Abstract
CD19-targeted chimeric antigen receptor (CAR) T cells are currently being tested in the clinic with very promising outcomes. However, limitations to CAR T cell therapy exist. These include lack of efficacy against some tumors, specific targeting of tumor cells without affecting normal tissue and retaining activity within the suppressive tumor microenvironment. Whilst promising clinical trials are in progress, preclinical development is focused on optimizing CAR design, to generate “armored CAR T cells” which are protected from the inhibitory tumor microenvironment. Studies investigating the expression of cytokine transgenes, combination therapy with small molecule inhibitors or monoclonal antibodies are aimed at improving the anti-tumor efficacy of CAR T cell therapy. Other strategies aimed at improving CAR T cell therapy include utilizing dual CARs and chemokine receptors to more specifically target tumor cells. This review will describe the current clinical data and some novel “armored CAR T cell” approaches for improving anti-tumor efficacy therapy.
Clinical experience with CAR T cell treatment of B cell malignancies
Despite the promising anti-tumor activity of CD19 or CD20-targeted CAR modified T cells in animal models, limited clinical response was observed in initial clinical trials with first-generation autologous CAR modified T cells lacking co-stimulatory signal, leading to limited persistence of the CAR T cells1.
To overcome the lack of T cell co-stimulation in the first-generation CARs, two approaches have been used. Expression of CARs in antigen-specific T cells such as Epstein-Barr virus-specific T cells2, and incorporation of co-stimulatory signaling domains into the CAR (second-generation CAR). By incorporating co-stimulatory domains such as CD28, CD137 (4-1BB), or CD134 (OX40) to the CARs, several groups demonstrated increased persistence and anti-tumor efficacy in animal models3-6. Similarly, significantly enhanced expansion and persistence of the second-generation CAR T cells have been demonstrated in humans when CD19-targeted first and second generation CAR T cells were simultaneously infused in patients with B cell lymphoma7. However, it remains unclear whether any particular co-stimulatory molecule is superior to another, and the current ongoing clinical trial wherein patients with relapsed chronic lymphocytic leukemia (CLL) are simultaneously infused CD19-tarteted second-generation CARs comparing CD28 and 4-1BB costimulation will partly address the question (NCT 00466531).
CD28z CARs in CLL and indolent B cell lymphoma
The anti-tumor efficacy of second-generation CAR T cells in patients with B-cell malignancies was first reported in 2010. A patient with advanced follicular lymphoma experienced a partial remission (PR) and long-term B-cell aplasia following infusion of CD19-targeted CD28/CD3ζ CAR8. Subsequently, the same group of investigators reported the outcome of 4 relapsed CLL patients treated with CD19-targeted CD28/CD3ζ CAR T cells. All patients received nonmyeloablative conditioning therapy consisting of fludarabine and cyclophosphamide prior to T cell infusion, and one patient achieved a CR, and 3 patients achieved PR9.
We have reported the similar encouraging results in 8 patients with purine-analog refractory or relapsed CLL with bulky lymphadenopathy who received the autologous CD19-targeted CD28/CD3ζ CAR T cells. Of the 6 evaluable patients, one patient achieved minimal residual disease (MRD) negative complete remission (CR), 2 patients achieved PR, and 2 patients had stable disease despite rapid tumor progression before therapy10,11. In order to better assess the efficacy of CAR T cells in minimal disease setting, we are conducting a phase I study of CD19-targeted CD28/CD3ζ CAR T cells in patients with previously untreated CLL who have residual disease following frontline chemotherapy (NCT01416974)12.
CD28z CARs in acute lymphoblastic leukemia
Striking activity of the CD28/CD3ζ CAR T cells was observed in patients with relapsed B-cell acute lymphoblastic leukemia (ALL), and first reported in 201313. Five relapsed ALL patients received CD19-targeted CD28/CD3ζ CAR T cells, and all patients experienced rapid tumor eradication and achieved MRD negative CR. Therapy was well tolerated, although significant cytokine release syndrome was observed in those patients with large tumor burden at the time of T cell infusion. Updated results from this trial report CRs in 10 out of 12 treated patients with chemo-refractory ALL including patients with Philadelphia-chromosome positive ALL14.
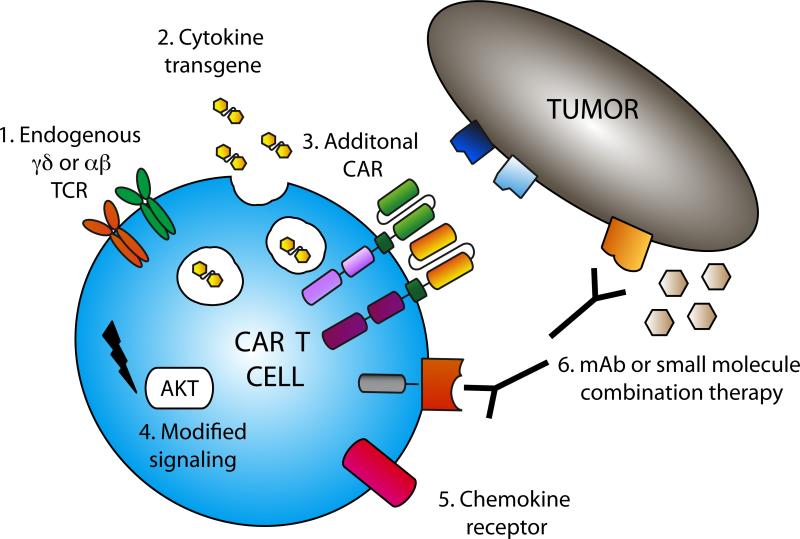
Despite the promising results of CAR T cell therapy in patients with ALL, there remains room for improvement in order to achieve equivalent results in CLL patients. Novel preclinical studies aimed at improving this therapy include utilization of different cells, combination therapies and modification of T cells with cytokine transgenes (Fig 1).
Figure 1. Armored CARs for improved anti-tumor therapy.
Numerous strategies exist to improve CAR T cell therapy. These include 1. Modification of alterntive cell types, eg. γδ T cells or T cells derived from immature precursors. 2. Expression of immune stimulatory cytokine transgenes in CAR T cells, eg. IL-2, IL-15 or IL-12. 3. Expression of 2 CARs on a single T cell to improve specificity and safety. 4. Modified T cell signaling to generate more sustained T cell responses or resistance to immune-suppressives effects, eg. constitutively active AKT or knock-down of Dgk. 5. Expression of chemokine receptors to increase T cell survival and trafficking to tumor, eg. Transgenic expression of IL-7 Rα or CCR4 on CAR T cells. 6. Combination therapy with monoclonal antibodies (mAb), e.g. anti-PD-1 or Rituximab, or small molecule inhibitors e.g. ABT-737 or rapamycin.
CAR expressed on different cell types
To date, clinical application of CAR T cell therapy has utilized αβ T cells, that is a T cell expressing a T cell receptor (TCR) consisting of an α and a β chain. However, alternative T cell types have been investigated in preclinical studies. These include γδ T cells, precursor T cells and T cells differentiated from induced pluripotent stem cells (iPSCs).
CARs γδ T cells
In γδ T cells, the TCR is composed of γ and δ molecules, which recognize a limited number of antigens in a MHC-unrestricted fashion.15 These cells have an inherent anti-tumor activity and lack allo-reactivity, and have lower potential for causing graft versus host disease making them ideal for adoptive transfer therapy. Deniger and colleagues modified γδ T cells to express a CD19-targeted CD3ζ/CD28 CAR16. The CAR γδ T cells were subsequently expanded on K562 artificial antigen presenting cells (aAPCs) resulting in a polyclonal population of CAR γδ T cells. Expression of the CAR mediated lysis of normally γδ T cell-resistant cell lines, including human pre B-ALL Nalm6 tumor cells. The anti-tumor efficacy of CAR+ γδ T cells was tested using a xenogeneic murine model wherein weekly infusions of γδ CAR+ T cells led to delayed growth of tumor. It remains to be determined whether γδ T cells provide a superior anti-tumor efficacy compared to αβ T cells and whether alternate signaling domains may stimulate a more effective anti-tumor response from these cells.
CAR T cells from precursor or stem cells
Differentiation of hematopoietic stem cells into committed T-lineage precursor cells resulted in “off the shelf” allogeneic T cells for adoptive cell therapy. Using mouse models, Zakrzewski and colleagues demonstrate that transfer of MHC-disparate T cell precursors into irradiated recipients resulted in some anti-tumor efficacy17. Retroviral transduction resulted in expression of a CD19-targeted first generation CAR and was shown to increase the anti-tumor efficacy of these cells. Importantly, these T cell precursors develop into mature T cells in the recipient and were recipient-tolerant as no evidence of GVHD was observed. In another attempt to generate a large population of tumor-targeted T cells, Themeli and colleagues utilized iPSCs to generate human T cells (T-iPSCs-T) that are phenotypically defined, functional and lentivirally transduced to express a CD19-targeted CD3ζ/CD28 CAR.18 T-iPSCS-T expressing the CD19-targeted CAR, 1928z, secreted IL-2, TNFα, IFNγ and were cytotoxic against human CD19-positive tumors in vitro and in vivo. The T-iPSCs-T cells resembled γδ T cells, functionally and phenotypically, and like CAR γδ T cells, CAR T-iPSC-T induced rapid eradication of human Burkitt's lymphoma Raji tumors in a preclinical murine model. While there remains a significant amount of investigation into the phenotype, function and safety of these cells, these studies provide a proof of principle that stem cells can be differentiated into T cells and targeted to tumor for effective anti-cancer therapy.
CAR plus cytokine/ transgene
Several strategies to improve the anti-tumor effect of adoptive T cell therapy employ expression of a cytokine transgene in CAR T cells. CAR T cells which provide autocrine growth factors and stimulatory cytokines is one approach to protect or “armor” the T cells from the suppressive tumor environment. Immune stimulatory cytokines including IL-2, IL-15 and IL-12 have been explored in this context.
IL-2 transgenes
Interleukin (IL)-2 is an important growth factor for T cells.19 Early clinical trials demonstrated the importance of IL-2 for effective adoptive T cell transfer therapy. However, the levels of IL-2 required are high and often result in significant toxicity.20 In order to overcome this toxicity, Treisman and colleagues investigated an approach whereby tumor-specific T cells were transduced with an IL-2 transgene to provide autocrine IL-2 stimulation.21 A murine tumor-specific T cell line 14.1 modified to secrete IL-2 was shown to produce high levels of IL-2 and proliferate in the absence of exogenous IL-2. Further to these studies, Liu and colleagues describe the modification of a CD8 T cell clone isolated from tumor infiltrating lymphocytes (TIL) from a metastatic melanoma lesion. When modified to express an IL-2 transgene, this clone was shown to proliferate in the absence of exogenous IL-2.22 A subsequent publication demonstrated that bulk TIL from metastatic melanoma lesions from two patients were modified to secrete IL-223. These modified TIL were shown to destroy tumor and proliferate in the absence of exogenous cytokine support and CD4+ T cell help, in response to autologous tumor cells. This group then investigated the clinical application of adoptive transfer of IL-2 secreting TILs. Heemskerk and colleagues treated metastatic melanoma patients with autologous TIL modified to constitutively produce IL-2.24 Patients had symptoms of toxicity and this therapy was not shown to have increased clinical efficacy. Utilization of an IL-2 transgene has lost favor due to the toxicity associated with this potent cytokine.
IL-12 transgene
Several studies have investigated the effects of tumor specific T cells modified to produce IL-12, a potent immune stimulatory cytokine. Unfortunately, systemic infusion of IL-12 in cancer patients was found to be highly toxic25. However, targeting the IL-12 to the site of the tumor may allow exploitation of its potent effects without systemic toxicity. IL-12 is a heterodimeric protein consisting of a p35 and p40 subunit, which together form active IL-12p7026. T cells do not usually secrete IL-12, however, modification of these cells with genes encoding the two subunits linked with a flexible hinge region allows the secretion of functional IL-12 by T cells. Ideally, the tumor targeted T cells traffic to the site of the tumor, where they produce IL-12 within the tumor microenvironment, therefore limiting systemic exposure and potentially reducing toxicity.
Tumor specific T cells that secrete IL-12 have been tested in several preclinical models. One extensively studied model utilizes transgenic pmel T cells, where the CD8+ T cells are specific for melanoma antigen, gp100, expressed on B16 tumors27. IL-12 producing pmel T cells were shown to have enhanced anti-tumor function against subcutaneous B16 tumors28. These cells had increased accumulation at tumor sites, though did not persist in treated mice. This group subsequently reported that pmel T cells constitutively producing IL-12 failed to proliferate, underwent apoptosis and that higher doses of these cells were toxic to mice29. By utilizing the nuclear factor of activated T cells (NFAT) promoter, it was demonstrated that IL-12 secretion could be induced following T cell activation. The authors showed that inducible IL-12 secretion resulted in reduced toxicity whilst maintaining anti-tumor efficacy in vivo. This was also confirmed in another study utilizing a CAR specific for vascular endothelial growth factor receptor 230. In a third publication using the pmel model, it was shown that IL-12 secreting T cells “reprogrammed” tumor resident myeloid derived stromal cells, including myeloid derived suppressor cells (MDSCs), dendritic cells (DCs) and macrophages31. The authors propose that IL-12/pmel T cells allowed MDSCs, DCs and macrophages to cross present natural TAAs and allow transferred cells to infiltrate the tumor and mediate regression. In a fourth study, Kerkar and colleagues demonstrated that IL-12/pmel T cells mediated increased levels of Fas expression on macrophages, DCs and MDSCs within the tumor microenvironment32. IL-12/pmel T cells form a Fas/FasL synapse leading to apoptosis of stromal cells and stromal collapse of subcutaneous B16 tumors. It is unclear how these two mechanisms work together for eradication of tumor or which is the more dominant of the two. In addition, these conclusions come with the caveats associated with the pmel model, including the absence of tumor-targeted CD4+ T cells, which may respond to IL-12. These findings in these solid tumor models may be relevant to masses seen in some lymphoma patients, though more investigation is required.
We utilized a syngeneic murine model where tumor targeted T cells were infused into immune-competent mice to treat systemic EL4 tumor modified to express human CD1933. Mouse T cells were modified to express a CD19-specific first generation CAR, 19mz, and constitutively secrete IL-12. These T cells had increased cytokine secretion, increased cytotoxic capacity, as well as complete tumor eradication in the absence of toxicity. Using murine knockout studies, we demonstrate that anti-tumor efficacy was dependent on autocrine IL-12 stimulation and subsequent IFNγ production. Furthermore, CAR/IL-12 T cells were shown to be resistant to regulatory T cell (Treg)-mediated suppression, obviating the need for conditioning pre-treatment for effective therapy.
It is very clear that IL-12 provides a potent stimulation for tumor-targeted T cells and may subvert the inhibitory microenvironment. The optimal control of IL-12 production (constitutive or inducible) remains to be determined for maximal efficacy and safety. However, given the impressive anti-tumor effects in multiple tumor models, treatment with IL-12 secreting, tumor-targeted T cells holds much promise for the treatment of cancer.
IL-15
IL-15 is normally produced by monocytes, macrophages and dendritic cells and stimulates the proliferation and survival of T and NK cells34-36. Transgeneic expression of this cytokine in tumor-targeted T cells may result in uncontrolled T cell proliferation unless tightly regulated.
Initial studies demonstrated that exogenous IL-15 injections could increase the anti-tumor efficacy of adoptively transferred pmel T cells37. These authors then crossed human IL-15 transgenic mice with pmel mice to generate tumor-targeted T cells that constitutively secreted IL-15. Adoptive transfer of these cells into tumor bearing mice resulted in enhanced survival.
Following these promising studies, Hsu and colleagues modified T cells to constitutively produce IL-1538. These cells had enhanced proliferation and resistance to apoptosis while maintaining antigen specificity, ideal for adoptive transfer therapy. However, subsequent studies demonstrated that expression of the IL-15 transgene in human T cells could be leukemogenic, as T cells from one donor (out of 23) retained proliferative capacity without the addition of exogenous cytokines39. These cells stained hetereogenously for CD5 and CD38, inconsistent with T cell leukemia, but they were demonstrated to be clonal. Further studies showing that short hairpin RNA-mediated knockdown of the IL-15 receptor decreased proliferation of these cells, proving that the IL-15 was responsible for the uncontrolled T cell growth.
Cytokine transgenes can be coexpressed with a suicide gene (e.g inducible Caspase9), which provides a reliable method of eradicating transferred cells in the case of adverse events. This “safety switch” has encouraged the use of IL-15 in adoptive T cell transfer therapy. Hoyos and colleagues modified human T cells to express a CD19-specific CD3ζ/CD28 CAR, an IL-15 transgene and the iCaspase9 suicide gene40. As predicted, these cells produced IL-15 upon stimulation. Murine models revealed that the CAR/IL-15 cells had increased in vivo expansion and better control of Burkitt's lymphoma Raji and Daudi tumor growth, although these T cells did not eradicate systemic tumor in treated mice. The iCaspase9 suicide gene effectively eliminated T cells in vitro and in vivo, demonstrating that this approach could be safer due to the ability to potentially eradicate the T cells in the event of uncontrolled T cell proliferation or other adverse events.
IL-21
Combining CAR T cell therapy with cytokine transgenes may present serious concerns as discussed above. One strategy to avoid this is to culture CAR T cells in the context of stimulatory cytokines ex vivo to provide benefits without the risk of toxicity. Exogenous IL-21 was added to the culture of CD19-targeted CAR T cells upon expansion with K562 aAPCs to provide “signal 3” to the T cells for full activation41. Inclusion of IL-21 resulted in increased T cell yield as well as increased GzmB expression and IFNγ secretion. In vivo, IL-21 cultured T cells have increased efficacy against Nalm6 tumors in an immune-compromised murine model. It remains to be seen whether an IL-21 transgene will be utilized to ensure that T cells receive autologous IL-21 stimulation and whether this approach is safe.
Dual targeted T cells
Dual-targeted CAR T cells have been proposed to improve safety and ensure that CAR T cell activation only occurs in contact with tumor antigens. Expression of two CARs on a T cell, each with a different specificity, is proposed to increase the specificity and therefore safety of this approach. Despite the following studies describing glioblastoma and prostate cancer models, this approach could be applied to B cell malignancies. For example, targeting CLL could be achieved with CARs specific for CD5 and CD19.
Hegde and colleagues used a mathematical model to determine the best two TAAs to target T cells to glioblastoma42. Based on screening of patient tumor cells, targeting both Her2 and IL-13Rα was reported to result in maximal anti-tumor efficacy. Furthermore, treatment of tumor bearing mice with dual-specific T cells was able to offset antigen escape, resulting in disease eradication and long term survival of treated mice. In similar studies, Duong and colleagues targeted both folate binding protein and erbB2 with two CARs expressed on a single cell43. These authors reported an increased response to model tumor (expressing both antigens) compared to model “normal” tissue (expressing either FBP or erbB2 alone).
A more complex strategy involves separating the activation and costimulatory domains on two different CARs expressed in one T cell. Wilkie and colleagues utilized a first generation CAR targeted ErbB2 and a Muc-1-targeted CAR with a CD28 signaling domain44. Unfortunately, on T cells expressing both CARs, the authors demonstrate that the costimulatory signal delivered by the second CAR was impaired. It was proposed that engagement of the CD28 containing CAR was affected by steric hindrance of the zeta-containing CAR binding to its target. In similar study, Kloss and colleagues utilized a first generation CAR targeted to human CD19, 19z1, and a second costimulatory chimeric receptor (CCR) targeted to prostate specific membrane antigen (PSMA) that contained both 4-1BB and CD28 signaling domains45. T cells were modified to express either CAR or CCR or both (CCR/CAR). Despite effective tumor elimination by CAR/CCR T cells, murine studies demonstrated that CAR/CCR T cells did not need the second antigen and were able to eradicate tumors that were only positive for human CD19. The authors then generated a suboptimal CAR to be expressed alongside the CCR. It was demonstrated that only mice treated with T cells expressing both the suboptimal CAR and CCR survived. T cells expressing CCR and a suboptimal CAR can only respond to cells expressing both tumor antigens, but not either target antigen alone. This strategy proves the principle that targeting two antigens with separate CARs can make this therapy safer.
One unique strategy to target T cells is to attack the tissues supporting the tumor, or the tumor stroma. Karkarla and colleagues designed a CAR to target cancer associated fibroblasts (CAFs)46. This CAR was specific for fibroblast activation protein alpha (FAP), which is expressed on cancer-associated fibroblasts on over 90% of epithelial cancers. The authors investigated a dual-targeted therapy where tumor-bearing mice were treated with FAP-CAR T cells and T cells bearing a CAR that was specific for EphA2, an antigen expressed on the lung tumor cells. Treatment with FAP-CAR T cells and EphA2-CAR T cells resulted in tumor control and enhanced the survival of tumor-bearing mice. This report demonstrates the efficacy of targeting both the tumor and its supporting stromal tissue.
CARs with modified signaling
Modifying the T cell signaling pathways also been proposed for enhancing anti-tumor efficacy of adoptive T cell therapy. Intrinsic modifications to allow improved proliferation and anti-tumor function, as well as mediate resistance to Tregs, have been reported.
Tregs are often recruited to tumor sites, and these cells may suppress any endogenous T cells and CAR T cell function47. Altering T cell signaling to render CAR T cells resistant to Treg mediated suppression has been proposed to improve CAR T cell function. Sun and colleagues demonstrated that expression of a constitutively active serine/threonine kinase, AKT (caAKT), increased T cell proliferation and cytokine production48. T cells expressing caAkt retained the ability to proliferate in the presence of Tregs and were resistant to TGFβ-mediated conversion to suppressive T cells. Using T cells expressing a CAR specific for GD2 (neuroblastoma TAA) and caAkt, it was shown that this strategy could result in increased response to tumors. Another publication reported the modification of CD28 signaling domain to create CAR T cells that are resistant to Tregs49. The lck-binding domain in the CD28 signaling moiety of the CAR was mutated, preventing the secretion of IL-2. The lck mutated CAR T cell retained IFNγ secreting capacity and was cytotoxic to tumor cells in vitro. The mutated CAR T cell was able to eradicate the tumor in presence of Tregs and resulted in decreased Treg accumulation at the tumor site, compared to mice treated with a wild type CAR. This study shows that CAR T cell derived IL-2 may allow for the accumulation of Tregs and subsequent suppression of both CAR and endogenous T cells.
Given that CAR T cells require proteins that are essential for TCR signal transduction, relieving negative regulators of TCR signaling may improve CAR T cell function. Diacyglycerol kinase (Dgk) is an enzyme that limits Ras/Erk activation, essential for T cell activation. By utilizing T cells from Dgk knockout (Dgk−/−) mice, it was found that Dgk−/− CAR T cells have increased levels of Erk activation in response to antigenic stimulation and increased anti-tumor function in vitro50. Adoptive transfer of Dgk−/− CAR T cells into tumor bearing mice revealed increased anti-tumor function in vivo. These findings were extended to show that pharmacological inhibition of Dgk augmented the function of human CAR T cells. Another study describing the mutation of a negative regulator targeted CblB, which increases the threshold for T cell activation by modulating TCR and CD28 signaling. Stromnes and colleagues demonstrated that CblB abrogation can increase the function of tumor reactive T cells51. The authors demonstrate that Cbl-B knockout (cblb−/−) T cells had increased proliferation and cytokine secretion, their cytolytic function was unaffected, and extend this finding by transducing human CD8+CD28− T cell clones with siRNA targeting CblB, with similar results. These studies show that targeting negative regulator of TCR signaling can improve CAR T cell mediated anti-tumor efficacy, though caution is warranted so as not to confer uncontrolled T cell activation.
CAR T cells modified to express chemokine receptors/costimulatory ligands
Survival, proliferation and trafficking of CAR T cells can be improved through the expression of chemokine receptors. Endowing CAR T cells with an increased ability to respond to growth factors or traffic to tumors may permit function in the inhibitory tumor microenvironment.
Vera and colleagues modified T cells to express IL-7 receptor alpha chain (IL-7Rα), which restores their responsiveness to IL-7, a cytokine important for T cell homeostasis52. Subsequent studies by Perna and colleagues described the further modification of Epstein Barr virus specific T cells with both a GD2-specific CAR and IL-7Rα53. Transgenic IL-7Rα expression resulted in increased proliferation and resistance to Treg–mediated suppression in vitro and in vivo. It is important to note that treatment of patients with IL-7 is well tolerated even at high doses, compared to IL-2 or IL-12, making this a clinically relevant strategy. While this model is designed to improve neuroblastoma T cell therapy, this strategy could be applied to T cells targeted to B cell malignancies.
Di Stasi and colleagues demonstrated that Reed-Stemberg cells of Hodgkin Lymphoma produce CCL17 and CCL2, which results in the recruitment of Th2 and Treg cells54. T cells were modified to express a CD30-specific CAR and CCR4 to increase recruitment to the Reed Stemberg cells. Using an immunocompromised murine model, expression of both the CD30-targeted CAR and CCR4 resulted in increased in vivo migration and anti-tumor efficacy with an overall cure rate of 57% in treated mice. Similar studies have been described modifying T cells with CXCR255. This study demonstrates that trafficking of T cells can be manipulated to ensure tumor localization and on-target effects.
Constitutive expression of costimulatory ligands has also been proposed to improve the anti-tumor function of CAR T cells. Stephan and colleagues modified T cells to express a prostate specific membrane antigen (PSMA)-targeted CAR and CD80 and 4-1BB ligand (41BBL)56. These cells have robust proliferative responses to stimulation in vitro and in vivo, and mediated rejection of established systemic tumor in an immunodeficient preclinical murine model. Furthermore, the CAR/CD80/41BBL T cells mediated bystander activation of tumor-specific bystander cells, indicating the potential of these cells to further recruit effective anti-tumor responses from TIL.
Combination treatments and other approaches
Combining CAR T cells with other agents may increase the anti-tumor efficacy of adoptive therapy. Here, we discuss a few strategies to further activate the T cells, dampen the inhibitory microenvironment, or sensitize the tumor to the T cells.
The hostile tumor microenvironment includes the upregulation of ligands that bind inhibitory receptors on T cells. Programmed death receptor 1 (PD-1), is expressed on activated T cells and interaction with its ligands, PD-L1 or L2, can induce anergy or apoptosis in the T cells. John and colleagues modified T cells with a Her2-specific CAR and demonstrated that culture of targeted T cells with PD-L1+ tumors led to increased PD-1 expression on the T cell57. Monoclonal antibodies (mAb) were utilized to block PD-1 from interacting with PD-L1 resulting in increased IFNγ, proliferation marker Ki67 and GzmB in CAR T cells. This combined therapy had increased in vivo anti-tumor efficacy utilizing two orthotopic murine tumor models. While this model doesn't investigate B cell malignancies, it is well documented that B cell lymphomas can express PD-L1, therefore this approach could improve CD19 targeted T cell therapy.
Monoclonal antibody therapy has also been combined with dual specific CAR T cells. Second generation CARs specific for CD19 or CD38 were used for treating non-Hodgkin lymphoma58. The authors noted an additive effect of utilizing two CARs, though complete eradication of tumor was only observed when the mice were also treated with Rituxumab. This combination has a high potential for clinical translation given that both CAR therapy and Rituximab therapy are currently individually used in the clinic.
Small molecule inhibitors may sensitize tumors to T cell mediated destruction, therefore combination therapy of CAR T cells and existing drugs may improve overall anti-tumor efficacy. Small molecule histone deacetylase inhibitors can restore the apoptotic pathway in tumor cells and are currently being tested clinically. Karlsson and colleagues investigated the combination of CD19-specific CAR T cells with Bcl-2 family inhibitor, ABT-73759. ABT737 blocks apoptosis inhibitors that are often over expressed in tumor cells, and as a single agent has shown efficacy against CLL and B-ALL. Combination ABT 737/CAR T cell treatment had a synergistic effect on apoptosis induction in tumor cells. Furthermore, pretreatment with ABT737 improved the anti-tumor efficacy of CAR T cells. AKT/mTOR pathway is often dysregulated in lymphomas and promotes tumor growth by contributing to proliferation and angiogeneis. Small molecule inhibitors like rapamycin can be utilized to destabilize tumors by decreasing expression of anti-apoptotic molecules and downregulating inhibitory cytokines and ligands. Huye and colleagues investigated the strategy of making tumor-specific T cells resistant to Rapamycin by transducing them with both a CAR and mutated mTOR that was resistant to Rapamycin60. In the presence of rapamycin, cells expressing both the CAR and mTorRR had increased anti-tumor function (cytotoxicity and IFNγ secretion) compared to cells expressing the only the CAR. CAR/mTorRR T cell lysed rapamycin pretreated Burkitt's lymphoma Raji cells at a higher rate than untreated Raji tumor cells. While these strategies require some optimization, it highlights the notion of combining small molecule drugs with adoptive T cell therapy.
Conclusion
CAR T cell therapy has recently demonstrated very promising clinical activity in B cell hematologic malignancies. However, limitations to CAR T cell therapy exist. Relapses do occur in patients with ALL following CAR T cell therapy, and second-generation CAR T cells remain relatively ineffective in eradicating bulky lymph nodes in patients with B cell lymphoma/leukemia. As discussed in this review, several approaches are being explored to generate armored CAR T cells to overcome the hostile tumor microenvironment and further enhance clinical activity of adoptive T cell therapy. It remains to be seen which of these approaches would be most effective and safe in the clinical setting, and well-designed clinical trials and close collaboration between the institutions will be needed to successfully bring these next generation CARs to the clinic.
References
- 1.Jensen MC, Popplewell L, Cooper LJ, et al. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2010;16(9):1245–1256. doi: 10.1016/j.bbmt.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pule MA, Savoldo B, Myers GD, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nature medicine. 2008;14(11):1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brentjens RJ, Latouche JB, Santos E, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nature medicine. 2003;9(3):279–286. doi: 10.1038/nm827. [DOI] [PubMed] [Google Scholar]
- 4.Carpenito C, Milone MC, Hassan R, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(9):3360–3365. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finney HM, Akbar AN, Lawson AD. Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCR zeta chain. J Immunol. 2004;172(1):104–113. doi: 10.4049/jimmunol.172.1.104. [DOI] [PubMed] [Google Scholar]
- 6.Hombach A, Wieczarkowiecz A, Marquardt T, et al. Tumor-specific T cell activation by recombinant immunoreceptors: CD3 zeta signaling and CD28 costimulation are simultaneously required for efficient IL-2 secretion and can be integrated into one combined CD28/CD3 zeta signaling receptor molecule. J Immunol. 2001;167(11):6123–6131. doi: 10.4049/jimmunol.167.11.6123. [DOI] [PubMed] [Google Scholar]
- 7.Savoldo B, Ramos CA, Liu E, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. The Journal of clinical investigation. 2011;121(5):1822–1826. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kochenderfer JN, Wilson WH, Janik JE, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116(20):4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119(12):2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brentjens RJ, Riviere I, Park JH, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118(18):4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park JH, Riviere I, Wang X, et al. Impact of the Conditioning Chemotherapy On Outcomes in Adoptive T Cell Therapy: Results From a Phase I Clinical Trial of Autologous CD19-Targeted T Cells for Patients with Relapsed CLL. ASH Annual Meeting Abstracts. 2012;120(21):1797. [Google Scholar]
- 12.Park JH, Rivière I, Wang X, et al. Phase I Trial Of Autologous CD19-Targeted CAR-Modified T Cells As Consolidation After Purine Analog-Based First-Line Therapy In Patients With Previously Untreated CLL. Blood. 2013;122(21):874. [Google Scholar]
- 13.Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Science translational medicine. 2013;5(177):177ra138. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davila ML, Riviere I, Wang X, et al. Safe and Effective Re-Induction Of Complete Remissions In Adults With Relapsed B-ALL Using 19-28z CAR CD19-Targeted T Cell Therapy. Blood. 2013;122(21):69. [Google Scholar]
- 15.Carding SR, Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nat Rev Immunol. 2002;2(5):336–345. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 16.Deniger DC, Switzer K, Mi T, et al. Bispecific T-cells expressing polyclonal repertoire of endogenous gammadelta T-cell receptors and introduced CD19-specific chimeric antigen receptor. Mol Ther. 2013;21(3):638–647. doi: 10.1038/mt.2012.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zakrzewski JL, Suh D, Markley JC, et al. Tumor immunotherapy across MHC barriers using allogeneic T-cell precursors. Nat Biotechnol. 2008;26(4):453–461. doi: 10.1038/nbt1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Themeli M, Kloss CC, Ciriello G, et al. Generation of tumor-targeted human T lymphocytes from induced pluripotent stem cells for cancer therapy. Nat Biotechnol. 2013;31(10):928–933. doi: 10.1038/nbt.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waldmann TA. The structure, function, and expression of interleukin-2 receptors on normal and malignant lymphocytes. Science. 1986;232(4751):727–732. doi: 10.1126/science.3008337. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg SA, Lotze MT, Yang JC, et al. Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Ann Surg. 1989;210(4):474–484. doi: 10.1097/00000658-198910000-00008. discussion 484-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Treisman J, Hwu P, Minamoto S, et al. Interleukin-2-transduced lymphocytes grow in an autocrine fashion and remain responsive to antigen. Blood. 1995;85(1):139–145. [PubMed] [Google Scholar]
- 22.Liu K, Rosenberg SA. Transduction of an IL-2 gene into human melanoma-reactive lymphocytes results in their continued growth in the absence of exogenous IL-2 and maintenance of specific antitumor activity. J Immunol. 2001;167(11):6356–6365. doi: 10.4049/jimmunol.167.11.6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu K, Rosenberg SA. Interleukin-2-independent proliferation of human melanoma-reactive T lymphocytes transduced with an exogenous IL-2 gene is stimulation dependent. J Immunother. 2003;26(3):190–201. doi: 10.1097/00002371-200305000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heemskerk B, Liu K, Dudley ME, et al. Adoptive cell therapy for patients with melanoma, using tumor-infiltrating lymphocytes genetically engineered to secrete interleukin-2. Hum Gene Ther. 2008;19(5):496–510. doi: 10.1089/hum.2007.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leonard JP, Sherman ML, Fisher GL, et al. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood. 1997;90(7):2541–2548. [PubMed] [Google Scholar]
- 26.Carra G, Gerosa F, Trinchieri G. Biosynthesis and posttranslational regulation of human IL-12. J Immunol. 2000;164(9):4752–4761. doi: 10.4049/jimmunol.164.9.4752. [DOI] [PubMed] [Google Scholar]
- 27.Overwijk WW, Theoret MR, Finkelstein SE, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198(4):569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kerkar SP, Muranski P, Kaiser A, et al. Tumor-specific CD8+ T cells expressing interleukin-12 eradicate established cancers in lymphodepleted hosts. Cancer Res. 2010;70(17):6725–6734. doi: 10.1158/0008-5472.CAN-10-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L, Kerkar SP, Yu Z, et al. Improving adoptive T cell therapy by targeting and controlling IL-12 expression to the tumor environment. Mol Ther. 2011;19(4):751–759. doi: 10.1038/mt.2010.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chinnasamy D, Yu Z, Kerkar SP, et al. Local delivery of interleukin-12 using T cells targeting VEGF receptor-2 eradicates multiple vascularized tumors in mice. Clin Cancer Res. 2012;18(6):1672–1683. doi: 10.1158/1078-0432.CCR-11-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kerkar SP, Goldszmid RS, Muranski P, et al. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J Clin Invest. 2011;121(12):4746–4757. doi: 10.1172/JCI58814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kerkar SP, Leonardi AJ, van Panhuys N, et al. Collapse of the tumor stroma is triggered by IL-12 induction of Fas. Mol Ther. 2013;21(7):1369–1377. doi: 10.1038/mt.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pegram HJ, Lee JC, Hayman EG, et al. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood. 2012;119(18):4133–4141. doi: 10.1182/blood-2011-12-400044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doherty TM, Seder RA, Sher A. Induction and regulation of IL-15 expression in murine macrophages. J Immunol. 1996;156(2):735–741. [PubMed] [Google Scholar]
- 35.Stoklasek TA, Colpitts SL, Smilowitz HM, Lefrancois L. MHC class I and TCR avidity control the CD8 T cell response to IL-15/IL-15Ralpha complex. J Immunol. 2010;185(11):6857–6865. doi: 10.4049/jimmunol.1001601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cooper MA, Bush JE, Fehniger TA, et al. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood. 2002;100(10):3633–3638. doi: 10.1182/blood-2001-12-0293. [DOI] [PubMed] [Google Scholar]
- 37.Klebanoff CA, Finkelstein SE, Surman DR, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101(7):1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsu C, Hughes MS, Zheng Z, et al. Primary human T lymphocytes engineered with a codon-optimized IL-15 gene resist cytokine withdrawal-induced apoptosis and persist long-term in the absence of exogenous cytokine. J Immunol. 2005;175(11):7226–7234. doi: 10.4049/jimmunol.175.11.7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsu C, Jones SA, Cohen CJ, et al. Cytokine-independent growth and clonal expansion of a primary human CD8+ T-cell clone following retroviral transduction with the IL-15 gene. Blood. 2007;109(12):5168–5177. doi: 10.1182/blood-2006-06-029173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoyos V, Savoldo B, Quintarelli C, et al. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia. 2010;24(6):1160–1170. doi: 10.1038/leu.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh H, Figliola MJ, Dawson MJ, et al. Reprogramming CD19-specific T cells with IL-21 signaling can improve adoptive immunotherapy of B-lineage malignancies. Cancer Res. 2011;71(10):3516–3527. doi: 10.1158/0008-5472.CAN-10-3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hegde M, Corder A, Chow KK, et al. Combinational targeting offsets antigen escape and enhances effector functions of adoptively transferred T cells in glioblastoma. Mol Ther. 2013;21(11):2087–2101. doi: 10.1038/mt.2013.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duong CP, Westwood JA, Berry LJ, Darcy PK, Kershaw MH. Enhancing the specificity of T-cell cultures for adoptive immunotherapy of cancer. Immunotherapy. 2011;3(1):33–48. doi: 10.2217/imt.10.81. [DOI] [PubMed] [Google Scholar]
- 44.Wilkie S, van Schalkwyk MC, Hobbs S, et al. Dual targeting of ErbB2 and MUC1 in breast cancer using chimeric antigen receptors engineered to provide complementary signaling. J Clin Immunol. 2012;32(5):1059–1070. doi: 10.1007/s10875-012-9689-9. [DOI] [PubMed] [Google Scholar]
- 45.Kloss CC, Condomines M, Cartellieri M, Bachmann M, Sadelain M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat Biotechnol. 2013;31(1):71–75. doi: 10.1038/nbt.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kakarla S, Chow K, Mata M, et al. Antitumor effects of chimeric receptor engineered human T cells directed to tumor stroma. Mol Ther. 2013;21(8):1611–1620. doi: 10.1038/mt.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee JC, Hayman E, Pegram HJ, et al. In vivo inhibition of human CD19-targeted effector T cells by natural T regulatory cells in a xenotransplant murine model of B cell malignancy. Cancer Res. 2011;71(8):2871–2881. doi: 10.1158/0008-5472.CAN-10-0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun J, Dotti G, Huye LE, et al. T cells expressing constitutively active Akt resist multiple tumor-associated inhibitory mechanisms. Mol Ther. 2010;18(11):2006–2017. doi: 10.1038/mt.2010.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kofler DM, Chmielewski M, Rappl G, et al. CD28 costimulation Impairs the efficacy of a redirected t-cell antitumor attack in the presence of regulatory t cells which can be overcome by preventing Lck activation. Mol Ther. 2011;19(4):760–767. doi: 10.1038/mt.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Riese MJ, Wang LC, Moon EK, et al. Enhanced effector responses in activated CD8+ T cells deficient in diacylglycerol kinases. Cancer Res. 2013;73(12):3566–3577. doi: 10.1158/0008-5472.CAN-12-3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stromnes IM, Blattman JN, Tan X, et al. Abrogating Cbl-b in effector CD8(+) T cells improves the efficacy of adoptive therapy of leukemia in mice. J Clin Invest. 2010;120(10):3722–3734. doi: 10.1172/JCI41991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vera JF, Hoyos V, Savoldo B, et al. Genetic manipulation of tumor-specific cytotoxic T lymphocytes to restore responsiveness to IL-7. Mol Ther. 2009;17(5):880–888. doi: 10.1038/mt.2009.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perna SK, Pagliara D, Mahendravada A, et al. Interleukin-7 Mediates Selective Expansion of Tumor-redirected Cytotoxic T Lymphocytes (CTLs) without Enhancement of Regulatory T-cell Inhibition. Clin Cancer Res. 2014;20(1):131–139. doi: 10.1158/1078-0432.CCR-13-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Di Stasi A, De Angelis B, Rooney CM, et al. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood. 2009;113(25):6392–6402. doi: 10.1182/blood-2009-03-209650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peng W, Ye Y, Rabinovich BA, et al. Transduction of tumor-specific T cells with CXCR2 chemokine receptor improves migration to tumor and antitumor immune responses. Clin Cancer Res. 2010;16(22):5458–5468. doi: 10.1158/1078-0432.CCR-10-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stephan MT, Ponomarev V, Brentjens RJ, et al. T cell-encoded CD80 and 4-1BBL induce auto- and transcostimulation, resulting in potent tumor rejection. Nat Med. 2007;13(12):1440–1449. doi: 10.1038/nm1676. [DOI] [PubMed] [Google Scholar]
- 57.John LB, Devaud C, Duong CP, et al. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin Cancer Res. 2013;19(20):5636–5646. doi: 10.1158/1078-0432.CCR-13-0458. [DOI] [PubMed] [Google Scholar]
- 58.Mihara K, Yanagihara K, Takigahira M, et al. Synergistic and persistent effect of T-cell immunotherapy with anti-CD19 or anti-CD38 chimeric receptor in conjunction with rituximab on B-cell non-Hodgkin lymphoma. Br J Haematol. 2010;151(1):37–46. doi: 10.1111/j.1365-2141.2010.08297.x. [DOI] [PubMed] [Google Scholar]
- 59.Karlsson SC, Lindqvist AC, Fransson M, et al. Combining CAR T cells and the Bcl-2 family apoptosis inhibitor ABT-737 for treating B-cell malignancy. Cancer Gene Ther. 2013;20(7):386–393. doi: 10.1038/cgt.2013.35. [DOI] [PubMed] [Google Scholar]
- 60.Huye LE, Nakazawa Y, Patel MP, et al. Combining mTor inhibitors with rapamycin-resistant T cells: a two-pronged approach to tumor elimination. Mol Ther. 2011;19(12):2239–2248. doi: 10.1038/mt.2011.179. [DOI] [PMC free article] [PubMed] [Google Scholar]