the cluster predictors by using only demographic and first-year predictors in the multinomial regression to minimize reverse causation. Our final-year CD4 measure is also an absolute measure that does not account for CD4 at study enrollment, therefore probability of achieving lower CD4 is subject to variability in duration of observation between patients. We used an absolute (versus relative change from baseline) measure because a 350 cut-point was being used clinically as a minimum level to which immunosuppression was allowed prior to providing ART during the study period.
The site reported here is a Ryan White funded academic clinic and generalizations made to patient populations in other care settings should be made with caution. Our study design also necessitated the exclusion of individuals with less than one year of follow-up time from cluster analysis, a group comprising both patients with extremely early dropout and those seeking care elsewhere (e.g., non-local patients who are referred to other outpatient sites for care). Similarly, we could not observe emergency, inpatient or outpatient HIV visits sought at other healthcare sites and cannot make conclusions about actual care received by these patients during periods of poor retention at the UNC ID clinic. However, patients who receive care at multiple sites have poorer outcomes than those who receive care at a single site [28]. Nonetheless, our results have limited generalizability to patients at risk for early dropout, which is a clinically important group that warrants further study, and we cannot infer with certainty the care behaviors of patients out of care at this clinic. Finally, this is an observation cohort and the conclusions are subject to the effect of unmeasured confounding, for example, from income, employment or other socioeconomic characteristics that could affect both access to care and attendance, as well as medical and psychiatric co-morbidities and diminished physiological responses to treatment that would trigger increased referral to care and probability of negative outcomes.
In the context of current US treatment guidelines, achievement of HIV viral suppression is the primary goal of care and treatment for HIV-infected individuals [29]. Yet, despite recent advances in HIV treatment options, individuals with HIV are still experiencing very low rates of viral suppression (28 %) due to inadequate entry into and retention in care and adherence to ART [6, 30]. Evidence has shown that decreased life expectancy and other negative outcomes result from poor retention and early discontinuation of therapy, and these outcomes exhibit disparities by sex and race [31]. Thus, it is of paramount importance to identify patients who are most at risk for falling out of care and failing to achieve viral suppression early in care and refer them for additional support. A recent systematic literature review of interventions to improve retention in HIV primary care in the US resulted in recommendations to use evidence based strategies comprising a broad range of services: strengths-based case management, patient navigation, appointment facilitation, transportation assistance, co-location of other services, bilingual/bicultural health care teams, and peer outreach and education, among others [32]. Successful programs were observed to feature a multi-component approach tailored specifically to patients with the greatest need, for which these early prediction models could provide assistance. Further study to adapt and validate the utility of risk prediction models with tailored programs is necessary. With increased life expectancy due to improved HIV treatment options, PLWH are living longer and the resource burden on providers to deliver HIV care is strained by these growing populations. Thus, it is important to determine accurately what HIV care means and how often it should be delivered.
Acknowledgments
This research was supported by funds from HRSA’s Special Projects of National Significance program (HA15148, H9HA15152), UNC Centers for AIDS Research (P30-AI50410) and the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (1UL1TR001111). This study was conducted with the approval of the University of North Carolina at Chapel Hill Institutional Review. The authors also acknowledge the clinic staff, providers, and patients for their invaluable contributions to this research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or HRSA.
Contributor Information
Anton Palma, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY, USA, ap3223@columbia.edu; Department of Health Policy and Management, Columbia University, New York, NY, USA.
David W. Lounsbury, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY, USA
Lynne Messer, School of Community Health, Portland State University, Portland, OR, USA.
Evelyn Byrd Quinlivan, Division of Infectious Disease, University of North Carolina - Chapel Hill, 130 Mason Farm Road, Chapel Hill, NC 27599-7030, USA, ebq@med.unc.edu.
References
- 1.Lewden C, Bouteloup V, De Wit S, Sabin C, Mocroft A, Collaboration of Observational HIV Epidemiological Research Europe (COHERE) et al. All-cause mortality in treated HIV-infected adults with CD4 C500/mm3 compared with the general population: evidence from a large European observational cohort collaboration. Int J Epidemiol. 2012;41(2):433–45. doi: 10.1093/ije/dyr164. [DOI] [PubMed] [Google Scholar]
- 2.Mayer KH. Introduction: linkage, engagement, and retention in HIV care: essential for optimal individual- and community-level outcomes in the era of highly active antiretroviral therapy. Clin Infect Dis. 2011;52(Suppl 2):S205–7. doi: 10.1093/cid/ciq043. [DOI] [PubMed] [Google Scholar]
- 3.Cohen SM, Van Handel MM, Branson BM, Blair JM, Hall HI, Hu X, et al. Vital signs: hIV prevention through care and treatment — United States. Morb Mortal Wkly Rep. 2011;60(47):1618–23. [PubMed] [Google Scholar]
- 4.Doshi RK, Matthews T, Isenberg D, Matosky M, Milberg J, Malitz F, et al., editors. Continuum of HIV care among Ryan White HIV/AIDS Program clients, United States, 2010. Conference on Retroviruses and Opportunistic Infections; Atlanta, GA. 2013. [Google Scholar]
- 5.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52(6):793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall HI, Frazier EL, Rhodes P, Holtgrave DR, Furlow-Parmley C, Tang T, et al., editors. Continuum of HIV care: differences in care and treatment by sex and race/ethnicity in the United States. Internatinal AIDS Conference; Washington, D.C.. 2012. [Google Scholar]
- 7.Horberg M, Hurley L, Towner W, Gambatese R, Klein D, Antoniskis D, et al., editors. HIV spectrum of engagement cascade in a large integrated care system by gender, age, and methodologies. Conference on Retroviruses and Opportunistic Infections; Atlanta, GA. 2013. [Google Scholar]
- 8.Althoff K, Rebeiro P, Brooks JT, et al. Disparities in the quality of HIV care when using US Department of Health and Human Services indicators. Clin Infect Dis. 2014;58(8):1185–9. doi: 10.1093/cid/ciu044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giordano TP, Gifford AL, White AC, Jr, Suarez-Almazor ME, Rabeneck L, Hartman C, et al. Retention in care: a challenge to survival with HIV infection. Clin Infect Dis. 2007;44(11):1493–9. doi: 10.1086/516778. [DOI] [PubMed] [Google Scholar]
- 10.Mugavero MJ, Lin HY, Willig JH, Westfall AO, Ulett KB, Routman JS, et al. Missed visits and mortality among patients establishing initial outpatient HIV treatment. Clin Infect Dis. 2009;48(2):248–56. doi: 10.1086/595705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crawford TN, Sanderson WT, Thornton A. Impact of poor retention in HIV medical care on time to viral load suppression. J Int Assoc Provid AIDS Care. 2014;13(3):242–9. doi: 10.1177/2325957413491431. [DOI] [PubMed] [Google Scholar]
- 12.Hanna DB, Selik RM, Tang T, Gange SJ. Disparities among US states in HIV-related mortality in persons with HIV infection, 2001–2007. Aids. 2012;26(1):95–103. doi: 10.1097/QAD.0b013e32834dcf87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crawford TN, Sanderson WT, Thornton A. A comparison study of methods for measuring retention in HIV medical care. AIDS Behav. 2013;17(9):3145–51. doi: 10.1007/s10461-013-0559-0. [DOI] [PubMed] [Google Scholar]
- 14.Mugavero MJ, Amico KR, Westfall AO, Crane HM, Zinski A, Willig JA, et al. Early retention in HIV care and viral load suppression: implications for a test and treat approach to HIV prevention. J Acquir Immune Defic Syndr. 2012;59:86–93. doi: 10.1097/QAI.0b013e318236f7d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shepherd BE, Blevins M, Vaz LM, Moon TD, Kipp AM, Jose E, et al. Impact of definitions of loss to follow-up on estimates of retention, disease progression, and mortality: application to an HIV program in Mozambique. Am J Epidemiol. 2013;178(5):819–28. doi: 10.1093/aje/kwt030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yehia BR, Fleishman JA, Metlay JP, Korthuis PT, Agwu AL, Berry SA, et al. Comparing different measures of retention in outpatient HIV care. Aids. 2012;26(9):1131–9. doi: 10.1097/QAD.0b013e3283528afa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panel on Antiretroviral Guidelines for Adults and Adolescents Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents 2013 6/23/2013. Available from: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- 18.Human Resources and Services Administration HIV/AIDS Bureau Performance Measure for Adult/Adolescent Clients: Group 12008 August 5, 2013. Available from: http://hab.hrsa.gov/deliverhivaidscare/habperformmeasures.html.
- 19.Mugavero M, Westfall AO, Zinski A, Davila J, Drainoni ML, Gardner LI, et al. Measuring retention in HIV care: the elusive gold standard. J Acquir Immune Defic Syndr. 2012;61(5):574–80. doi: 10.1097/QAI.0b013e318273762f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madec Y, Boufassa F, Porter K, Prins M, Sabin C, d’Arminio Monforte A, et al. Natural history of HIV-control since seroconversion. Aids. 2013;27(15):2451–60. doi: 10.1097/01.aids.0000431945.72365.01. [DOI] [PubMed] [Google Scholar]
- 21.Hosmer DW, Lemeshow S. Applied logistic regression. Wiley; New York City: 2000. [Google Scholar]
- 22.Buscher A, Mugavero M, Westfall AO, Keruly J, Moore R, Drainoni ML, et al. The association of clinical follow-up intervals in HIV-infected persons with viral suppression on subsequent viral suppression. AIDS Patient Care STDS. 2013;27(8):459–66. doi: 10.1089/apc.2013.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adeyemi OM, Livak B, McLoyd P, Smith KY, French AL. Racial/ethnic disparities in engagement in care and viral suppression in a large urban HIV clinic. Clin Infect Dis. 2013;56(10):1512–4. doi: 10.1093/cid/cit063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore RD, Keruly JC, Bartlett JG. Improvement in the health of HIV-infected persons in care: reducing disparities. Clin Infect Dis. 2012;55(9):1242–51. doi: 10.1093/cid/cis654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horberg M, Hurley LB, Silverberg MJ, Klein DB, Quesenberry CP, Mugavero MJ. Missed office visits and risk of mortality among HIV-infected subjects in a large healthcare system in the United States. AIDS Patient Care STDS. 2013;27(8):442–9. doi: 10.1089/apc.2013.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keller SC, Yehia BR, Eberhart MG, Brady KA. Accuracy of definitions for linkage to care in persons living with HIV. J Acquir Immune Defic Syndr. 2013;63(5):622–30. doi: 10.1097/QAI.0b013e3182968e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krentz HB, Worthington H, Gill MJ. Adverse health effects for individuals who move between HIV care centers. J Acquir Immune Defic Syndr. 2011;57(1):51–4. doi: 10.1097/QAI.0b013e318214feee. [DOI] [PubMed] [Google Scholar]
- 28.Thompson MA, Mugavero MJ, Amico KR, Cargill VA, Chang LW, Gross R, et al. Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: evidence-based recommendations from an International Association of Physicians in AIDS Care panel. Ann Intern Med. 2012;156(11):817–33. doi: 10.7326/0003-4819-156-11-201206050-00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall HI, Gray KM, Tang T, Li J, Shouse L, Mermin J. Retention in care of adults and adolescents living with HIV in 13 U.S. areas. J Acquir Immune Defic Syndr. 2012;60(1):77–82. doi: 10.1097/QAI.0b013e318249fe90. [DOI] [PubMed] [Google Scholar]
- 30.Losina E, Schackman BR, Sadownik SN, Gebo KA, Walensky RP, Chiosi JJ, et al. Racial and sex disparities in life expectancy losses among HIV-infected persons in the united states: impact of risk behavior, late initiation, and early discontinuation of anti-retroviral therapy. Clin Infect Dis. 2009;49(10):1570–8. doi: 10.1086/644772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higa DH, Marks G, Crepaz N, Liau A, Lyles CM. Interventions to improve retention in HIV primary care: a systematic review of U.S. studies. Current HIV/AIDS Rep. 2012;9(4):313–25. doi: 10.1007/s11904-012-0136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]