Abstract
Background
MiRNAs play important roles in regulating many fundamental biological processes. Deregulation of miRNAs is involved in the initiation and progression of cancer. MiR-193b is regarded as tumor suppressor in many types of cancers. However, the role of miR-193b in ovarian cancer is poorly understood.
Material/Methods
The expression level of miR-193b in ovarian cancer cell lines and ovarian cancer samples was evaluated using quantitative real-time reverse transcription-PCR (qRT-PCR). The ovarian cancer patients were categorized into a high miR-193b expression group and a low miR-193b expression group according to the median miR-193b expression level. The correlation between tissue miR-193b expression and the patients’ clinicopathological factors, as well as survival, was also analyzed.
Results
The results showed that the miR-193b expression was significantly down-regulated in ovarian cancer cell lines and tumor tissues compared with normal controls. In addition, tissue miR-193b expression was positively correlated with FIGO stage (P=0.001), histological grade (P=0.032), ascites (P=0.019), lymph node metastasis (P=0.003), and tumor size (P=0.041). Among 116 patients with ovarian cancer examined, the 5-year overall survival (OS) rates were 62.5% and 22.01% in patients with high and low miR-193b expression, respectively (P=0.003). Multivariate analysis showed that tissue miR-193b is an independent prognostic factor in patients with ovarian cancer (HR=4.219; P=0.015).
Conclusions
Reduction of miR-193b was found in ovarian cancer and its lower expression was associated with poorer prognosis. Tissue miR-193b showed potential as novel biomarker for ovarian cancer.
MeSH Keywords: Early Detection of Cancer, MicroRNAs, Ovarian Neoplasms, Prognosis
Background
Ovarian cancer is the 8th most prevalent cancer in females worldwide and has the highest mortality rate of all gynecological cancers [1]. Although there has been great progress in surgery techniques and chemotherapeutic treatment, improvement in survival in the past few decades has been minimal [2], mostly because ovarian cancer is asymptomatic until it has significantly progressed. The prognosis is very unfavorable if this malignant disease has spread to other organs. If diagnosed and treated at the early clinical stage, the 5-year overall survival (OS) rate for ovarian cancer is up to 90%. However, patients in the advanced stage have extremely poor OS (less than 20%) [3]. Thus, it is urgent to develop effective screening strategies for detecting ovarian cancer at an early, treatable stage.
MicroRNAs (MiRNAs) are a family of small, evolutionary conserved, noncoding RNAs, 18–25 nucleotides in length, which regulate the target genes post-transcriptionally. MiRNAs have been proved to play important roles in regulation of many physiological processes, including, but not limit to, proliferation, differentiation, survival, and apoptosis [4,5]. Deregulation of miRNAs is implicated in the initiation and progression of many diseases, including cancer. MiRNAs may serve as oncogenes or tumor suppressors in tumorigenesis; they rely heavily on the downstream signal pathways they regulate and the cancer types [6]. A number of miRNAs have been identified to promote or repress the development of ovarian cancer. Chen et al. showed that miR-370 could not only inhibit cellular viability and colony formation in ovarian cancer cell lines by targeting Endoglin, but also enhance chemosensitivity to cisplatin; indicating that miR-370 might function as tumor suppressor in ovarian cancer [7]. MiR-181b was upregulated in ovarian cancer tissues and exerted its oncogenic effects by suppressing the expression level of large tumor suppressor 2[8].
Aberrant expression of miR-193b is frequently observed in cancer and it acts as a tumor suppressor in many types of cancers. MiR-193b is down-regulated in pancreatic cancer and can promote tumorigenesis by inhibiting stathmin 1 and urokinase-type plasminogen activator (uPA) [9]. Rauhala showed that miR-193b was methylated and thus epigenetically silenced in prostate cancer. Enforced expression of miR-193b can significantly suppress proliferative capacity of prostate cancer cell lines [10]. However, little information is currently available on the role of miR-193b in ovarian cancer. In this study we investigated the expression level of miR-193b in ovarian cancer cell lines and ovarian cancer tissues to determine if tissue miR-193b could be a useful biomarker for ovarian cancer.
Material and Methods
Patients and specimens
The study was approved by the Research Ethics Committee of Zhongnan Hospital of Wuhan University. After obtaining written informed consent from all patients, surgical samples (tumor tissues and matched adjacent normal tissues) were collected from 116 patients with ovarian cancer in the Department of Gynecology and Obstetrics, Zhongnan Hospital of Wuhan University. The staging was made based on the 2014 Federation of Obstetrics and Gynecology (FIGO) classification. The clinical features of the ovarian cancer patients are listed in Table 1.
Table 1.
Associations of tissue miR-193b clinicopathological characteristics in 116 ovarian cancer samples.
| Characteristics | N | miR-193b expression | χ2 | P value | |
|---|---|---|---|---|---|
| Low 68 | High 48 | ||||
| Age | |||||
| ≤50 | 54 | 31 | 23 | 0.061 | 0.804 |
| >50 | 62 | 37 | 25 | ||
| FIGO stage | |||||
| Stage I | 25 | 8 | 17 | 15.510 | 0.001 |
| Stage II | 37 | 19 | 18 | ||
| Stage III | 31 | 22 | 9 | ||
| Stage IV | 23 | 19 | 4 | ||
| Histological grade | |||||
| Grade 1 | 36 | 15 | 21 | 6.874 | 0.032 |
| Grade 2 | 45 | 28 | 17 | ||
| Grade 3 | 35 | 25 | 10 | ||
| Ascites | |||||
| No | 55 | 26 | 29 | 5.553 | 0.019 |
| Yes | 61 | 42 | 19 | ||
| LN metastasis | |||||
| No | 49 | 21 | 28 | 8.691 | 0.003 |
| Yes | 67 | 47 | 20 | ||
| Serum CA-125(U/ml) | |||||
| ≤35 | 51 | 29 | 22 | 0.116 | 0.734 |
| >35 | 65 | 39 | 26 | ||
| Tumor size (cm) | |||||
| ≤5 | 57 | 28 | 29 | 4.168 | 0.041 |
| >5 | 59 | 40 | 19 | ||
| Histological type | |||||
| Serous | 56 | 34 | 22 | 0.924 | 0.921 |
| Mucinous | 16 | 8 | 8 | ||
| Endometrioid | 18 | 11 | 7 | ||
| Clear-cell | 15 | 8 | 7 | ||
| Mixed type | 11 | 7 | 4 | ||
Cell culture
All ovarian cancer cells (SKOV3, HO8910PM, HO8910, and OVCAR-3) were obtained from the American Type Culture Collection and cultured in RPMI-1640 (Corning, Corning, NY, USA) containing 10% fetal bovine serum (FBS) and incubated at 37°C in a humidified atmosphere of 5% CO2. The human normal ovarian epithelial cell line (NEOC), which has been transfected hTERT, was maintained in MCDB105 and M199 medium supplemented with 10% FBS.
RNA extraction
Total RNA of tissues and cells were both isolated by TRIzol reagent (Invitrogen) according to the manufacturer’s protocol. The purity of the samples was measured by the ratio of OD260/OD280 using NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA).
The extracted RNA was stored at −80°C.
Reverse transcription and real-time PCR
Briefly, 20 μL of total RNA sample was transcribed to cDNA using miScript-II-RT-Kit (Qiagen, Germany) following manufacturer’s protocols. Then real-time PCR was performed with the Applied Biosystems 7500 real-time PCR system (Applied Biosystems). Cycling conditions were 1 cycle of 95°C for 1 min, 40 cycles of 95°C for 5 s, and 60°C for1 min. Reactions were performed in triplicate using e 2−Δ ΔCt method; U6 small nuclear RNA was used as an endogenous control.
Statistical analysis
One-way ANOVA was conducted to compare the miR-193b expression level among the cell lines investigated. The difference regarding the tissue miR-193b expression between ovarian cancer samples and matched normal tissues was compared using the Mann-Whitney U test. Associations between clinicopathological parameters and tissue miR-193b expression were evaluated using χ2 tests. Overall survival was analyzed using Kaplan-Meier method and log-rank test. Univariate and multivariate Cox proportional hazard models were used to identify the independent risk factors for ovarian cancer. All analyses were performed using the SPSS.21 software package (SPSS Inc., Chicago, IL, USA) and P<0.05 was considered to indicate the difference was statistically significant.
Results
The expression level of miR-193b in ovarian cancer tissues and ovarian cancer cell lines
To reveal the role of miR-193b in ovarian cancer, we evaluated miR-193b expression level in 4 ovarian cancer cell lines and NEOC using real-time PCR. All ovarian cancer lines had significantly decreased expression of miR-193b compared with the normal controls (** P<0.01[HO8910, OVCAR-3 vs. NEOC]; *** P<0.001[SKOV3/HO8910PM vs. NEOC]). In addition, lower expression of miR-193b was detected in the 2 cell lines (SKOV3 and HO8910PM) with highly metastasis capacity in comparison to the other 2 less invasive cell lines (HO8910 and OVCAR-3) (## P<0.01); indicating miR-193b might be closely correlated with the metastasis process of ovarian cancer (Figure 1).
Figure 1.
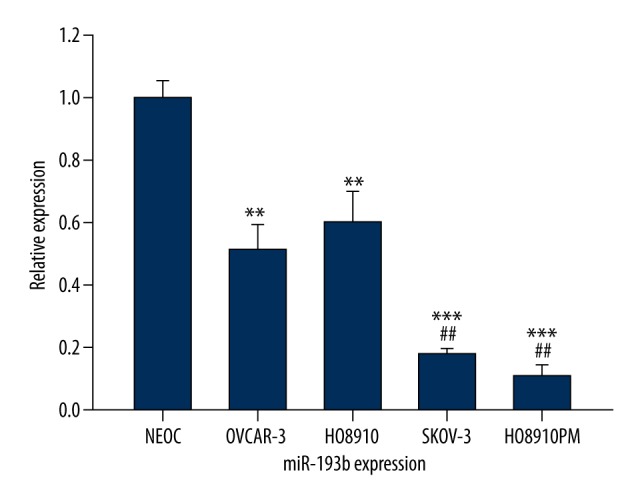
The expression level of miR-193b in ovarian cancer cell lines.
As regards to the expression level of tissue miR-193b in ovarian cancer, the results revealed that tissue miR-193b expression level in the ovarian cancer tissues was significantly lower than that in the matched adjacent normal ovarian tissues (P<0.01) (Figure 2).
Figure 2.
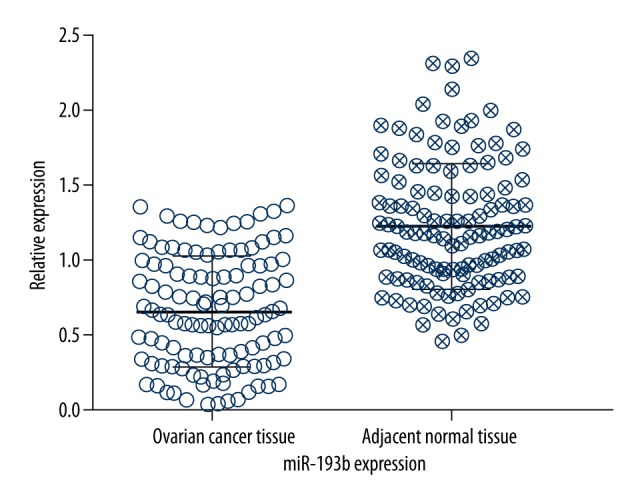
The expression level of miR-193b in ovarian cancer tissues.
The association between tissue miR-193b expression and clinical parameters of ovarian cancer
All patients with ovarian cancer were assigned into 2 groups (high tissue miR-193b group and low tissue-193b group) based on the median expression level of tissue miR-193b (0.63 fold). As shown in Table 1, the level of tissue miR-193b was strongly associated with FIGO stage (P=0.001), histological grade (P=0.032), ascites (P=0.019), lymph node metastasis (LN metastasis) (P=0.003), and tumor size (P=0.041). However, no significant associations were found between tissue miR-193b expression and other clinical features, including age, serum CA-125, and histological type. The results from the above analysis suggest that tissue miR-193b is reduced in ovarian cancer and is associated with the progression of this malignant disease.
Down-regulation of tissue miR-193b was correlated with poor overall survival
Kaplan-Meier survival analysis indicated that the 5-year OS rate was 22.01% in the low tissue miR-193b expression group, and 62.5% in the high tissue miR-193b expression group (Figure 3). The log-rank test showed that the patients with low expression of miR-193b had significantly worse OS (P=0.003).
Figure 3.
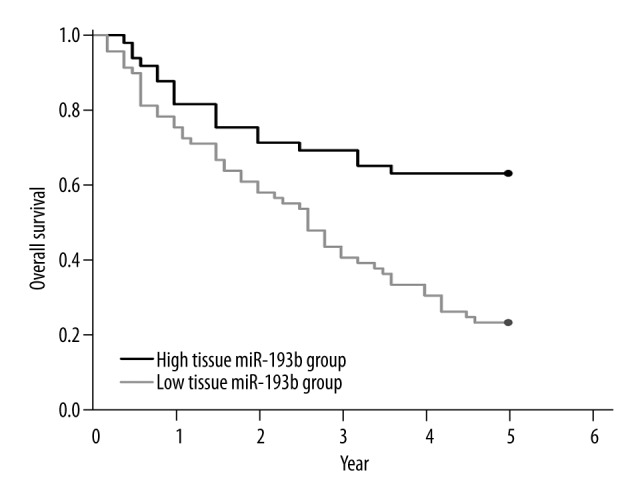
Down-regulation of tissue miR-193b was correlated with poor overall survival.
Tissue miR-193b was an independent risk factor for ovarian cancer
The univariate analyses showed statistically significant associations between OS and FIGO stage (HR=5.369; P=0.004), ascites (HR=2.542; P=0.046), LN metastasis (HR=4.695; P=0.012), and miR-193b expression (HR=4.364; P=0.028) (Table 2).
Table 2.
Univariate analysis of OS in ovarian cancer patients.
| Prognostic variables | OS | |
|---|---|---|
| HR (95% CI) | P value | |
| Age (>50 vs. ≤50) | 1.282 (0.342–3.218) | 0.522 |
| FIGO Stage (III–IV vs. I–II) | 5.369 (3.426–11.63) | 0.004 |
| Histological grade (3 vs. 1/2) | 2.387 (0.863–4.294) | 0.069 |
| Ascites (Yes vs. No) | 2.542 (0.975–4.821) | 0.046 |
| LN metastasis (Yes vs. No) | 4.695 (2.621–8.610) | 0.012 |
| Serum CA-125(U/ml) (>35 vs. ≤35) | 1.638 (0.503–3.746) | 0.367 |
| Tumor size (cm) (>5 vs. ≤5) | 1.856 (0.684–3.911) | 0.187 |
| Histological type (Serous vs. Non serous) | 1.089 (0.417–2.019) | 0.762 |
| miR-193b expression (High vs. Low) | 4.364 (2.514–7.589) | 0.028 |
FIGO stage (HR=6.266; P=0.006), LN metastasis (HR=3.143; P=0.038), and miR-193b expression (HR=4.219; P=0.015) maintained their significance as independent prognostic factors using multivariate Cox regression analyses (Table 3).
Table 3.
Multivariate analysis of OS in ovarian cancer patients.
| Prognostic variables | OS | |
|---|---|---|
| HR (95% CI) | P value | |
| FIGO Stage (III–IV vs. I–II) | 6.266 (2.961–12.521) | 0.006 |
| Ascites (Yes vs. No) | 1.702 (0.538–2.650) | 0.453 |
| LN metastasis (Yes vs. No) | 3.143 (1.475–5.742) | 0.038 |
| miR-193b expression (High vs. Low) | 4.219 (1.813–8.365) | 0.015 |
Discussion
Annually, about 140 200 women die from ovarian cancer globally, and an additional 225 000 are diagnosed [11]. Tumor metastasis is a complex multi-step process, which is common for advanced stage of cancer and is the major reason for the high mortality rate of ovarian cancer. Although the CA125 test has been used for ovarian cancer screening and predicting recurrence, its elevation is not specific to ovarian cancer. Thus, finding biomarkers with high specificity and sensitivity for detecting ovarian cancer is important.
Our study demonstrated that expression of miR-193b was significantly reduced in ovarian cancer and in cell lines compared with the controls. In addition, low-level expression of miR-193b is much lower in highly metastatic ovarian cancer cells. Expression of tissue miR-193b was higher in tumors with FIGO stage, histological grade, ascites, LN metastasis, and tumor size. High tissue miR-193b expression significantly correlated with worse 5-year overall survival and was an independent prognostic risk factor in patients with ovarian cancer. These results indicate miR-193b might be closely associated with the metastatic capacity of ovarian cancer cells, which is in line with recently published findings reported by Mitra et al. They used a 3-dimensional culture model to show that the interaction between ovarian cancer cells and mesothelial cells could down-regulated miR-193b expression in ovarian cancer cells. Aberrant reduction of miR-193b is crucial for maintaining the metastasis capacity of ovarian cancer cells in ex vivo and in vivo models by increasing uPA expression [12]. Multiple studies have also revealed the tumor suppressive role of miR-193b in ovarian cancer. Nakano et al. showed that overexpression of miR-193b in ovarian cancer cells could not only inhibit cell proliferation by affecting cell cycle, but also induce apoptosis via activating caspase 3 and 7 [13]. Deletion of miR-193b in serous ovarian tumors was closely correlated with increased loss of heterozygosity events, BRCA1 upregulation, and overall promotion of genomic instability, suggesting that alteration in miR-193b expression might play important roles in driving the tumorigenesis of ovarian cancer [14]. Ectopic expression of miR-193b resulted in increased cellular resistance capability of ovarian cancer cells to chemotherapy drugs by suppressing CRIM1expression [15]. MiR-193b is probably a tumor suppressor in ovarian cancer, based on current findings.
MiR-193b has also been shown to be a negative regulator of tumor progression in various types of cancers. In T-cell acute lymphoblastic leukemia, miR-193b-3p was identified as a tumor suppressor miRNA by targeting MYB oncogene, which is essential for normal and malignant human hematopoiesis [16]. MiR-193b expression was reduced in hepatocellular carcinoma tissues. Moreover, enforced expression of miR-193b could inhibit proliferation, colony formation, migration, and invasion of hepatoma cells by regulating cyclin D1 and ETS1 [17]. Li et al. reported that overexpression of miR-193b inhibited uPA expression in breast cancer cell lines, while anti-miR-193b could induce uPA expression and increase cell invasion capacity, suggesting that miR-193b might be closely associated with clinical metastasis in breast cancer [18].
Although the tumor suppressive role of miR-193b has been reported in many of the cancers investigated so far, it might also function as an oncogene and promote the progression of cancer.
Zhong et al. showed that the expression level of miR-193b was up-regulated in glioma tissues and glioma cells. In addition, miR-193b could promote the proliferation capacity of gliomas cells through the transforming growth factor-β (TGF-β) pathway by regulating Smad3 [19]. Lenarduzzi et al. revealed that inhibition of miR-193b suppressed tumor cell proliferation, migration, invasion, and tumor formation in head and neck squamous cell carcinomas. Moreover, the patients with high expression of miR-193b had a lower disease-free survival [20]. MiR-193b was down-regulated in melanoma tissues and could suppress the proliferation of melanoma cells in vitro [21]. However, Caramuta et al. reported that melanoma patients who had high miR-193b expression had poor melanoma-specific survival [22], indicating that miR-193b might act as an oncogene in melanoma. The contradictory findings regarding the role of miR-193b in the development of different cancers or even the same type of cancer indicates that miR-193b might have many downstream targeted genes. Whether the targeted genes are activated or suppressed might depend on the tumor microenvironment in which the cancer cells reside.
Conclusions
MiR-193b expression was reduced in ovarian cancer and lower expression of tissue miR-193b was associated with worse clinical outcome. Our data also indicated that miR-193b might be correlated with clinical metastasis of ovarian cancer and could be used as a promising biomarker. Future investigations are needed to understand how miR-193b down-regulation contributes to progression of ovarian cancer.
Footnotes
Conflict of interest
None declared.
Source of support: Departmental sources
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. Cancer J Clin. 2011;1:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Liu X, Chong Y, Liu H, et al. Novel reversible selective inhibitor of CRM1 for targeted therapy in ovarian cancer. J Ovarian Res. 2015;8:35. doi: 10.1186/s13048-015-0166-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suh DH, Kim K, Kim JW. Major clinical research advances in gynecologic cancer in 2011. J Gynecol Oncol. 2012;23:53–64. doi: 10.3802/jgo.2012.23.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winter J, Jung S, Keller S, et al. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol. 2009;11:228–34. doi: 10.1038/ncb0309-228. [DOI] [PubMed] [Google Scholar]
- 5.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 12:861–74. doi: 10.1038/nrg3074. 201. [DOI] [PubMed] [Google Scholar]
- 6.Zhang B, Pan X, Cobb GP, et al. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302:1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 7.Chen XP, Chen YG, Lan JY, et al. MicroRNA-370 suppresses proliferation and promotes endometrioid ovarian cancerchemosensitivity to cDDP by negatively regulating ENG. Cancer Lett. 2014;353:201–10. doi: 10.1016/j.canlet.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 8.Xia Y, Gao Y. MicroRNA-181b promotes ovarian cancer cell growth and invasion by targeting LATS2. Biochem Biophys Res Commun. 2014;447:446–51. doi: 10.1016/j.bbrc.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Kong F, Wu K, et al. miR-193b directly targets STMN1 and uPA genes and suppresses tumor growth and metastasis in pancreaticcancer. Mol Med Rep. 2014;10:2613–20. doi: 10.3892/mmr.2014.2558. [DOI] [PubMed] [Google Scholar]
- 10.Rauhala HE, Jalava SE, Isotalo J, et al. miR-193b is an epigenetically regulated putative tumor suppressor in prostate cancer. Int J Cancer. 2010;127:1363–72. doi: 10.1002/ijc.25162. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Matulonis UA. New strategies in ovarian cancer: translating the molecular complexity of ovarian cancer into treatment advances. Clin Cancer Res. 2014;20:5150–56. doi: 10.1158/1078-0432.CCR-14-1312. [DOI] [PubMed] [Google Scholar]
- 12.Mitra AK, Chiang CY, Tiwari P, et al. Microenvironment-induced downregulation of miR-193b drives ovarian cancer metastasis. Oncogene. 2015 doi: 10.1038/onc.2015.43. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakano H, Yamada Y, Miyazawa T, et al. Gain-of-function microRNA screens identify miR-193a regulating proliferation and apoptosis in epithelial ovarian cancer cells. Int J Oncol. 2013;42:1875–82. doi: 10.3892/ijo.2013.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi YE, Pan Y, Park E, et al. MicroRNAs down-regulate homologous recombination in the G1 phase of cycling cells to maintain genomic stability. Elife. 2014;3:e02445. doi: 10.7554/eLife.02445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ziliak D, Gamazon ER, Lacroix B, et al. Genetic variation that predicts platinum sensitivity reveals the role of miR-193b* in chemotherapeutic susceptibility. Mol Cancer Ther. 2012;11:2054–61. doi: 10.1158/1535-7163.MCT-12-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mets E, Van der Meulen J, Van Peer G, et al. MicroRNA-193b-3p acts as a tumor suppressor by targeting the MYB oncogene in T-cell acute lymphoblastic leukemia. Leukemia. 2015;29:798–806. doi: 10.1038/leu.2014.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu C, Liu S, Fu H, et al. MicroRNA-193b regulates proliferation, migration and invasion in human hepatocellular carcinoma cells. Eur J Cancer. 2010;4:2828–36. doi: 10.1016/j.ejca.2010.06.127. [DOI] [PubMed] [Google Scholar]
- 18.Li XF, Yan PJ, Shao ZM. Downregulation of miR-193b contributes to enhance urokinase-type plasminogen activator (uPA) expression and tumor progression and invasion in human breast cancer. Oncogene. 2009;28:3937–48. doi: 10.1038/onc.2009.245. [DOI] [PubMed] [Google Scholar]
- 19.Zhong Q, Wang T, Lu P, et al. miR-193b promotes cell proliferation by targeting Smad3 in human glioma. J Neurosci Res. 2014;92:619–26. doi: 10.1002/jnr.23339. [DOI] [PubMed] [Google Scholar]
- 20.Lenarduzzi M, Hui AB, Alajez NM, et al. MicroRNA-193b enhances tumor progression via down regulation of neurofibromin 1. PLoS One. 2013;8:e53765. doi: 10.1371/journal.pone.0053765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J, Feilotter HE, Paré GC, et al. MicroRNA-193b represses cell proliferation and regulates cyclin D1 in melanoma. Am J Pathol. 2010;176:2520–29. doi: 10.2353/ajpath.2010.091061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caramuta S, Egyházi S, Rodolfo M, et al. MicroRNA expression profiles associated with mutational status and survival in malignant melanoma. J Invest Dermatol. 2010;130:2062–70. doi: 10.1038/jid.2010.63. [DOI] [PubMed] [Google Scholar]


