Abstract
Recent research on hepatic stellate cells (HSCs) has spotlighted the involvement of morphogens in their cell fate determination in liver fibrosis. Temporally and spatially expressed during embryonic development, morphogens are involved in regulation of cell proliferation and differentiation, and tissue patterning. In normal adult liver, morphogens are generally expressed at low levels. However, in liver disease, myofibroblastic HSCs express morphogens such as Wnt, Shh, Necdin, DLK1, and Notch as part of their participation in fibrogenesis and wound healing. Liver regeneration involves cell proliferation and differentiation akin to embryonic liver development where the cells appear to undergo similar fates, and not surprisingly the morphogens are re-activated for the regenerative purpose in adult liver injury. Evidence also points to crosstalk of these morphogens in regulation of HSC fate determination. Genetic ablation or pharmacologic inhibition of morphogens reverts activated HSC to quiescent cells in culture and attenuates progression of hepatic fibrosis. However, positive regulation of liver regeneration by the morphogens needs to be spared. Therapeutically, manipulation of morphogen activities in a cell type and phase-specific manner, should offer new modalities for chronic liver disease.
Role of hepatic stellate cells (HSCs) in liver fibrosis
Remarkably, the liver is the only internal organ with the ability to regenerate. A liver mass loss triggers well-orchestrated signaling cascades involving growth factors, cytokines, hormones, and neurotransmitters to restore the tissue to its original size and function [1,2]. However, this unique regenerative ability is often impaired in chronic liver disease resulting from sustained or repeated injury. Central to this defect is liver fibrosis characterized by accumulation of excessive extracellular matrix (ECM) proteins which compromises normal liver regeneration, functions and intrahepatic circulation. Progression of liver fibrosis results in cirrhosis, which markedly increases the risk of developing liver failure and cancer [3].
HSCs make up approximately 5-8% of a total liver population. Residing in the perisinusoidal space of Disse, HSCs are the body's major storage site for vitamin A and serve as pericytes for hepatic sinusoids. They also fulfill the role of the major mesenchymal cell type in mesenchyme-epithelial interactions in the liver via maintenance of normal ECM milieu and production of hepatotrophic soluble factors [4]. HSCs also store neutral lipids resembling adipocytes [5] as they were once called “fat-storing cells” [6]. Upon injury to the liver, HSCs are transdifferentiated or “activated” toward a myofibroblast-like phenotype with induced expressions of cytokines, growth factors, and ECM components required for wound repair [4]. Activation of HSCs involves the coordination of multiple signaling events including down-regulation of PPARγ much like de-differentiation of mature adipocytes to preadipocytic fibroblasts [7,8].
Morphogen-associated signaling in liver regeneration
During development, morphogens are secreted from primordial cells and are recognized by specific receptors in distant cells to activate signaling cascades in support of organ growth and development [9]. Typically, morphogens form a concentration gradient to pass positional information to responding cells and guide the differentiation of cells into specific patterns and morphologies [10,11]. Embryonic tissue recombination experiments established the roles of molecular signals from the adjacent mesoderm in inducing hepatic commitment of the foregut endoderm [12]. Subsequent genetic studies revealed these molecular cues as members belonging to the fibroblast growth factors (FGF) and transforming growth factors β (TGFβ) families of morphogens [13]. FGF released from cardiac mesoderm binds to the newly formed endodermal cells and leads to induction of two FoxA transcription factors, FoxA1 and FoxA2, securing the fate of these cells to become future liver cells [14]. BMP released from septum transversum mesenchyme cooperates with FGF to support hepatic specification [15]. A recent lineage tracing definitively demonstrates that HSCs arise from mesoderm-derived septum transversum [16], suggesting a possibility that HSCs may retain this role in mesenchyme-epithelial interaction even in adult liver.
In the normal adult liver, morphogen signaling is not generally required due to the low cell turnover rate [17]. The damaged liver, on the other hand, has a vigorous injury response including activation of quiescent HSCs. Numerous mediators are released by different cell types to facilitate hepatic wound response, and those known to activate HSCs include growth factors (PDGF, TGF-α, IGF, HGF), acute phase cytokines (IL-1, TNF-α, IL-6), ECM inducers (TGF-β, CTGF), hormones (leptin, angiotensin), and lipid metabolites (PAF) [4]. Activated HSCs also serve as the source of many of these mediators to recruit inflammatory cells to the injured liver and to commence the wound healing and regeneration processes As HSC activation is considered as a manifestation of cell fate regulation which recapitulates the program known for liver development, the role of morphogens derived from HSCs can obviously be a subject of interest. Indeed, the morphogens known for liver morphogenesis are shown expressed by activated HSCs in adult liver injury.
Wnt family
The molecular name Wnt is derived from combining Wingless, the segment-polarity gene from Drosophila melangonster, and Integrase-1, the vertebrate homologue. The Wnt pathway is a highly conserved signaling in regulating tissue development and regeneration, and cell differentiation. The canonical pathway is activated when the Wnt protein forms a complex with the transmembrane Frizzled receptor (Fz) and the low-density lipoprotein receptor related protein (LRP) 5/6 co-receptor. The formation of the Wnt-Fz-LRP complex attracts the scaffolding protein Disheveled (Dvl) and recruits Axin and glycogen synthase kinase 3β (GSK3β) from the β-catenin destruction complex. As a consequence, this inhibits phosphorylation and subsequent degradation of β-catenin, allowing it to translocate into the nucleus for transcriptional activation of target genes with the T cell factor/lymphocyte enhancer factor (TCF/LEF) family of transcription factors. Non-canonical Wnt pathways including the Wnt/Ca2+ pathway and planar cell polarity (PCP) pathway are independent of regulation by β-catenin. Wnt5a, the ligand for Wnt/Ca2+ pathways, causes the release of intracellular Ca2+ through the interaction of the cytosolic protein Dvl1 and activates protein kinase C (PKC) and calmodulin kinase II (CAMKII). Wnt5a may also play a critical role in inflammatory processes associated with skeletal tissues through receptor tyrosine kinaselike orphan receptor (Ror) proteins [18]. Wnt5a/Frz5 can also activate the transcriptional factor Nuclear Factor-KappaB (NF-κb) that in turn stimulates the expression of chemokines and pro-inflammatory cytokines [19]. Activation of the PCP pathway supports cytoskeletal organization for cell proliferation and differentiation through the recruitment of Dvl by Frz which then further transduces the activation of Rho family of GTPases [20].
Wnt pathway enhances the survival of activated HSC and thereby promotes hepatic fibrosis [21]. Global changes in gene expression from culture-activated rat HSCs and CCl4-treated liver reveal the upregulation of Wnt signaling components [22]. Both the components of the Wnt pathway including Wnt5a, Wnt4, Frz1 and Frz7 and genes downstream of the Wnt pathway (Gfg18, Sox9, Twist, and Fibronectin) are increased in activated HSCs compared to quiescent HSCs. The involvement of the canonical Wnt pathway in activated HSCs is validated by our study in culture-activated HSCs as well as HSCs from cholestatic rat livers. Wnt and Fz mRNA expressions are increased in activated HSCs relative to their quiescent counterparts [23]. The importance of Wnt signaling in HSC activation is supported by a reversal of activated HSCs to quiescent cells with the Wnt co-receptor antagonist Dickkopff 1 (Dkk-1). TOPFLASH activity as a measure of TCF/β-catenin-dependent transcription in activated HSCs is blocked by expression of Chibby, a nuclear protein which blocks p-catenin interaction with TCF, further supporting the functional existence of the canonical Wnt pathway. Experimental liver fibrosis induced by bile duct ligation in mice is attenuated with the administration of an adenovirus expressing Dkk-1, providing the evidence for physiological relevance. Canonical Wnt pathway is known to suppress adipocyte differentiation [24], and these results enforce the concept of anti-adipogenic mode of HSC activation. As shown for adipocytes, Wnt pathway antagonizes adipogenic transcription factors, C/EBPα and PPARγ [25]. As predicted, the restoration of HSC quiescence with Dkk-1 is accompanied by enhanced PPARγ expression. In contrast, the role of canonical Wnt pathway in the maintenance of quiescent HSCs has also been proposed [26]. In this study, inhibition of the GSK3β activity by the small molecule TWS119, which activated the canonical Wnt pathway, reduced the expression of α-smooth muscle actin (αSMA) and DNA synthesis.
Necdin
Necdin is a family member of immunoglobulin-like (Ig) cell adhesion molecules and the melanoma antigen family (MAGE) of proteins [27]. Necdin promotes neuronal and myogenic differentiation [28] but also inhibits adipocyte differentiation [29]. In the liver, necdin is selectively expressed in HSCs and induced in culture and in liver fibrosis models [30]. In culture-activated HSC, knockdown of necdin with an adenovirus expressing small hairpin RNA effectively reverts activated HSCs to the quiescent state in a manner dependent on PPARγ upregulation, highlighting again the role of this morphogen in providing anti-adipogenic regulation for HSC activation much like Wnt. In fact, necdin binds to guanosine (G)-rich motif, termed GN boxes in the proximal promoter of Wnt10b to transactivate the gene, activates canonical Wnt pathway, and thus epigenetically represses PPARγ to activate HSCs [30].
Notch family
The role of the Notch pathway in cholangiocyte differentiation is firmly established [31]. During liver development, activation of Notch signaling in hepatoblasts stimulates transcriptional regulators RBP-Jk and Hes-1 to regulate pathways for biliary commitment and ductal plate remodeling and tuberization [32,33]. In mammals, the Notch genes encode four single pass transmembrane receptors (Notch 1-4) for their canonical ligands, Jagged 1 and 2 and Delta-like ligand (Dll) 1, 3 and 4. The defining feature of Notch from the other morphogens described in this review is that Notch signaling requires cell-cell contacts [34]. Interaction with Notch ligands expressed by adjacent cells, leads to the activation of γ-secretase and cleavage and release of the Notch intracellular domain (NICD). NICD translocates to the nucleus where it binds to a CSL-corepressor complex, releases corepressors, recruits co-activators to form a trans-activating complex. In activated HSCs as well as in livers after partial hepatectomy, Notch 1 and 3 are expressed following the epigenetic repression of Nestin, a class IV intermediate filament protein that is expressed in progenitor-like cells [35,36]. In intrahepatic lesions in polycystic kidney rats, periportal myofibroblasts express Jagged1 and proliferating cholangiocytes express Notch2, suggesting the role of activated Notch pathway in formation of bile ducts in these lesions [37].
DLK1
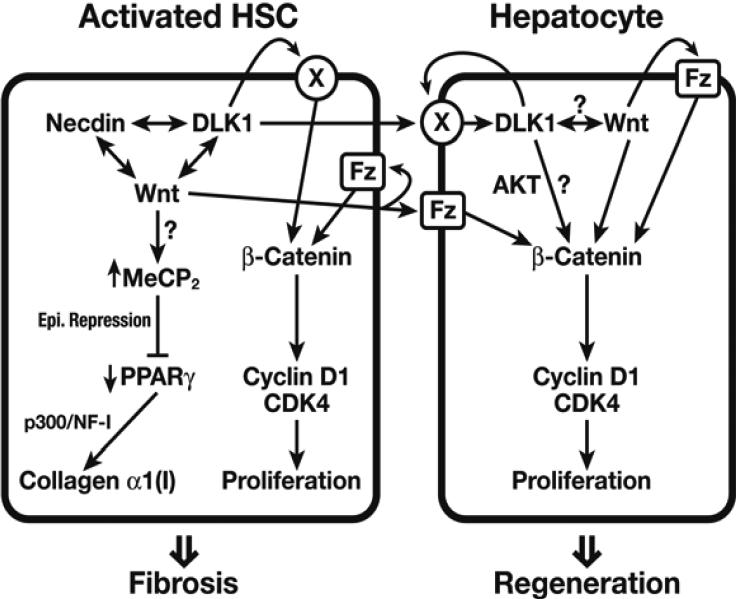
Recently, we reported the identification of a non-canonical Notch ligand, delta like homolog 1 (Dlk1) that is selectively expressed in HSCs and induced in activation in vitro and in vivo [38]. Dlk1, also known as Pref-1 from earlier studies, was identified as an inhibitor of adipogenic differentiation expressed in preadipocytes [39]. As part of the Dlk1-Dios locus, Dlk1 is a paternally imprinted gene that regulates cellular differentiation including adipogenesis, osteogenesis, and neurogenesis. Highly expressed in embryonic hepatoblasts [40] and some malignant cells [41], it has been implicated in tumorigenesis and stemness [42]. Structurally similar to canonical Notch ligands, Dlk1 is a single-pass transmembrane protein whose extracellular region- consisting of multiple EGF-binding domains, is released upon cleavage by the metalloproteinase ADAM-17 (TNF-α-converting enzyme, TACE). Our study demonstrates Dlk1 guides activation of HSCs by epigenetically repressing PPARγ. Adenovirally expressed shRNA specifically designed to suppress Dlk1 mRNA expression achieves the reversal of culture-activated HSCs to quiescent cells with restored vitamin A and lipid content. Moreover, knockdown of Dlk1 leads to the induction of the PPARγ and C/EBPα, the phenotype identical to that observed by suppression of necdin or Wnt. This is explained in part by DLK1's ability to upregulate canonical Wnt pathway although the mechanisms are not known. This DLK-Wnt link is depicted well in liver regeneration induced by partial hepatectomy (PH) in mice. Dlk1 is induced in regenerating liver right after PH along with other genes involved in cell proliferation (i.e. p75Ntr, Wnt10b, and Ptn). Administration of neutralizing antibody against DLK1, to these mice results in impaired Wnt10b induction; an overall decrease in non-phosphorylated β-catenin (S33/S37/T41), total β-catenin, and phosphorylated β-catenin (S552); and suppresses early liver growth. Co-culture studies using HSCs and hepatocytes isolated from one day after PH, suggest DLK1 derived from HSCs may upregulate hepatocyte DLK1 to promote hepatocyte proliferation (Figure 1).
Figure 1.
The hypothetical Wnt-DLK1 pathway in HSC-hepatocyte crosstalk in liver regeneration is depicted. Necdin, Wnt, DLK1 positively cross-interact to cause epigenetic Pparγrepression and activate HSCs. Wnt and DLK1 produced by HSCs stimulate HSC proliferation while exerting paracrine stimulation of hepatocyte proliferation. An unidentified receptor for DLK1 is depicted as X.
Hedgehog family
In vertebrates, the hedgehog family is represented by at least three members: Desert hedgehog (Dhh), Indian hedgehog (Ihh) and Sonic hedgehog (Shh). The Hedgehog gene was originally identified in segment polarity based on Drosophila development studies [43]. In activated HSC, Shh is upregulated and enhances the survival of transdifferentiated HSCs [44]. During wound healing, Shh may interact with bone-marrow derived mesenchymal stem cells which may migrate into the liver as part of the injury response program [45]. Hepatocytes undergoing apoptosis may secrete Hh ligands to promote myofibroblast proliferation [46]. Aberrant Shh signaling is also implicated in malignancies. In our studies in characterizing Dlk1 in HSC activation, Dlk1 knockdown in HSC is accompanied by decreased Shh mRNA expression. In a separate study, the administration of curcumin dose-dependently attenuated both Dlk1 and Shh expression and as a result, reversed activated HSC to quiescent cells [47]. These coordinated regulations of Shh and Dlk1 may involve a mechanistic relationship such as the ability of DLK1 to upregulate the expression of Gli1, the Shh effector molecule, via activation of β1 integrin and ERK1/2.
In the absence of a Shh ligand, its receptor Patched (Ptc) negatively regulates the coreceptor Smoothened (Smo) and the expression of Shh target genes involved in cell proliferation and differentiation. Shh binding to Ptc releases this inhibition on Smo which in turn causes stabilization and nuclear-translocation of the Gli family of transcription factors. Highlighting a recent study from Michelotti et al. [48], conditional deletion of Smo in αSMA expressing myofibroblasts disrupts Shh signaling and attenuates cholestatic liver fibrosis through the up-regulation of Pparγ and Gfap - genes known to maintain quiescent HSCs. This genetic manipulation to block Shh pathway in activated HSCs also reduces the accumulation of myofibroblasts in mice fed methionine choline deficient diet, supporting the role of Shh pathway in myofibroblastic activation in liver fibrosis. The study further points to the role of Shh pathway in activated HSCs in generating liver progenitor cells.
Future considerations concerning morphogens as therapeutic targets
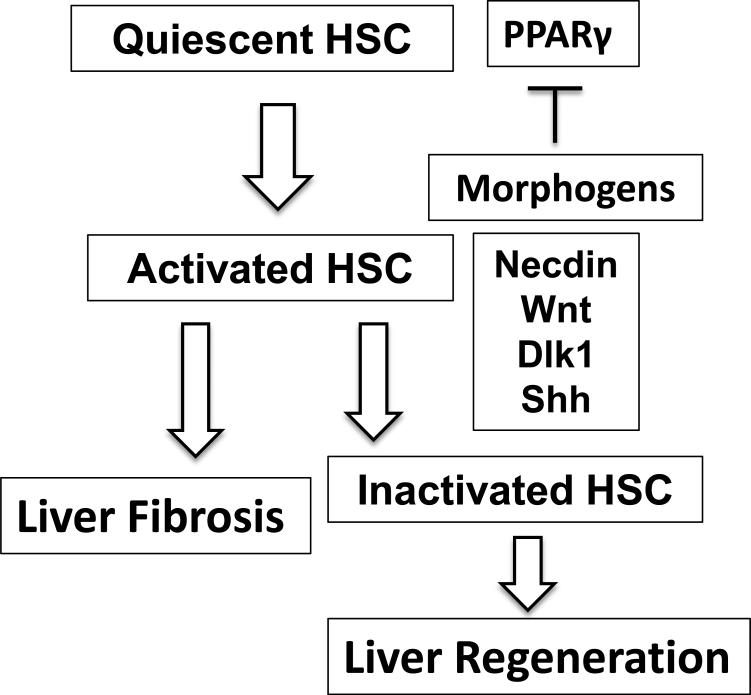
Compelling evidence exists for the roles of HSC-derived morphogens in HSC activation and liver fibrogenesis. At the same time, these morphogens may support a regenerative response of injured liver. From the viewpoint of wound healing, these dual functions make sense as the wound-repairing mesenchymal cells need to lay down ECM for scar formation while stimulating regeneration of the parenchymal cells which will eventually replace the scar. That is what is expected to proceed in normal wound healing, and interference of morphogen pathways in this context may be detrimental. Then how is this repair process altered in liver fibrosis and how can we approach it? From analysis of HSCs isolated from chronic liver fibrosis models, the morphogens are still upregulated, yet the fibrotic livers are commonly refractory to regeneration. Logically, changes may be taking place in hepatocytes, preventing them from the normal response to morphogen-mediated regulation. If this is the case, the treatment should be directed to correction of such defect. In early fibrosis, transiently targeting the morphogens to prevent progression of liver fibrosis via inactivation of HSCs, may be a potential therapeutic option as preclinically shown. In normal resolution from liver fibrosis, activated HSCs have two fates: a half of activated HSCs die while another half become “inactivated” to behave almost like but not exactly same as normal quiescent HSCs [49]. It is possible that the latter group may have a functional significance in liver regeneration (Figure 2). If activated HSCs are transiently inactivated, a half may fulfill this possible role as in spontaneous recovery. In advanced liver fibrosis, compensatory proliferation of progenitor-type of cells may ensue as hepatocyte proliferation is suppressed. These cells may be prone to transformation via genotoxicity and may actively respond to morphogens derived from activated HSCs leading to liver cancer development, the phenotype often characterized by aberrant expression of and responsiveness to morphogens. Here, more aggressive suppression of the morphogens in activated HSCs, may be plausible.
Figure 2.
A simplified schematic diagram depicting the roles of activated morphogens in facilitating activation of HSCs via suppression of PPARγ in liver fibrogenesis and the potential role of inactivated HSCs contributing to a regenerative response.
Acknowledgments
The studies described in this review have been supported by NIAAA grants (P50AA011999, R24AA012885, U01AA018663) and Medical Research Service of Department of Veterans Affairs (VA Merit Review: 1I01BX001991 and senior research career scientist award).
References
- 1.Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol. 2010;176(1):2–13. doi: 10.2353/ajpath.2010.090675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stanger BZ. Cellular homeostasis and repair in the mammalian liver. Annu Rev Physiol. 2015;77:179–200. doi: 10.1146/annurev-physiol-021113-170255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38(Suppl 1):S38–53. doi: 10.1016/s0168-8278(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 4.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88(1):125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsukamoto H, She H, Hazra S, Cheng J, Wang J. Fat paradox of steatohepatitis. J Gastroenterol Hepatol. 2008;23(Suppl 1):S104–107. doi: 10.1111/j.1440-1746.2007.05294.x. [DOI] [PubMed] [Google Scholar]
- 6.Ito T, Shibasaki S. Electron microscopic study on the hepatic sinusoidal wall and the fat-storing cells in the normal human liver. Arch Histol Jpn. 1968;29(2):137–192. doi: 10.1679/aohc1950.29.137. [DOI] [PubMed] [Google Scholar]
- 7.Miyahara T, Schrum L, Rippe R, et al. Peroxisome proliferator-activated receptors and hepatic stellate cell activation. J Biol Chem. 2000;275(46):35715–35722. doi: 10.1074/jbc.M006577200. [DOI] [PubMed] [Google Scholar]
- 8.Hazra S, Xiong S, Wang J, et al. Peroxisome proliferator-activated receptor gamma induces a phenotypic switch from activated to quiescent hepatic stellate cells. J Biol Chem. 2004;279(12):11392–11401. doi: 10.1074/jbc.M310284200. [DOI] [PubMed] [Google Scholar]
- 9.Lecuit T, Goff LL. Orchestrating size and shape during morphogenesis. Nature. 2007;450(7167):189–192. doi: 10.1038/nature06304. [DOI] [PubMed] [Google Scholar]
- 10.Turing AM. The Chemical Basis of Morphogenesis. Philosophical Transactions. 1952 [Google Scholar]
- 11.Wolpert L. Positional information and the spatial pattern of cellular differentiation. J Theor Biol. 1969;25(1):1–47. doi: 10.1016/s0022-5193(69)80016-0. [DOI] [PubMed] [Google Scholar]
- 12.Wessells NK, Cohen JH. Early Pancreas Organogenesis: Morphogenesis, Tissue Interactions, and Mass Effects. Dev Biol. 1967;15(3):237–270. doi: 10.1016/0012-1606(67)90042-5. [DOI] [PubMed] [Google Scholar]
- 13.Zaret KS, Grompe M. Generation and regeneration of cells of the liver and pancreas. Science. 2008;322(5907):1490–1494. doi: 10.1126/science.1161431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee CS, Friedman JR, Fulmer JT, Kaestner KH. The initiation of liver development is dependent on Foxa transcription factors. Nature. 2005;435(7044):944–947. doi: 10.1038/nature03649. [DOI] [PubMed] [Google Scholar]
- 15.Rossi JM, Dunn NR, Hogan BL, Zaret KS. Distinct mesodermal signals, including BMPs from the septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Genes Dev. 2001;15(15):1998–2009. doi: 10.1101/gad.904601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asahina K, Zhou B, Pu WT, Tsukamoto H. Septum transversum-derived mesothelium gives rise to hepatic stellate cells and perivascular mesenchymal cells in developing mouse liver. Hepatology. 2011;53(3):983–995. doi: 10.1002/hep.24119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macdonald RA. “Lifespan” of liver cells. Autoradio-graphic study using tritiated thymidine in normal, cirrhotic, and partially hepatectomized rats. Arch Intern Med. 1961;107:335–343. doi: 10.1001/archinte.1961.03620030023003. [DOI] [PubMed] [Google Scholar]
- 18.Maeda K, Kobayashi Y, Udagawa N, et al. Wnt5a-Ror2 signaling between osteoblast-lineage cells and osteoclast precursors enhances osteoclastogenesis. Nature Medicine. 2012;18:405–412. doi: 10.1038/nm.2653. [DOI] [PubMed] [Google Scholar]
- 19.Naskar D, Maiti G, Chakraborty A, Roy A, Chattopadhyay D, Sen M. Wnt5a–Rac1–NF-κB Homeostatic Circuitry Sustains Innate Immune Functions in Macrophages. J Immunol. 2014;192(9):4386–4397. doi: 10.4049/jimmunol.1302817. [DOI] [PubMed] [Google Scholar]
- 20.Schlessinger K, Hall A, Tolwinski N. Wnt signaling pathways meet Rho GTPases. Genes Dev. 2009;23(3):265–277. doi: 10.1101/gad.1760809. [DOI] [PubMed] [Google Scholar]
- 21.Myung SJ, Yoon JH, Gwak GY, et al. Wnt signaling enhances the activation and survival of human hepatic stellate cells. FEBS Lett. 2007;581(16):2954–2958. doi: 10.1016/j.febslet.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 22.Jiang F, Parsons CJ, Stefanovic B. Gene expression profile of quiescent and activated rat hepatic stellate cells implicates Wnt signaling pathway in activation. J Hepatol. 2006;45(3):401–409. doi: 10.1016/j.jhep.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 23.Cheng JH, She H, Han YP, et al. Wnt antagonism inhibits hepatic stellate cell activation and liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2008;294(1):G39–49. doi: 10.1152/ajpgi.00263.2007. [DOI] [PubMed] [Google Scholar]
- 24.Bennett CN, Ross SE, Longo KA, et al. Regulation of Wnt signaling during adipogenesis. J Biol Chem. 2002;277(34):30998–31004. doi: 10.1074/jbc.M204527200. [DOI] [PubMed] [Google Scholar]
- 25.Ross SE, Hemati N, Longo KA, et al. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289(5481):950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 26.Kordes C, Sawitza I, Haussinger D. Canonical Wnt signaling maintains the quiescent stage of hepatic stellate cells. Biochem Biophys Res Commun. 2008;367(1):116–123. doi: 10.1016/j.bbrc.2007.12.085. [DOI] [PubMed] [Google Scholar]
- 27.Barker PA, Salehi A. The MAGE proteins: emerging roles in cell cycle progression, apoptosis, and neurogenetic disease. J Neurosci Res. 2002;67(6):705–712. doi: 10.1002/jnr.10160. [DOI] [PubMed] [Google Scholar]
- 28.Kuwajima T, Taniura H, Nishimura I, Yoshikawa K. Necdin interacts with the Msx2 homeodomain protein via MAGE-D1 to promote myogenic differentiation of C2C12 cells. J Biol Chem. 2004;279(39):40484–40493. doi: 10.1074/jbc.M404143200. [DOI] [PubMed] [Google Scholar]
- 29.Tseng YH, Butte AJ, Kokkotou E, et al. Prediction of preadipocyte differentiation by gene expression reveals role of insulin receptor substrates and necdin. Nat Cell Biol. 2005;7(6):601–611. doi: 10.1038/ncb1259. [DOI] [PubMed] [Google Scholar]
- 30.Zhu NL, Wang J, Tsukamoto H. The Necdin-Wnt pathway causes epigenetic peroxisome proliferator-activated receptor gamma repression in hepatic stellate cells. J Biol Chem. 2010;285(40):30463–30471. doi: 10.1074/jbc.M110.156703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zong Y, Panikkar A, Xu J, et al. Notch signaling controls liver development by regulating biliary differentiation. Development. 2009;136(10):1727–1739. doi: 10.1242/dev.029140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanimizu N, Miyajima A. Notch signaling controls hepatoblast differentiation by altering the expression of liver-enriched transcription factors. J Cell Sci. 2004;117(Pt 15):3165–3174. doi: 10.1242/jcs.01169. [DOI] [PubMed] [Google Scholar]
- 33.Sparks EE, Perrien DS, Huppert KA, Peterson TE, Huppert SS. Defects in hepatic Notch signaling result in disruption of the communicating intrahepatic bile duct network in mice. Dis Model Mech. 2011;4(3):359–367. doi: 10.1242/dmm.005793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D'Souza B, Meloty-Kapella L, Weinmaster G. Canonical and non-canonical Notch ligands. Curr Top Dev Biol. 2010;92:73–129. doi: 10.1016/S0070-2153(10)92003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kohler C, Bell AW, Bowen WC, Monga SP, Fleig W, Michalopoulos GK. Expression of Notch-1 and its ligand Jagged-1 in rat liver during liver regeneration. Hepatology. 2004;39(4):1056–1065. doi: 10.1002/hep.20156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reister S, Kordes C, Sawitza I, Haussinger D. The epigenetic regulation of stem cell factors in hepatic stellate cells. Stem Cells Dev. 2011;20(10):1687–1699. doi: 10.1089/scd.2010.0418. [DOI] [PubMed] [Google Scholar]
- 37.Furubo S, Sato Y, Harada K, Nakanuma Y. Roles of myofibroblasts and notch and hedgehog signaling pathways in the formation of intrahepatic bile duct lesions in polycystic kidney rats. Pediatr Dev Pathol. 2013;16(3):177–190. doi: 10.2350/12-11-1267-OA.1. [DOI] [PubMed] [Google Scholar]
- 38.Zhu NL, Asahina K, Wang J, et al. Hepatic stellate cell-derived delta-like homolog 1 (DLK1) protein in liver regeneration. J Biol Chem. 2012;287(13):10355–10367. doi: 10.1074/jbc.M111.312751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smas CM, Sul HS. Pref-1, a protein containing EGF-like repeats, inhibits adipocyte differentiation. Cell. 1993;73(4):725–734. doi: 10.1016/0092-8674(93)90252-l. [DOI] [PubMed] [Google Scholar]
- 40.Tanimizu N, Nishikawa M, Saito H, Tsujimura T, Miyajima A. Isolation of hepatoblasts based on the expression of Dlk/Pref-1. J Cell Sci. 2003;116(Pt 9):1775–1786. doi: 10.1242/jcs.00388. [DOI] [PubMed] [Google Scholar]
- 41.Yanai H, Nakamura K, Hijioka S, et al. Dlk-1, a cell surface antigen on foetal hepatic stem/progenitor cells, is expressed in hepatocellular, colon, pancreas and breast carcinomas at a high frequency. J Biochem. 2010;148(1):85–92. doi: 10.1093/jb/mvq034. [DOI] [PubMed] [Google Scholar]
- 42.Kim Y, Lin Q, Zelterman D, Yun Z. Hypoxia-regulated delta-like 1 homologue enhances cancer cell stemness and tumorigenicity. Cancer Res. 2009;69(24):9271–9280. doi: 10.1158/0008-5472.CAN-09-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287(5785):795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 44.Yang L, Wang Y, Mao H, et al. Sonic hedgehog is an autocrine viability factor for myofibroblastic hepatic stellate cells. J Hepatol. 2008;48(1):98–106. doi: 10.1016/j.jhep.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin N, Tang Z, Deng M, et al. Hedgehog-mediated paracrine interaction between hepatic stellate cells and marrow-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2008;372(1):260–265. doi: 10.1016/j.bbrc.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 46.Omenetti A, Yang L, Li YX, et al. Hedgehog-mediated mesenchymal-epithelial interactions modulate hepatic response to bile duct ligation. Lab Invest. 2007;87(5):499–514. doi: 10.1038/labinvest.3700537. [DOI] [PubMed] [Google Scholar]
- 47.Qiu J, Zhou Q, Zhai X, Jia X, Zhou Y. Curcumin regulates delta-like homolog 1 expression in activated hepatic stellate cell. Eur J Pharmacol. 2014;728:9–15. doi: 10.1016/j.ejphar.2014.01.074. [DOI] [PubMed] [Google Scholar]
- 48.Michelotti GA, Xie G, Swiderska M, et al. Smoothened is a master regulator of adult liver repair. J Clin Invest. 2013;123(6):2380–2394. doi: 10.1172/JCI66904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kisseleva T, Cong M, Paik Y, et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci U S A. 2012;109(24):9448–9453. doi: 10.1073/pnas.1201840109. [DOI] [PMC free article] [PubMed] [Google Scholar]