Abstract
Purpose of review
This review provides an update on current developments with prevention, treatment and cure strategies in the field of pediatric HIV.
Recent findings/Summary
There has been tremendous progress in the prevention and treatment of pediatric HIV infection. With new strategies for prevention of mother-to-child transmission, we are growing ever closer towards elimination of pediatric HIV, though challenges with retention of pregnant woman and their HIV-exposed infants remain. Ongoing vigilance regarding the potential hazards of in utero ART exposure to infants continues with no significant alarms yet identified. Though cure has not been achieved, evidence of the impact of early treatment on reducing HIV-1 reservoir size with subsequent prolonged remission has enlivened efforts to rapidly identify and treat HIV-infected newborns. There is an increasing array of treatment options for pediatric patients and reassuring evidence regarding long-term complications of ART. Unfortunately, despite evidence suggesting the benefit of early treatment, timely identification and treatment of children remains a challenge. Better strategies for effective case-finding and engagement in care are urgently needed in addition to an improved understanding of how to retain HIV-positive children and adolescents on treatment. However, further emboldened by recent international commitments and robust global support, the future is hopeful.
Keywords: Pediatric HIV, PMTCT, review
INTRODUCTION
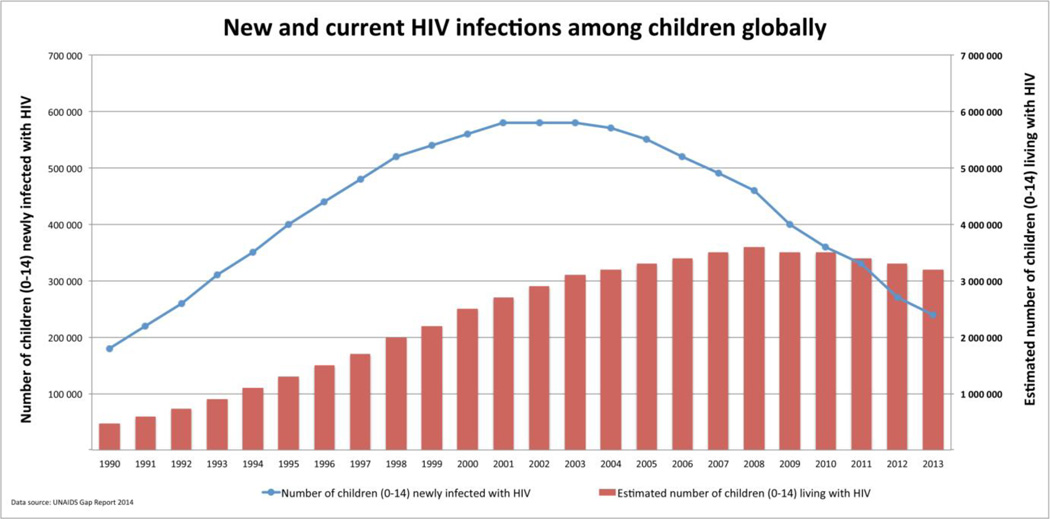
There has been tremendous progress in the prevention and treatment of pediatric HIV infection. In well-resourced settings, comprehensive prevention of mother-to-child transmission of HIV (PMTCT) efforts have resulted in few new perinatal infections (Figure 1) [1**] and an aging cohort of children with established infection surviving into adolescence and adulthood [2*]. We are also finally seeing a shift in the same direction globally alongside an unprecedented international commitment to eliminate new pediatric HIV infections, as well as identify and treat those with established HIV infection.
Figure 1.
New and current HIV infections among children globally
In 2013, nearly 70% of the estimated 1.5 million HIV-positive pregnant women globally received antiretroviral drugs (ARVs) with a 58% decrease in new pediatric infections from 2002 [3]. The number of HIV-infected children receiving care and antiretroviral treatment (ART) also increased [4] and there is a widening array of antiretroviral medications for pediatric treatment. Finally, there is new interest in very early ART initiation, close to the time of birth, as a way to improve outcomes among HIV-infected infants, minimize viral reservoirs, and inform effort towards achieving a cure [5*,6*].
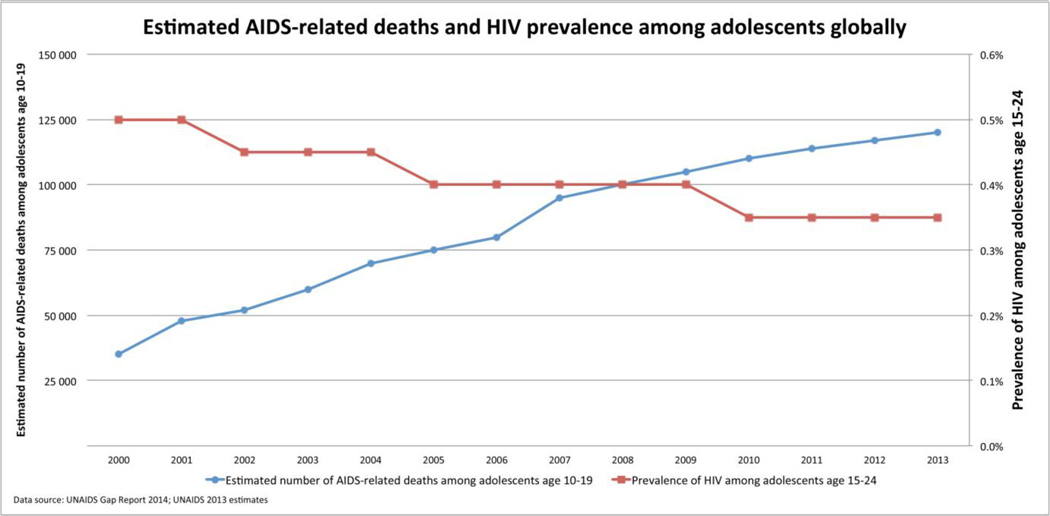
However, despite improving ART coverage, only 22% of HIV-infected children are currently receiving treatment [4], and a staggering number of childhood deaths, 190 000 [170 000 – 220 000] in 2013, continue to be attributed to HIV infection [7]. Furthermore, large numbers of adolescents are newly acquiring HIV, and HIV has become the second-leading cause of death among adolescents globally (Figure 2) [8]. This review outlines the progress towards prevention, treatment and cure in the field of pediatric HIV.
Figure 2.
Estimated AIDS-related deaths and HIV prevalence among adolescents globally
PREVENTION OF MOTHER-TO-CHILD HIV TRANSMISSION
The number of new pediatric infections globally continues to decrease with 240 000 [210 000–280 000] children less than 15 years of age newly acquiring HIV in 2013 (Figure 1) [4]. Improved access to HIV testing and ART along with better implementation of PMTCT services have likely contributed to this decline. Option B+ has provided a simplified PMTCT approach [9] with increasing evidence supporting its impact on improving maternal access to ART and retention across the PMTCT cascade. However, complex challenges to long-term retention of mothers and their infants throughout the period of MTCT risk are being described. There is also a growing body of research examining the potential adverse effects of in utero ART exposure and the cumulative evidence is increasingly reassuring.
Option B+: uptake and retention along the PMTCT cascade
One of the most notable recent developments in PMTCT globally has been the introduction of lifelong ART for all pregnant and breast-feeding women, the Option B+ approach [9]. Option B+ (B+) emerged at a time when there were significant losses along the PMTCT cascade due to the complexity of determining which pregnant women needed ART for their own health from those who required only ARV prophylaxis for PMTCT (Options A and B) [10]. Option B+ simplified the PMTCT implementation strategy by introducing a test and treat approach and reframing PMTCT as lifelong treatment for all pregnant and breastfeeding women, thereby improving access to ART and reducing opportunities for dropout along the cascade. A recent report on B+ implementation in 11 Sub Saharan African countries, from January 2013–March 2014, demonstrated that at least 50% of HIV-positive women in antenatal care received ART in 8/9 countries, with >80% receiving ART in 4/9 countries [11*]. Another report [12*] from several health centers in urban central Malawi confirmed improved enrollment into PMTCT services after the introduction of Option B+ (68.3% vs. 92.6% post-Option B+; p<0.001) as well as a shorter time to ART initiation (median, IQR 48 days [19,130] vs. 0 days [0,15.5] post-Option B+; p<0.001) [12*]. The only published prospective study [13] comparing Option B+ and Option A, conducted in the Kingdom of Swaziland, reported that a higher proportion of women with CD4 <350 cells/mm3 initiated ART in health zones implementing Option B+ versus those zones where Option A was the standard PMTCT approach (86% vs. 74%, p=0.032).
However, retention of women and infants within PMTCT services remains problematic. A recent study from Malawi (where Option B+ was first introduced) using aggregate data from 540 health facilities (n=21,939) and patient-level data from 19 facilities (n=11,534) found that loss-to-follow-up (LTFU) at 6 months was 17% amongst women starting ART under Option B+. Moreover, compared to women who initiated ART for their own health, women who started ART in PMTCT services were five times more likely not to return to clinic [OR 5.0, 95% confidence interval (CI) 4.2–6.1] [14**]. A study from northern Malawi also found that women starting ART for Option B+ experienced higher attrition than women of childbearing age starting treatment for other reasons [15]. Amongst women newly initiating ART post B+ at health facilities in central Malawi, a smaller proportion was alive-on-ART six-months after initiation (89.3% vs. 78.8% post-; p=0.0004) and a higher proportion stopped ART (2.2% vs. 8.2%, p=0.002) under Option B+ conditions [12*]. By comparison, in Uganda, retention at 6 months for women who initiated ART was 88%, similar to the 87% seen among other adults, in 149 health facilities supported by the Elizabeth Glazer Pediatric AIDS Foundation, an implementing partner for HIV service delivery in Sub Saharan Africa [11*].
Studies examining these losses reveal the complexity of retaining mothers and babies in HIV services and the myriad of inter-related factors that influence outcomes including health facility and patient-level factors, as well as patient-provider relations and quality of counseling.
A study from southeastern Malawi [16*] examined the relationship between models of service delivery at 141 health facilities and retention among pregnant women initiating ART. Factors independently associated with ART retention were district location, patient volume, and the model of care: facilities where women were referred from the antenatal clinic (ANC) to the ART clinic for initiation and follow-up were five times more likely to have higher retention rates than facilities where women initiated ART in the ANC and were subsequently referred to ART clinic for follow-up [adjusted odds ratios (aOR) 5.4(1.2–28)] [16*]. Another study from central Malawi [17*] described patient-level factors influencing retention. Of 577 HIV+ pregnant women classified as LTFU, 229 were successfully traced; common reasons for stopping ART included travel (38%), lack of transport money (16%), not understanding the initial ARV education session (10%), being too weak/sick (10%), and ARV side effects (10%) [17*]. Qualitative studies from South Africa [18*] and Tanzania [19] have found that negative experienced and even anticipated [19] clinic staff treatment were important barriers to retention in PMTCT care. A cross-sectional study amongst 277 HIV+ pregnant women in Tigray, Ethiopia [20] found that after controlling for other factors, the odds of adhering to Option B+ PMTCT were 4.7 times higher among women who received medication counseling as compared to those who did not (aOR 4.7, 95% CI 1.98–11.35). Much research is currently underway exploring how best to optimize uptake and retention along the PMTCT cascade [21,22,23,24,25,26,27]. However, much work remains to be done to better understand the complexity of retention, measure long-term retention, and ultimately evaluate impact on MTCT and infant HIV-free survival.
Efficacy and complications of antiretroviral regimens for PMTCT
The use of a once daily regimen, tenofovir (TDF) + emtricitibine (FTC) + efavirenz (EFV), for all pregnant and breast feeding women, the same regimen recommended for non-pregnant adults initiating ART in low and middle income countries is an essential component of the Option B+ approach [9]. The first evidence of the efficacy of EFV-based ART during pregnancy and breastfeeding was provided by the PROMOTE trial (ClinicalTrials.gov, NCT00993031) conducted in rural Uganda [28**]. Among 389 study participants randomized to one of two ART regimens, the proportion of women with virologic suppression (HIV-1 RNA </=400 copies/ml) at delivery was higher with an EFV- vs. lopinavir/ritonavir(LPV/r)-based regimen (97.6% in the EFV arm and 86.0% in the LPV/r arm, p< 0.001) and similar at 48 weeks postpartum (91% and 88.4% for EFV- and LPV/r-based regimens respectively, p=0.49). HIV-free infant survival was also similar between study arms: 97.2% (EFV) versus 92.9% (LPV/r), p=0.10. Effectiveness under non-trial conditions has not yet been evaluated.
There is also an increasingly large body of research examining the impact to infants of HIV and ARV exposure in utero and through breastfeeding [28**,29**,30,31*,32*,33,34*,35*,36*,37*,38*,39**,40*,41,42,43*]. Thus far there have been no significant safety alarms or ARVs identified as especially hazardous to the fetus. However, while the substantial benefits of combination ARVs for prevention of pediatric infection and maternal treatment have been repeatedly demonstrated, these drug exposures are not without some cost, albeit modest, to the fetus and infant. Historically efavirenz was contraindicated during pregnancy due to concerns around first trimester exposure and possible central nervous system (CNS) congenital anomalies. A recent meta-analysis confirmed the safety of this drug during pregnancy and found no evidence of increased risk of CNS anomalies with early EFV exposure [29**]. The association between ART during pregnancy and adverse birth outcomes (prematurity, stillbirth, low birth weight and small for gestational age) remains open to debate though emerging evidence suggests that ART during pregnancy, particularly PI-based treatment, may increase the risk of some of these outcomes [29**,30,31*,32*,33]. Protease inhibitor use has been associated with decreased progesterone levels during pregnancy possibly contributing to these outcomes. In an elegant set of experiments, investigators examined the association between lower birth weight and PI-based ART [32*,34*]. PI exposure was associated with reduced trophoblast progesterone production in vitro; lower progesterone levels and fetal weight in pregnant mice and; lower progesterone levels and smaller infants in 27-HIV-infected vs. 17 HIV-uninfected pregnant women.
Several studies provide reassurance regarding the potential adverse impact of ARV exposure on congenital anomalies [35*], cardiac toxicities [36*], cognitive and language developmental outcomes [37*,38*], hematologic [41,42], mitochondrial and metabolic outcomes [31*]. However, ongoing vigilance will be important, especially as new drugs are introduced into adult treatment regimens, as there will likely be new fetal exposures as women become pregnant. For example, while the Pediatric HIV/AIDS Cohort Study’s Surveillance Monitoring of ART Toxicities (SMARTT) Study, a prospective observational cohort study of HIV and ARV exposed uninfected children, found no association of first-trimester exposures with congenital anomalies for any ARV, combination ARV regimens or any drug class. However, among protease inhibitors, first trimester atazanavir (ATZ) was associated with increased risk for skin and musculoskeletal anomalies [35*].
In addition to potential ARV-associated toxicities, there are emerging concerns that HIV- exposed uninfected infants may have other vulnerabilities [40*]. A recent study demonstrated significantly increased risk of invasive pneumococcal disease and mortality among HIV-exposed compared to unexposed young infants in South Africa [43*]. However, many well described, often preventable and easily treated health conditions such as pneumonia, diarrhea, malnutrition, and malaria continue to pose the greatest threats to survival among populations of HIV-exposed, uninfected infants.
TREATMENT OF HIV INFECTION IN CHILDREN
Due to the tremendous success of PMTCT efforts, the perinatal pediatric epidemic in high and many middle income countries is predominantly adolescent with many already transitioned into adulthood. While there is also a growing population of aging adolescents with perinatal HIV in high burden, low resource settings, adolescent issues are still often overshadowed by the need to address the still too many [4] new pediatric infections and substantial unmet treatment need. A new global movement aims to close the treatment gap, but evidence to guide approaches to pediatric HIV case finding is very limited. Substantial delays in both diagnosis and treatment of HIV infection amongst infants and children continue alongside new studies demonstrating the benefit of ART earlier initiation. Amongst adolescents, large numbers are newly acquiring HIV, and are being recognized as a particularly vulnerable group (Figure 2) [8]. At the same time studies determining drug dosing and efficacy of new agents as well as long and short term toxicities and complications continue to shape treatment options for children.
The pediatric treatment cascade
Early infant diagnosis, case finding, ART initiation and retention
Despite improved coverage and uptake of PMTCT, early infant HIV diagnosis (EID) programs continue to underperform. An MMWR report [44*] documented results from the EID program in Francistown, Botswana from 2005–2012. Despite being among the most successful PMTCT programs in sub-Saharan Africa, the report identified substantial challenges in the EID program. Almost one third of the 10,923 HIV-exposed infants did not receive a virologic test to determine infection status and of the 202 infants identified as HIV-infected, only 123 (60%) initiated ART. Similarly, a study from Malawi reported that in the context of Option B+, only 52% of 513 HIV-exposed infants were tested at 6–12 weeks [45]. Results from a national assessment of missed EID opportunities in South Africa [46*] found that 68% of immunization clinics provided EID testing for infants with reported or documented HIV exposure but only 9% offered testing to infants with undocumented/unknown HIV exposure. Finally, a cohort study described HIV-exposed infant outcomes in Kinshasa, Democratic Republic of Congo, between 2007 and 2013 [47*]. Despite improvements over time, in 2011–2012 only 63% of infants had EID testing by 2 months of age (95% CI: 59–68%).
Beyond infancy, evidence on the most effective strategies to identify and link HIV-infected children to care is scarce [48,49] and delays in diagnosis and ART initiation persist. One study from Malawi [50*] introduced dedicated community health workers to improve identification and enrollment into care of HIV-infected infants and children by expanding both facility and community based HIV testing. Following the intervention there was a six-fold increase in rate of enrollment of children into HIV care from 3.2 to 19.8 per month. Provider initiated testing and counseling on inpatient wards has been shown to be an effective approach to identify HIV-infected children in high HIV prevalence settings but is rarely implemented routinely. Additional efforts to identify HIV-exposed and infected children and research on the most effective approaches to case finding are warranted.
Timely ART can dramatically reduce morbidity and mortality in HIV-infected children [51]. However, timely ART initiation remains a challenge particularly in countries with the highest burden of pediatric infections [7]. A multi-study collaboration [52*] described trends and determinants of CD4 cell count at ART initiation amongst 34,706 children less than 16 years of age spanning sub-Saharan Africa, Asia, Latin America, and the United States. The study found that although the estimated percentage of children starting treatment with severe immunodeficiency declined over time, in 2010, 70% of children less than 2 years still started ART with severe immunodeficiency despite guidelines recommending universal treatment for all children in this age group.
Unfortunately, not only are children initiating ART late, but they are not optimally retained in care. A study from Mozambique [53*] examined 2-year mortality and LTFU for children <15 years of age initiating ART between June 2006–July 2011. Of 753 children, 29.0% (95% CI: 24.5, 33.2) were confirmed dead by 2 years and 39.0% (95% CI: 34.8, 42.9) were LTFU with unknown clinical outcomes. Treatment was initiated late (WHO stage III/IV) among 48% of those with WHO stage recorded, emphasizing the need for earlier diagnosis and treatment. Similarly, a systematic review including 55,904 pediatric patients receiving first-line ART in routine settings in 23 countries found that retention at 12, 24, and 36 months was estimated at 88, 72, and 67% [54**].
Alongside reports of late treatment initiation is a burgeoning body of evidence suggesting the need for earlier treatment across all ages. The CHER study [51] demonstrated that early antiretroviral therapy, started at 6–12 weeks of age, reduced mortality and HIV progression by 75% [5*]. However, a recent study from South Africa suggests that ART initiation by three months of age may not adequately prevent disease progression [55**]. The investigators extracted records of all infants started on ART by three months of age between June 2007 and September 2010 at 20 public clinics and one large ART hospital service. On logistic regression, each month increase in age at ART initiation lowered the odds of initiating ART in an optimal state (OR: 0.56, CI: 0.36–0.94) and increased the odds of advanced HIV disease at ART initiation (OR: 1.69, CI: 1.05–2.71).
Results from several studies are building a case for universal treatment for all HIV-infected children independent of age, CD4 cell count or viral load. A secondary analysis [56] of data from the ARROW trial, a strategy trial comparing clinical and laboratory monitoring among children on ART, examined patterns of long-term CD4 reconstitution in HIV-infected children starting ART in sub-Saharan Africa. Using nonlinear mixed-effects models the study found that higher long-term CD4 counts were predicted for children starting ART at younger ages, and with higher CD4 counts (p<0.001) suggesting that children who remain ART näive beyond 10 years of age are unlikely to achieve a normalized CD4 count. Another ARROW analysis demonstrated that delaying ART initiation until older childhood substantially delayed pubertal development and menarche, independent of immunosuppression [57*]. These findings suggest that earlier ART initiation may lead to better long-term outcomes among HIV-infected children.
At the same time as evidence emerges to support early treatment initiation, studies are delineating the particular challenges of engaging and retaining adolescents with HIV in treatment. In a retrospective cohort study of 16,421 patients aged ≥15 years starting ART in seven African countries, 2004–2012, higher rates of LTFU were recorded among adolescents and young adults, aged 15–24 years, compared with adults, >/=50 years [58*]. Another cohort study utilizing routinely collected patient-level data from pre and post ART patients at least 10 years of age from 160 HIV clinics in Kenya, Mozambique, Tanzania, and Rwanda found that youth (15–24 years) experienced substantially higher attrition before and after ART initiation compared with younger adolescents and older adults [59*]. Optimizing adherence outcomes among youth are equally challenging. A recent meta-analysis including over 10,725 patients globally demonstrated that only 62.3% [95% CI 57.1–67.6; I:97.2%] of the adolescent population were adherent to therapy [60**]. In a study from the Adolescent Treatment Network, adherence and viral suppression were measured among youth with behaviorally and perinatally acquired HIV infection receiving care at 20 adolescent specialty centers in the US. Of 488 perinatally HIV-infected youth and 517 behaviorally infected youth who reported taking an ART regimen consecutively for at least the past 6 months, virologic suppression rates were 45.9% and 63.6% respectively [61**].
Antiretroviral treatment options: efficacy and safety
Antiretroviral drug options for children continue to lag behind adults for whom a number of new drug classes and formulations have become available in recent years. However, there is a slowly expanding array of treatment options for pediatric HIV. Once-daily atazanavir (ATV), a protease inhibitor (PI), has been studied down to infancy amongst both treatment experienced and naïve children and was shown to be safe and well tolerated with acceptable levels of viral suppression. The Pediatric AIDS Clinical Trials Group 1020A study of ATV [62*] enrolled participants from 91 days to 21 years of age, and at week 96 found respective rates of VL suppression of 57.0% in treatment naïve and 39.3% in treatment experienced patients. Raltegravir, an integrase inhibitor (INI), available as a film-coated tablet 400 mg twice daily (6 to <19 years, and >/= 25 kg) and chewable tablet 6 mg/kg (maximum dose 300 mg) twice daily (2 to <12 years), was shown to be well tolerated with favorable virologic and immunologic responses in a multicenter trial [63*] of drug experienced children and is now approved for children 4 weeks of age and older [64]. Once-daily darunavir/ritonavir 800/100 mg, a PI, has been shown to be to be effective and well-tolerated for treatment of HIV-1-infected, antiretroviral-naive adolescents (>/=12 to <18 years) [65*]. Dolutegravir, another INI, was the first ARV to receive simultaneous approval in young adolescents and adults [66]. Unfortunately none of these new drugs are yet widely available in the global marketplace for children.
There is increasing evidence confirming the benefit of starting LPV/r-based ART as first-line therapy in infants regardless of PMTCT history [67**,68]. Unfortunately in low resource settings programs have been slow to incorporate LPV/r into their first line regimens and nevirapine-based ART is more often prescribed for infants and young children [69]. This is particularly worrisome in light of new evidence on resistance among ART naïve HIV-infected children. A study of 230 newly diagnosed children under 2 years of age in South Africa [69] in 2011 found high rates of drug resistance: among the 67.4% of children with reported PMTCT exposure, 56.8% had NNRTI and 14.8% NRTI mutations; of those with no reported or recorded PMTCT exposures, resistance to NNRTI was detected in 24.0%, NRTI in 10.7%. It is anticipated that new formulations of LPV/r as sprinkles [70*] may improve uptake of PI-based treatment among infants by addressing issues of palatability, storage, transport and cost.
More information is also becoming available on the long term safety of ARVs commonly used to treat pediatric HIV infection. The ARROW study found that long-term zidovudine (ZDV)-based ART was associated with similar body circumference and skinfold thickness as abacavir-based ART, with low rates of lipid abnormalities and clinical lipodystrophy [71]. A prospective, open-label study evaluated the efficacy, safety and pharmacokinetics of TDF (given as TDF/3TC/EFV once daily) in treatment-experienced children 3–18 years of age in Thailand found no evidence of increased renal glomerular or renal tubular toxicity. However, the investigators reported a statistically significant decrease in spine bone mineral density Z-score in the TDF vs. non-TDF group [72*,73*]. A Gilead sponsored trial [74*] in the United States, United Kingdom and Panama randomized HIV-infected children (2 to <16 years) on a stavudine (d4T) or ZDV containing regimen with HIV-1 RNA <400 copies/mL to continue d4T or ZDV or switch to TDF. In this study TDF did not demonstrate non-inferiority in maintaining HIV-1 RNA <400 copies/mL at week 48 as compared to d4T or ZDV and 4/79 patients discontinued TDF due to late occurrence (study weeks 84–156) of renal tubular dysfunction. Additional surveillance for the long term, especially bone effects, of TDF in children are needed [73*].
Very early treatment and prospects for CURE
The report of a functional cure in the ‘Mississippi baby,’ brought great optimism to the HIV community. The possibility that very early treatment could result in sustained virologic control off ART (remission) for an infection that necessitates a lifelong commitment to treatment was very compelling. In the case of the ‘Mississippi baby,’ triple ARV therapy was initiated very close to the time of birth (at 30 hours) and discontinued after 18 months of treatment, with no evidence of viral rebound including undetectable proviral DNA, plasma HIV-1 RNA and HIV-1 antibodies [75]. Unfortunately, more than two years after treatment cessation, the period of remission ended and HIV RNA was detectable at 16,750 copies/mL, dashing hopes that the child may have attained permanent virologic control off ART [76**]. Thus far, additional cases of early treatment have also failed to demonstrate evidence of sustained viral remission off ART. A British child was initiated on triple ARV therapy within hours of life and virally suppressed on treatment for four years, but had a rapid viral rebound when ART was temporarily discontinued [77*].
Although there has been great disappointment around the failure of early treatment initiators to sustain viral remission off treatment, very early treatment appears to reduce replication competent HIV-1 reservoir size. Reduction of latent replication-competent HIV reservoirs is believed to be critical to attain a sustained remission [6*,78*,79*]. A report on outcomes of early treated children from three centers in Canada described four vertically-infected children with sustained viral suppression on ART [78*]. At 2–5 years on treatment, levels of proviral HIV-1 DNA and replication-competent virus in the peripheral blood were dramatically reduced: HIV serology, HIV specific cell-mediated immune response, and ultrasensitive viral load were negative, cell-associated HIV-1 DNA in plasma and virion-associated HIV-1 RNA in stimulated CD4 +T cells were undetectable. Only one of two children tested had a low level of replication-competent HIV-1 in CD4+ T cells. These findings provide evidence to the hypothesis that early treatment can lead to reduced replication competent reservoirs. However, treatment interruption is required to determine if any of these children can sustain viral suppression off treatment and achieve a period of remission. Several studies are currently in progress to examine the impact of very early treatment of the HIV-infected newborns to prevent HIV disease progression, limit and possibly prevent establishment of latent reservoirs, and test approaches to viral eradication [5*,6*,79*].
CONCLUSION
There is enormous optimism that the future holds opportunities for more effective prevention, treatment, and possible cure of pediatric HIV infection. With new PMTCT implementation strategies bolstered by robust global support, we are growing ever closer towards elimination of pediatric HIV. PMTCT uptake is being further optimized and there is increasing interest in understanding and addressing challenges with retention. Ongoing vigilance regarding the potential hazards of in utero ART exposure to infants continues with no significant alarms yet identified. Though cure has not been achieved, evidence of the impact of early treatment on reducing HIV-1 reservoir size has enlivened efforts to rapidly identify and treat HIV-infected newborns. There is an increasing array of treatment options and reassuring evidence regarding long term complications of ART. Unfortunately, alongside burgeoning evidence suggesting the benefit of even earlier treatment, timely identification and treatment of children remains a challenge. Evidence regarding most effective case-finding and linkage strategies are urgently needed in addition to an improved understanding of how to engage and retain HIV-positive children and adolescents on treatment. However, further emboldened by recent international commitments, the future is hopeful.
ACKNOWLEDGEMENTS
MHK was supported by the Fogarty International Center of the National Institutes of Health under award number K01 TW009644
The authors would like to thank Rachael Sabelli for her assistance with the references and Robbie Flick for his assistance in developing Figures 1 and 2.
Footnotes
COMPLIANCE WITH ETHICS GUIDELINES
Conflicts of Interest
Maria H. Kim, Saeed Ahmed, and Elaine J. Abrams declare they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
REFERENCES
Recent papers of particular interest have been denoted as:
* “of importance”
** “of major importance”
- 1. Townsend CL, Byrne L, Cortina-Borja M, et al. Earlier initiation of ART and further decline in mother-to-child HIV transmission rates, 2000–2011. Aids. 2014;28(7):1049–1057. doi: 10.1097/QAD.0000000000000212. Analysis of mother-to-child HIV transmission rates over time using population-based surveillance data in the UK and Ireland demonstrated a continued decline in MTCT from 2006 to 2011. The authors delineated the trajectory of viral suppression during pregnancy. Low transmission rates were associated with a variety of factors including earlier ART initiation.
- 2. Bamford A, Lyall H. Paediatric HIV grows up: recent advances in perinatally acquired HIV. Arch Dis Child. 2015;100(2):183–188. doi: 10.1136/archdischild-2014-306079. Description of current status of pediatric HIV in the UK and Ireland including the epidemiology, perinatal prevention efforts, and treatment outcomes.
- 3.UNAIDS. The Gap Report. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS; 2014. [Google Scholar]
- 4.UNAIDS. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS; 2014. 2014 Progress Report on the Global Plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive. [Google Scholar]
- 5. Cotton MF, Rabie H. Impact of earlier combination antiretroviral therapy on outcomes in children. Curr Opin HIV AIDS. 2015;10(1):12–17. doi: 10.1097/COH.0000000000000117. Review of the findings of studies and case reports of early ART initiation during infancy as well as challenges and implications of treatment initiation close to the time of birth.
- 6. Rainwater-Lovett K, Luzuriaga K, Persaud D. Very early combination antiretroviral therapy in infants: prospects for cure. Curr Opin HIV AIDS. 2015;10(1):4–11. doi: 10.1097/COH.0000000000000127. Review of the rationale and feasibility of very early combination antiretroviral therapy to achieve sustained virologic remission in perinatal infection. The authors describe several cases of virologic remission and rebound, the impact of early ART on reservoir establishment, reservoir size and immune response, and describe challenges to initiation of very early diagnosis and treatment in infants.
- 7.World Health Organization. Geneva, Switzerland: World Health Organization; 2014. Optimizing Treatment Options and Improving Access to Priority Products for Children. [Google Scholar]
- 8.World Health Organization. Geneva, Switzerland: World Health Organization; 2014. Global Update on the Health Sector Response to HIV. [Google Scholar]
- 9.World Health Organization. Geneva Switzerland: 2013. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV infection: recommendations for a public health approach. [PubMed] [Google Scholar]
- 10.WHO. Geneva, Switzerland: WHO Press; 2010. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: recommendations for a public health approach. [PubMed] [Google Scholar]
- 11. Kieffer MP, Mattingly M, Giphart A, et al. Lessons learned from early implementation of option B+: the Elizabeth Glaser Pediatric AIDS Foundation experience in 11 African countries. J Acquir Immune Defic Syndr. 2014;67(Suppl 4):S188–S194. doi: 10.1097/QAI.0000000000000372. A descriptive summary of Option B+ implementation using routinely collected program data to describe uptake and retention in 11 Sub Saharan African countries.
- 12. Kim MH, Ahmed S, Hosseinipour MC, et al. Implementation and Operational Research: The Impact of Option B+ on the Antenatal PMTCT Cascade in Lilongwe, Malawi. J Acquir Immune Defic Syndr. 2015;68(5):e77–e83. doi: 10.1097/QAI.0000000000000517. Pre/post evaluation of the impact of Option B+ on the antenatal PMTCT cascade in Lilongwe, Malawi using patient level data.
- 13.Parker LA, Jobanputra K, Okello V, et al. Barriers and facilitators to combined ART initiation in pregnant women with HIV: lessons learnt from a PMTCT B+ pilot program in Swaziland. Journal of acquired immune deficiency syndromes (1999) 2015 doi: 10.1097/QAI.0000000000000537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tenthani L, Haas AD, Tweya H, et al. Retention in care under universal antiretroviral therapy for HIV-infected pregnant and breastfeeding women (‘Option B+’) in Malawi. Aids. 2014;28(4):589–598. doi: 10.1097/QAD.0000000000000143. Description of the impact of Option B+ on retention using routinely collected nationwide data in Malawi from 540 health facilities including 21939 patients. The loss- to-follow-up rate at 6 months was 17% amongst women starting ART under Option B+ and women who started ART through PMTCT were five times more likely not to return to clinic as compared to women beginning treatment for their own health.
- 15.Koole O, Houben RM, Mzembe T, et al. Improved retention of patients starting antiretroviral treatment in Karonga District, northern Malawi, 2005–2012. Journal of acquired immune deficiency syndromes (1999) 2014;67(1):e27–e33. doi: 10.1097/QAI.0000000000000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van Lettow M, Bedell R, Mayuni I, et al. Towards elimination of mother-to-child transmission of HIV: performance of different models of care for initiating lifelong antiretroviral therapy for pregnant women in Malawi (Option B+) J Int AIDS Soc. 2014;17:18994. doi: 10.7448/IAS.17.1.18994. A study from southeastern Malawi examined associations between models of service delivery under Option B+ service delivery models and program performance at 141 facilities. Model of care was independently associated with ART retention highlighting the importance of facility level characteristics and how services are implemented on patient outcomes.
- 17. Tweya H, Gugsa S, Hosseinipour M, et al. Understanding factors, outcomes and reasons for loss to follow-up among women in Option B+ PMTCT programme in Lilongwe, Malawi. Trop Med Int Health. 2014;19(11):1360–1366. doi: 10.1111/tmi.12369. The investigators traced women categorized as lost-to-follow-up from an Option B+ programme at one large antenatal clinic in Lilongwe Malawi. Outcomes suggest the importance of improving counselling strategies and psychosocial services to optimize retention and patient outcomes.
- 18. Clouse K, Schwartz S, Van Rie A, et al. "What they wanted was to give birth; nothing else": barriers to retention in option B+ HIV care among postpartum women in South Africa. J Acquir Immune Defic Syndr. 2014;67(1):e12–18. doi: 10.1097/QAI.0000000000000263. Qualitative study from a primary care facility in Johannesburg, South Africa, offering Option B+ in South Africa. Women in care were described reasons women might chose not to continue in HIV postpartum, highlighting the complexity of factors influencing retention.
- 19.Gourlay A, Wringe A, Birdthistle I, et al. "It is like that, we didn't understand each other": exploring the influence of patient-provider interactions on prevention of mother-to-child transmission of HIV service use in rural Tanzania. PLoS One. 2014;9(9):e106325. doi: 10.1371/journal.pone.0106325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ebuy H, Yebyo H, Alemayehu M. Adherence level to and predictors of Option B+ PMTCT programme in Tigray, northern Ethiopia. Int J Infect Dis. 2014 doi: 10.1016/j.ijid.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 21.Sando D, Geldsetzer P, Magesa L, et al. Evaluation of a community health worker intervention and the World Health Organization's Option B versus Option A to improve antenatal care and PMTCT outcomes in Dar es Salaam, Tanzania: study protocol for a cluster-randomized controlled health systems implementation trial. Trials. 2014;15:359. doi: 10.1186/1745-6215-15-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lema IA, Sando D, Magesa L, et al. Community health workers to improve antenatal care and PMTCT uptake in Dar es Salaam, Tanzania: a quantitative performance evaluation. Journal of acquired immune deficiency syndromes (1999) 2014;67(Suppl 4):S195–S201. doi: 10.1097/QAI.0000000000000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mwapasa V, Pro G, Chinkhumba J, et al. Mother-infant pair clinic and SMS messaging as innovative strategies for improving access to and retention in eMTCT care and Option B+ in Malawi: a cluster randomized control trial (the PRIME study) Journal of acquired immune deficiency syndromes (1999) 2014;67(Suppl 2):S120–S124. doi: 10.1097/QAI.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 24.Foster G, Kangwende A, Magezi V, et al. Cluster randomized trial on the effect of mother support groups on retention-in-care and PMTCT outcomes in Zimbabwe: study design, challenges, and national relevance. Journal of acquired immune deficiency syndromes (1999) 2014;67(Suppl 2):S145–S149. doi: 10.1097/QAI.0000000000000325. [DOI] [PubMed] [Google Scholar]
- 25.Rosenberg NE, van Lettow M, Tweya H, et al. Improving PMTCT uptake and retention services through novel approaches in peer-based family-supported care in the clinic and community: a 3-arm cluster randomized trial (PURE Malawi) Journal of acquired immune deficiency syndromes (1999) 2014;67(Suppl 2):S114–S119. doi: 10.1097/QAI.0000000000000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sam-Agudu NA, Cornelius LJ, Okundaye JN, et al. The impact of mentor mother programs on PMTCT service uptake and retention-in-care at primary health care facilities in Nigeria: a prospective cohort study (MoMent Nigeria) Journal of acquired immune deficiency syndromes (1999) 2014;67(Suppl 2):S132–S138. doi: 10.1097/QAI.0000000000000331. [DOI] [PubMed] [Google Scholar]
- 27.Oyeledun B, Oronsaye F, Oyelade T, et al. Increasing retention in care of HIV-positive women in PMTCT services through continuous quality improvement-breakthrough (CQI-BTS) series in primary and secondary health care facilities in Nigeria: a cluster randomized controlled trial. The Lafiyan Jikin Mata Study. Journal of acquired immune deficiency syndromes (1999) 2014;67(Suppl 2):S125–S131. doi: 10.1097/QAI.0000000000000320. [DOI] [PubMed] [Google Scholar]
- 28. Cohan D, Natureeba P, Koss CA, et al. Efficacy and safety of lopinavir/ritonavir versus efavirenz-based antiretroviral therapy in HIV-infected pregnant Ugandan women. Aids. 2015;29(2):183–191. doi: 10.1097/QAD.0000000000000531. Planned secondary analysis comparing viral load suppression, safety, and HIV transmission to infants in a randomized controlled trial to test the hypothesis that lopinavir/ritonavir versus efavirenz-based ART would reduce placental malaria (PROMOTE, ClinicalTrials.gov, NCT00993031). First study to evaluate the efficacy of efavirenz-based ART for PMTCT, demonstrating high rates of viral suppression and infant HIV-free survival.
- 29. Ford N, Mofenson L, Shubber Z, et al. Safety of efavirenz in the first trimester of pregnancy: an updated systematic review and meta-analysis. Aids. 2014;28(Suppl 2):S123–S131. doi: 10.1097/QAD.0000000000000231. An updated systematic review and meta-analysis examining the safety of EFV use during pregnancy providing further evidence of no increased risk of overall or central nervous system congenital anomalies with first-trimester exposure to efavirenz. Ongoing monitoring is important given the low incidence of central nervous system anomalies in the overall population and relatively small number of exposures in the current literature.
- 30.Chen JY, Ribaudo HJ, Souda S, et al. Highly Active Antiretroviral Therapy and Adverse Birth Outcomes Among HIV-Infected Women in Botswana. Journal of Infectious Diseases. 2012;206(11):1695–1705. doi: 10.1093/infdis/jis553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jao J, Abrams EJ. Metabolic complications of in utero maternal HIV and antiretroviral exposure in HIV-exposed infants. Pediatr Infect Dis J. 2014;33(7):734–740. doi: 10.1097/INF.0000000000000224. The authors conducted a systematic literature review to examine complications of in utero HIV and ARV exposures among HIV exposed uninfected infants and reported on outcomes including intrauterine and early postnatal growth, bone health, metabolic complications and mitochondrial toxicity.
- 32. Papp E, Mohammadi H, Loutfy MR, et al. HIV protease inhibitor use during pregnancy is associated with decreased progesterone levels, suggesting a potential mechanism contributing to fetal growth restriction. J Infect Dis. 2015;211(1):10–18. doi: 10.1093/infdis/jiu393. The investigators demonstrate an association between lower birth weight and protease inhibitor based antiretroviral therapy in vitro and among HIV-infected pregnant women.
- 33.Watts DH, Mofenson LM. Antiretrovirals in pregnancy: a note of caution. J Infect Dis. 2012;206(11):1639–1641. doi: 10.1093/infdis/jis581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Powis KM, Shapiro RL. Protease inhibitors and adverse birth outcomes: is progesterone the missing piece to the puzzle? J Infect Dis. 2015;211(1):4–7. doi: 10.1093/infdis/jiu397. Commentary on Papp et al (reference #33) on association between progesterone, perinatal protease inhibitor exposure and adverse birth outcomes. The authors review and synthesize findings on risk of adverse pregnancy outcomes and proposed underlying mechanisms.
- 35. Williams PL, Crain MJ, Yildirim C, et al. Congenital anomalies and in utero antiretroviral exposure in human immunodeficiency virus-exposed uninfected infants. JAMA Pediatr. 2015;169(1):48–55. doi: 10.1001/jamapediatrics.2014.1889. Pediatric HIV/AIDS Cohort Study’s Surveillance Monitoring of ART Toxicities (SMARTT) Study, includes 2580 HIV-exposed uninfected children in the US. There was no association of first-trimester exposures with congenital anomalies for any ARV, combination ARV regimens, or any drug class.
- 36. Lipshultz SE, Williams PL, Zeldow B, et al. Cardiac effects of in-utero exposure to antiretroviral therapy in HIV-uninfected children born to HIV-infected mothers. Aids. 2015;29(1):91–100. doi: 10.1097/QAD.0000000000000499. An examination of echocardiographic parameters among 417 HIV-exposed uninfected and 98-HIV-unexposed children aged 2–7 years in the Pediatric HIV/AIDS Cohort Study’s Surveillance Monitoring of ART Toxicities (SMARTT) Study revealed no significant differences in echocardiographic Z-scores, suggesting no significant cardiac toxicity of perinatal exposure to combination antiretroviral therapy.
- 37. Ngoma MS, Hunter JA, Harper JA, et al. Cognitive and language outcomes in HIV-uninfected infants exposed to combined antiretroviral therapy in utero and through extended breast-feeding. Aids. 2014;28(Suppl 3):S323–S330. doi: 10.1097/QAD.0000000000000357. Nonverbal problem-solving and language skills were examined in 97 HIV- and perinatal ART-exposed, uninfected and 103 unexposed 15–36 month old children in Zambia. Language delay was associated with low birth weight; there was no evidence of an adverse effect of perinatal ART exposure on cognitive and language development.
- 38. Nozyce ML, Huo Y, Williams PL, et al. Safety of in utero and neonatal antiretroviral exposure: cognitive and academic outcomes in HIV-exposed, uninfected children 5–13 years of age. Pediatr Infect Dis J. 2014;33(11):1128–1133. doi: 10.1097/INF.0000000000000410. Study of HIV-exposed, uninfected children, ages 5–13 years, in Pediatric HIV/AIDS Cohort Study Surveillance Monitoring for Antiretroviral Treatment Toxicities (SMARTT), found no significant association between any ARV regimen or class and any cognitive or academic outcome.
- 39. Koss CA, Natureeba P, Plenty A, et al. Risk factors for preterm birth among HIV-infected pregnant Ugandan women randomized to lopinavir/ritonavir- or efavirenz-based antiretroviral therapy. J Acquir Immune Defic Syndr. 2014;67(2):128–135. doi: 10.1097/QAI.0000000000000281. The PROMOTE-Pregnant Women and Infants Study was an open-label, randomized controlled trial comparing the risk of placental malaria among HIV-infected, ART-naïve pregnant Ugandan women assigned to initiate lopoinavir/ritonavir vs. efavirenz-based ART. ART regimen was not associated with preterm birth outcomes.
- 40. Mofenson LM. New Challenges in the Elimination of Pediatric HIV Infection: The Expanding Population of HIV-Exposed but Uninfected Children. Clin Infect Dis. 2015 doi: 10.1093/cid/civ064. Reviews the recent literature compiling evidence around the possible association between in utero HIV and ART exposure with immunologic and biologic abnormalities predisposing HIV-exposed uninfected infants to increased risk of morbidity and mortality.
- 41.Dryden-Peterson S, Shapiro RL, Hughes MD, et al. Increased risk of severe infant anemia after exposure to maternal HAART, Botswana. J Acquir Immune Defic Syndr. 2011;56(5):428–436. doi: 10.1097/QAI.0b013e31820bd2b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lahoz R, Noguera A, Rovira N, et al. Antiretroviral-related hematologic short-term toxicity in healthy infants: implications of the new neonatal 4-week zidovudine regimen. Pediatr Infect Dis J. 2010;29(4):376–379. doi: 10.1097/INF.0b013e3181c81fd4. [DOI] [PubMed] [Google Scholar]
- 43. von Mollendorf C, von Gottberg A, Tempia S, et al. Increased Risk for and Mortality From Invasive Pneumococcal Disease in HIV-Exposed but Uninfected Infants Aged <1 Year in South Africa, 2009–2013. Clin Infect Dis. 2015 doi: 10.1093/cid/civ059. Using data from a national, laboratory-based surveillance program for invasive pneumococcal disease in South Africa, investigators demonstrated an increased risk of invasive pneumococcal disease in HIV-exposed uninfected compared with HIV-unexposed infants.
- 44. Motswere-Chirwa C, Voetsch A, Lu L, et al. Follow-up of infants diagnosed with HIV - Early Infant Diagnosis Program, Francistown, Botswana, 2005–2012. MMWR Morb Mortal Wkly Rep. 2014;63(7):158–160. A descriptive analysis of the pediatric care cascade including early infant diagnosis, linkage to care, ART initiation, retention and mortality amoung HIV-exposed infants in Francistown, Botswana, 2005–2012.
- 45.Martínez Pérez G, Metcalf C, Garone D, et al. HIV testing and retention in care of infants born to HIV- infected women enrolled in ‘Option B+’. Thyolo, Malawi. Public Health Action. 2014;4(2):102–104. doi: 10.5588/pha.14.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Woldesenbet SA, Jackson D, Goga AE, et al. Missed opportunities for early infant HIV diagnosis: results of a national study in South Africa. J Acquir Immune Defic Syndr. 2015;68(3):e26–e32. doi: 10.1097/QAI.0000000000000460. A national assessment of early infant diagnosis services in South Africa underscores missed opportunities for obtaining early infant HIV testing.
- 47. Feinstein L, Edmonds A, Chalachala JL, et al. Temporal changes in the outcomes of HIV-exposed infants in Kinshasa, Democratic Republic of Congo during a period of rapidly evolving guidelines for care (2007–2013) Aids. 2014;28(Suppl 3):S301–S311. doi: 10.1097/QAD.0000000000000331. In a cohort study of mother-infant pairs enrolled in family-centered comprehensive HIV care in Kinshasa, Democratic Republic of Congo, the investigators described temporal changes in HIV-exposed infant outcomes between 2007 and 2013 in early infant diagnosis, HIV transmission, mortality and ART initiation.
- 48.Busza J, Dauya E, Bandason T, et al. "I don't want financial support but verbal support" How do caregivers manage children's access to and retention in HIV care in urban Zimbabwe? J Int AIDS Soc. 2014;17:18839. doi: 10.7448/IAS.17.1.18839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahmed S, Kim MH, Sugandhi N, et al. Beyond early infant diagnosis: case finding strategies for identification of HIV-infected infants and children. Aids. 2013;27(Suppl 2):S235–S245. doi: 10.1097/QAD.0000000000000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ahmed S, Kim MH, Dave AC, et al. Improved identification and enrolment into care of HIV-exposed and -infected infants and children following a community health worker intervention in Lilongwe, Malawi. J Int AIDS Soc. 2015;18(1):19305. doi: 10.7448/IAS.18.1.19305. Innovative community health worker intervention to improve identification and enrolment into care of HIV-exposed and –infected infants and children in Lilongwe Malawi. Authors report a six-fold increase in rate of enrollment of children into HIV care from 3.2 to 19.8 per month.
- 51.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359(21):2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Koller M, Patel K, Chi BH, et al. Immunodeficiency in children starting antiretroviral therapy in low-, middle-, and high-income countries. J Acquir Immune Defic Syndr. 2015;68(1):62–72. doi: 10.1097/QAI.0000000000000380. Describes trends of CD4 measures at ART initiation in children from 9 low-income, 6 lower middle-income, 4 upper middle-income countries, and 1 high-income country (United States), 2004–2010. Children in low and middle-income countries continue to start ART with severe immunodeficiency emphasizing the need for earlier diagnosis and treatment.
- 53. Vermund SH, Blevins M, Moon TD, et al. Poor clinical outcomes for HIV infected children on antiretroviral therapy in rural Mozambique: need for program quality improvement and community engagement. PLoS One. 2014;9(10):e110116. doi: 10.1371/journal.pone.0110116. The investigators report on mortality and loss to follow-up among children starting ART in Zambezia Province, Mozambique, 2006–2011. At two years, 29.0% were confirmed dead and 39% lost-to-follow-up with substantial variation by district. Poor programmatic outcomes persist for children with HIV in rural Mozambique.
- 54. Fox MP, Rosen S. Systematic review of retention of pediatric patients on HIV treatment in low and middle-income countries 2008–2013. Aids. 2015;29(4):493–502. doi: 10.1097/QAD.0000000000000559. Systematic review of pediatric retention on ART in low and middle-income countries during 2008–2013; includes 39 reports from 45 patient cohorts and 55,904 patients in 23 countries. Authors found that pediatric ART retention was similar to that among adults: estimated retention at 12, 24, and 36 months at 88, 72, and 67% from life-table analysis.
- 55. Innes S, Lazarus E, Otwombe K, et al. Early severe HIV disease precedes early antiretroviral therapy in infants: Are we too late? J Int AIDS Soc. 2014;17:18914. doi: 10.7448/IAS.17.1.18914. Investigators described HIV disease progression among 403 children initiating ART by three months of age, 2007–2010, in 20 public clinics in Cape Town and a large ART service in Soweto, South Africa. 62% had advanced disease at ART start suggesting that initiation by three months of age may not be early enough to adequately prevent disease progression.
- 56.Picat MQ, Lewis J, Musiime V, et al. Predicting patterns of long-term CD4 reconstitution in HIV-infected children starting antiretroviral therapy in sub-Saharan Africa: a cohort-based modelling study. PLoS Med. 2013;10(10):e1001542. doi: 10.1371/journal.pmed.1001542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Szubert AJ, Musiime V, Bwakura-Dangarembizi M, et al. Pubertal development in HIV-infected African children on first-line antiretroviral therapy. Aids. 2015;29(5):609–618. doi: 10.1097/QAD.0000000000000590. In this observational analysis within the ARROW trial, a randomized study of monitoring strategies for children on ART, the investigators found that delaying ART initiation until older age substantially delays pebertal development and menarche independently of immune status.
- 58. Auld AFAS, Shiraishi RW, Wabwire-Mangen F, Kwesigabo G, Mulenga M, et al. Antiretroviral Therapy Enrollment Characteristics and Outcomes Among HIV-Infected Adolescents and Young Adults Compared with Older Adults - Seven African Countries, 204–2013. Morbidity and Mortality Weekly Report: Centers for Disease Control and Prevention. 2014 The investigators analyzed age-related differences in enrollment characteristics and outcomes among 16,421 patients aged ≥15 years initiating ART in seven African countries, 2004–2012. Adolescents and young adults (aged 15–24 years) had higher LTFU rates in all seven countries and reached statistical significance in three countries in crude and multivariable analyses compared with older adults (aged >/=50 years).
- 59. Lamb MR, Fayorsey R, Nuwagaba-Biribonwoha H, et al. High attrition before and after ART initiation among youth (15–24 years of age) enrolled in HIV care. Aids. 2014;28(4):559–568. doi: 10.1097/QAD.0000000000000054. Cohort study utilizing routinely collected patient-level data from 160 HIV clinics in four African countries. The investigators compared pre and post-ART attrition between youth (15–24 years) and other patients in HIV care. Youth experienced significantly higher attrition before and after ART initiation as compared to younger adolescents and older adults.
- 60. Kim SH, Gerver SM, Fidler S, Ward H. Adherence to antiretroviral therapy in adolescents living with HIV: systematic review and meta-analysis. Aids. 2014;28(13):1945–1956. doi: 10.1097/QAD.0000000000000316. The authors performed a systematic review and mata-analysis of published studies reporting adherence to ART among adolescents with HIV ages 12–24 years. Approximately two-thirds of 10,725 patients were reported as adherent with rates higher in Africa and Asia than Europe and North America.
- 61. Kahana SY, Fernandez MI, Wilson PA, et al. Rates and correlates of antiretroviral therapy use and virologic suppression among perinatally and behaviorally HIV-infected youth linked to care in the United States. J Acquir Immune Defic Syndr. 2015;68(2):169–177. doi: 10.1097/QAI.0000000000000408. Cross-sectional study of rates and correlates of ART use and virologic suppression among 649 perinatally HIV-infected youth and 1547 behaviorally HIV-infected youth in 20 Adolescent Medicine Trials Network for HIV/AIDS Interventions sites in the United States, 2009–2012. Overall only 37.0% of perinatally HIV-infected youth and 27.1% of behaviorally HIV-infected youth were virologically suppressed, increasing to 45.9% and 63.6% among those reporting having taken an ART regimen consecutively for at least 6 months.
- 62. Rutstein RM, Samson P, Fenton T, et al. Long-term Safety and Efficacy of Atazanavir-based Therapy in HIV-infected Infants, Children and Adolescents: The Pediatric AIDS Clinical Trials Group Protocol 1020A. Pediatr Infect Dis J. 2015;34(2):162–167. doi: 10.1097/INF.0000000000000538. Report on the phase I/II open label study of long-term safety and virologic and immunologic responses of atazanavir (ATV) (with/without ritonavir [RTV] boosting) amongst HIV-infected children.
- 63. Nachman S, Zheng N, Acosta EP, et al. Pharmacokinetics, safety, and 48-week efficacy of oral raltegravir in HIV-1-infected children aged 2 through 18 years. Clin Infect Dis. 2014;58(3):413–422. doi: 10.1093/cid/cit696. Report of the pharmacokinetics, safety and 48-week efficacy of oral raltegravir in HIV-infected children 2–18 years old.
- 64.Panel on Antiretroviral Therapy and Medical Management of HIV-Infected Children. [Retrieved March 5, 2015];Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. from http://aidsinfo.nih.gov/contentfiles/lvguidelines/pediatricguidelines.pdf.
- 65. Flynn P, Komar S, Blanche S, et al. Efficacy and safety of darunavir/ritonavir at 48 weeks in treatment-naive, HIV-1-infected adolescents: results from a phase 2 open-label trial (DIONE) Pediatr Infect Dis J. 2014;33(9):940–945. doi: 10.1097/INF.0000000000000308. Results from a phase II, 48-week, open-label, single-arm study of once-daily darunavir/ritonavir 800/100 mg in treatment-naive, HIV-1-infected adolescents, aged 12 to <18 years.
- 66.Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; [Retrieved March 5, 2015]. Panel on Antiretroviral Guidelines for Adults and Adolescents. from http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. [Google Scholar]
- 67. Penazzato M, Prendergast AJ, Muhe LM, et al. Optimization of antiretroviral therapy in HIV-infected children under 3 years of age: a systematic review. Aids. 2014;28(Suppl 2):S137–S146. doi: 10.1097/QAD.0000000000000240. Systematic review and meta-analysis critically assessing RCTS that evaluated treatment strategies in perinatally HIV-infected children under 3 years of age.
- 68.Lindsey JC, Hughes MD, Violari A, et al. Predictors of virologic and clinical response to nevirapine versus lopinavir/ritonavir-based antiretroviral therapy in young children with and without prior nevirapine exposure for the prevention of mother-to-child HIV transmission. Pediatr Infect Dis J. 2014;33(8):846–854. doi: 10.1097/INF.0000000000000337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kuhn L, Hunt G, Technau KG, et al. Drug resistance among newly diagnosed HIV-infected children in the era of more efficacious antiretroviral prophylaxis. Aids. 2014;28(11):1673–1678. doi: 10.1097/QAD.0000000000000261. Among 230 newly diagnosed children less than 2 years of age in South Africa in 2011, the investigators found high rates drug resistance mutations, primarily NNRTI and NRTI, among children with and without reported PMTCT exposure.
- 70. Musiime V, Fillekes Q, Kekitiinwa A, et al. The pharmacokinetics and acceptability of lopinavir/ritonavir minitab sprinkles, tablets, and syrups in african HIV-infected children. J Acquir Immune Defic Syndr. 2014;66(2):148–154. doi: 10.1097/QAI.0000000000000135. In this open label randomized crossover study assessing the pharmacokinetics and acceptability of lopinavir/ ritonavir minitab sprinkles, tablets, and syrups in HIV-infected children in Uganda, minitabs were found to be more acceptable than syrups for younger children while older children preferred tablets.
- 71.Bwakura-Dangarembizi M, Musiime V, Szubert AJ, et al. Prevalence of Lipodystrophy and Metabolic Abnormalities in HIV-Infected African Children after Three Years on First-Line Antiretroviral Therapy. Pediatr Infect Dis J. 2014 doi: 10.1097/INF.0000000000000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Aurpibul L, Cressey TR, Sricharoenchai S, et al. Efficacy, Safety and Pharmacokinetics of Tenofovir Disoproxil Fumarate in Virologic-Suppressed HIV-infected Children Using Weight-Band Dosing. Pediatr Infect Dis J. 2015;34(4):392–397. doi: 10.1097/INF.0000000000000633. This prospective, open-label study of efficacy, safety and pharmacokinetics of tenofovir (TDF) (in combination with lamivudine/efavirenz once daily) in virologically suppressed HIV infected children/adolescents, 3–18 years of age and >/=15 kg, receiving first-line regimen without TDF. Adequate TDF exposures were achieved without clinically significant renal or bone adverse events over 96 weeks.
- 73. Havens PL, Hazra R. Commentary: the place of tenofovir disoproxil fumarate in pediatric antiretroviral therapy. Pediatr Infect Dis J. 2015;34(4):406–408. doi: 10.1097/INF.0000000000000650. The authors review four studies of tenofovir use in children and adolescents and discuss study results, including safety and efficacy as well as indications for use in the pediatric population.
- 74. Saez-Llorens X, Castano E, Rathore M, et al. A Randomized, Open-Label Study of the Safety and Efficacy of Switching Stavudine or Zidovudine to Tenofovir Disoproxil Fumarate in HIV-1-infected Children With Virologic Suppression. Pediatr Infect Dis J. 2015;34(4):376–382. doi: 10.1097/INF.0000000000000289. The investigative team presents results from a randomized, open-label Gilead supported study evaluating the safety and efficacy of switching stavudine or zidovudine to tenofovir in HIV-1-infected children (2 to <16 years) with virologic suppression. Overall safety and tolerability of TDF in children were similar to adults but tenofovir did not attain the prespecified noninferiority margin for efficacy when compared with zidovudine or stavudine.
- 75.Persaud D, Gay H, Ziemniak C, et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med. 2013;369(19):1828–1835. doi: 10.1056/NEJMoa1302976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Luzuriaga K, Gay H, Ziemniak C, et al. Viremic relapse after HIV-1 remission in a perinatally infected child. N Engl J Med. 2015;372(8):786–788. doi: 10.1056/NEJMc1413931. The investigators previously reported sustained viral remission (undetectable proviral DNA in peripheral-blood mononuclear cells, plasma HIV-1 RNA, and HIV-1 antibodies) after ART discontinuation in a child who was diagnosed and initiated treatment within 30 hours of birth. Approximately 27 months after ART discontinuation plasma viral load rebounded to 16,750 copies/mL. The child was re-initiated on treatment resulting in a drop in viral load and increase in CD4 cell count. The investigators conclude that the return of viremia after a sustained period of viral quiescence supports the model of HIV-1 latency in long-lived resting memory CD4 cells.
- 77. Butler KM, Gavin P, Coughlan S, et al. Rapid Viral Rebound after 4 Years of Suppressive Therapy in a Seronegative HIV-1 Infected Infant Treated from Birth. Pediatr Infect Dis J. 2014 doi: 10.1097/INF.0000000000000570. The case of a child initiated on antiretroviral drugs within 30 minutes of birth, who sustained viral suppression with undetectable HIV-1 RNA and DNA but experienced rapid viral rebound within days of treatment discontinuation at 48 months of life.
- 78. Bitnun A, Samson L, Chun TW, et al. Early initiation of combination antiretroviral therapy in HIV-1-infected newborns can achieve sustained virologic suppression with low frequency of CD4+ T cells carrying HIV in peripheral blood. Clin Infect Dis. 2014;59(7):1012–1019. doi: 10.1093/cid/ciu432. Investigators report on the experience from a Canadian cohort: 136 children born to HIV-positive women were initiated on ART; 12 were determined to be HIV-infected and 4 achieved sustained virologic suppression. Detailed characterization of the treated children suggest that early treatment can result in reduced HIV-1 reservoirs.
- 79. Persaud D, Patel K, Karalius B, et al. Influence of age at virologic control on peripheral blood human immunodeficiency virus reservoir size and serostatus in perinatally infected adolescents. JAMA Pediatr. 2014;168(12):1138–1146. doi: 10.1001/jamapediatrics.2014.1560. In a cross-sectional study of 144 perintally HIV-infected youth, median age 14.3 years, proviral reservoir size was associated with age and duration of viral suppression. Proviral reservoir size was smallest among children who achieved viral control before 1 year of age.