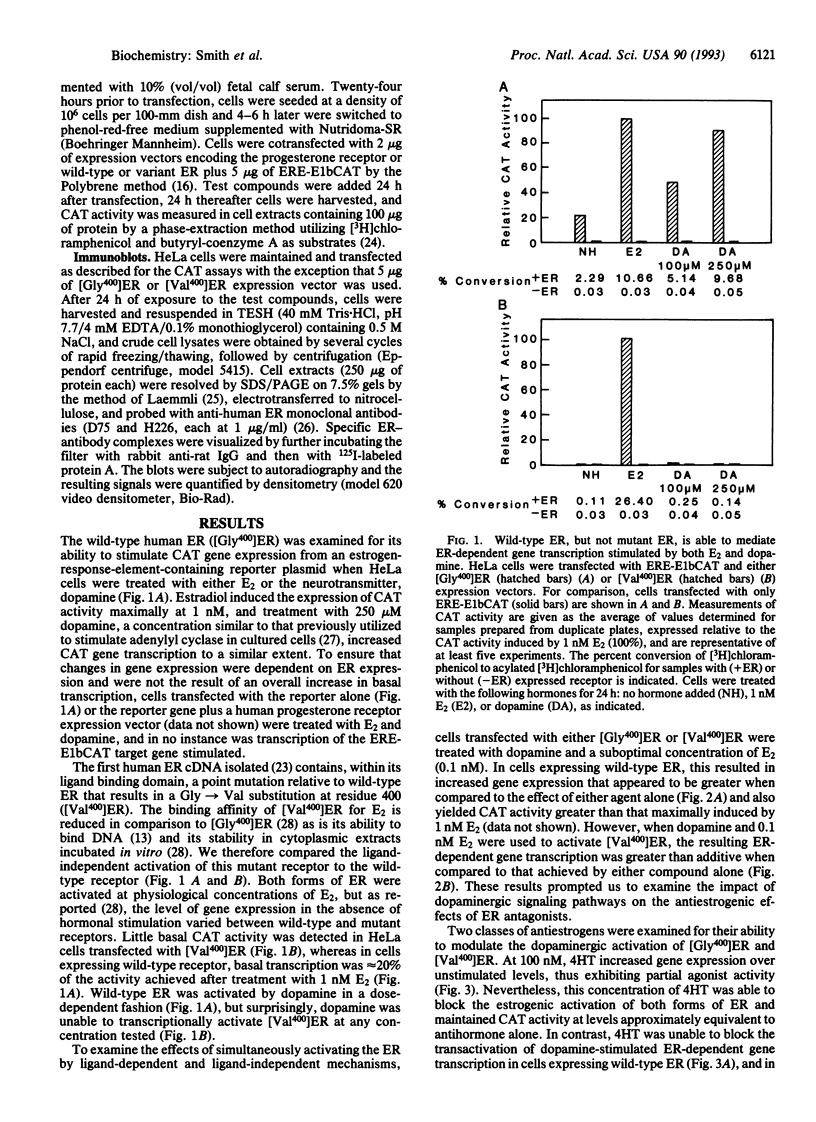
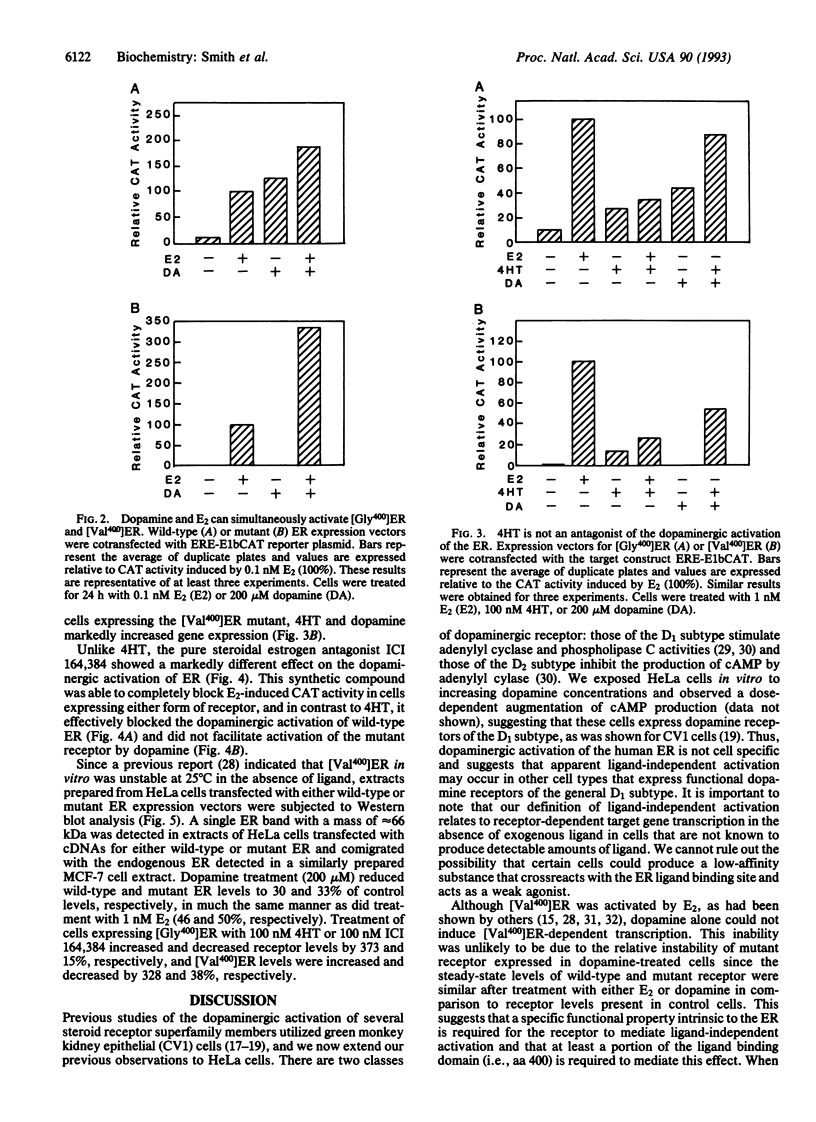
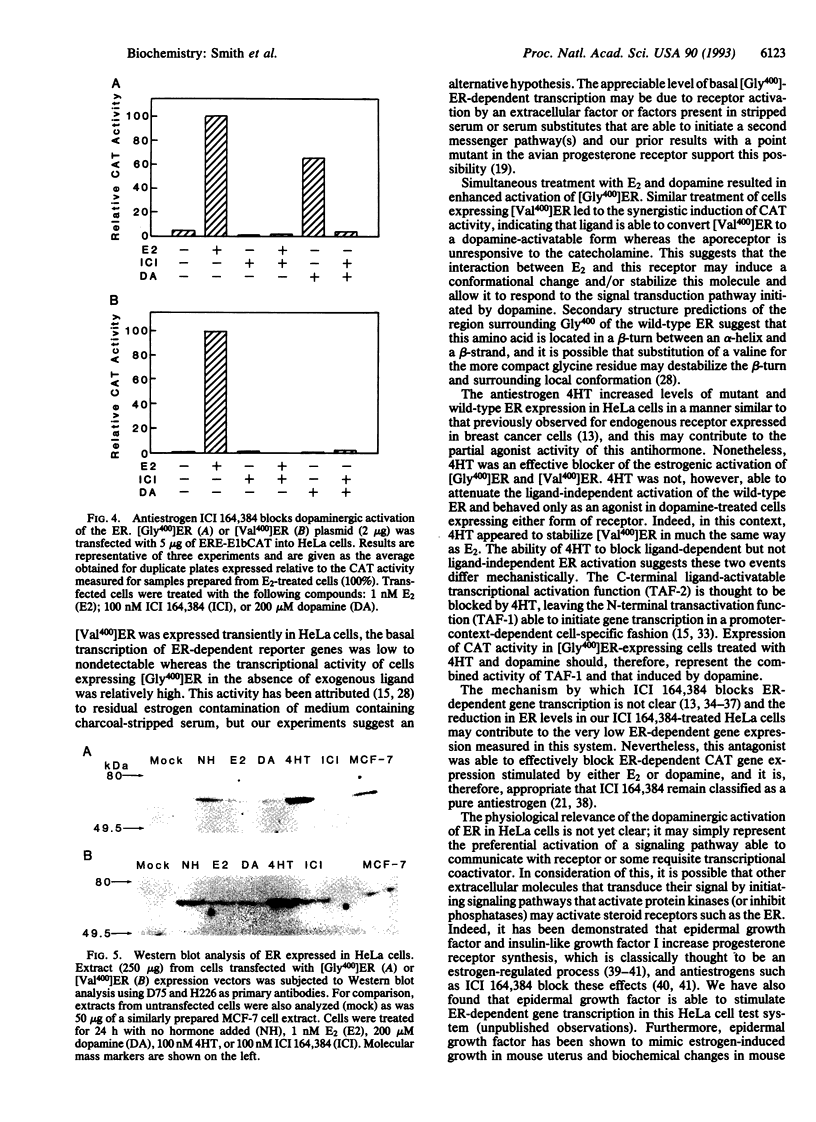
Abstract
It has been previously demonstrated that several members of the steroid receptor superfamily may be activated by the neurotransmitter dopamine in the apparent absence of cognate ligand. We have examined wild-type and mutant human estrogen receptors (ERs, [Gly400]ER and [Val400]ER, respectively) for their abilities to activate ER-dependent transcription of a transgene in a ligand-independent manner. In cells expressing the wild-type ER, dopamine was nearly as effective as 17 beta-estradiol at inducing the chloramphenicol acetyltransferase activity of the reporter gene in a dose-dependent manner; simultaneous addition of suboptimal concentrations of 17 beta-estradiol and dopamine stimulated transcription more than either compound alone. Dopamine alone was unable to induce gene expression in cells expressing [Val400]ER mutant receptors, but concomitant treatment with 17 beta-estradiol produced a synergistic increase in transcription, suggesting that the ligand may alter the mutant receptor's conformation such that it can be activated subsequently by a dopaminergic signaling mechanism. In the presence of the antiestrogen ICI 164,384, dopamine-stimulated gene expression was undetectable in cells expressing either form of ER. However, simultaneous treatment of cells expressing wild-type ER with trans-4-hydroxytamoxifen and dopamine resulted in transgene expression that was additive in nature compared to either compound alone; similar treatment of cells expressing [Val400]ER produced a synergistic increase. Our results suggest that ligand and ligand-independent activation of the ER initiate from distinct pathways and that the latter may occur in a variety of target tissues subject to modulation by receptor ligands.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan G. F., Leng X., Tsai S. Y., Weigel N. L., Edwards D. P., Tsai M. J., O'Malley B. W. Hormone and antihormone induce distinct conformational changes which are central to steroid receptor activation. J Biol Chem. 1992 Sep 25;267(27):19513–19520. [PubMed] [Google Scholar]
- Arbuckle N. D., Dauvois S., Parker M. G. Effects of antioestrogens on the DNA binding activity of oestrogen receptors in vitro. Nucleic Acids Res. 1992 Aug 11;20(15):3839–3844. doi: 10.1093/nar/20.15.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronica S. M., Katzenellenbogen B. S. Progesterone receptor regulation in uterine cells: stimulation by estrogen, cyclic adenosine 3',5'-monophosphate, and insulin-like growth factor I and suppression by antiestrogens and protein kinase inhibitors. Endocrinology. 1991 Apr;128(4):2045–2052. doi: 10.1210/endo-128-4-2045. [DOI] [PubMed] [Google Scholar]
- Bagchi M. K., Elliston J. F., Tsai S. Y., Edwards D. P., Tsai M. J., O'Malley B. W. Steroid hormone-dependent interaction of human progesterone receptor with its target enhancer element. Mol Endocrinol. 1988 Dec;2(12):1221–1229. doi: 10.1210/mend-2-12-1221. [DOI] [PubMed] [Google Scholar]
- Bagchi M. K., Tsai S. Y., Tsai M. J., O'Malley B. W. Identification of a functional intermediate in receptor activation in progesterone-dependent cell-free transcription. Nature. 1990 Jun 7;345(6275):547–550. doi: 10.1038/345547a0. [DOI] [PubMed] [Google Scholar]
- Bagchi M. K., Tsai S. Y., Tsai M. J., O'Malley B. W. Ligand and DNA-dependent phosphorylation of human progesterone receptor in vitro. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2664–2668. doi: 10.1073/pnas.89.7.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M., Metzger D., Chambon P. Role of the two activating domains of the oestrogen receptor in the cell-type and promoter-context dependent agonistic activity of the anti-oestrogen 4-hydroxytamoxifen. EMBO J. 1990 Sep;9(9):2811–2818. doi: 10.1002/j.1460-2075.1990.tb07469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthois Y., Dong X. F., Martin P. M. Regulation of epidermal growth factor-receptor by estrogen and antiestrogen in the human breast cancer cell line MCF-7. Biochem Biophys Res Commun. 1989 Feb 28;159(1):126–131. doi: 10.1016/0006-291x(89)92413-3. [DOI] [PubMed] [Google Scholar]
- Bradshaw M. S., Tsai M. J., O'Malley B. W. A far upstream ovalbumin enhancer binds nuclear factor-1-like factor. J Biol Chem. 1988 Jun 15;263(17):8485–8490. [PubMed] [Google Scholar]
- Dauvois S., Danielian P. S., White R., Parker M. G. Antiestrogen ICI 164,384 reduces cellular estrogen receptor content by increasing its turnover. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4037–4041. doi: 10.1073/pnas.89.9.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearry A., Gingrich J. A., Falardeau P., Fremeau R. T., Jr, Bates M. D., Caron M. G. Molecular cloning and expression of the gene for a human D1 dopamine receptor. Nature. 1990 Sep 6;347(6288):72–76. doi: 10.1038/347072a0. [DOI] [PubMed] [Google Scholar]
- Denis M., Poellinger L., Wikstöm A. C., Gustafsson J. A. Requirement of hormone for thermal conversion of the glucocorticoid receptor to a DNA-binding state. Nature. 1988 Jun 16;333(6174):686–688. doi: 10.1038/333686a0. [DOI] [PubMed] [Google Scholar]
- Denner L. A., Weigel N. L., Maxwell B. L., Schrader W. T., O'Malley B. W. Regulation of progesterone receptor-mediated transcription by phosphorylation. Science. 1990 Dec 21;250(4988):1740–1743. doi: 10.1126/science.2176746. [DOI] [PubMed] [Google Scholar]
- DiAugustine R. P., Petrusz P., Bell G. I., Brown C. F., Korach K. S., McLachlan J. A., Teng C. T. Influence of estrogens on mouse uterine epidermal growth factor precursor protein and messenger ribonucleic acid. Endocrinology. 1988 Jun;122(6):2355–2363. doi: 10.1210/endo-122-6-2355. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder C. C., Blecher M., Jose P. A. Dopamine-1-mediated stimulation of phospholipase C activity in rat renal cortical membranes. J Biol Chem. 1989 May 25;264(15):8739–8745. [PubMed] [Google Scholar]
- Gibson M. K., Nemmers L. A., Beckman W. C., Jr, Davis V. L., Curtis S. W., Korach K. S. The mechanism of ICI 164,384 antiestrogenicity involves rapid loss of estrogen receptor in uterine tissue. Endocrinology. 1991 Oct;129(4):2000–2010. doi: 10.1210/endo-129-4-2000. [DOI] [PubMed] [Google Scholar]
- Green S., Walter P., Kumar V., Krust A., Bornert J. M., Argos P., Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986 Mar 13;320(6058):134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- Greene G. L., Sobel N. B., King W. J., Jensen E. V. Immunochemical studies of estrogen receptors. J Steroid Biochem. 1984 Jan;20(1):51–56. doi: 10.1016/0022-4731(84)90188-2. [DOI] [PubMed] [Google Scholar]
- Henderson B. E., Ross R. K., Pike M. C., Casagrande J. T. Endogenous hormones as a major factor in human cancer. Cancer Res. 1982 Aug;42(8):3232–3239. [PubMed] [Google Scholar]
- Ignar-Trowbridge D. M., Nelson K. G., Bidwell M. C., Curtis S. W., Washburn T. F., McLachlan J. A., Korach K. S. Coupling of dual signaling pathways: epidermal growth factor action involves the estrogen receptor. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4658–4662. doi: 10.1073/pnas.89.10.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan V. C. Biochemical pharmacology of antiestrogen action. Pharmacol Rev. 1984 Dec;36(4):245–276. [PubMed] [Google Scholar]
- Katzenellenbogen B. S., Norman M. J. Multihormonal regulation of the progesterone receptor in MCF-7 human breast cancer cells: interrelationships among insulin/insulin-like growth factor-I, serum, and estrogen. Endocrinology. 1990 Feb;126(2):891–898. doi: 10.1210/endo-126-2-891. [DOI] [PubMed] [Google Scholar]
- Kebabian J. W., Calne D. B. Multiple receptors for dopamine. Nature. 1979 Jan 11;277(5692):93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- Klein-Hitpass L., Tsai S. Y., Weigel N. L., Allan G. F., Riley D., Rodriguez R., Schrader W. T., Tsai M. J., O'Malley B. W. The progesterone receptor stimulates cell-free transcription by enhancing the formation of a stable preinitiation complex. Cell. 1990 Jan 26;60(2):247–257. doi: 10.1016/0092-8674(90)90740-6. [DOI] [PubMed] [Google Scholar]
- Kumar V., Chambon P. The estrogen receptor binds tightly to its responsive element as a ligand-induced homodimer. Cell. 1988 Oct 7;55(1):145–156. doi: 10.1016/0092-8674(88)90017-7. [DOI] [PubMed] [Google Scholar]
- Kumar V., Green S., Stack G., Berry M., Jin J. R., Chambon P. Functional domains of the human estrogen receptor. Cell. 1987 Dec 24;51(6):941–951. doi: 10.1016/0092-8674(87)90581-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lydon J. P., Power R. F., Conneely O. M. Differential modes of activation define orphan subclasses within the steroid/thyroid receptor superfamily. Gene Expr. 1992;2(3):273–283. [PMC free article] [PubMed] [Google Scholar]
- Mukku V. R., Stancel G. M. Regulation of epidermal growth factor receptor by estrogen. J Biol Chem. 1985 Aug 15;260(17):9820–9824. [PubMed] [Google Scholar]
- Murphy L. J., Murphy L. C., Friesen H. G. Estrogen induces insulin-like growth factor-I expression in the rat uterus. Mol Endocrinol. 1987 Jul;1(7):445–450. doi: 10.1210/mend-1-7-445. [DOI] [PubMed] [Google Scholar]
- Nelson K. G., Takahashi T., Lee D. C., Luetteke N. C., Bossert N. L., Ross K., Eitzman B. E., McLachlan J. A. Transforming growth factor-alpha is a potential mediator of estrogen action in the mouse uterus. Endocrinology. 1992 Oct;131(4):1657–1664. doi: 10.1210/endo.131.4.1396310. [DOI] [PubMed] [Google Scholar]
- Payvar F., Wrange O., Carlstedt-Duke J., Okret S., Gustafsson J. A., Yamamoto K. R. Purified glucocorticoid receptors bind selectively in vitro to a cloned DNA fragment whose transcription is regulated by glucocorticoids in vivo. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6628–6632. doi: 10.1073/pnas.78.11.6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power R. F., Lydon J. P., Conneely O. M., O'Malley B. W. Dopamine activation of an orphan of the steroid receptor superfamily. Science. 1991 Jun 14;252(5012):1546–1548. doi: 10.1126/science.2047861. [DOI] [PubMed] [Google Scholar]
- Power R. F., Mani S. K., Codina J., Conneely O. M., O'Malley B. W. Dopaminergic and ligand-independent activation of steroid hormone receptors. Science. 1991 Dec 13;254(5038):1636–1639. doi: 10.1126/science.1749936. [DOI] [PubMed] [Google Scholar]
- Pratt W. B. Transformation of glucocorticoid and progesterone receptors to the DNA-binding state. J Cell Biochem. 1987 Sep;35(1):51–68. doi: 10.1002/jcb.240350105. [DOI] [PubMed] [Google Scholar]
- Reese J. C., Katzenellenbogen B. S. Differential DNA-binding abilities of estrogen receptor occupied with two classes of antiestrogens: studies using human estrogen receptor overexpressed in mammalian cells. Nucleic Acids Res. 1991 Dec 11;19(23):6595–6602. doi: 10.1093/nar/19.23.6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese J. C., Katzenellenbogen B. S. Examination of the DNA-binding ability of estrogen receptor in whole cells: implications for hormone-independent transactivation and the actions of antiestrogens. Mol Cell Biol. 1992 Oct;12(10):4531–4538. doi: 10.1128/mcb.12.10.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seed B., Sheen J. Y. A simple phase-extraction assay for chloramphenicol acyltransferase activity. Gene. 1988 Jul 30;67(2):271–277. doi: 10.1016/0378-1119(88)90403-9. [DOI] [PubMed] [Google Scholar]
- Sumida C., Pasqualini J. R. Antiestrogens antagonize the stimulatory effect of epidermal growth factor on the induction of progesterone receptor in fetal uterine cells in culture. Endocrinology. 1989 Feb;124(2):591–597. doi: 10.1210/endo-124-2-591. [DOI] [PubMed] [Google Scholar]
- Tora L., Mullick A., Metzger D., Ponglikitmongkol M., Park I., Chambon P. The cloned human oestrogen receptor contains a mutation which alters its hormone binding properties. EMBO J. 1989 Jul;8(7):1981–1986. doi: 10.1002/j.1460-2075.1989.tb03604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tora L., White J., Brou C., Tasset D., Webster N., Scheer E., Chambon P. The human estrogen receptor has two independent nonacidic transcriptional activation functions. Cell. 1989 Nov 3;59(3):477–487. doi: 10.1016/0092-8674(89)90031-7. [DOI] [PubMed] [Google Scholar]
- Tsai S. Y., Carlstedt-Duke J., Weigel N. L., Dahlman K., Gustafsson J. A., Tsai M. J., O'Malley B. W. Molecular interactions of steroid hormone receptor with its enhancer element: evidence for receptor dimer formation. Cell. 1988 Oct 21;55(2):361–369. doi: 10.1016/0092-8674(88)90059-1. [DOI] [PubMed] [Google Scholar]
- Wakeling A. E., Bowler J. Novel antioestrogens without partial agonist activity. J Steroid Biochem. 1988 Oct;31(4B):645–653. doi: 10.1016/0022-4731(88)90014-3. [DOI] [PubMed] [Google Scholar]
- Wakeling A. E. Novel pure antiestrogens. Mode of action and therapeutic prospects. Ann N Y Acad Sci. 1990;595:348–356. doi: 10.1111/j.1749-6632.1990.tb34308.x. [DOI] [PubMed] [Google Scholar]
- Webster N. J., Green S., Jin J. R., Chambon P. The hormone-binding domains of the estrogen and glucocorticoid receptors contain an inducible transcription activation function. Cell. 1988 Jul 15;54(2):199–207. doi: 10.1016/0092-8674(88)90552-1. [DOI] [PubMed] [Google Scholar]
- Weigel N. L., Carter T. H., Schrader W. T., O'Malley B. W. Chicken progesterone receptor is phosphorylated by a DNA-dependent protein kinase during in vitro transcription assays. Mol Endocrinol. 1992 Jan;6(1):8–14. doi: 10.1210/mend.6.1.1738374. [DOI] [PubMed] [Google Scholar]




