Summary
Background
We planned to investigate contribution of DWMR to the treatment efficacy with ADC values which were measured in acute and chronic plaque before and after MS treatment. ADC changes in normal appearing white matter (NAWM) in patients with MS and healthy volunteers were also evaluated in this study.
Material/Methods
25 patients with MS and 30 healthy subjects with normal brain MR findings were included to our study. Contrast enhancement in plaque was evaluated as an acute, and non-contrast enhancement in plaque was evaluated as a chronic. Also, ADC measurements were performed using the same parameters in NAWM in plaque neighborhood and volunteers. Results were compared with appropriate statistical methods.
Results
ADC values in acute and chronic plaques were decreased after the treatment, and these reductions were statistically significant for acute plaqus in b500 and for chronic plaques in b500 and b1000. The mean ADC values were measured as 1.53±0.49×10−3 and 1.43±0.58×10−3 in acute plaques and 1.40±0.35×10−3 and 1.34±0.36×10−3 mm2/sec in chronic plaques before and after the treatment.
Conclusions
We think that DWMR have important role due to quantitative measurement ability in the evaluation of the treatment efficacy of the MS patients with acute attack in addition to contrast-enhanced MR sequence.
MeSH Keywords: Diabetes Mellitus, Type 2; Fatty Acid-Binding Proteins; PTEN Phosphohydrolase
Background
Multiple Sclerosis (MS) is one of the demyelinating disorders of the central nervous system (CNS). The etiology of MS is yet not clear but genetic and environmental factors are two of the causes [1]. The most commonly affected population is young adults (range from 20 to 40 years). MS is seen 2–3 times more common in women than in men [2].
The diagnosis of MS is based on the clinical symptoms and history. Detailed neurological examination, magnetic resonance imaging (MRI), visual evoked potential (VEPs) and the blood tests assist the diagnosis. But there is no laboratory test to make a definite diagnosis [3,4].
The diagnosis of MS is based on the McDonald criteria which were revised by Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology in 2005. The McDonald criteria include the Barkhof-Tintore imaging criteria [5–7]. These are (1) at least one gadolinium-enhancing lesion or nine T2-hyperintense lesions, (2) at least one infratentorial lesion, (3) at least one juxtacortical lesion and (4) at least three periventricular lesions. Three of four is sufficient for the diagnosis. In 2005 the spinal cord lesions were assigned the same status as the infratentorial lesions [5,8].
The sensitivity of MRI is high in early and asymptomatic MS plaques. The sensitivity of MRI for spinal cord lesions varies from 68 to 89% in different papers [2,9]. MS plaques generally occur in the white matter although 10% of the plaques are seen in the gray matter. The MS plaques are generally seen in the capsula interna, periventricular white matter, corpus callosum and pons [10].
Some conventional findings of MS are T2- and fast fluid attenuated inversion recovery (FLAIR)-hyperintense plaques perpendicular to the lateral ventricules and thinning of the corpus callosum. These plaques show similar signal characteristics to the cerebrospinal fluid (CSF) in T2-Weighted Images (WI) thus Proton Dansity (PD) and FLAIR sequences also help to differentiate MS plaques from small CSF areas. Gadolinium-enhanced post-contrast T1WI presents the stage and activity of plaques and disease [2,11].
Nowadays, it is popular to use diffusion weighted MRI to evaluate the lesions anywhere in the body [12–18]. Diffusion weighted imaging (DWI) is very sensitive to alterations of the Brownian movements of water molecules. Moreover, DWI does not need contrast media and takes very short time [9,11]. DWI describes the water molecular movements using a powerful diffusion gradient independent from T1 and T2 relaxation times. The disorder progress causes changes in the water molecular movements. In conventional MRI the effect of molecular movement of water on the images is very low. But in DWI the molecular movements of water create the images. The diffusion coefficient can be measured and mapped. DWI usually uses cytotoxic and vasogenic edema in the diagnostics [19]. However, potential contributions of DWI to the conventional sequences in non-ischemic disorders are important. The main disadvantage of DWI is a marked decrease in the signal-noise ratio and tissue contrast secondary to the powerful diffusion gradients [20]. The contrast resolution and anatomic borders are weakened in DWI. Thus DWI should be evaluated together with convantional MRI sequences.
In this study, we aimed to evaluate the effectiveness of treatment in acute and chronic plaques in MS patients with acute exacerbation, using Apparent Diffusion Coefficient (ADC) measurements before and after the acute attack treatment. Additionally, we evaluated the ADC values of the white matter nearby the acute and chronic plaques of MS patients and normal white matter of healthy individuals.
Material and Methods
Informed consent was obtained from all MS patients and control group. The study was performed in accordance with the ethical guidelines of the Helsinki Declaration and approved by the local ethics committee.
A total of 25 (12 men, 13 women) MS acute attack patients and 30 (9 men, 21 women) controls were included in this prospective study. The patients were referred to the Radiology Department from the Neurology Department. MS acute attack diagnosis was made in the Neurology Department according to the symptoms, history, physical examination, and laboratory tests including the lumbar puncture (mononuclear pleocytosis and increase in IgG). Cranial and spinal MRI of the patients was performed just before treatment and in the week after treatment. The treatmant included 500 mg of IV steroid per day for 5–10 days. The measurements were made from the acute and chronic plaques and white matter nearby the plaques in the patients and normal white matter in the control group. A standardized region of interest (ROI) (30–50 mm2) was used to measure the ADC (mm2/sec) values. Three different b values were used as b100, b500 and b1000. All the measurements were done for all b values. The control group was selected from the patients who were admitted to the Neurology Department with a headache but had no pathology on MRI or laboratory tests.
The MRI equipment was: a 1.5 T GE Signa Highspeed scanner Excite (General Electric, Milwaukee, WI, USA). Pre- and post-contrast standard cranial MRI, DWI and ADC mapping were performed. Standardized sagittal, coronal and axial images were done for T1 spin echo, T2 spin echo, and FLAIR sequences. Intravenous Gadolinium, 0.1 mmol/kg, was used for contrast enhancement. The plaques showing contrast enhancement were considered as acute plaques.
A total of 23 acute plaques and 73 chronic plaques were evaluated. The ADC values were measured for three different b values.
The measurements were also done from the normal white matter of MS patients before and after treatment; as well as in the control group.
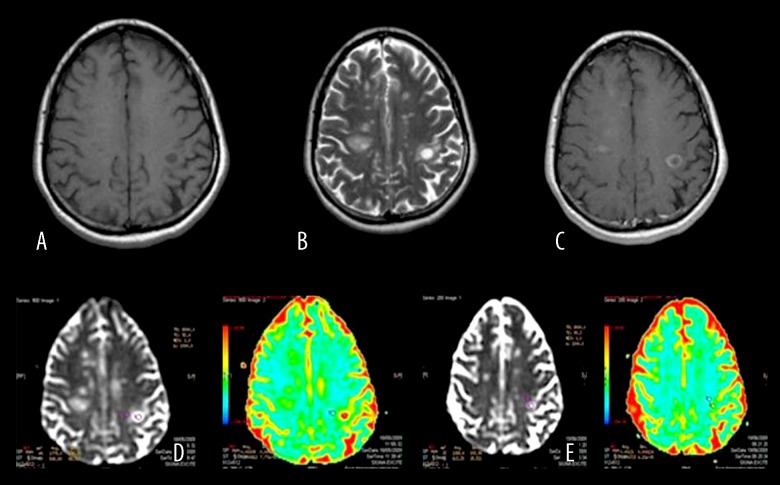
Colored and black-white ADC mapping were obtained by postprocessing DWI on a workstation (Advantage Windows, software version 2.0, GE Medical Systems). The ROIs were adjusted to the acute and chronic plaques and nearby normal white matter of the patients and normal white matter of the control group. In the control group, the ROIs were adjusted to the bifrontal subcortical white matter and to tissues adjacent to the bilateral lateral ventricul frontal horns paying attention to adjusting far away from the ventricular system and gray matter (Figure 1).
Fgure 1.
Pre- and post-treatment b: 1000 ADC values of an acute MS plaque and nearby NAWM in a patient with MS exacerbation. Acute plaque in the left parietal lobe (A) is hypointense on precontrast T1WI, (B) hyperintense on T2WI, (C) displays peripheral enhancement after IV contrast medium injection. (D) ADC map before treatment (E) ADC map after treatment.
Statistical analysis
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) version 12.0 for Windows. Correlations were assessed by Spearman’s correlation coefficient. Continuous variables were expressed as mean ± standard deviation. Student’s t-test and Paired Sample test were used to compare the means. P values lower than 0.05 were considered statistically significant.
Results
The white matter ADC values of acute and chronic plaques showed a significiant difference after treatment in b1000 value (Table 1). The ADC values of the white matter nearby the chronic plaques were higher than of the white matter nearby the acute plaques before treatment for all b values (Table 2). The ADC values of the white matter nearby the chronic plaques were higher than of the white matter nearby the acute plaques after treatment for b100 and b1000 values (Table 3). In Table 4 the mean ADC values of acute and chronic plaques are demonstrated.
Table 1.
Normal white matter ADC values of nearby acute and chronic plaques before and after treatment (×10−3mm2/sec).
| White matter nearby acute plaque (BT) | White matter nearby acute plaque (AT) | p | White matter nearby chronic plaque (BT) | White matter nearby chronic plaque (AT) | p | |
|---|---|---|---|---|---|---|
| b100 | 1.05±0.37 | 1.12±0.61 | 0.617 | 1.27±0.46 | 1.20±0.55 | 0.355 |
| b500 | 0.90±0.06 | 0.91±0.14 | 0.719 | 0.93±0.12 | 0.91±0.10 | 0.042 |
| b1000 | 0.78±0.08 | 0.78±0.07 | 0.950 | 0.83±0.08 | 0.80±0.09 | 0.000 |
BT – before treatment; AT – after treatment.
Tablo 2.
The ADC values (×10−3 mm2/sec) of the white matter nearby acute and chronic plaques before treatment and control group.
| White matter nearby acute plaque (BT) | White matter nearby chronic plaque Control Group | p | White matter nearby chronic plaque (BT) | White matter nearby chronic plaque Control Group | p | |
|---|---|---|---|---|---|---|
| b100 | 1.05±0.37 | 1.53±0.52 | 0.000 | 1.27±0.46 | 1.53±0.52 | 0.013 |
| b500 | 0.90±0.06 | 0.86±0.05 | 0.023 | 0.93±0.12 | 0.86±0.05 | 0.003 |
| b1000 | 0.78±0.08 | 0.72±0.10 | 0.001 | 0.83±0.08 | 0.72±0.10 | 0.000 |
BT – before treatment; AT – after treatment.
Tablo 3.
The ADC values (×10−3 mm2/sec) of the white matter nearby acute and chronic plaques after treatment and control group.
| White matter nearby acute plaque (AT) | White matter nearby chronic plaque Control Group | p | White matter nearby chronic plaque (AT) | White matter nearby chronic plaque Control Group | p | |
|---|---|---|---|---|---|---|
| b100 | 1.12±0.61 | 1.53±0.52 | 0.012 | 1.20±0.55 | 1.53±0.52 | 0.050 |
| b500 | 0.91±0.14 | 0.86±0.05 | 0.115 | 0.91±0.10 | 0.86±0.05 | 0.028 |
| b1000 | 0.78±0.07 | 0.72±0.10 | 0.048 | 0.80±0.09 | 0.72±0.10 | 0.010 |
BT – before treatment; AT – after treatment.
Table 4.
The mean ADC values of acute and chronic plaques before and after treatment (×10−3 mm2/sec).
| Acute plaque (BT) N=23 |
Acute plaque (AT) N=23 |
p | Chronic plaque (BT) N=73 |
Chronic plaque (AT) N=73 |
p | |
|---|---|---|---|---|---|---|
| b100 | 1.76±0.54 | 1.59±0.63 | 0.440 | 1.75±0.43 | 1.65±0.45 | 0.355 |
| b500 | 1.44±0.42 | 1.24±0.38 | 0.042 | 1.30±0.23 | 1.24±0.18 | 0.028 |
| b1000 | 1.30±0.38 | 1.16±0.35 | 0.093 | 1.19±0.21 | 1.15±0.19 | 0.040 |
BT – before treatment; AT – after treatment. In both acute and chronic plaques the ADC values were decreased in all b values after treatment.
Discussion
In attack patients the plaques show nodular and homogeneus enhancement. On control MRI after the treatment of an attack, the number and the enhancement of plaques decrease.
The MRI findings of MS attack activation include new plaque formation, increase in the diameter of an old plaque, or contrast enhancemet. It is hard to detect an active plaque in T2 WI although easy to use contrast enhancement. Contrast enhancement can indicate the activation of new and old plaques [21].
The treatment of MS is divided into two approaches: attack treatment and relaps-remission treatment. The corticosteroids shorten the attack time and accelerate the healing process [22–24].
MRI is the primary imaging modality to diagnose MS and follow up the plaques and has a high sensitivity to detect the plaques [25]. Contrast enhancement in MS plaques indicates activation and is superior to the clinical assessment [26]. The contrast enhancement shows peak after the 5th minute and reaches a platau within 20 minutes [27].
The disorders in the white matter and axonal membrane permeability cause an increase in ADC values [28]. Typically, MS plaques tend to have increased ADC values compared to contralateral white matter [29]. The increase of ADC is not specific and also seen in demyelinisation, gliosis, inflammation and axonal loss. The high ADC values in normal white matter in MS indicate microstructural changes [30]. In the research of Schmierer et al. it was reported that in postmortem MS cases there was a correlation between mean ADC values and demyelinisation [31].
In our study we demonstrated that the ADC values of acute and chronic plaques showed decrement after the therapy. This finding can be related with the decrease in the diffusion restriction due to the therapy. The ADC values of the white matter of chronic plaques are higher than the ADC values of acute plaques before and after treatment. Perhaps the plaques affect the circumjacent white matter and the diffusion restriction is increased according to the time of tissue being affected. Maybe increasing time of tissue being affected increases diffusion restriction.
To the best of our knowledge there is no study in the literature on the evaluation of the effectiveness of MS attack therapy using the DWI values. Werring et al. detected moderately and highly increased ADC values in just developing MS plaques. And they also found that contralateral normal white matter showed mildly increased ADC values [32]. It has been shown that the ADC values of MS plaques are higher than those of normal white matter and also the ADC values of acute plaques are higher than of the chronic ones [33]. In our study we found that the ADC values of acute plaques were higher than of the chronic plaques. And also the ADC values of acute and chronic plaques tended to decrease after treatment. The cause of that decrement could related to the decreasing edema in the plaques due to the steroids. Thus, the Brownian movements of water molecules show normality. Additionally, the normal white matter ADC values of MS patients were higher than those of control group ADC values. This was probably related to the micromolecular changes in the white matter of MS patients that were not detectable with conventional MRI sequences. Perhaps there was microscopic involvement in the normal white matter of MS patients.
The main limitation of our study was that some of the plaque measurements could not be carried out after treatment. Especially the formal changes of the plaques after treatment were difficult to measure.
We also detected that some of the contrast-enhanced plaques showed lower ADC values than chronic plaques. Thus, it is not objective enough to use DWI only. Contrast enhancement is essential and constitutive. Tekşam et l. reported that contrast-enhanced MR images were essential to diagnose the active plaques because of the missmatch between increased diffusion and active plaques [34].
Conclusions
In our study we demonstrated that the ADC values of acute and chronic plaques showed a decrease after the therapy. The ADC values of the white matter of chronic plaques were higher than the ADC values of acute plaques before and after treatment. Perhaps the plaques affect the circumjacent white matter and the diffusion restriction is increased according to the time of tissue being affected. Maybe, the higher the time affecting the tissue, the higher the diffusion restriction. We think that further research studies on MS and DWI are required.
References
- 1.Kahana E. Epidemiologic studies of multiple sclerosis: a review. Biomed Pharmacother. 2000;54(2):100–2. doi: 10.1016/S0753-3322(00)88859-9. [DOI] [PubMed] [Google Scholar]
- 2.Grossman RI, McGowan JC. Perspectives on multiple sclerosis. Am J Neuroradiol. 1998;19(7):1251–65. [PMC free article] [PubMed] [Google Scholar]
- 3.Marshall I, Wardlaw J, Cannon J, et al. Reproducibility of metabolite peak areas in 1H MRS of brain. Magn Reson Imaging. 1996;14(3):281–92. doi: 10.1016/0730-725x(95)02084-7. [DOI] [PubMed] [Google Scholar]
- 4.Ashwal S, Holshouser B, Tong K, et al. Proton spectroscopy detected myoinositol in children with traumatic brain injury. Pediatr Res. 2004;56(4):630–38. doi: 10.1203/01.PDR.0000139928.60530.7D. [DOI] [PubMed] [Google Scholar]
- 5.Inglese M. Multiple sclerosis: new insights and trends. Am J Neuroradiol. 2006;27(5):954–57. [PMC free article] [PubMed] [Google Scholar]
- 6.Barkhof F, Filippi M, Miller DH, et al. Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain. 1997;120(Pt 11):2059–69. doi: 10.1093/brain/120.11.2059. [DOI] [PubMed] [Google Scholar]
- 7.Tintoré M, Rovira A, Martínez MJ, et al. Isolated demyelinating syndromes: comparison of different MR imaging criteria to predict conversion to clinically definite multiple sclerosis. Am J Neuroradiol. 2000;21(4):702–6. [PMC free article] [PubMed] [Google Scholar]
- 8.Bot JC, Barkhof F, Polman CH, et al. Spinal cord abnormalities in recently diagnosed MS patients: added value of spinal MRI examination. Neurology. 2004;62(2):226–33. doi: 10.1212/wnl.62.2.226. [DOI] [PubMed] [Google Scholar]
- 9.Sayin B, et al. An assessment of clinical findings and cervical spinal magnetic resonance imaging in patients with multiple sclerosis. Turkiye Klinikleri Journal of Medical Sciences. 2005;25(1):58. [Google Scholar]
- 10.Ge Y. Multiple sclerosis: the role of MR imaging. Am J Neuroradiol. 2006;27(6):1165–76. [PMC free article] [PubMed] [Google Scholar]
- 11.Karabudak R. Evaluation of MR imaging in the diagnosis of multiple sclerosis. Turkiye Klinikleri Journal of Neurology Special Topics. 2009;2(2):45–49. [Google Scholar]
- 12.Hannoun S, Roch JA, Durand-Dubief F, et al. Weekly multimodal MRI follow-up of two multiple sclerosis active lesions presenting a transient decrease in ADC. Brain Behav. 2015;5(2):e00307. doi: 10.1002/brb3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klistorner A, Vootakuru N, Wang C, et al. Decoding diffusivity in multiple sclerosis: analysis of optic radiation lesional and non-lesional white matter. PLoS One. 2015;10(3):e0122114. doi: 10.1371/journal.pone.0122114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avcu S, Bulut MD, Yavuz A, et al. Value of DW-MRI ADC quantification of colonic wall lesions in differentiation of inflammatory bowel disease and colorectal carcinoma. Jpn J Radiol. 2014;32(1):6–13. doi: 10.1007/s11604-013-0260-2. [DOI] [PubMed] [Google Scholar]
- 15.Avcu S, Çetin FA, Arslan H, et al. The value of diffusion-weighted imaging and apparent diffusion coefficient quantification in the diagnosis of perforated and nonperforated appendicitis. Diagn Interv Radiol. 2013;19(2):106–10. doi: 10.4261/1305-3825.DIR.6070-12.1. [DOI] [PubMed] [Google Scholar]
- 16.Unal O, Koparan HI, Avcu S, et al. The diagnostic value of diffusion-weighted magnetic resonance imaging in soft tissue abscesses. Eur J Radiol. 2011;77(3):490–94. doi: 10.1016/j.ejrad.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 17.Avcu S, Arslan H, Unal O, et al. The role of diffusion-weighted MR imaging and ADC values in the diagnosis of gastric tumors. JBR-BTR. 2012;95(1):1–5. doi: 10.5334/jbr-btr.62. [DOI] [PubMed] [Google Scholar]
- 18.Avcu S, Koseoglu MN, Ceylan K, et al. The value of diffusion-weighted MRI in the diagnosis of malignant and benign urinary bladder lesions. Br J Radiol. 2011;84(1006):875–82. doi: 10.1259/bjr/30591350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sener RN. Diffusion MRI: apparent diffusion coefficient (ADC) values in the normal brain and a classification of brain disorders based on ADC values. Comput Med Imaging Graph. 2001;25(4):299–326. doi: 10.1016/s0895-6111(00)00083-5. [DOI] [PubMed] [Google Scholar]
- 20.Cercignani M, Horsfield MA. The physical basis of diffusion-weighted MRI. J Neurol Sci. 2001;186(Suppl 1):S11–14. doi: 10.1016/s0022-510x(01)00486-5. [DOI] [PubMed] [Google Scholar]
- 21.Gronseth GS, Ashman EJ. Practice parameter: the usefulness of evoked potentials in identifying clinically silent lesions in patients with suspected multiple sclerosis (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;54(9):1720–25. doi: 10.1212/wnl.54.9.1720. [DOI] [PubMed] [Google Scholar]
- 22.Offenbacher H, Fazekas F, Schmidt R, et al. Assessment of MRI criteria for a diagnosis of MS. Neurology. 1993;43(5):905–9. doi: 10.1212/wnl.43.5.905. [DOI] [PubMed] [Google Scholar]
- 23.Howe FA, Barton SJ, Cudlip SA, et al. Metabolic profiles of human brain tumors using quantitative in vivo 1H magnetic resonance spectroscopy. Magn Reson Med. 2003;49(2):223–32. doi: 10.1002/mrm.10367. [DOI] [PubMed] [Google Scholar]
- 24.Miller DH, Barkhof F, Nauta JJ. Gadolinium enhancement increases the sensitivity of MRI in detecting disease activity in multiple sclerosis. Brain. 1993;116(5):1077–94. doi: 10.1093/brain/116.5.1077. [DOI] [PubMed] [Google Scholar]
- 25.Polman CH, Uitdehaag BM. Drug treatment of multiple sclerosis. BMJ. 2000;321(7259):490–94. doi: 10.1136/bmj.321.7259.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnes D, Hughes RA, Morris RW, et al. Randomised trial of oral and intravenous methylprednisolone in acute relapses of multiple sclerosis. Lancet. 1997;349(9056):902–6. doi: 10.1016/s0140-6736(96)06453-7. [DOI] [PubMed] [Google Scholar]
- 27.Barkhof F, Polman C. Oral or intravenous methylprednisolone for acute relapses of MS? Lancet. 1997;349(9056):893–94. doi: 10.1016/S0140-6736(05)62691-8. [DOI] [PubMed] [Google Scholar]
- 28.Mehta RC, Pike GB, Enzmann DR. Improved detection of enhancing and nonenhancing lesions of multiple sclerosis with magnetization transfer. Am J Neuroradiol. 1995;16(9):1771–78. [PMC free article] [PubMed] [Google Scholar]
- 29.Knauth M, Forsting M, Hartmann M, et al. MR enhancement of brain lesions: increased contrast dose compared with magnetization transfer. Am J Neuroradiol. 1996;17(10):1853–59. [PMC free article] [PubMed] [Google Scholar]
- 30.Pagani E, Bammer R, Horsfield MA, et al. Diffusion MR imaging in multiple sclerosis: technical aspects and challenges. Am J Neuroradiol. 2007;28(3):411–20. [PMC free article] [PubMed] [Google Scholar]
- 31.Schmierer K, Wheeler-Kingshott CA, Boulby PA, et al. Diffusion tensor imaging of post mortem multiple sclerosis brain. Neuroimage. 2007;35(2):467–77. doi: 10.1016/j.neuroimage.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Werring DJ, Brassat D, Droogan AG, et al. The pathogenesis of lesions and normal-appearing white matter changes in multiple sclerosis: a serial diffusion MRI study. Brain. 2000;123(Pt 8):1667–76. doi: 10.1093/brain/123.8.1667. [DOI] [PubMed] [Google Scholar]
- 33.Rovira A1, Pericot I, Alonso J, et al. Serial diffusion-weighted MR imaging and proton MR spectroscopy of acute large demyelinating brain lesions: case report. Am J Neuroradiol. 2002;23(6):989–94. [PMC free article] [PubMed] [Google Scholar]
- 34.Teksam M, et al. Diffusion-weighted imaging in non ischemic lesions. Diagn Interv Radiol. 2002;8(1):31–37. [Google Scholar]