Abstract
This phase I trial evaluated two schedules of escalating vorinostat in combination with decitabine every 28 days: (i) sequential or (ii) concurrent. There were three dose-limiting toxicities: grade 3 fatigue and generalized muscle weakness on the sequential schedule (n = 1) and grade 3 fatigue on the concurrent schedule (n = 2). The maximum tolerated dose was not reached on both planned schedules. The overall response rate (ORR) was 23% (three complete response [CR], two CR with incomplete incomplete blood count recovery [CRi], one partial response [PR] and two morphological leukemic free state [MLFS]). The ORR for all and previously untreated patients in the sequential arm was 13% (one CRi; one MLFS) and 0% compared to 30% (three CR; one CRi; one PR; one MLFS) and 36% in the concurrent arm (p = 0.26 for both), respectively. Decitabine plus vorinostat was safe and has clinical activity in patients with previously untreated acute myeloid leukemia. Responses appear higher with the concurrent dose schedule. Cumulative toxicities may limit long-term usage on the current dose/schedules.
Keywords: Decitabine, vorinostat, hypomethylating agent, histone deacetylase inhibitor, acute myeloid leukemia
Introduction
The development and progression of cancer is due to both genetic and epigenetic changes in the malignant cell. A large number of genes are silenced by aberrant DNA methylation and/or histone deacetylation in different types of cancers, many of which are involved in the control of cell cycle progression, apoptosis, tissue invasion and metastasis, and genomic stability [1–3]. Aberrant histone deacetylase (HDAC) activity has been implicated in a variety of cancers. However, HDACs not only deacetylate histones to regulate gene transcription, they also affect the acetylation status and, thus, the function of non-histone proteins, such as the tumor suppressor p53, heat-shock protein 90 (hsp90), signal transducer and activator of transcription (STAT3) and the DNA-damage associated protein Ku70 [4]. Similarly, aberrant DNA methylation may also lead to genomic instability. While the mechanism behind this is unknown, it may involve increased activity or abnormal regulation of the DNA methyltransferases (DNMTs), which are responsible for DNA methylation of cytosine residues in CpG islands [5–7], and/or impaired function of a repair enzyme, such as a DNA demethylase [8,9].
Decitabine (5-aza-2’-deoxycytidine, Dacogen®; Eisai Co., Ltd.), a DNMT inhibitor, is currently approved by the United States Food and Drug Administration for the treatment of patients with myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML) with 20–30% bone marrow blasts by World Health Organization (WHO) classification [10,11] and the European Medicines Agency (EMA) for untreated older patients with AML with ≥ 20% marrow blasts [12]. Three multicenter trials (two phase II and one phase III) have reported overall response rates (ORRs) of 25–30% (complete response [CR] rate 13–25%) with single agent decitabine [12–14]. Similar to what has been observed in patients with MDS [15–18], higher CR rates appear to be achieved in patients with AML using a 5-day compared to a 3-day dosing regimen [13,14]. In a multicenter, randomized phase III trial comparing a 5-day dosing regimen of decitabine with best supportive and/or low dose cytarabine in older patients with intermediate- or poor-risk AML, higher response rates (CR plus CR with incomplete platelet recovery [CRp]) were obtained with decitabine (18% vs. 8%; p = 0.001) [12].
Biomarker analyses evaluating drug effect and predictors of response have been performed, such as gene-specific (e.g. p15 and estrogen receptor promoters) and genome-wide (e.g. long interspersed nucleotide element [LINE]) methylation during decitabine therapy. Although there was dose-dependent hypomethylation after therapy with decitabine at low doses, the degree of methylation of Alu repetitive elements, LINE repetitive elements and/or total 5-methylcytosine content (as surrogate markers for global methylation) did not correlate with response [15,19]. Similarly, promoter methylation status of the p15 gene did not correlate with response [15,19,20]; however, p15 expression levels at days 12 and 28 after therapy were significantly higher in responders than in non-responders [15]. More recently, the presence of DNMT3A mutations and higher pretreatment levels of miR-29b, a microRNA that down-regulates DNMT1, 3A and 3B expression [21,22], have been associated with clinical response to decitabine therapy [23,24].
A number of HDAC inhibitors have been used in patients with relapsed and/or refractory AML with modest activity [25–30]. Vorinostat (suberoylanilide hydroxamic acid, Zolinza®; Merck & Co., Inc.), a HDAC inhibitor, is approved for the treatment of skin manifestations of cutaneous T-cell lymphoma (CTCL) [31,32]. Single agent vorinostat has yielded responses (CR and/or CR with incomplete blood count recovery [CRi]) of 4.5–10% in patients with AML and/or MDS [25,26]. Increased histone acetylation was observed at all dose levels (DLs) in the phase I study [25]. Gene expression analysis indicated that transcript levels of proliferation-associated genes were down-regulated after therapy in the peripheral blood samples of patients with AML who had a hematologic improvement or response [25]. Furthermore, increased expression of an antioxidant gene signature at baseline was associated with vorinostat resistance [25].
In an attempt to improve response rates, quality of responses and duration of responses, and to minimize toxicities (by lowering doses) observed with single agent hypomethylating agents, DNMT inhibitors have been combined with a variety of agents. Hypomethylating agents have been shown to have additive or synergistic effects with HDAC inhibitors in reactivating epigenetically silenced genes and inducing apoptosis, differentiation and/or cell growth arrest in various cancer cell lines and primary samples [33–40]. Although transcriptional silencing is mediated by both methylation and histone deacetylase activity, it is currently not known which alteration is dominant [33,41–43]. Hence, it is unclear whether the sequence of administration of hypomethylating agents and HDAC inhibitors impacts on efficacy. Furthermore, the optimal dosing and scheduling of multiple dosage decitabine in combination with multiple doses of a HDAC inhibitor have not been established.
Therefore, a phase I study was conducted to determine the safety, tolerability and activity of vorinostat administered with decitabine in two different dose schedules: (i) sequential administration of decitabine followed by vorinostat, and (ii) concurrent administration of decitabine and vorinostat in patients with relapsed or refractory AML.
Patients and methods
Patients
Patients with histologically confirmed relapsed or refractory AML were eligible for enrollment. Patients with untreated AML were also considered eligible if they refused induction chemotherapy, had medical conditions that rendered them unfit to receive standard curative therapy as determined by their treating physician or required an allogeneic stem cell transplant (alloSCT) but lacked an appropriate donor. Other inclusion criteria were Eastern Cooperative Group (ECOG) performance status ≤ 2, adequate hepatorenal function (total bilirubin within normal limits, alanine transaminase [ALT] or aspartate transaminase [AST] ≤ 2.5 times the upper limit of normal [ULN], creatinine ≤ 150 μmol/L). Patients with cerebrospinal fluid (CSF) involvement were allowed to participate and could receive concurrent intrathecal chemotherapy. Women of childbearing potential must have had a negative pregnancy test and must not have been breast-feeding.
Exclusion criteria included systemic chemotherapy or radiotherapy within 3 weeks prior to entering the study, valproic acid or other HDAC inhibitors within 2 weeks prior to study enrollment, concurrent administration of cytotoxic and/or investigational agents (except hydroxyurea which was permitted up to 24 h prior to starting study medications), allergic reactions attributable to compounds of similar chemical or biologic composition to vorinostat, decitabine or any of their excipients, and serious medical or psychiatric conditions that could interfere with treatment. Prior use of decitabine was not permitted. All patients had to practice effective birth control and give written informed consent indicating that they were aware of the investigational nature of the study. All study procedures and informed consent forms were approved by the institutional review boards of the participating centers in accordance with federal and institutional guidelines.
Treatment protocol
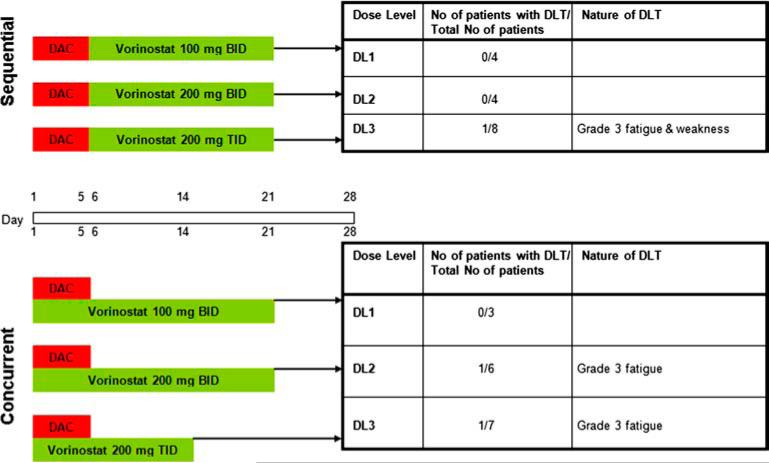
This was an open-label, phase I study evaluating two different dose schedules of vorinostat administered with decitabine: (i) sequential administration of decitabine followed by vorinostat, and (ii) concurrent administration of decitabine and vorinostat in patients with relapsed or refractory AML. The study began with dose escalation on the sequential schedule, and once the maximum tolerated dose (MTD) was established, accrual began on the concurrent schedule. In both the sequential and concurrent schedules, a fixed low dose of decitabine, 20 mg/m2/day, was administered as a 1 h intravenous infusion on days 1–5 (Figure 1). Patients received escalating doses of oral vorinostat administered either sequentially (100 mg twice a day [bid], 200 mg bid or 200 mg three times a day [tid] on days 6–21) or concurrently (100 mg or 200 mg bid on days 1–21 or 200 mg tid on days 1–14) with decitabine every 28 days. The dose/schedule of decitabine was selected as optimal based on previous data demonstrating good tolerability and high response rates in patients with MDS [15]. The starting dose of vorinostat was chosen based on previous studies showing that the MTD was 200 mg bid or 250 mg tid for 14 consecutive days every 21 days in patients with AML and MDS [25]. Based on data suggesting that alterations in methylation are dominant over changes in histone deacetylation [33,41,43], vorinostat was administered after completion of the infusion of decitabine, on days when both study drugs were given. Patients could receive supportive care measures as clinically indicated. Other anticancer agents, including hydroxyurea, were prohibited while on study.
Figure 1.
Dose escalation scheme for sequential and concurrent schedules and specification of dose-limiting toxicities (DLTs) at each dose level. DAC, decitabine.
A minimum of four cycles of therapy were planned. Patients with persistent disease could receive their next cycle of treatment regardless of peripheral blood counts. For those with CR, an absolute neutrophil count ≥ 1.0 × 109/L and platelet count ≥ 50 × 109/L were required. Treatment was stopped if any of the following events occurred: disease progression requiring the administration of hydroxyurea or cytotoxic chemotherapy, unacceptable toxicity, intercurrent illness or change in the patient's condition that rendered the patient unsuitable for further therapy in the judgement of the investigator, or patient withdrawal from the study.
Dose escalation proceeded on a standard 3 + 3 dose escalation design. Briefly, three patients were enrolled at a given DL. If two or more experienced a dose-limiting toxicity (DLT), escalation was stopped and three additional patients were enrolled at the next lowest dose. If one of the initial three patients at a given DL experienced a DLT, an additional three patients were enrolled at the same DL. Escalation proceeded if none of the initial three patients or no more than one of six patients at a given level experienced a DLT. The MTD was defined as the highest DL in which fewer than two of six patients developed first course DLT. This cohort could then be expanded by recruitment of up to four more (for a total of 10) patients to allow for further evaluation of toxicity at that level. Cumulative safety information will be considered for the final determination of the recommended phase II dose (RP2D), which may differ from the MTD.
Dose modification
Treatment was withheld in patients with National Cancer Institute Common Toxicity Criteria (NCI-CTC) grade 3 or more non-hematologic toxicity, except for alopecia or nausea and vomiting, diarrhea or biochemical abnormalities controlled by supportive care, until the side effects diminished to grade 1 or better or baseline. After resolution, treatment was resumed at the next lower DL. Dose interruption or reduction was permitted for patients with subjective, persistent, intolerable grade 2 toxicity not controlled by supportive therapy. Patients were removed from study if they developed treatment-related toxicities, leading to treatment interruption for greater than 3 weeks.
Assessment of toxicity and response
Baseline evaluation included medical history, vital signs, performance status of the patient, clinical examination, concurrent medications, bone marrow aspirate and/or biopsy, complete blood count (CBC) and differential, biochemistry, electrocardiogram, coagulation profile and serum pregnancy test if applicable. Patients were evaluated prior to the start of each cycle of therapy with repeat CBC and differential, and biochemistry. Bone marrow aspirate and/or biopsy were performed after each cycle of therapy, as clinically indicated, and at the time of coming off study. Patients completed a patient diary and returned the empty bottles and unused vorinostat at the end of each treatment cycle.
All patients were evaluable for toxicity if they received at least one dose of decitabine. Adverse events were assessed at each visit and graded according to the NCI-CTC Version 3.0. DLTs were assessed during the first cycle of therapy. Hematologic DLT was defined as myelosuppression with bone marrow hypoplasia (cellularity ≤ 5%) without evidence of leukemia for ≥ 42 days. Non-hematologic DLT was defined as any grade 3 or 4 toxicity except for the following: alopecia, grade 3 or 4 nausea or vomiting responsive to antiemetics, diarrhea controlled with medications and electrolyte abnormalities correctable with supportive therapy. Also included were any clinically significant grade 2 toxicities requiring treatment delay for > 2 weeks.
Patients were assessable for response if they completed at least one cycle of therapy. Responses were evaluated according to the revised guidelines of the International Working Group (IWG) for patients with AML [44]. Remission duration was calculated from the date of first response until relapse.
Pharmacokinetics analysis
Blood samples for evaluation of decitabine were collected on days 1 and 5 of cycle 1, before dosing, 30 min after the infusion had started, at the end of infusion and at 5, 20, 35, 45 and 60 min from the end of infusion. Blood was collected into a sodium heparin Vacutainer tube and centrifuged at 1500 × g for 15 min. The resulting plasma was transferred into polypropylene tubes and stored at − 70°C until analyzed for decitabine concentration, using a validated high performance liquid chromatography with tandem mass spectrometry (LC-MS/MS) [45]. On the sequential schedule, blood samples for vorinostat were collected on day 9 of cycle 1 before dosing and at 0.5, 1, 2, 2.5, 3, 4, 6 and 8 h after dosing. On the concurrent schedule, blood samples were collected on days 4 and 15 of cycle 1 at the same time points. Samples were allowed to clot at 4°C for 20–30 min, and then centrifuged at 2000 × g for 15 min at 4°C. The resulting serum was transferred to polypropylene cryotubes and stored at − 70°C until analyzed for vorinostat concentrations with a validated LC-MS/MS assay [46]. Pharmacokinetics (PK) parameters were calculated by non-compartmental methods using Win-Nonlin (Version 5.2; Pharsight Corp.)
Exploratory analyses were performed for PK parameters and adjustments made for multiple comparisons. t-Tests were used for independent group comparisons and paired t-tests were used to compare PK parameters of decitabine on day 1 versus day 5 within a DL.
Results
Study group
A total of 36 patients with AML were entered onto the study between March 2006 and February 2008. Patient characteristics are listed in Table I. Of those patients, 16 were treated on the sequential schedule and 20 on the concurrent schedule. Eight patients had received three or more treatment regimens for their disease, including four patients who received an alloSCT. No patients had received prior therapy with a HDAC inhibitor. Eighteen patients had not received prior therapy for their disease, including four patients who received only hydroxyurea: five on the sequential arm and 13 on the concurrent arm. During the course of the study, emerging data indicated that higher response rates were obtained when decitabine was administered to previously untreated patients; hence, a concerted effort was made to enroll untreated patients with AML on the concurrent schedule. Of the 21 patients with cytogenetic analyses available, two had favorable core-binding factor mutations, nine intermediate-risk cytogenetics (including five with normal karyotype) and 10 poor-risk cytogenetics (including four monosomal karyotypes).
Table I.
Patient characteristics.
| Characteristic | All (n = 36) | Sequential (n = 16) | Concurrent (n = 20) |
|---|---|---|---|
| Age, years, median (range) | 69 (32–82) | 67 (41–82) | 71.5 (32–82) |
| > 60 years | 26 (72) | 10 (62) | 16 (80) |
| Gender (%) | |||
| Female | 22 (61) | 7 (44) | 7 (35) |
| Male | 14 (39) | 9 (56) | 13 (65) |
| Diagnosis (%) | |||
| AML | 36 (100) | 16 (100) | 20 (100) |
| Prior MDS or MPD | 14 (39) | 8 (50) | 6 (30) |
| Treatment-related | 3 (8) | 1 (6) | 2 (10) |
| Duration of disease, months, median (range) | 2.8 (0.4–49.7) | 4.4 (0.6–15.3) | 1.4 (0.4–49.7) |
| ECOG performance status, median (range) | 1 (0–2) | 1 (1–2) | 1 (0–1) |
| 0–1 (%) | 33 (92) | 13 (81) | 20 (100) |
| 2 (%) | 3 (8) | 3 (19) | 0 |
| Median prior treatment regimens (range) | 1 (0–4) | 1 (0–4) | 0 (0–4) |
| Prior therapy (%) | |||
| Untreated | 14 (39) | 4 (25) | 10 (50) |
| Hydroxyurea | 4 (11) | 1 (6) | 3 (15) |
| Chemotherapy | 13 (36) | 11 (69) | 2 (10) |
| Targeted therapy | 2 (6) | 0 | 2 (10) |
| Allogeneic stem cell transplant | 3 (8) | 0 | 3 (15) |
| Primary refractory AML (%) | 6 (17) | 6 (38) | 0 |
| Relapsed AML (%) | 11 (31) | 5 (31) | 6 (30) |
| Cytogenetic risk (%) | |||
| Favorable | 2 (6) | 1 (6) | 1 (5) |
| Intermediate | 9 (25) | 3 (19) | 5 (25) |
| Poor | 10 (28) | 4 (25) | 7 (35) |
| Inconclusive/not available | 15 (42) | 8 (50) | 7 (35) |
| Baseline white blood cell count (× 109/L), median (range) | 3.4 (0.6–76.6) | 3.5 (0.7–74.6) | 3.2 (0.6–76.6) |
| Baseline platelet count (× 109/L), median (range) | 41 (3–391) | 25 (3–374) | 58 (9–391) |
| Baseline peripheral blast count (× 109/L L), median (range) | 0.26 (0–75.1) | 0.52 (0–66.4) | 0.26 (0–75.1) |
AML, acute myeloid leukemia; MDS, myelodysplastic syndromes; MPD, myeloproliferative disorder; ECOG, Eastern Cooperative Group.
Toxicities
All 36 patients were evaluable for toxicity. The frequency and grading of treatment-related adverse effects observed during cycle 1 and for cycles 2 and beyond (reported as percentages of patients) are summarized in Tables II–V, respectively. All patients experienced adverse events. The most common toxicities observed during the first cycle of therapy and at any time during the study were fatigue, nausea, diarrhea, anorexia and vomiting. Toxicities were similar in patients receiving treatment on the sequential or concurrent arm. Two patients died while on study due to sepsis and/or pneumonia.
Table II.
Non-hematologic toxicities possibly attributable to decitabine and/or vorinostat during cycle 1: sequential schedule (n = 16).
| Dose level 1 (n = 4) |
Dose level 2 (n = 4) |
Dose level 3 (n = 8) |
All (n = 16) |
|||||
|---|---|---|---|---|---|---|---|---|
| Adverse effect | All grades, no. (%) | Grade 3/4, no. (%) | All grades, no. (%) | Grade 3/4, no. (%) | All grades, no. (%) | Grade 3.4, no. (%) | All grades, no. (%) | Grade 3/4, no. (%) |
| Fatigue | 3 (75) | 0 | 2 (50) | 0 | 5 (62) | 1 (12) | 10 (62) | 1 (6) |
| Nausea | 2 (50) | 0 | 3 (75) | 0 | 7 (88) | 0 | 12 (75) | 0 |
| Diarrhea | 1 (25) | 0 | 3 (75) | 0 | 5 (62) | 0 | 9 (56) | 0 |
| Vomiting | 0 | 0 | 1 (25) | 0 | 5 (62) | 0 | 6 (38) | 0 |
| Anorexia | 1 (25) | 0 | 1 (25) | 0 | 1 (12) | 0 | 3 (18) | 0 |
| Abdominal pain | 0 | 0 | 0 | 0 | 1 (12) | 0 | 1 (6) | 0 |
| Constipation | 1 (25) | 0 | 1 (25) | 0 | 0 | 0 | 2 (12) | 0 |
| Dehydration | 0 | 0 | 0 | 0 | 1 (12) | 0 | 1 (6) | 0 |
| Dizziness | 1 (25) | 0 | 0 | 0 | 1 (12) | 0 | 2 (12) | 0 |
| Dyspepsia | 2 (50) | 0 | 1 (25) | 0 | 1 (12) | 0 | 4 (25) | 0 |
| Hyperglycemia | 1 (25) | 0 | 1 (25) | 0 | 0 | 0 | 2 (12) | 0 |
| Hyponatremia | 2 (50) | 0 | 0 | 0 | 0 | 0 | 2 (12) | 0 |
| Generalized muscle weakness | 1 (25) | 0 | 0 | 0 | 1 (12) | 1 (12) | 2 (12) | 1 (6) |
Table IV.
Non-hematologic toxicities possibly attributable to decitabine and/or vorinostat during cycles 2 and higher: sequential schedule (n = 16).
| Dose level 1 (n = 4) |
Dose level 2 (n = 4) |
Dose level 3 (n = 8) |
All (n = 16) |
|||||
|---|---|---|---|---|---|---|---|---|
| Adverse effect | All grades, no. (%) | Grade 3/4, no. (%) | All grades, no. (%) | Grade 3/4, no. (%) | All grades, no. (%) | Grade 3/4, no. (%) | All grades, no. (%) | Grade 3/4, no. (%) |
| Nausea | 2 (50) | 0 | 3 (75) | 1 (25) | 5 (62) | 0 | 10 (62) | 1 (6) |
| Fatigue | 3 (75) | 1 (25) | 2 (50) | 0 | 2 (25) | 1 (12) | 7 (44) | 2 (12) |
| Diarrhea | 0 | 0 | 3 (75) | 1 (25) | 3 (38) | 0 | 6 (38) | 1 (6) |
| Vomiting | 1 (25) | 0 | 2 (50) | 1 (25) | 2 (25) | 1 (12) | 5 (31) | 2 (12) |
| Anorexia | 1 (25) | 0 | 2 (50) | 0 | 3 (38) | 1 (12) | 6 (38) | 1 (6) |
| Mucositis | 1 (25) | 0 | 1 (25) | 0 | 2 (25) | 0 | 4 (25) | 0 |
| Dyspepsia | 1 (25) | 0 | 0 | 0 | 1 (12) | 0 | 2 (12) | 0 |
| Headache | 2 (50) | 0 | 1 (25) | 1 (25) | 1 (12) | 0 | 4 (25) | 1 (6) |
| Abdominal pain | 0 | 0 | 1 (25) | 0 | 1 (12) | 0 | 2 (12) | 0 |
| Infections | 0 | 0 | 1 (25) | 1 (25) | 1 (12) | 0 | 2 (12) | 1 (6) |
| Dehydration | 0 | 0 | 0 | 0 | 1 (12) | 0 | 1 (6) | 0 |
| Hyperglycemia | 0 | 0 | 1 (25) | 0 | 0 | 0 | 1 (6) | 0 |
| Weight loss | 0 | 0 | 0 | 0 | 2 (25) | 0 | 2 (12) | 0 |
To be evaluable for DLT, patients must have completed at least one cycle of therapy and been observed for at least 28 days or have experienced a DLT before day 28. Four patients did not complete one cycle of therapy on the sequential schedule and were replaced: one patient each on DL1 and DL2 (due to progressive disease requiring cytotoxic therapy) and two patients on DL3 (one due to non-compliance and one due to sepsis). As depicted in Figure 1, one DLT of grade 3 fatigue and generalized muscle weakness was observed on DL3. This patient died while on the first cycle of therapy due to pneumonia and sepsis. One patient on DL3 of the concurrent schedule did not complete one cycle of therapy (due to progressive disease) and was replaced. Two patients developed DLTs of grade 3 fatigue on DL2 and DL3 of the concurrent schedule (Figure 1).
The MTD was not reached on both the planned sequential and concurrent schedules. Dose delays and dose reductions of vorinostat due to toxicities occurred in seven patients: two in the sequential arm (one DL1 and one DL3) and five in the concurrent arm (three DL2 and two DL3). No dose reductions occurred with decitabine. Two additional patients on DL3 of the concurrent schedule developed grade 3 fatigue on cycle 2, while a third patient developed grade 3 anorexia and dehydration on cycle 3. Another two patients on DL3 of the concurrent schedule described grade 2 fatigue that necessitated short delays in treatment. As a result, the decision was made to halt further study at DL3 and expand enrollment to four more patients on DL2 of the concurrent schedule in order to obtain more safety and pharmacodynamics data. A total of three of 10 patients (30%) treated at DL2 of the concurrent schedule developed a DLT (grade 3 fatigue). Prior studies have demonstrated that continuous therapy with hypomethylating agents is required to achieve and maintain any response. However, given the concern of chronic toxicities (i.e. ongoing nausea, vomiting and fatigue) and tolerability with repetitive cycles of decitabine and vorinostat with the current dose/schedules, the RP2D is not well-defined in this study population; only 10 patients were treated on DL2 of the concurrent arm, with just four patients having received > 4 cycles of therapy (range, 1–11).
Response to treatment
Patients completed a total of 159 cycles of therapy, with a median of 2 cycles (range, 0–23). Seventeen patients (47%) received three or more cycles of therapy (13 of whom received four or more cycles of therapy). Patients in the sequential group completed a median of 2 cycles (range, 0–13) and those in the concurrent group 4 (range, 0–23) (p = 0.44). A higher proportion of patients in the concurrent group received four or more cycles of therapy compared with the sequential group (50% vs. 20%, p = 0.09). One patient was taken off study before completing one cycle of therapy due to non-compliance. Therefore, 35 patients were evaluable for response.
The ORR was 23% (eight of 35), with three CRs, two CRis, one partial response (PR) and two morphological leukemic free states (MLFS) (Tables VI and VII). This included five (29%) of the 17 previously untreated evaluable patients enrolled on the study, four of whom had received prior hydroxyurea for count control. The ORR for all and previously untreated patients in the sequential arm was 13% (one CRi and one MLFS) and 0% compared to 30% (three CR, one CRi, one PR and one MLFS) and 36% in the concurrent arm, respectively (p = 0.26 for both). Responses were observed on all DLs except for DL3 on the sequential arm. Median number of cycles to best response was 4.5 (range, 2–12). Median duration of response for patients achieving a CR and CRi was 6 months (range, 3.9–23.3 + months). Of the 27 remaining patients, there were three patients who had a reduction in blast counts by 25–51%. All patients eventually relapsed or had disease progression, with the exception of one, who demonstrated a poor recovery of counts after the 23rd cycle of treatment necessitating a treatment delay of more than 3 weeks; therefore, he was taken off study.
Table VI.
Clinical response: sequential schedule (n = 16).
| Dose level | Dose of vorinostat | Patient | No. of prior therapies | Best response | Duration of response (weeks) | No. of cycles to best response | Total no. of cycles completed |
|---|---|---|---|---|---|---|---|
| 1 | 100 mg bid days 6–21 | 901 | 0 | NR | NA | NA | 0 |
| 902 | 1 | NR | NA | NA | 2 | ||
| 903 | 2 | CRi | 26.7 | 6 | 12 | ||
| 904 | 1 | NR | NA | NA | 3 | ||
| 2 | 200 mg bid days 6–21 | 905 | 3 | NR | NA | NA | 0 |
| 906 | 0 | NR | NA | NA | 2 | ||
| 907 | 1 | MLFS | 16.3 | 12 | 13 | ||
| 908 | 3 | NR | NA | NA | 2 | ||
| 3 | 200 mg tid days 6–21 | 909 | 0 | NR | NA | NA | 0 |
| 910 | 1 | NR | NA | NA | 3 | ||
| 911 | 1 | NR | NA | NA | 2 | ||
| 912 | 3 | NR | NA | NA | 3 | ||
| 913 | 0 | NR | NA | NA | 10 | ||
| 914 | 0 | NR | NA | NA | 1 | ||
| 915 | 4 | NR | NA | NA | 0 | ||
| 918 | 1 | NR | NA | NA | 2 |
bid, twice a day; tid, three times a day; NR, no response; CRi, complete response with incomplete blood count recovery; MLFS, morphologic leukemic free state; NA, not applicable.
Table VII.
Clinical response: concurrent schedule (n = 20).
| Dose level | Dose of vorinostat | Patient | No. of prior therapies | Best response | Duration of response | No. of cycles to best response | Total no. of cycles received |
|---|---|---|---|---|---|---|---|
| 1 | 100 mg bid days 1–21 | 916 | 2 | NR | NA | NA | 1 |
| 917 | 0 | CR | 114.6 | 8 | 23 | ||
| 919 | 0 | PR | 25.6 | 4 | 9 | ||
| 2 | 200 mg bid days 1–21 | 920 | 0 | NR | NA | NA | 0 |
| 921 | 2 | NR | NA | NA | 1 | ||
| 922 | 3 | NR | NA | NA | 8 | ||
| 923 | 0 | CR | 60.3 | 4 | 11 | ||
| 924 | 4 | MLFS | 15.7 | 2 | 5 | ||
| 925 | 3 | NR | NA | NA | 1 | ||
| 933 | 0 | CRi | 22.7 | 5 | 6 | ||
| 934 | 0 | NR | NA | NA | 1 | ||
| 935 | 0 | NR | NA | NA | 2 | ||
| 936 | 0 | NR | NA | NA | 2 | ||
| 3 | 200 mg tid days 1–14 | 926 | 3 | NR | NA | NA | 3 |
| 927 | 0 | CR | 16.8 | 2 | 6 | ||
| 928 | 0 | NR | NA | NA | 0 | ||
| 929 | 0 | NR | NA | NA | 2 | ||
| 930 | 0 | NR | NA | NA | 5 | ||
| 931 | 1 | NR | NA | NA | 13 | ||
| 932 | 0 | NR | NA | NA | 5 |
bid, twice a day; tid, three times a day; NR, no response; CR, complete response; PR, partial remission; MLFS, morphologic leukemic free state; CRi, complete response with incomplete blood count recovery; NA, not applicable.
To determine whether differences in patient characteristics could account for the differences in response rates between the previously untreated patients with AML in the sequential schedule and the concurrent schedule, the t-test and Fisher's exact test were used to assess continuous and categorical patient variables, respectively. The only significant difference was ECOG status. There was no difference in the proportion of patients with de novo versus secondary or therapy-related AML, age, cytogenetic risk group, disease duration or percentage of bone marrow blasts between the two groups. Meaningful multivariate analyses could not be performed due to the small sample size.
Reasons for discontinuation from the study included: disease progression (n = 21), toxicity (n = 5), patient request (n = 3), physician discretion (n = 2), death due to sepsis while on study (n = 2), non-compliance (n = 1), treatment delay for more than 3 weeks due to prolonged myelosuppression (n = 1) and complicating medical condition (n = 1). At the time of data cut-off on 7 November 2011, with a median follow-up of 5.2 months (range, 1.1–37.5 + months) from the time of study entry, 21 patients had died. The median overall survival (OS) for all patients was 7.5 months (95% confidence interval [CI]: 4.1 months–not reached).
Pharmacokinetics analyses
PK results (Table VIII) of decitabine were similar to those reported in previous studies [47]. Decitabine is rapidly eliminated with a half-life of approximately 20 min. No significant differences were found when comparing PK parameters on day 1 and day 5 within the same DL in either schedule.
Table VIII.
Decitabine pharmacokinetics parameters.
| PK parameter | Day 1 | Day 5 |
|---|---|---|
| Sequential schedule: decitabine 20 mg/m2/day (n = 3) | ||
| Mean Cmax (ng/mL) (SD) | 137.1 (126.4) | 129.9 (87.1) |
| Mean AUCt (ng·h/mL) (SD) | 102 (76.1) | 107.1 (67.3) |
| Mean AUCinf (ng·h/mL) (SD) | 105.4 (77.3) | 111 (66.2) |
| Mean t1/2 (h) (SD) | 0.36 (0.05) | 0.42 (0.18) |
| Mean CL (L/h/m2) (SD) | 254.8 (134.3) | 226.2 (121.6) |
| Concurrent schedule: decitabine 20 mg/m2/day (n = 6) | ||
| Mean Cmax (ng/mL) (SD) | 192.6 (164.1) | 235.9 (187.5) |
| Mean AUCt (ng·h/mL) (SD) | 149.1 (95.8) | 165.7 (85.8) |
| Mean AUCinf (ng·h/mL) (SD) | 152 (96.6) | 169.5 (85.2) |
| Mean t1/2 (h) (SD) | 0.36 (0.12) | 0.40 (0.17) |
| Mean CL (L/h/m2) (SD) | 202.8 (153.7) | 139.6 (55.1) |
PK, pharmacokinetics; Cmax, maximum plasma concentration; SD, standard deviation; AUCt, area under the plasma decitabine concentration versus time curve to the last sampling time; AUCinf, area under the plasma decitabine concentration versus time curve from zero to infinity; t½, terminal half-life; CL, clearance.
The PK parameters of vorinostat (Table IX) were similar to those reported previously [48,49]. There was no difference among different levels in volume of distribution and clearance among DLs. Furthermore, no significant differences were found when comparing PK parameters on day 4 and day 9 of the concurrent schedule. As only a small number of patients had PK of either decitabine and/or vorinostat determined, no meaningful correlation of PK with adverse events or responses could be obtained.
Table IX.
Vorinostat pharmacokinetics parameters.
| PK parameter | 100 mg bid days 6–21 (n = 4) | 200 mg bid days 6–21 (n = 4) | 200 mg tid days 6–21 (n = 8) |
|---|---|---|---|
| Sequential schedule | |||
| Mean Cmax (ng/mL) (SD) | 116.8 (82.5) | 125.5 (56.1) | 263.4 (170.8) |
| Mean AUCt (ng·h/mL) (SD) | 276.3 (87.9) | 555.9 (354.7) | 743.2 (421.4) |
| Mean AUCinf (ng·h/mL) (SD) | 352.1 (57.9) | 974.1 (599.2) | 910.4 (633.9) |
| Mean t½ (h) (SD) | 1.23 (0.02) | 3.34 (0.66) | 1.65 (1.02) |
| Mean CL/F (L/min) (SD) | 4.80 (0.79) | 5.57 (5.26) | 5.89 (4.48) |
| 100 mg bid days 1–21 |
200 mg bid days 1–21 |
200 mg tid days 1–14 |
||||
|---|---|---|---|---|---|---|
| Day 4 (n = 3) | Day 15 (n = 1) | Day 4 (n = 10) | Day 15 (n = 7) | Day 4 (n = 7) | Day 15 (n = 0) | |
| Concurrent schedule | ||||||
| Mean Cmax (ng/mL) (SD) | 150.8 (40.5) | 226.3 | 251.5 (96.0) | 245.6 (115.3) | 352.9 (110.9) | NA |
| Mean AUCt (ng·h/mL) (SD) | 399.9 (127.1) | 607.5 | 733.8 (362.9) | 778.5 (401.7) | 1062.9 (262.7) | NA |
| Mean AUCinf (ng·h/mL) (SD) | 406.7 (121.4) | 608.6 | 930.0 (357.8) | 1007.2 (649.1) | 1239.4 (403.5) | NA |
| Mean t½ (h) (SD) | 1.04 (0.52) | 0.62 | 1.94 (0.64) | 2.34 (1.05) | 2.68 (1.93) | NA |
| Mean CL/F (L/min) (SD) | 4.35 (1.26) | 2.74 | 4.02 (1.30) | 4.70 (2.45) | 2.86 (0.81) | NA |
PK, pharmacokinetics; Cmax, maximum plasma concentration; SD, standard deviation; AUCt, area under the plasma decitabine concentration versus time curve to the last sampling time; AUCinf, area under the plasma decitabine concentration versus time curve from zero to infinity; t½, terminal half-life; CL/F, oral clearance.
Discussion
Both decitabine and azacitidine are approved for the treatment of WHO-defined AML with up to 30% blasts [10,50]. In Europe, decitabine is also approved for older untreated patients with AML with > 20% blasts [12]. However, response rates are low, with CR rates of 9–18% in randomized phase III clinical trials [12,15,50].
Although the addition of vorinostat to decitabine did not increase the ORR for all patients treated on the study, it did yield a higher response rate in patients with previously untreated AML compared to historical responses observed with single agent decitabine (29% vs. 18%, respectively) [12]. Furthermore, subgroup analysis demonstrated a higher response rate for patients with previously untreated AML who received decitabine concurrently with vorinostat. This is consistent with observations from a phase I study of sequential decitabine and vorinostat in patients with relapsed and/or refractory acute leukemia and MDS [51]. Increased dose intensity has been associated with improved and prolonged responses in patients receiving hypomethylating agents; however, the median number of courses administered in this study was only 2.
Although transcriptional silencing is mediated by both methylation and histone deacetylase activity, methylation is dominant; therefore, transcription cannot occur without first inhibiting DNA methylation [33,41]. Hence, in preclinical studies, initial treatment has been with a hypomethylating agent followed by a HDAC inhibitor. Preclinical data suggest that the sequence of administration of hypomethylating agents and HDAC inhibitors does not impact efficacy [42]. Clinical studies with hypomethylating agents and HDAC inhibitors have administered the drugs concurrently or sequentially (with administration of the HDAC inhibitor after the hypomethylating agent) [52–54]. However, minimal responses (CR 4%) were observed in a phase I study of sequential decitabine and vorinostat in patients with relapsed and/or refractory acute leukemia and MDS [51]. Similarly, in the present study, despite a higher proportion of patients with untreated AML (including those who received hydroxyurea) receiving treatment on the concurrent schedule compared to the sequential schedule (60% vs. 32%, respectively), concurrent administration of decitabine and vorinostat appears more efficacious than sequential administration.
Confirmatory evidence for the role of vorinostat in combination with decitabine was subsequently evaluated in a separate phase I study evaluating a concurrent and sequential schedule in previously treated and untreated patients with AML and MDS [55]. In this study, once-a-day dosing and shorter durations of vorinostat are being used in an attempt to decrease the risk of cumulative toxicities (predominantly, fatigue and gastrointestinal) associated with this drug combination and, hence, permit prolonged drug administration. Responses were observed more frequently on the concurrent schedule compared with the sequential schedule in all patient groups. However, data on efficacy and optimal dose schedule are limited by the proportion of previously treated and untreated patients enrolled onto the study.
Studies have failed to conclusively demonstrate that the degree of global and/or gene specific DNA hypomethylation and histone deacetylation are markers for clinical response [15,47,52]. Some studies have suggested that earlier expression of certain genes (e.g. estrogen receptor [ER]) or higher levels of gene expression (e.g. p15) are associated with achievement of a CR and can serve as a biomarker of response [15,47]. It will be important to determine whether the addition of a deacetylase inhibitor to decitabine increases not only the degree of DNA hypomethylation but also gene re-expression and whether this correlates with response.
In summary, the combination of decitabine and vorinostat has activity in patients with previously untreated AML. Responses appear higher with the concurrent schedule. However, given the concern of chronic toxicities and tolerability with repetitive cycles of decitabine 20 mg/m2/day on days 1–5 and vorinostat 200 mg bid on days 1–21 every 28 days, the RP2D is not well defined in this study population. Hence, exploration of the agents administered at different doses and schedules is warranted.
Table III.
Non-hematologic toxicities possibly attributable to decitabine and/or vorinostat during cycle 1: concurrent schedule (n = 20).
| Dose level 1 (n = 3) |
Dose level 2 (n = 10) |
Dose level 3 (n = 7) |
All (n = 20) |
|||||
|---|---|---|---|---|---|---|---|---|
| Adverse effect | All grades, no. (%) | Grade 3/4, no. (%) | All grades, no. (%) | Grade 3/4, no. (%) | All grades, no. (%) | Grade 3/4, no. (%) | All grades, no. (%) | Grade 3/4, no. (%) |
| Fatigue | 1 (33) | 0 | 7 (70) | 3 (30) | 5 (71) | 1 (14) | 13 (65) | 4 (20) |
| Nausea | 1 (33) | 0 | 7 (70) | 0 | 5 (71) | 0 | 13 (65) | 0 |
| Diarrhea | 1 (33) | 0 | 6 (60) | 0 | 4 (57) | 1 (14) | 11 (55) | 1 (5) |
| Vomiting | 0 | 0 | 7 (70) | 0 | 2 (28) | 0 | 9 (45) | 0 |
| Anorexia | 0 | 0 | 6 (60) | 0 | 2 (28) | 0 | 8 (40) | 0 |
| Abdominal pain | 0 | 0 | 2 (20) | 0 | 1 (14) | 0 | 3 (15) | 0 |
| Constipation | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dehydration | 0 | 0 | 2 (20) | 0 | 0 | 0 | 2 (10) | 0 |
| Dizziness | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dyspepsia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hyperglycemia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hyponatremia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Generalized muscle weakness | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Table V.
Non-hematologic toxicities possibly attributable to decitabine and/or vorinostat during cycles 2 and higher: concurrent schedule (n = 20).
| Dose level 1 (n = 3) |
Dose level 2 (n = 10) |
Dose level 3 (n = 7) |
All (n = 20) |
|||||
|---|---|---|---|---|---|---|---|---|
| Adverse effect | All grades, no. (%) | Grade 3/4, no. (%) | All grades, no. (%) | Grade 3/4, no. (%) | All grades, no. (%) | Grade 3/4, no. (%) | All grades, no. (%) | Grade 3/4, no. (%) |
| Nausea | 1 (33) | 0 | 4 (40) | 0 | 4 (57) | 1 (14) | 9 (45) | 1 (5) |
| Fatigue | 0 | 0 | 4 (40) | 1 (10) | 5 (71) | 2 (28) | 9 (45) | 3 (15) |
| Diarrhea | 1 (33) | 0 | 4 (40) | 0 | 3 (43) | 0 | 8 (40) | 0 |
| Vomiting | 0 | 0 | 2 (20) | 0 | 4 (57) | 0 | 6 (30) | 0 |
| Anorexia | 0 | 0 | 3 (30) | 0 | 5 (71) | 1 (14) | 8 (40) | 1 (5) |
| Mucositis | 1 (33) | 0 | 1 (10) | 0 | 1 (14) | 0 | 3 (15) | 0 |
| Dyspepsia | 1 (33) | 0 | 0 | 0 | 1 (14) | 0 | 2 (10) | 0 |
| Headache | 0 | 0 | 1 (10) | 0 | 1 (14) | 0 | 2 (10) | 0 |
| Abdominal pain | 0 | 0 | 1 (10) | 0 | 2 (28) | 0 | 3 (15) | 0 |
| Infections | 0 | 0 | 2 (20) | 1 (10) | 3 (43) | 1 (14) | 5 (25) | 2 (10) |
| Dehydration | 0 | 0 | 1 (10) | 0 | 1 (14) | 1 (14) | 2 (10) | 1 (5) |
| Hyperglycemia | 0 | 0 | 2 (20) | 2 (20) | 0 | 0 | 2 (10) | 2 (10) |
| Weight loss | 0 | 0 | 1 (10) | 0 | 1 (14) | 0 | 2 (10) | 0 |
Acknowledgement
This work was supported by the NCI Contract Number: N01-CM-62203 and U01-CA-132123.
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
References
- 1.Costello JF, Fruhwald MC, Smiraglia DJ, et al. Aberrant CpG-island methylation has non-random and tumour-type-specific patterns. Nat Genet. 2000;24:132–138. doi: 10.1038/72785. [DOI] [PubMed] [Google Scholar]
- 2.Shi H, Wei SH, Leu YW, et al. Triple analysis of the cancer epigenome:an integrated microarray system for assessing gene expression, DNA methylation, and histone acetylation. Cancer Res. 2003;63:2164–2171. [PubMed] [Google Scholar]
- 3.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 4.Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006;6:38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- 5.Mizuno S, Chijiwa T, Okamura T, et al. Expression of DNA methyltransferases DNMT1, 3A, and 3B in normal hematopoiesis and in acute and chronic myelogenous leukemia. Blood. 2001;97:1172–1179. doi: 10.1182/blood.v97.5.1172. [DOI] [PubMed] [Google Scholar]
- 6.Fournel M, Sapieha P, Beaulieu N, et al. Down-regulation of human DNA-(cytosine-5) methyltransferase induces cell cycle regulators p16(ink4A) and p21(WAF/Cip1) by distinct mechanisms. J Biol Chem. 1999;274:24250–24256. doi: 10.1074/jbc.274.34.24250. [DOI] [PubMed] [Google Scholar]
- 7.Bakin AV, Curran T. Role of DNA 5-methylcytosine transferase in cell transformation by fos. Science. 1999;283:387–390. doi: 10.1126/science.283.5400.387. [DOI] [PubMed] [Google Scholar]
- 8.Patra SK, Patra A, Zhao H, et al. DNA methyltransferase and demethylase in human prostate cancer. Mol Carcinog. 2002;33:163–171. doi: 10.1002/mc.10033. [DOI] [PubMed] [Google Scholar]
- 9.Kanai Y, Ushijima S, Nakanishi Y, et al. Reduced mRNA expression of the DNA demethylase, MBD2, in human colorectal and stomach cancers. Biochem Biophys Res Commun. 1999;264:962–966. doi: 10.1006/bbrc.1999.1613. [DOI] [PubMed] [Google Scholar]
- 10.Gore SD, Jones C, Kirkpatrick P. Decitabine. Nat Rev Drug Discov. 2006;5:891–892. doi: 10.1038/nrd2180. [DOI] [PubMed] [Google Scholar]
- 11.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 12.Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30:2670–2677. doi: 10.1200/JCO.2011.38.9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lubbert M, Ruter B, Claus R, et al. A multicenter phase II trial of decitabine as first-line treatment of older AML patients judged unfit for induction chemotherapy. Haematologica. 2012;97:393–401. doi: 10.3324/haematol.2011.048231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cashen AF, Schiller GJ, O'Donnell MR, et al. Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J Clin Oncol. 2010;28:556–561. doi: 10.1200/JCO.2009.23.9178. [DOI] [PubMed] [Google Scholar]
- 15.Kantarjian H, Oki Y, Garcia-Manero G, et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 2007;109:52–57. doi: 10.1182/blood-2006-05-021162. [DOI] [PubMed] [Google Scholar]
- 16.Steensma DP, Baer MR, Slack JL, et al. Multicenter study of decitabine administered daily for 5 days every 4 weeks to adults with myelodysplastic syndromes: the alternative dosing for outpatient treatment (ADOPT) trial. J Clin Oncol. 2009;27:3842–3848. doi: 10.1200/JCO.2008.19.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lubbert M, Suciu S, Baila L, et al. Low-dose decitabine versus best supportive care in elderly patients with intermediate- or high-risk myelodysplastic syndrome (MDS) ineligible for intensive chemotherapy: final results of the randomized phase III study of the European Organisation for Research and Treatment of Cancer Leukemia Group and the German MDS Study Group. J Clin Oncol. 2011;29:1987–1996. doi: 10.1200/JCO.2010.30.9245. [DOI] [PubMed] [Google Scholar]
- 18.Kantarjian H, Issa JP, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes:results of a phase III randomized study. Cancer. 2006;106:1794–1803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 19.Yang AS, Doshi KD, Choi SW, et al. DNA methylation changes after 5-aza-2′-deoxycytidine therapy in patients with leukemia. Cancer Res. 2006;66:5495–5503. doi: 10.1158/0008-5472.CAN-05-2385. [DOI] [PubMed] [Google Scholar]
- 20.Issa JP, Garcia-Manero G, Giles FJ, et al. Phase 1 study of low-dose prolonged exposure schedules of the hypomethylating agent 5-aza-2′-deoxycytidine (decitabine) in hematopoietic malignancies. Blood. 2004;103:1635–1640. doi: 10.1182/blood-2003-03-0687. [DOI] [PubMed] [Google Scholar]
- 21.Garzon R, Liu S, Fabbri M, et al. MicroRNA-29b induces global DNA hypomethylation and tumor suppressor gene reexpression in acute myeloid leukemia by targeting directly DNMT3A and 3B and indirectly DNMT1. Blood. 2009;113:6411–6418. doi: 10.1182/blood-2008-07-170589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fabbri M, Garzon R, Cimmino A, et al. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc Natl Acad Sci USA. 2007;104:15805–15810. doi: 10.1073/pnas.0707628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blum W, Garzon R, Klisovic RB, et al. Clinical response and miR-29b predictive significance in older AML patients treated with a 10-day schedule of decitabine. Proc Natl Acad Sci USA. 2010;107:7473–7478. doi: 10.1073/pnas.1002650107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Metzeler KH, Walker A, Geyer S, et al. DNMT3A mutations and response to the hypomethylating agent decitabine in acute myeloid leukemia. Leukemia. 2012;26:1106–1107. doi: 10.1038/leu.2011.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Manero G, Yang H, Bueso-Ramos C, et al. Phase 1 study of the histone deacetylase inhibitor vorinostat (suberoylanilide hydroxamic acid [SAHA]) in patients with advanced leukemias and myelodysplastic syndromes. Blood. 2008;111:1060–1066. doi: 10.1182/blood-2007-06-098061. [DOI] [PubMed] [Google Scholar]
- 26.Schaefer EW, Loaiza-Bonilla A, Juckett M, et al. A phase 2 study of vorinostat in acute myeloid leukemia. Haematologica. 2009;94:1375–1382. doi: 10.3324/haematol.2009.009217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giles F, Fischer T, Cortes J, et al. A phase I study of intravenous LBH589, a novel cinnamic hydroxamic acid analogue histone deacetylase inhibitor, in patients with refractory hematologic malignancies. Clin Cancer Res. 2006;12:4628–4635. doi: 10.1158/1078-0432.CCR-06-0511. [DOI] [PubMed] [Google Scholar]
- 28.Gojo I, Jiemjit A, Trepel JB, et al. Phase 1 and pharmacologic study of MS–275, a histone deacetylase inhibitor, in adults with refractory and relapsed acute leukemias. Blood. 2007;109:2781–2790. doi: 10.1182/blood-2006-05-021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klimek VM, Fircanis S, Maslak P, et al. Tolerability, pharmacodynamics, and pharmacokinetics studies of depsipeptide (romidepsin) in patients with acute myelogenous leukemia or advanced myelodysplastic syndromes. Clin Cancer Res. 2008;14:826–832. doi: 10.1158/1078-0432.CCR-07-0318. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Manero G, Assouline S, Cortes J, et al. Phase 1 study of the oral isotype specific histone deacetylase inhibitor MGCD0103 in leukemia. Blood. 2008;112:981–989. doi: 10.1182/blood-2007-10-115873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mann BS, Johnson JR, He K, et al. Vorinostat for treatment of cutaneous manifestations of advanced primary cutaneous T-cell lymphoma. Clin Cancer Res. 2007;13:2318–2322. doi: 10.1158/1078-0432.CCR-06-2672. [DOI] [PubMed] [Google Scholar]
- 32.Mann BS, Johnson JR, Cohen MH, et al. FDA approval summary: vorinostat for treatment of advanced primary cutaneous T-cell lymphoma. Oncologist. 2007;12:1247–1252. doi: 10.1634/theoncologist.12-10-1247. [DOI] [PubMed] [Google Scholar]
- 33.Cameron EE, Bachman KE, Myohanen S, et al. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet. 1999;21:103–107. doi: 10.1038/5047. [DOI] [PubMed] [Google Scholar]
- 34.Zhu WG, Dai Z, Ding H, et al. Increased expression of unmethylated CDKN2D by 5-aza-2′-deoxycytidine in human lung cancer cells. Oncogene. 2001;20:7787–7796. doi: 10.1038/sj.onc.1204970. [DOI] [PubMed] [Google Scholar]
- 35.Zhu WG, Lakshmanan RR, Beal MD, et al. DNA methyltransferase inhibition enhances apoptosis induced by histone deacetylase inhibitors. Cancer Res. 2001;61:1327–1333. [PubMed] [Google Scholar]
- 36.Boivin AJ, Momparler LF, Hurtubise A, et al. Antineoplastic action of 5-aza-2′-deoxycytidine and phenylbutyrate on human lung carcinoma cells. Anticancer Drugs. 2002;13:869–874. doi: 10.1097/00001813-200209000-00013. [DOI] [PubMed] [Google Scholar]
- 37.Kikuchi T, Toyota M, Itoh F, et al. Inactivation of p57KIP2 by regional promoter hypermethylation and histone deacetylation in human tumors. Oncogene. 2002;21:2741–2749. doi: 10.1038/sj.onc.1205376. [DOI] [PubMed] [Google Scholar]
- 38.Bovenzi V, Momparler RL. Antineoplastic action of 5-aza- 2′-deoxycytidine and histone deacetylase inhibitor and their effect on the expression of retinoic acid receptor beta and estrogen receptor alpha genes in breast carcinoma cells. Cancer Chemother Pharmacol. 2001;48:71–76. doi: 10.1007/s002800100294. [DOI] [PubMed] [Google Scholar]
- 39.Takai N, Kawamata N, Walsh CS, et al. Discovery of epigenetically masked tumor suppressor genes in endometrial cancer. Mol Cancer Res. 2005;3:261–269. doi: 10.1158/1541-7786.MCR-04-0110. [DOI] [PubMed] [Google Scholar]
- 40.Zhu WG, Otterson GA. The interaction of histone deacetylase inhibitors and DNA methyltransferase inhibitors in the treatment of human cancer cells. Curr Med Chem Anticancer Agents. 2003;3:187–199. doi: 10.2174/1568011033482440. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki H, Gabrielson E, Chen W, et al. A genomic screen for genes upregulated by demethylation and histone deacetylase inhibition in human colorectal cancer. Nat Genet. 2002;31:141–149. doi: 10.1038/ng892. [DOI] [PubMed] [Google Scholar]
- 42.Yang H, Hoshino K, Sanchez-Gonzalez B, et al. Antileukemia activity of the combination of 5-aza-2′-deoxycytidine with valproic acid. Leuk Res. 2005;29:739–748. doi: 10.1016/j.leukres.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 43.Fiskus W, Buckley K, Rao R, et al. Panobinostat treatment depletes EZH2 and DNMT1 levels and enhances decitabine mediated de-repression of JunB and loss of survival of human acute leukemia cells. Cancer Biol Ther. 2009;8:939–950. doi: 10.4161/cbt.8.10.8213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 45.Liu Z, Marcucci G, Byrd JC, et al. Characterization of decomposition products and preclinical and low dose clinical pharmacokinetics of decitabine (5-aza-2′-deoxycytidine) by a new liquid chromatography/ tandem mass spectrometry quantification method. Rapid Commun Mass Spectrom. 2006;20:1117–1126. doi: 10.1002/rcm.2423. [DOI] [PubMed] [Google Scholar]
- 46.Parise RA, Holleran JL, Beumer JH, et al. A liquid chromatography-electrospray ionization tandem mass spectrometric assay for quantitation of the histone deacetylase inhibitor, vorinostat (suberoylanilide hydroxamicacid, SAHA), and its metabolites in human serum. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;840:108–115. doi: 10.1016/j.jchromb.2006.04.044. [DOI] [PubMed] [Google Scholar]
- 47.Blum W, Klisovic RB, Hackanson B, et al. Phase I study of decitabine alone or in combination with valproic acid in acute myeloid leukemia. J Clin Oncol. 2007;25:3884–3891. doi: 10.1200/JCO.2006.09.4169. [DOI] [PubMed] [Google Scholar]
- 48.Stathis A, Hotte SJ, Chen EX, et al. Phase I study of decitabine in combination with vorinostat in patients with advanced solid tumors and non-Hodgkin's lymphomas. Clin Cancer Res. 2011;17:1582–1590. doi: 10.1158/1078-0432.CCR-10-1893. [DOI] [PubMed] [Google Scholar]
- 49.Kelly WK, O'Connor OA, Krug LM, et al. Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol. 2005;23:3923–3931. doi: 10.1200/JCO.2005.14.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ravandi F, Faderl S, Thomas D, et al. Phase I study of suberoylanilide hydroxamic acid (SAHA) and decitabine in patients with relapsed, refractory or poor prognosis leukemia. Blood. 2007;110(Suppl. 1) Abstract 897. [Google Scholar]
- 52.Garcia-Manero G, Kantarjian HM, Sanchez-Gonzalez B, et al. Phase ½ study of the combination of 5-aza-2′-deoxycytidine with valproic acid in patients with leukemia. Blood. 2006;108:3271–3279. doi: 10.1182/blood-2006-03-009142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maslak P, Chanel S, Camacho LH, et al. Pilot study of combination transcriptional modulation therapy with sodium phenylbutyrate and 5-azacytidine in patients with acute myeloid leukemia or myelodysplastic syndrome. Leukemia. 2006;20:212–217. doi: 10.1038/sj.leu.2404050. [DOI] [PubMed] [Google Scholar]
- 54.Gore SD, Baylin S, Sugar E, et al. Combined DNA methyltransferase and histone deacetylase inhibition in the treatment of myeloid neoplasms. Cancer Res. 2006;66:6361–6369. doi: 10.1158/0008-5472.CAN-06-0080. [DOI] [PubMed] [Google Scholar]
- 55.Kirschbaum M, Gojo I, Goldberg SL, et al. A phase 1 clinical trial of vorinostat in combination with decitabine in patients with acute myeloid leukaemia or myelodysplastic syndrome. Br J Haematol. 2014;167:185–193. doi: 10.1111/bjh.13016. [DOI] [PubMed] [Google Scholar]