Abstract
Doses of 2 × 1012 virus particles/kilogram (vp/kg) and higher of recombinant human adenovirus serotype 5 (HAdV-5) given via the tail vein induce significant toxicity and mortality in the rat. This was not observed when doses of 5.7 × 1012 vp/kg were given through a surgically implanted jugular catheter. Here we assess how the manner by which HAdV-5 is introduced into the systemic circulation affects biodistribution, transgene expression, toxicity and mortality 0.25, 1, and 4 days after treatment in the rat. Animals were given 5.7 × 1012 vp/kg of HAdV-5 expressing beta-galactosidase or saline through a jugular catheter or by direct tail-vein injection. All animals survived after jugular vein dosing. Tail-vein injection of HAdV-5 increased the mortality rate to 42% (p ≤ 0.01). All deaths occurred within 4 hours. Animals dosed through the jugular vein had significantly higher levels of transgene expression in the liver and spleen and significantly more viral genomes in these tissues and kidney and lung within the first 24 hours of viral infection compared to those dosed by tail-vein injection (p ≤ 0.01). There was no significant difference between the groups thereafter. Samples from animals that died contained even higher levels of viral genomes and serum transaminases were elevated on average by a factor of 4 at the time of death. There was no significant difference between the two dosing methods with respect to changes in hepatic cytochrome P450 expression and activity throughout the study. These findings suggest that the method of systemic administration should be carefully considered when assessing toxicity data and other parameters at early time points after virus administration in the rat and possibly other animal models.
Keywords: adenovirus, toxicity, liver, drug metabolism, route of administration
Introduction
Recombinant adenoviruses are currently under development for the treatment of various monogenetic, hereditary and infectious diseases. Although adenoviruses have been used clinically since the 1970s (Gooch 1972; Top 1975), continual use of their recombinant counterparts in successful therapeutic regimens for the treatment of several cancers (Cattaneo 2008; Vähä-Koskela 2007; Yang 2007), is hindered by a narrow, non-linear therapeutic threshold dictated by an acute, innate immune response when they are introduced into the systemic circulation (Seiler 2007). Significant work in the area of adenovirus biology has revealed that this effect is largely due to the ability of the one component of the virus that makes it so attractive as a gene delivery vector, the protein capsid, to directly interact with and activate blood-borne factors (complement proteins C3 and C4b and clotting factors IX and X) and antigen presenting cells (macrophages, Kupffer cells and dendritic cells) (Baker 2007; Hartman 2008). This response, which can have severe manifestations within several minutes to hours after administration, is characterized by thrombocytopenia and elevated liver enzymes in response to the release of large amounts of cytokines (IL-1, IL-6, TNF-α) and chemokines (MIP-2, MCP-1, IP-10 and RANTES) into the general circulation by antigen presenting cells in the liver, spleen and peritoneum. The dose as well as the route of administration can often dictate whether this resolves quickly or has a fatal outcome (Ben-Gary 2002; Bessis 2004).
Development of physical and biochemical methods to prevent interaction of the virus with components of the immune system to reduce its toxicological profile without compromising transduction efficiency is currently an intensely active area of research. Direct injection of the virus into target tissues, use of immunosuppressive reagents prior to administration of virus, genetic modification of the capsid to include proteins from other adenovirus serotypes and covalent attachment of molecules to target specific organs and shield the virus from recognition by the immune system represent only a few of the current approaches to alter the therapeutic index and improve the utility of adenovirus-mediated gene transfer (Campos 2007; Kreppel 2008; Wu 2008; Zaldumbide 2008). As with any novel biological therapeutic, pre-clinical testing of these vectors includes characterization of the pharmacological and toxicological effects of a series of doses after administration by different routes in rodents prior to testing in higher species.
Research in our laboratory involves the development of recombinant adenoviruses capable of evading the immune response and the mechanistic study of how viral infection affects the expression and function of key cytochrome P450 enzymes in the liver, kidney and intestine (Callahan 2006, 2005, 2008; Le 2006). Discussion of our results with others in the field has revealed that doses of 2 × 1012 virus particles/kilogram (vp/kg) and higher of recombinant adenovirus serotype 5 given via the tail vein induce significant toxicity and mortality in the rat (A. Byrnes and J. Smith, personal communication, June 6, 2003). This was not observed in any of our studies when 5.7 × 1012 vp/kg were given through a surgically implanted jugular catheter which allows for easy, relatively stress-free serial blood sampling in the rat (Cocchetto 1983; Thrivikraman 2002). Animals given this much virus did not exhibit any signs of toxicity and survived for up to 14 days when they were sacrificed at this pre-determined time point. Careful evaluation of the literature revealed that documented reports of adenovirus-induced deaths in rodents were limited and were not the primary subject of any given study. We did find some reports, however, describing marked signs of virus-induced toxicity including bloody urine, black ocular discharge, weight loss, lethargy and subsequent mortality when similar viruses were given by direct injection in the tail vein at much lower concentrations in healthy animals and those with pre-existing liver disease (Garcia-Banuelos 2002; Morrissey 2002; Smith 2004a, b).
Given this information, we initiated a series of experiments designed to determine how slight changes in the manner by which recombinant adenoviruses are administered could influence the toxicological profile associated with the virus, something that is of great concern in and out of the clinic. We also tested the hypothesis that long-term affects associated with the virus such as transgene expression or changes in the expression and function of hepatic cytochrome P450 enzymes would not be affected by the different methods of virus administration. Male Sprague Dawley rats were given 5.7 × 1012 vp/kg of a first generation adenovirus serotype 5 expressing the E. coli beta-galactosidase transgene by either direct tail vein injection or through a cannula implanted in the jugular vein. Animals were closely monitored for the first eight hours for visible signs of distress and toxicity. Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were measured 0.25, 1, and 4 days after administration. Distribution of viral genomes and transgene expression were also measured in key tissues (liver, spleen, kidney, lung and heart) at the same time points. Changes in hepatic CYP3A2 and 2C11 over the course of four days after administration of virus by either route is also discussed.
Materials and Methods
Amplification and Production of Recombinant Adenovirus
First generation E1/E3 deleted adenovirus serotype 5 containing the E. coli beta-galactosidase transgene under the control of a CMV promoter was amplified in HEK 293 cells (CRL-1573, ATCC, Manassas, VA) and purified from cell lysates by two rounds of cesium chloride density gradient ultracentrifugation according to established protocols (Altaras 2005). Viral bands isolated after the final centrifugation step were desalted on Econo-Pac 10DG disposable columns (BioRad, Hercules, CA) equilibrated with phosphate buffered saline (pH 7.4, Sigma Aldrich, St. Louis, MO). Fractions containing virus were collected and the number of virus particles determined using the method of Maizel et al. with the following formula (Maizel Jr. 1968):
All animals were treated with freshly purified virus.
Plaque assay
The amount of active virus in a given preparation was determined by plaque assay according to an established protocol (Graham 1973). Assays were performed on the day animals were treated. Plaque forming units (pfu) were calculated according to the following formula:
The particle to pfu ratio was calculated by dividing the number of particles obtained from the absorbance reading of a preparation at 260 nm by the number of active particles (pfu/ml) detected by the plaque assay. The average virus particle to pfu ratio for the virus preparations used in these studies was 53:1.
Assay for Detection of Replication Competent Adenovirus (RCA)
A two-cell line bioassay was performed on each preparation to determine the presence of RCA as described previously (Murakami 2002). One RCA was detected for every 3 × 1012 virus particles tested.
Administration of Recombinant Adenovirus
All procedures were approved by the Institutional Animal Care and Use Committee of The University of Texas at Austin and are in accordance with the guidelines established by the National Institutes of Health for the humane treatment of animals. Male Sprague-Dawley rats (7–8 weeks, Charles River Laboratories, Wilmington, MA) were housed in individual cages and given unrestrained access to standard rodent chow (Harlan Teklad, Indianapolis, IN) and tap water. A single intra-muscular injection of a 1:1:1 (v/v/v) ratio of ketamine (100 mg/ml, Wyeth, Fort Dodge, Animal Health, Overland Park, KS), xylazine (20 mg/ml, Sigma Aldrich), and acetopromazine (10 mg/ml, Sigma Aldrich) achieved deep plane anesthesia for placement of catheters into the right jugular vein. Aside from a single dose of heparinized saline (0.3 ml, 20 U/ml, Heparin Sodium, Baxter Healthcare, Deerfield, IL) to maintain the cannula immediately following surgery, animals in this study did not receive any additional medications prior to or after administration of recombinant adenovirus. Twenty-four hours after surgery, rats were placed in polycarbonate plastic restraining devices (Braintree Scientific, Braintree, MA) and given a single intravenous dose of either 5.7 × 1012 viral particles per kilogram (vp/kg), bacterial lipopolysaccharides (LPS, 1 mg/kg, from Escherichia coli serotype 0127:B8, Sigma Aldrich) or phosphate buffered saline each in a 0.5 ml volume followed by 0.5 milliliters of saline to ensure that each reagent was effectively flushed from the catheter and into the bloodstream.
A separate group of animals that did not undergo jugular cannulation were placed in polycarbonate restraining devices and given each of these agents in the same volume (0.5 ml) by direct injection in the lateral tail vein according to established procedures (Cocchetto 1983). Care was taken to ensure that each preparation was administered at a constant rate of 1 ml/min regardless of the manner in which it was given. Surviving animals from each group were sacrificed 0.25, 1 or 4 days after treatment. Serum was collected for assessment of alanine aminotransferase (ALT) and aspartate aminotransferase (AST). A section of liver, spleen, kidney, lung and heart tissue was placed in Tissue-tek® embedding medium (Fisher Scientific, Pittsburgh, PA) for X-gal histochemistry. Remaining liver tissue was excised, rinsed in saline, snap frozen in liquid nitrogen, and stored at −80°C for microsome preparation and PCR analysis.
Microsome Isolation
Hepatic microsomal proteins were isolated by differential centrifugation as described previously (Callahan 2005). Microsomes were stored at −80°C prior to analysis.
Gel Electrophoresis and Immunoblot Analysis
Microsomal proteins (20 μg) and isoform-specific CYP protein standards (XenoTech, LLC, Lenexa, KS) were separated by size by sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE) as described (Callahan 2005). Detection of putative proteins was achieved with a 1:3000 dilution of the specific primary CYP antibody (BD Gentest, Woburn, MA) in 3% NFDM followed by a second incubation with a corresponding horseradish peroxidase conjugated secondary antibody (1:3000, ICN Pharmaceuticals, Inc. Aurora, OH). Immune complexes for CYP3A1/2 and CYP2C11 were detected by chemiluminescence (Western Lightning Detection Kit, PerkinElmer, Boston, MA). Protein band densities were analyzed using Kodak 1D image analysis software (Eastman Kodak, Rochester, NY). CYP3A1 and CYP3A2 co-migrate during electrophoresis. The antibody used to detect CYP3A2 was polyclonal with cross reactivity to CYP3A1, therefore all protein levels for CYP3A2 are reported as CYP3A1/2.
In vitro Testosterone Hydroxylation Assay
Metabolic activity for CYP3A2 and 2C11 was determined by in vitro analysis of testosterone hydroxylation as described (Callahan 2006). Samples were incubated with testosterone (250 μM in ethanol, Sigma Aldrich) for 18 minutes at 37°C with gentle agitation after addition of glucose-6-phosphate dehydrogenase (1 unit/μl, Sigma) and then quenched with dichloromethane (5 ml). 11α-hydroxyprogesterone (1.2 μg, Sigma) was added as an internal standard. The organic phase was evaporated under a constant stream of air, dissolved in 200 μl of methanol and stored in a sealed tube at 4°C until analysis. Testosterone metabolites were separated and quantified by HPLC according to an established method (Shaw 2002). Peak areas of corresponding hydroxylation metabolites were measured and compared to peak areas of the internal standard within the same run.
RT-PCR
Hepatic RNA was isolated with TRIzol (Invitrogen Co., Carlsbad, CA) according to the manufacturer’s instructions. Isolated RNA was adjusted to a concentration of 100 μg/ml and one microliter reverse-transcribed using RETROscript, (Ambion, Austin, TX) following the manufacturer’s instructions. PCR was performed using primers specifically for CYP3A2 (5′-TTG ATC CGT TGT TCT TGT CA-3′ (sense) and 5′-GGC CAG GAA ATA CAA GAC AA-3′ (anti-sense)) and 2C11 (5′-CTG CTG CTG CTG AAA CAC GTC-3′ (sense) and 5′-GGA TGA CAG CGA TAC TAT CAC-3′ (anti-sense)) as described previously (Callahan 2006). QuantumRNA™ 18S internal standards (Ambion, Austin, TX) were co-amplified in CYP 3A2 and 2C11 reaction tubes. The ratio of 18S primer to competimer for all duplex PCR reactions was 4:6. Amplicons were visualized on a 1.5% agarose gel containing ethidium bromide and band intensity determined by densitometric analysis using Kodak 1D image analysis software.
Real Time PCR
Genomic DNA was extracted from ~ 25 mg (liver, lung, kidney) and 10 mg (spleen) tissue using a QIAamp DNA Mini Kit (Qiagen, Valencia, CA), according to the manufacturer’s instructions. Quantification of viral DNA was determined by real time PCR according to established methods (Callahan 2006; Demers 2003). Primer sequences, used to amplify a region of the adenovirus hexon protein, were 5′-ACT ATA TGG ACA ACG TCA ACC CAT T-3′ (forward) and 5′-ACC TTC TGA GGC ACC TGG ATG T-3′ (reverse). The internal probe sequence, tagged with 6FAM fluorescence dye at the 5′ end and TAMRA quencher at the 3′ end, was 5′-ACC ACC GCA ATG CTG GCC TGC-3′. Each sample was run in triplicate. The standard virus preparation used for the real time PCR assay was obtained from a lot of virus produced in our laboratory specifically to serve as a standard reference lot by which to compare other viral preparations for subsequent studies. Assays for assignment of the particle number and infectious titer of this lot of virus were validated using the Adenovirus Reference Material (ATCC VR-1516). Standard curves were used to estimate the number of adenovirus particles in test samples, which were expressed as genome copies per 100 nanograms of genomic DNA.
Evaluation of Gene Expression and Toxicity
The amount of beta-galactosidase in tissue samples was quantified by an enzyme-linked immunosorbent assay (ELISA, Roche Applied Science, Indianapolis, IN) and visually localized by histochemical staining as described previously (Croyle 2002). Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were measured with Vitros ALT slides on a Vitros DT60 autoanalyzer (Ortho-Clinical Diagnostics, Rochester, NY).
Data analysis
Statistical analysis of data was performed using SigmaStat software (Systat Software, Inc., San Jose, CA). One-way analysis of variance with a Bonferonni/Dunn post-hoc analysis was used to determine statistical differences between individual groups for all data collected except survival. Survival curve data comparison was performed using a LogRank survival analysis test. For each of these tests, differences were determined to be significant when the probability of chance explaining the results was reduced to less than 5% (p<0.05).
Results
The primary goal of the studies outlined in this manuscript was to compare the toxicological and pharmacological effect of a single dose of recombinant adenovirus (AdlacZ) after administration via a catheter surgically implanted in the jugular vein, a technique established in our laboratory (Callahan 2006, 2005, 2008), with that induced by direct injection in the lateral tail vein of male Sprague Dawley rats. Additional animals were treated with bacterial lipopolysaccharides (LPS, 1 mg/kg) given by either route to confirm that our findings were not specific to our adenovirus construct. These animals also served as positive controls for studies characterizing the expression and function of hepatic CYP since it has been shown that LPS affects CPY3A2 and 2C11 in a manner similar to adenovirus (Callahan 2005; Morgan 2008). A third group of animals given phosphate buffered saline (PBS) by either route served as negative controls. There was no significant difference between any of the parameters assessed in these studies between animals given saline with respect to route of administration. Thus, data shown for this group throughout the manuscript are from animals dosed by the jugular cannula. Each preparation (AdlacZ, LPS, PBS) was dispensed in a volume of 500 microliters.
Method by which a Preparation is Introduced into the Systemic Circulation and Mortality in the Rat
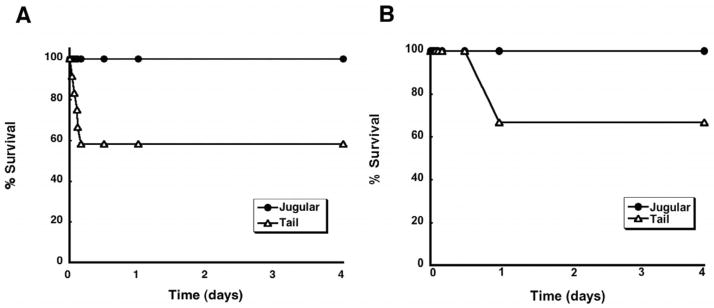
To date, we have found that doses of up to 5.7 × 1012 vp/kg of AdlacZ given by infusion into jugular catheter were well tolerated by male Sprague Dawley rats (Callahan 2006, 2005, 2008). Visible signs of toxicity (prostration, labored breathing, cyanosis), previously reported in this animal model after systemic administration via the tail vein, were not observed in animals treated in this manner (Garcia-Banuelos 2002; Morrissey 2002; Smith 2004a, b). This outcome was also observed in the current set of experiments since all of the animals given the virus by this method survived (Figure 1A). Animals given the same dose of virus by the tail vein, however, exhibited signs of morbundity (loud, labored breathing, lying prostrate on one side) as early as 50 minutes after treatment. This phenomenon was very animal specific with some animals not responding in this manner until 4 hours after administration. Every animal that developed these symptoms did not recover and expired within minutes after symptoms became obvious. All told, 42% of the animals given the virus by tail vein injection died. Every animal given LPS through the jugular cannula survived (Figure 1B). Animals given LPS by tail vein injection, however, exhibited signs of morbundity much later than those given adenovirus (~12 hours after treatment). All animals that died (33.3%) did so within 24 hours after dosing.
Figure 1. Systemic Administration of (A) recombinant adenovirus or (B) bacterial lipopolysaccharide (LPS) by Tail Vein Injection Causes Significant Mortality in the Male Sprague Dawley Rat within 24 Hours.
Rats were given either a single intravenous dose of a first generation recombinant adenovirus expressing beta-galactosidase (5.7 × 1012 vp/kg) or bacterial LPS (1 mg/kg) via the lateral tail vein (Tail) or a catheter surgically implanted in the jugular vein (Jugular). Each point on the chart represents the time an animal or a group of animals died. Percent survival was determined by dividing the total number of animals alive at each time point within the four day interval by the total of number animals dosed (n=12/group) and multiplying by 100. Animals given adenovirus exhibited overt signs of toxicity and died as early as 50 minutes after administration while those given LPS did not experience any toxicity until 24 hours after treatment. Regardless of the agent administered, survival of animals dosed via the tail vein was significantly lower than those dosed by the jugular cannula (p = 0.01, LogRank survival analysis). All animals given saline by either route survived (data not shown for clarity).
Method by which a Preparation is Introduced into the Circulation and Toxicity in the Rat
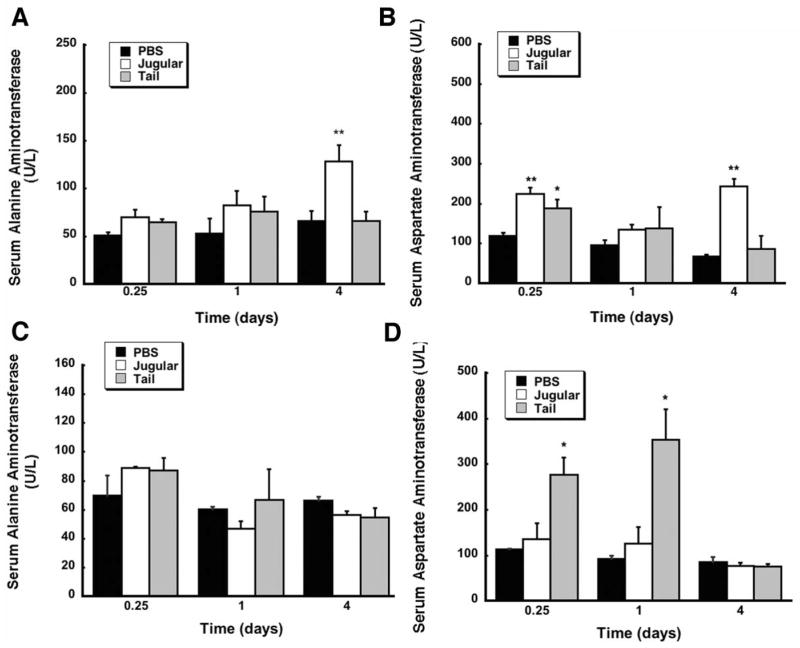
Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels, often used as indicators of liver function during inflammation, injury, or disease (Ozer 2008), were used to monitor hepatotoxicity associated with administration adenovirus and LPS by either route (Figure 2). There was no significant difference in serum ALT levels between animals given adenovirus at the 6 hour and 1 day time points (p=0.1, Figure 2A). ALT levels of animals given the virus through the jugular cannula, however, were significantly higher than those given saline in the same manner 4 days after treatment (128.3 ± 17 vs. 66 ± 10.4 U/L, p = 0.006) but were less than the average ALT level measured in samples obtained from animals that died after tail vein injection of virus (180.7 U/L). Serum AST was significantly elevated 6 hours after administration of virus regardless of the manner in which it was delivered (224.3 ± 15.5 U/L (Jugular), 188.7 ± 21.6 U/L (Tail) vs. 119.3 ± 7.4 U/L (PBS), Figure 2B). AST in samples from animals dosed via the cannula was also elevated to approximately 5 times that seen in samples from animals given saline 4 days after treatment (p = 0.008). This was significantly lower, however, than the average serum AST of animals that died after administration of virus via the tail vein (320.3 U/L). Serum ALT levels of animals given bacterial LPS were not significantly different from those given saline by either route throughout the course of the study (Figure 2C). Animals given LPS by tail vein injection, however, had significantly higher levels of serum AST than those given saline at 6 hours (277 ± 37 vs. 113 ± 2 U/L) and 24 hours (354 ± 66 vs. 93 ± 7 U/L) (Figure 2D). Samples obtained from animals that died after administration of LPS by the tail vein had an average ALT value of 616 U/L and AST value of 2,176 U/L.
Figure 2. Route of Administration of Bacterial Lipopolysaccharide Significantly Affects Serum Aspartate Aminotransferase (AST) Levels.
Serum alanine aminotransferase (ALT) and serum aspartate aminotransferase (AST) levels were assessed 0.25, 1, and 4 days after administration of either phosphate buffered saline (PBS), 5.7 × 1012 vp/kg adenovirus expressing beta-galactosidase (Panels A and B) or 1 mg/kg bacterial LPS (Panels C and D) by injection into the jugular vein through an implanted catheter (Jugular) or by direct injection into lateral tail vein (Tail). Values represent the mean ± standard error of 4 animals/treatment/time point. **p ≤ 0.01, *p ≤ 0.5, one-way analysis of variance with a Bonferonni/Dunn post-hoc test.
Effect of Method of Systemic Administration on Transgene Expression and Biodistribution of Adenovirus
In order to determine if the manner by which adenovirus was introduced into the systemic circulation influenced the time at which the virus reached specific target organs and transgene expression patterns, tissue samples were screened for virus genomes using real-time PCR (Table 2). Transgene expression was quantified by ELISA (Table 1) and visualized by histochemical staining (Figure 3). The beta-galactosidase transgene was detected in the liver of surviving animals as early as 6 hours and peaked 4 days after dosing regardless of the treatment method. Samples obtained from animals dosed through the jugular vein, however, contained significantly higher levels of the transgene at 6 hours (415.8 ± 57.1 ng/mg protein) than those given the virus by tail-vein injection (193.1 ± 23.4 ng/mg protein, p= 0.008). After four days, transgene expression was high and not significantly different between the two groups (p = 0.075, Table 1). This was also reflected in samples histochemically stained for transgene expression (Figure 3, panels B and C). The number of viral genomes followed the same trend with samples from animals dosed through the cannula containing significantly more virus at 6 hours (97,025.1 vs. 9,939 virus genomes/100 ng genomic DNA, Table 2) and then leveling out by day four.
Table 2.
Biodistribution of Virus Genomes following Systemic Administration of Recombinant Adenoviruses by Different Routes as determined by Quantitative Real-Time PCR.
| Time (days) | Jugular* | Tail* | |
|---|---|---|---|
| Liver | 0.25 | 97,025 ± 5,198 | 9,939 ± 1,653 |
| 1 | 323,743 ± 9,804 | 3,393 ± 1,398 | |
| 4 | 62,162 ± 8,506 | 57,262 ± 2,707 | |
| Spleen | 0.25 | 36,701 ± 1,169 | 7,971 ± 3,013 |
| 1 | 13,007 ± 876 | 4,558 ± 709 | |
| 4 | 1,509 ± 716 | 593 ± 189 | |
| Kidney | 0.25 | 102 ± 25.7 | 18.8 ± 11.8 |
| 1 | 71.6 ± 31.2 | N.D. | |
| 4 | 10.9 ± 1.87 | N.D. | |
| Lung | 0.25 | 15,291 ± 1,048 | 13.63 ± 5.15 |
| 1 | 1,396 ± 758 | 40.52 ± 21.2 | |
| 4 | 662 ± 336 | 13.8 ± 2.22 |
Data are reported as the average viral genome copy number detected/100 ng of genomic DNA ± the standard error of the mean for 4 animals/treatment/time point. N.D. - None detected. Sample fell below the detection limit of the assay (10 viral genomes/100 ng DNA).
Jugular: Data obtained from animals given 5.7 × 1012 vp/kg of adenovirus expressing beta-galactosidase into the jugular vein through an implanted catheter.
Tail: Data obtained from animals given 5.7 × 1012 vp/kg of adenovirus expressing beta-galactosidase by direct injection into the lateral tail vein.
Table 1.
Quantitative Analysis of Beta-Galatosidase Transgene Expression After Systemic Administration of Recombinant Adenovirus by Different Routes.a
| ng beta-galactosidase/mg protein | |||
|---|---|---|---|
|
| |||
| Time (days) | Jugular | Tail | |
|
| |||
| Liver | 0.25 | 415.8 ± 57.06 | 193.1 ± 23.36 |
| 1 | 3,731 ± 1,163 | 3,157 ± 1,444 | |
| 4 | 54,306 ± 7,265 | 62,273 ± 10,560 | |
| Spleen | 0.25 | 227.7 ± 47.58 | 32.62 ± 19.57 |
| 1 | 367.1 ± 144.0 | 110.3 ± 41.06 | |
| 4 | 44.79 ± 11.37 | 41.48 ± 2.43 | |
| Kidney | 0.25 | N.D. | N.D. |
| 1 | 6.52 ± 2.46 | N.D. | |
| 4 | 10.34 ± 1.41 | 1.89 ± 0.49 | |
Data were obtained from tissue homogenates and reflect average values ± standard errors of the means from 4 animals per treatment group. N.D. - None detected. Sample fell below the detection limit of the assay (30 pg/mg protein).
Jugular: Data obtained from animals given 5.7 × 1012 vp/kg of adenovirus expressing beta-galactosidase into the jugular vein through an implanted catheter.
Tail: Data obtained from animals given 5.7 × 1012 vp/kg of adenovirus expressing beta-galactosidase by direct injection into the lateral tail vein.
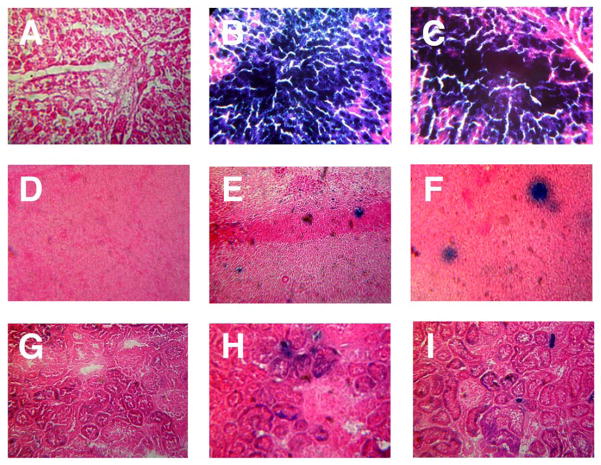
Figure 3. Transgene Expression in Key Tissues Four Days After Administration of a Single Dose of Recombinant Adenovirus by Different Routes.
Male Sprague Dawley rats were given either phosphate buffered saline (vehicle control, Column 1) or 5.7 × 1012 vp/kg of adenovirus expression beta-galactosidase by injection into the jugular vein through an implanted catheter (Column 2) or by direct injection into lateral tail vein (Column 3). Animals were sacrificed 4 days after treatment and liver (Panels A–C), spleen (Panels D–F) and kidney (Panels G–I) were evaluated for transgene expression by X-gal histochemistry. Morphological assessment was performed from serial sections of three rats for each treatment group and transgene expression assessed by visual inspection of the tissue for the blue product generated by active beta-galactosidase in the presence of the substrate 5-bromo-4-chloro-3-indolyl-β-D-galactoside (X-gal). Sections from animals given saline did not stain positive for the transgene while those from animals dosed via the jugular cannula contained slightly more of the transgene than those dosed by the tail vein. Magnification: Panels A–C, 400 ×, Panels D–F, 300 ×, G–I, 150 ×.
The transgene could also be detected in splenic samples obtained from both treatment groups as early as 6 hours and, as observed with the hepatic samples, those obtained from animals given the virus through the jugular cannula contained significantly higher levels of beta-galactosidase at this time point (227.7 ± 47.6 vs. 32.6 ± 19.6 ng/mg protein, p = 0.03). Transgene expression peaked at the 1 day time point in this tissue regardless of method of administration and declined significantly by day 4. This trend was also observed in samples histochemically stained for transgene expression (Figure 3, panels E and F). In general, transgene expression in the kidney following systemic administration of recombinant adenoviruses is extremely low (Le 2006). Transgene expression was detected in kidney tissue of animals dosed through the jugular cannula at the one day time point and was absent in those dosed by tail vein injection. Samples from animals dosed through the cannula contained significantly more beta-galactosidase at 4 days (10.3 ± 5.4 vs. 1.9 ± 0.99 ng/mg protein, p= 0.008, Table 1). This trend was also reflected by histochemical staining (Figure 3, panels H and I) and real-time PCR (Table 2).
Although transgene expression could not be detected in pulmonary and cardiac tissues obtained from the surviving animals in each of the treatment groups throughout the study, viral DNA could be detected in the lungs. The number of genomes in tissue obtained from animals dosed through the jugular vein was significantly higher at each time point than those in tissue from animals dosed via the tail vein (p ≤ 0.05). The number of viral genomes in the lung peaked at 6 hours in this treatment group (15,291 virus genomes/100 ng genomic DNA) while samples from animals dosed via the tail vein contained peak amounts, yet still significantly less viral DNA with respect to those dosed via the cannula, at the 1 day time point (40.5 virus genomes/100 ng genomic DNA, Table 2).
It is also important to note that significant amounts of viral genomes were detected in samples obtained from animals that received virus by tail vein injection but died within 6 hours after treatment. At the time of death, substantial increases in the average number of genomes were found in the liver (133,766 ± 1,037), spleen (58,543 ± 1,974), kidney (3,259 ± 317) and lung (6,231 ± 1,343) with respect to that found in samples from each of the treatment groups at the 6 hour time point. Viral DNA was also found in cardiac tissue of these animals (37.9 ± 2.4 virus genomes/100 ng genomic DNA).
Effect of Method of Systemic Administration of Recombinant Adenovirus on Hepatic CYP3A2 Expression and Function
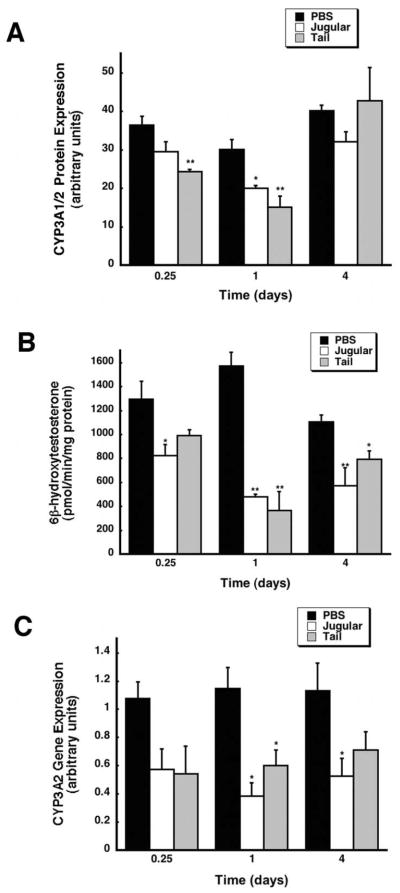
Western blot analysis revealed that hepatic CYP3A2 was significantly suppressed in samples obtained from animals given virus by either method with respect to saline controls (PBS, Figure 4A). CYP3A1/2 protein was reduced by 66% as early as 6 hours after administration in rats dosed by tail-vein injection. Protein levels fell further in this treatment group to 50% of control one day after treatment while samples obtained from animals dosed though the jugular vein contained CYP3A1/2 levels that were 66% of control at the same time point. Protein levels in both groups approached baseline levels by day four. Changes in hepatic CYP3A2 were confirmed by an in vitro catalytic assay measuring 6β-hydroxytestosterone, the major product of CYP3A2 testosterone hydroxylation (Waxman et al. 1983). The most profound suppression of CYP3A2 activity occurred one day after administration of virus by either method (jugular, 30.6% and tail, 23.4% of control) (Figure 4B, p ≤ 0.01). CYP3A2 activity remained suppressed at day 4 in rats dosed through the jugular catheter (53.8%) as well as in those dosed by tail-vein injection (74.2% of control, p ≤ 0.05). Recombinant adenovirus given by either method also significantly suppressed CYP3A2 at the transcriptional level. Significant reductions in 3A2 mRNA were observed at the 1 and 4 day time points in animals given the virus through the jugular cannula (33% and 52% of control respectively, Figure 4C, p ≤ 0.05). A similar trend was also observed in animals dosed by tail vein injection at the same time points (52.4% day 1, 62.8% of control day 4).
Figure 4. Recombinant Adenovirus Significantly Suppresses CYP3A2 in a Manner Independent of the Method of Administration.
(A) Western blot analysis of hepatic CYP3A1/2 protein expression at 0.25, 1, and 4 days after treatment. Male Sprague-Dawley rats were given an intravenous dose of either PBS or 5.7 × 1012 vp/kg of recombinant adenovirus expressing beta-galactosidase into the jugular vein through an implanted catheter (Jugular) or by direct injection into the lateral tail vein (Tail). Protein expression is reported as arbitrary units of relative density compared to a CYP3A2 protein standard. (B) In vitro catalytic activity of hepatic CYP3A2 microsomal proteins, measured by the production of the enzyme-specific testosterone metabolite, 6β-hydroxytestosterone, in samples obtained 0.25, 1, and 4 days after treatment. (C) Hepatic CYP3A2 mRNA levels after treatment with either PBS or virus administered via the jugular or tail vein. Data are reported as band densities of gene-specific RT-PCR products with respect to the density of products obtained from an internal control (18S rRNA) in arbitrary units. In all panels, values represent the mean ± standard error of 4 animals/treatment/time point. Animals dosed with PBS served as vehicle controls. **p ≤ 0.01, *p ≤ 0.5, one-way analysis of variance with a Bonferonni/Dunn post-hoc test.
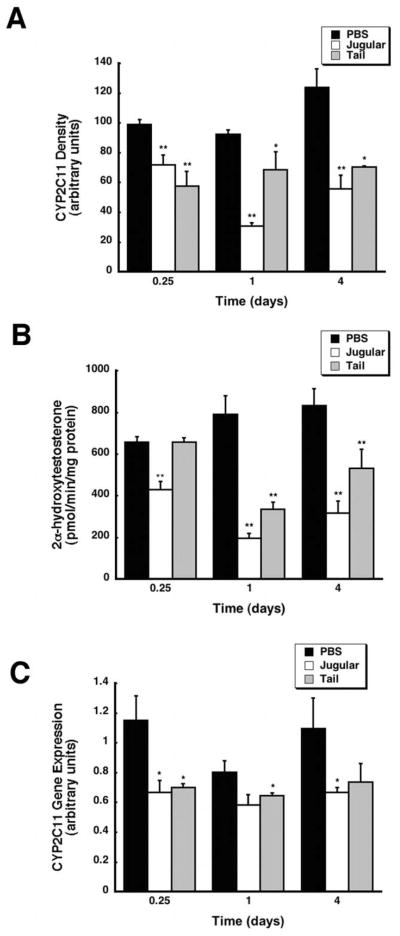
Effect of Method of Systemic Administration of Recombinant Adenovirus on Hepatic CYP2C11 Expression and Function
Assessment of the band densities of CYP2C11 protein via Western blot revealed that administration of the virus by either method also significantly suppressed this isoform throughout the course of the study (Figure 5A). Protein levels were reduced to 72.3 and 58.3% of control values as early as 6 hours in animals dosed via the jugular or tail veins respectively and continued to decline to 45% of control (jugular) and 56% (tail) four days after treatment. Suppression of CYP2C11 was confirmed by an in vitro catalytic assay measuring the amount of the isoform-specific testosterone metabolite, 2α-hydroxytestosterone, present in each sample (Cheng and Schenkman 1983). Animals given the virus through the jugular cannula exhibited CYP2C11 activity that was 65% of that of saline treated animals 6 hours after treatment (Figure 5B). Administration of the virus by the tail vein significantly suppressed CYP 2C11 activity to 25% and 38% of control at the 1 and 4 day time points (p ≤ 0.01). CYP 2C11 activity was also significantly reduced to 42% and 64% of control 1 and 4 days after administration of the virus by the tail vein (p ≤ 0.01). Administration of adenovirus into the systemic circulation by either method significantly suppressed CYP2C11 mRNA levels to 58% (jugular vein) and 60% (tail vein) of control 6 hours after treatment (p ≤ 0.05, Figure 5C). CYP2C11 mRNA remained at 58% of control at 4 days in animals given virus via the jugular vein while mRNA in samples from those given virus via the tail vein began to return to baseline.
Figure 5. Method of Administration Has Some Effect on the Degree By Which Recombinant Adenovirus Suppresses Hepatic CYP2C11 Activity.
(A) Immunoblot analysis of hepatic CYP2C11 protein levels 0.25, 1, and 4 days after treatment. Male Sprague-Dawley rats were given an intravenous dose of either PBS or 5.7 × 1012 vp/kg of recombinant adenovirus expressing beta-galactosidase into the jugular vein through an implanted catheter (Jugular) or by direct injection into the lateral tail vein (Tail). Protein expression is reported as arbitrary units of relative density compared to a CYP2C11 protein standard. (B) In vitro catalytic activity of hepatic CYP2C11 microsomal proteins, measured by the production of the enzyme-specific testosterone metabolite, 2α-hydroxytestosterone, in samples obtained 0.25, 1, and 4 days after treatment. (C) Hepatic CYP2C11 mRNA levels after treatment with either PBS or virus administered via the jugular or tail vein. Data are reported as band densities of gene-specific RT-PCR products with respect to the density of products obtained from an internal control (18S rRNA) in arbitrary units. In all panels, values represent the mean ± standard error of 4 animals/treatment/time point. Animals dosed with PBS served as vehicle controls. **p ≤ 0.01, *p ≤ 0.05, one-way analysis of variance with a Bonferonni/Dunn post-hoc test.
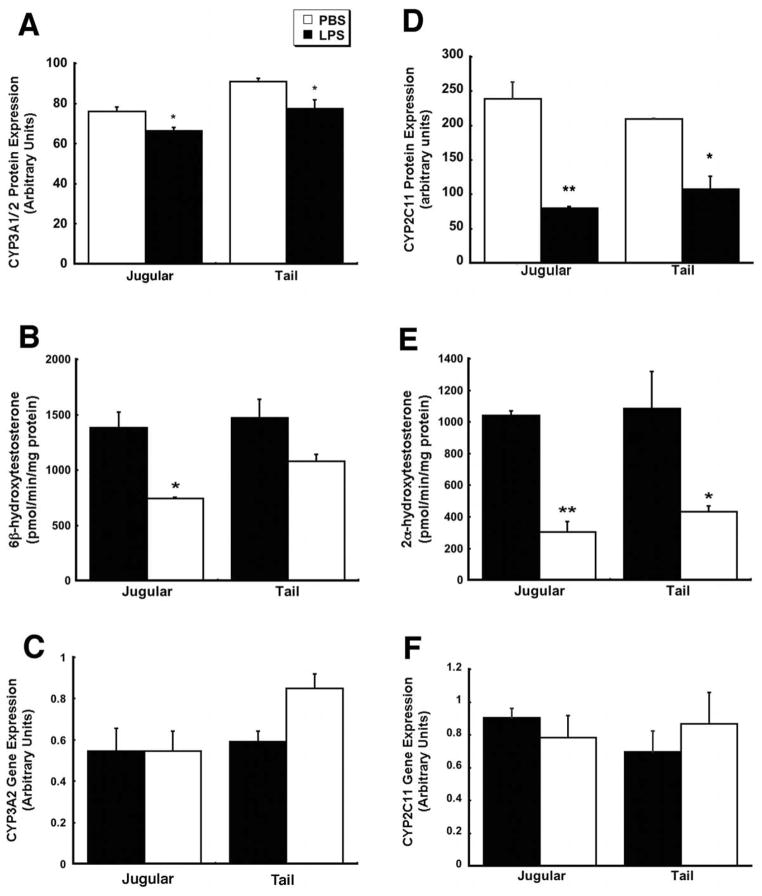
Effect of Method of Systemic Administration of Bacterial Lipopolysaccharide on Hepatic CYP Expression and Function
As stated previously, animals were also treated with bacterial lipopolysaccharides (LPS, 1 mg/kg) given by either route to confirm that our findings were not specific to our adenovirus construct. Data presented in Figure 6 represents that collected during a pilot study during which animals were sacrificed and CYP activity and expression assessed 4 days after treatment. CYP3A1/2 protein levels were reduced by approximately 15% in both treatment groups (Figure 6A) while administration of the virus by the cannula reduced CYP2C11 protein levels to 35.2% of control (Figure 6D). Administration of virus by the tail vein reduced CYP2C11 protein levels to 51.5% of control. Only administration of virus via the jugular cannula significantly reduced CYP3A2 activity (53.9% of control, Figure 6B, p ≤ 0.05) while treatment by either method significantly suppressed CYP2C11 activity (jugular 29.2% and tail 39.9% of control, Figure 6E, p ≤ 0.05). mRNA levels of either isoform were unaffected by administration of LPS by either route (Figures 6C and 6F).
Figure 6. Bacterial Lipopolysaccharide Suppresses Hepatic CYP2C11 in a Manner Independent of the Method of Administration.
Male Sprague Dawley rats were given either phosphate buffered saline (PBS) or 1 mg/kg bacterial lipopolysaccharides (LPS, from Escherichia coli serotype 0127:B8) by injection into the jugular vein through an implanted catheter or by direct injection into lateral tail vein. Animals were sacrificed 4 days after treatment and liver tissue process for assessment of CYP3A2 and 2C11 protein levels by Western blot (Panels A and D), activity by an in vitro assay (Panels B and E) and mRNA levels by RT-PCR (Panels C and F). In all panels, values represent the mean ± standard error of 4 animals/treatment/time point. Animals dosed with phosphate buffered saline served as vehicle controls. **p ≤ 0.01, *p ≤ 0.05, one-way analysis of variance with a Bonferonni/Dunn post-hoc test.
Discussion
Different methods of intravenous dosing and sampling from various regions of the body can significantly affect distribution patterns and measured blood levels of many medicinal compounds (Cocchetto 1983; Hui 2007; Tse et al. 1984). We have found this to be the case with respect to recombinant adenoviral vectors, particularly within the first 24 hours after administration. Significant differences in the distribution of viral genomes to all major organs (liver, spleen, kidney, and lung) were noted soon (0.25 and 1 day) after administration with respect to the manner by which the virus was introduced into the systemic circulation. Transgene expression in the liver and spleen at 6 hours was also significantly lower in rats dosed by tail-vein injection. We did not, however, find any significant difference in virus distribution, transgene expression and CYP expression patterns between the two treatment methods at the 4 day time point regardless of treatment.
None of the rats treated with 5.7 × 1012 vp/kg of adenovirus administered through an implanted jugular catheter died nor exhibited any signs associated with virus-induced toxicity. Direct injection of the same dose into the lateral tail vein did significantly alter the survival profile yielding a 42% incidence of mortality, comparable to that reported by Smith et al. in male Sprague-Dawley rats given 2.4 × 1012 vp/kg of a similar virus (Smith 2004b). Parallel results were obtained when another immunogenic compound, bacterial LPS, was also given by tail vein injection. Thirty-three percent died while all dosed through a jugular cannula survived. These results not only confirm our previous observations, but support the fact that the differences in survival between our experiments and those in the literature are not necessarily due to lab-to-lab variation of adenovirus production, purification, or quantification techniques and are not unique to the adenovirus alone. Instead, the difference in survival rate most likely can be attributed to the manner by which each preparation was introduced into the systemic circulation.
The primary reason for differences in mortality between the two methods for administration may be due to differences in the blood flow rate between the distal tail and jugular veins (Cocchetto 1983; Johannessen et al. 1982; Tse et al. 1984). The jugular vein is in close proximity to the heart, allowing the virus to quickly mix with blood and blood components, rapidly enter the circulation and distribute evenly throughout the body. In contrast, the smaller diameter and the corresponding slower mean blood flow rate of the tail vein (0.02 ml/min tail at 25 °C vs 4.1 ml/min jugular (Harrison 1977; Johannessen et al. 1982; Rand 1965)) limits the virus to a confined space for an extended period of time, which could promote aggregation, a phenomenon inherent to recombinant adenovirus and bacterial LPS (Galdiero 1979; Gutsmann 2007). Aggregates that form are then slowly released into the circulation from the tail vein region. This and the fact that the tail vein is more distally located from the general systemic circulation may explain the significant difference in the time it took for the number of viral genomes to peak in the livers of animals given recombinant adenovirus by this route with respect to those given the virus through the jugular cannula. When aggregates do reach the circulation, those that distribute into tissues rapidly activate strong local and systemic inflammatory immune responses which can progress to anaphylactic shock, similar to what was observed in animals dosed with either preparation via the tail vein (Brange 1997; Pace 1990; Thornton 1993). Any remaining aggregates are sequestered by circulating antigen presenting cells which further potentiate this effect. It has also been shown that serum samples obtained from the tail vein consistently contain higher numbers of lymphocytes and neutrophils than those obtained from a jugular cannula (Smith 1986), suggesting that this area is already somewhat primed to initiate the innate immune response against any microorganism introduced in this region. The fact that significantly higher quantities of viral genomes were found in the liver, spleen, kidney, and lung of animals that died compared to those that survived tail-vein injection and that each animal had significantly elevated serum transaminase levels at time of death further support this hypothesis.
Another aspect of this study that must be considered is the surgical procedure itself. This is illustrated by the fact that LPS given via tail vein elicits a biphasic fever response in the rat while that given via a jugular cannula elicits a monophasic response (Romanovsky 1998; Rudaya 2005). Although jugular cannulation is touted as a method that is relatively non-stressful to the animal with respect to dosing and sample collection, this procedure can induce physiological changes that may evoke a mild stress response such as an increase in plasma corticosteroid levels and fluxuations in the relative amounts of specific serum proteins (Ling 2003; Terao 1983). In cannulated rats, slightly elevated levels of serum corticosteroids may dampen or mask the innate immune response to either preparation. The kinetics and the strength of the innate immune response against both adenovirus and LPS and their associated toxicity profiles correlate with their appearance in the liver and subsequent uptake by Kupffer cells (Li 2004; Smith 2008). It has recently been shown that the presence of specific blood components and serum proteins are responsible for facilitating this interaction in the context of adenovirus infection (Baker 2007). Changes in protein levels may alter this process and, in turn, change the dynamics and intensity of the immune response initiated against a given immunogen. Additional studies are currently underway in our laboratory to determine if these changes are truly happening in the cannulated rat.
During the developmental process of any potential therapeutic agent, numerous studies are initiated to provide a complete pharmacological and toxicological profile of the drug in several animal species. Regardless of the intended application, studies employing intravenous dosing methods are executed so that important pharmacokinetic parameters associated with the novel compound such as the volume of distribution and absolute bioavailability can be determined. Parameters such as this are extremely important in the context of recombinant adenovirus based therapeutics given their success in the clinic and their inherent toxicological profile. In this report, we have demonstrated that the manner by which bacterial and viruses are introduced into the systemic circulation can significantly impact overall mortality and mobundity associated with a given agent and other parameters that measured within the first 24 hours. Transgene expression in the liver after systemic administration of adenovirus generally peaks four days after administration and changes in the expression and function of hepatic CYP3A2 and 2C11 can be assessed over fourteen days in the rat (Callahan 2005). These parameters are not affected by the dosing method. Although it is known that mice can easily tolerate administration of recombinant adenoviruses at doses of 1 × 1013 vp/kg or higher (Sakurai 2008), it is not clear if the phenomenon is specific to the rat or can be applied to larger animal models. It is also not limited to the Sprague-Dawley rat since Garcia-Banuelos et al. reported similar effects in Wistar rats (Garcia-Banuelos 2002) and should be taken into consideration when designing and analyzing data obtained from toxicity studies employing rat models.
Acknowledgments
The authors would like to thank Andrew Faso, Erin Clark and Courtney Clemens for expert technical assistance with the experiments outlined in this manuscript. This work was supported by research grant R21GM69870 from the National Institutes of Health (MAC). MPB was the recipient of a University of Texas at Austin Graduate School Recruitment Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altaras NE, Aunins JG, Evans RK, Kamen A, Konz JO, Wolf JJ. Production and formulation of adenovirus vectors. Adv Biochem Eng Biotechnol. 2005;99:193–260. doi: 10.1007/10_008. [DOI] [PubMed] [Google Scholar]
- Baker AH, Mcvey JH, Waddington SN, Di Paolo NC, Shayakhmetov DM. The influence of blood on in vivo adenovirus bio-distribution and transduction. Mol Ther. 2007;15:1410–1416. doi: 10.1038/sj.mt.6300206. [DOI] [PubMed] [Google Scholar]
- Ben-Gary H, McKinney RL, Rosengart T, Lesser ML, Crystal RG. Systemic interleukin-6 responses following administration of adenovirus gene transfer vectors to humans by different routes. Mol Ther. 2002;6:287–297. doi: 10.1006/mthe.2002.0658. [DOI] [PubMed] [Google Scholar]
- Bessis N, GarciaCozar FJ, Boissier MC. Immune responses to gene therapy vectors: influence on vector function and effector mechanisms. Gene Ther. 2004;11:S10–S17. doi: 10.1038/sj.gt.3302364. [DOI] [PubMed] [Google Scholar]
- Brange J, Andersen L, Laursen ED, Meyn G, Rasmussen E. Toward understanding insulin fibrillation. J Pharm Sci. 1997;86:517–525. doi: 10.1021/js960297s. [DOI] [PubMed] [Google Scholar]
- Callahan SM, Boquet MP, Ming X, Brunner LJ, Croyle MA. Impact of transgene expression on drug metabolism following systemic adenoviral vector administration. J Gene Med. 2006;8:566–576. doi: 10.1002/jgm.884. [DOI] [PubMed] [Google Scholar]
- Callahan SM, Ming X, Lu SK, Brunner LJ, Croyle MA. Considerations for use of recombinant adenoviral vectors: dose effect on hepatic cytochromes P450. J Pharmacol Exp Ther. 2005;312:492–501. doi: 10.1124/jpet.104.075374. [DOI] [PubMed] [Google Scholar]
- Callahan SM, Wonganan P, Croyle MA. Molecular and Macromolecular Alterations of Recombinant Adenoviral Vectors Do Not Resolve Changes in Hepatic Drug Metabolism During Infection. Eur J Pharm Biopharm. 2008 doi: 10.1186/1743-422X-5-111. (in review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos SK, Barry MA. Current advances and future challenges in Adenoviral vector biology and targeting. Curr Gene Ther. 2007;7:189–204. doi: 10.2174/156652307780859062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R, Miest T, Shashkova EV, Barry MA. Reprogrammed viruses as cancer therapeutics: targeted, armed and shielded. Nat Rev Microbiol. 2008;6:529–540. doi: 10.1038/nrmicro1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K, Schenkman J. Testosterone metabolism by cytochrome P-450 isozymes RLM3 and RLM5 and by microsomes. Metabolite identification. J Biol Chem. 1983;258:11738–11744. [PubMed] [Google Scholar]
- Cocchetto DM, Bjornsson TD. Methods for vascular access and collection of body fluids from the laboratory rat. J Pharm Sci. 1983;72:465–492. doi: 10.1002/jps.2600720503. [DOI] [PubMed] [Google Scholar]
- Croyle MA, Chirmule N, Zhang Y, Wilson JM. PEGylation of E1-deleted adenovirus vectors allows significant gene expression on readministration to liver. Hum Gene Ther. 2002;13:1887–1900. doi: 10.1089/104303402760372972. [DOI] [PubMed] [Google Scholar]
- Demers GW, Johnson DE, Tsai V, Wen SF, Quijano E, Machemer T, Philopena J, Ramachandra M, Howe JA, Shabram P, Ralston R, Engler H. Pharmacologic indicators of antitumor efficacy for oncolytic virotherapy. Cancer Res. 2003;63:4003–4008. [PubMed] [Google Scholar]
- Galdiero F. Adenovirus aggregation and preservation in extracellular environment. Arch Virol. 1979;59:99–105. doi: 10.1007/BF01317899. [DOI] [PubMed] [Google Scholar]
- Garcia-Banuelos J, Siller-Lopez F, Miranda A, Aguilar LK, Aguilar-Cordova E, Armendariz-Borunda J. Cirrhotic rat livers with extensive fibrosis can be safely transduced with clinical-grade adenoviral vectors. Evidence of cirrhosis reversion. Gene Ther. 2002;9:127–134. doi: 10.1038/sj.gt.3301647. [DOI] [PubMed] [Google Scholar]
- Gooch WM, 3rd, Mogabgab WJ. Simultaneous oral administration of live adenovirus types 4 and 7 vaccines. Protection and lack of emergence of other types. Arch Environ Health. 1972;25:388–394. doi: 10.1080/00039896.1972.10666192. [DOI] [PubMed] [Google Scholar]
- Graham FL, van der Eb AJ. A New Technique for the Assay of Infectivity of Human Adenovirus 5 DNA. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Gutsmann T, Schromm AB, Brandenburg K. The physicochemistry of endotoxins in relation to bioactivity. Int J Med Microbiol. 2007;297:341–352. doi: 10.1016/j.ijmm.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Harrison LI, Gibaldi M. Physiologically based pharmacokinetic model for digoxin distribution and elimination in the rat. J Pharm Sci. 1977;66:1138–1142. doi: 10.1002/jps.2600660822. [DOI] [PubMed] [Google Scholar]
- Hartman ZC, Appledorn DM, Amalfitano A. Adenovirus vector induced innate immune responses: impact upon efficacy and toxicity in gene therapy and vaccine applications. Virus Res. 2008;132:1–14. doi: 10.1016/j.virusres.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui YH, Huang NH, Ebbert L, Bina H, Chiang A, Maples C, Pritt M, Kern T, Patel N. Pharmacokinetic comparisons of tail-bleeding with cannula- or retro-orbital bleeding techniques in rats using six marketed drugs. J Pharmacol Toxicol Methods. 2007;56:256–264. doi: 10.1016/j.vascn.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Johannessen W, Tyssebotn T, Aarbakke J. Antipyrine and acetaminophen kinetics in the rat: comparision of data on blood samples from the cut tail and a cannulated femoral artery. J Pharm Sci. 1982;71:1352–1356. doi: 10.1002/jps.2600711211. [DOI] [PubMed] [Google Scholar]
- Kreppel F, Kochanek S. Modification of adenovirus gene transfer vectors with synthetic polymers: a scientific review and technical guide. Mol Ther. 2008;16:16–29. doi: 10.1038/sj.mt.6300321. [DOI] [PubMed] [Google Scholar]
- Le HT, Boquet MP, Clark EA, Callahan SM, Croyle MA. Renal pathophysiology after systemic administration of recombinant adenovirus: changes in renal cytochromes P450 based on vector dose. Hum Gene Ther. 2006;17:1095–1111. doi: 10.1089/hum.2006.17.1095. [DOI] [PubMed] [Google Scholar]
- Li Z, Blatteis CM. Fever onset is linked to the appearance of lipopolysaccharide in the liver. J Endotoxin Res. 2004;10:39–53. doi: 10.1179/096805104225003825. [DOI] [PubMed] [Google Scholar]
- Ling S, Jamali F. Effect of cannulation surgery and restraint stress on the plasma corticosterone concentration in the rat: application of an improved corticosterone HPLC assay. J Pharm Pharm Sci. 2003;6:246–251. [PubMed] [Google Scholar]
- Maizel JV, Jr, White DO, Scharff MD. The polypeptides of adenovirus I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virol. 1968;36:115–125. doi: 10.1016/0042-6822(68)90121-9. [DOI] [PubMed] [Google Scholar]
- Morgan ET, Goralski KB, Piquette-Miller M, Renton KW, Robertson GR, Chaluvadi MR, Charles KA, Clarke SJ, Kacevska M, Liddle C, Richardson TA, Sharma R, Sinal CJ. Regulation of drug-metabolizing enzymes and transporters in infection, inflammation, and cancer. Drug Metab Dispos. 2008;36:205–216. doi: 10.1124/dmd.107.018747. [DOI] [PubMed] [Google Scholar]
- Morrissey RE, Horvath C, Snyder EA, Patrick J, MacDonald JS. Rodent nonclinical safety evaluation studies of SCH 58500, an adenoviral vector for the p53 gene. Toxicol Sci. 2002;65:266–275. doi: 10.1093/toxsci/65.2.266. [DOI] [PubMed] [Google Scholar]
- Murakami P, Pungor E, Files J, Do L, van Rijnsoever R, Vogels R, Bout A, McCaman M. A single short stretch of homology between adenoviral vector and packaging cell line can give rise to cytopathic effect-inducing helper dependent E1 positive particles. Hum Gene Ther. 2002;13:909–920. doi: 10.1089/10430340252939023. [DOI] [PubMed] [Google Scholar]
- Ozer J, Ratner M, Shaw M, Bailey W, Schomaker S. The current state of serum biomarkers of hepatotoxicity. Toxicology. 2008;245:194–205. doi: 10.1016/j.tox.2007.11.021. [DOI] [PubMed] [Google Scholar]
- Pace CN. Conformational stability of globular proteins. Trends Biochem Sci. 1990;15:14–17. doi: 10.1016/0968-0004(90)90124-t. [DOI] [PubMed] [Google Scholar]
- Rand RP, Burton AC, Ing T. The Tail of The Rat: In Temperature, Regulation and Acclimatization. Can J Physiol Pharmacol. 1965;43:257–267. doi: 10.1139/y65-025. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Kulchitsky VA, Simons CT, Sugimoto N. Methodology of fever research: why are polyphasic fevers often thought to be biphasic? Am J Physiol. 1998;275:R332–R338. doi: 10.1152/ajpregu.1998.275.1.R332. [DOI] [PubMed] [Google Scholar]
- Rudaya AY, Steiner AA, Robbins JR, Dragic AS, Romanovsky AA. Thermoregulatory responses to lipopolysaccharide in the mouse: dependence on the dose and ambient temperature. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1244–R1252. doi: 10.1152/ajpregu.00370.2005. [DOI] [PubMed] [Google Scholar]
- Sakurai H, Kawabata K, Sakurai F, Nakagawa S, Mizuguchi H. Innate immune response induced by gene delivery vectors. Int J Pharm. 2008;354:9–15. doi: 10.1016/j.ijpharm.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Seiler MP, Cerullo V, Lee B. Immune response to helper dependent adenoviral mediated liver gene therapy: challenges and prospects. Curr Gene Ther. 2007;7:297–305. doi: 10.2174/156652307782151452. [DOI] [PubMed] [Google Scholar]
- Shaw AA, Hall SD, Franklin MR, Galinsky RE. The influence of L-glutamine on the depression of hepatic cytochrome P450 activity in male rats caused by total parenteral nutrition. Drug Metab Dispos. 2002;30:177–182. doi: 10.1124/dmd.30.2.177. [DOI] [PubMed] [Google Scholar]
- Smith CN, Neptun DA, Irons RD. Effect of sampling site and collection method on variations in baseline clinical pathology parameters in Fischer-344 rats. II Clinical hematology. Fundam Appl Toxicol. 1986;7:658–663. doi: 10.1016/0272-0590(86)90115-6. [DOI] [PubMed] [Google Scholar]
- Smith JS, Tian J, Muller J, Byrnes AP. Unexpected pulmonary uptake of adenovirus vectors in animals with chronic liver disease. Gene Ther. 2004a;11:431–438. doi: 10.1038/sj.gt.3302149. [DOI] [PubMed] [Google Scholar]
- Smith JS, Tian J, Lozier JN, Byrnes AP. Severe pulmonary pathology after intravenous administration of vectors in cirrhotic rats. Mol Ther. 2004b;9:932–941. doi: 10.1016/j.ymthe.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Smith JS, Xu Z, Tian J, Stevenson SC, Byrnes AP. Interaction of systemically delivered adenovirus vectors with Kupffer cells in mouse liver. Hum Gene Ther. 2008;19:547–554. doi: 10.1089/hum.2008.004. [DOI] [PubMed] [Google Scholar]
- Terao N, Shen DD. Alterations in serum protein binding and pharmacokinetics of l-propranolol in the rat elicited by the presence of an indwelling venous catheter. J Pharmacol Exp Ther. 1983;227:369–375. [PubMed] [Google Scholar]
- Thornton CA, Ballow M. Safety of intravenous immunoglobulin. Arch Neurol. 1993;50:135–136. doi: 10.1001/archneur.1993.00540020013009. [DOI] [PubMed] [Google Scholar]
- Thrivikraman KV, Huot RL, Plotsky PM. Jugular vein catheterization for repeated blood sampling in the unrestrained conscious rat. Brain Res Brain Res Protoc. 2002;10:84–94. doi: 10.1016/s1385-299x(02)00185-x. [DOI] [PubMed] [Google Scholar]
- Top FH., Jr Control of adenovirus acute respiratory disease in U.S. Army trainees. Yale J Biol Med. 1975;48:185–195. [PMC free article] [PubMed] [Google Scholar]
- Tse F, Chang T, Finkelstein B, Ballard F, Jaffe JM. Influence of Mode of Intravenous Administration and Blood Sample Collection on Rat Pharmacokinetic Data. J Pharm Sci. 1984;73:1599–1601. doi: 10.1002/jps.2600731128. [DOI] [PubMed] [Google Scholar]
- Vähä-Koskela MJ, Heikkilä JE, Hinkkanen AE. Oncolytic viruses in cancer therapy. Cancer Lett. 2007;254:178–216. doi: 10.1016/j.canlet.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman D, Ko A, Walsh C. Regioselectivity and stereoselectivity of androgen hydroxylations catalyzed by cytochrome P-450 isozymes purified from phenobarbital-induced rat liver. J Biol Chem. 1983;258:11937–11947. [PubMed] [Google Scholar]
- Wu H, Curiel DT. Fiber-modified Adenoviruses for Targeted Gene Therapy. Methods Mol Biol. 2008;434:113–132. doi: 10.1007/978-1-60327-248-3_8. [DOI] [PubMed] [Google Scholar]
- Yang ZR, Wang HF, Zhao J, Peng YY, Wang J, Guinn BA, Huang LQ. Recent developments in the use of adenoviruses and immunotoxins in cancer gene therapy. Cancer Gene Ther. 2007;14:599–615. doi: 10.1038/sj.cgt.7701054. [DOI] [PubMed] [Google Scholar]
- Zaldumbide A, Hoeben RC. How not to be seen: immune-evasion strategies in gene therapy. Gene Ther. 2008;15:239–246. doi: 10.1038/sj.gt.3303082. [DOI] [PubMed] [Google Scholar]