Synopsis
Elevated serum eosinophil levels have been associated with multiple disorders of immune deficiency or immune dysregulation. Although primary immunodeficiency diseases (PIDD) are rare, it is important to consider these in the differential diagnosis of patients with eosinophilia. This review discusses the clinical features, laboratory findings, diagnosis, and genetic basis of disease of several disorders of immune deficiency or dysregulation – all which have documented eosinophilia as part of the syndrome. The article includes autosomal dominant hyper IgE syndrome, DOCK8 deficiency, PGM3 deficiency, ADA-SCID, Omenn syndrome, Wiskott-Aldrich syndrome, Loeys-Dietz syndrome, autoimmune lymphoproliferative syndrome, immunodysregulation, polyendocrinopathy, enteropathy, X-linked syndrome, Comel-Netherton syndrome, and severe dermatitis, multiple allergies, and metabolic wasting syndrome (SAM).
Keywords: eosinophilia, immune, deficiency, dysregulation
1 Introduction
While elevated peripheral eosinophilia can be found in patients with parasitic infection, significant atopic disease, drug hypersensitivity reactions, connective tissue disorders, malignancy, and rare hypereosinophilic syndromes, monogenic disorders of immune deficiency or dysregulation should be considered, particularly in the pediatric age group. Some of these syndromes include clinical manifestations of atopy, such as atopic dermatitis or food allergy, which may contribute to the eosinophilia; however the mechanism driving the eosinophilia is not well understood. Many of these monogenic diseases are characterized by increased production of Th2 cytokines, such as IL-5, which is an essential promoter of eosinophil differentiation, maturation and survival [1].
This article reviews several disorders of immune deficiency or dysregulation that have documented eosinophilia as part of the syndrome (Figure 1). The clinical features, common infections, laboratory findings, diagnostic methods, and genetic basis of disease of each syndrome will be discussed.
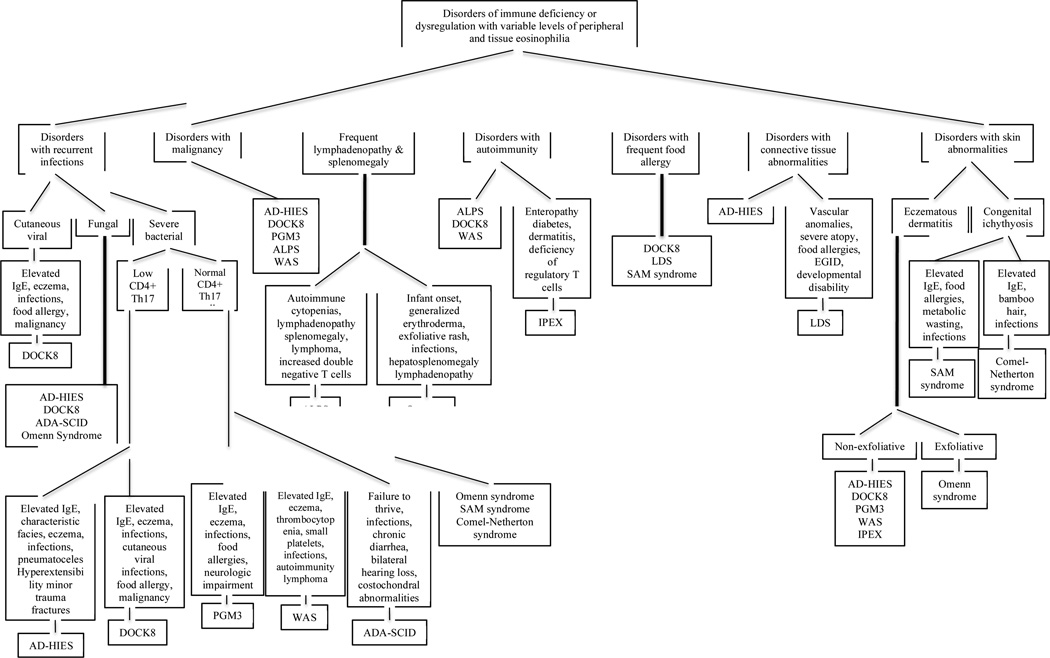
Figure 1.
Flow diagram showing disorders of immune deficiency or dysregulation with variable levels of peripheral and tissue eosinophilia. Characteristics of each disorder are listed once under the most prevalently featured category. AD-HIES: autosomal dominant hyper IgE syndrome, DOCK8: Dedicator of cytokinesis 8, PGM3: Phosphoglucomutase 3, ADA-SCID: Adenosine deaminase-severe combined immunodeficiency, ALPS: autoimmune lymphoproliferative syndrome, IPEX: Immunodysregulation, polyendocrinopathy, enteropathy, X-linked syndrome, LDS: Loeys-Dietz syndrome, SAM: severe dermatitis, multiple allergies, and metabolic wasting, WAS: Wiskott-Aldrich syndrome
2 Syndromic causes of elevated IgE and eosinophilia
2.1 Autosomal dominant HIES
Job’s syndrome was first described in 1966 with two patients who had recurrent staphylococcal abscesses, similar to the boils borne by the prophet Job in the Bible [2]. This clinical syndrome, which was first characterized as a triad of recurrent staphylococcal abscesses, pulmonary infections, and an eczematous dermatitis, was later found to be associated with elevated serum IgE levels leading to the name autosomal dominant Hyper IgE syndrome (AD-HIES) [3].
2.1.1 Clinical features, infections, and management
AD-HIES typically presents within the first few days of life as neonatal acne or erythema toxicum neonatorum secondary to the pustular rash that often encompasses the face, scalp, and upper body [4, 5]. Histologically, the skin infiltration is predominantly eosinophils [6]. The rash usually evolves to resemble an eczematous dermatitis, which is papular, pruritic, lichenified, and typically driven by Staphylococcus aureus colonization and superinfection [7].
Patients with AD-HIES classically have recurrent, “cold” S. aureus abscesses, which have frank pus when excised despite their lack of dolor, rubor, and calor [2]. Recurrent sinopulmonary infections generally start in the first several years of life with S. aureus being the most common pathogen implicated in the pneumonias. Streptococcus pneumoniae and Haemophilus influenzae also occur frequently, and the first presentation of pneumonia in infancy may be caused by Pneumocystis jirovecii [8, 9]. As with the cold abscesses, AD-HIES patients with pneumonia lack systemic signs of inflammation, including fever, frequently delaying diagnosis leading to parenchymal lung damage (Figure 2). Pneumatoceles and bronchiectasis increase the patients’ susceptibility to difficult to treat microbes, like Aspergillus, Scedosporium, Pseudomonas, and nontuberculous mycobacteria, which contribute significantly to their morbidity and mortality [9–11].
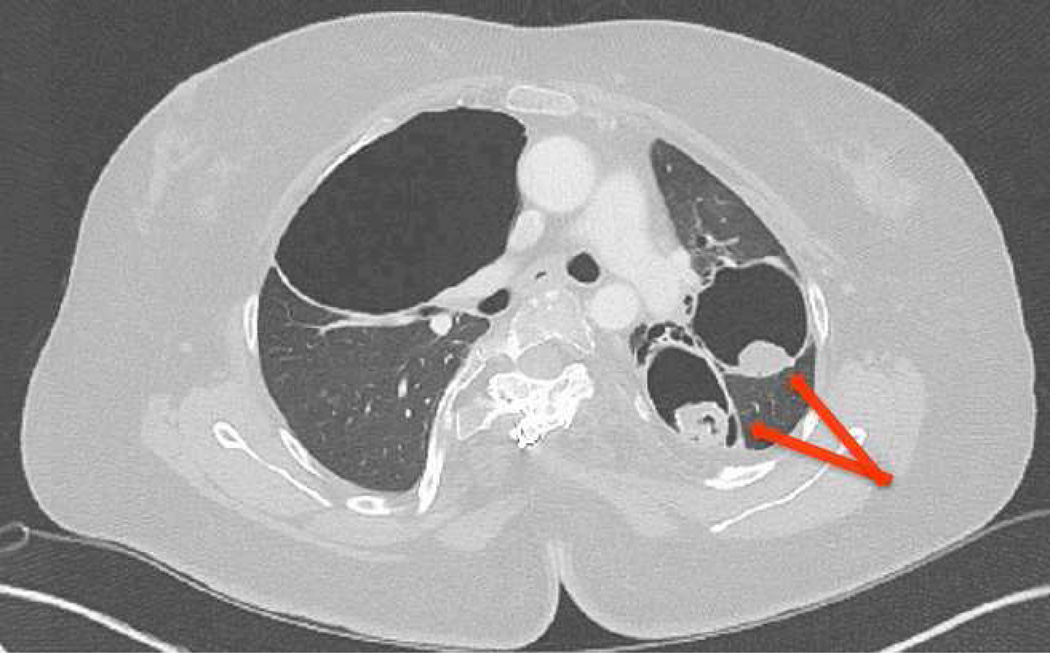
Figure 2.
Parenchymal lung findings and complications in AD-HIES. Chest CT from a 43-year-old woman with AD-HIES, multiple pneumatoceles and an evident aspergilloma (arrows).
Fungal susceptibility is apparent with more than 80% of patients having chronic mucocutaneous candidiasis [12] (Figure 3). Unlike patients with DOCK8 deficiency, those with AD-HIES do not commonly have severe viral infections (Table 1) [13, 14].
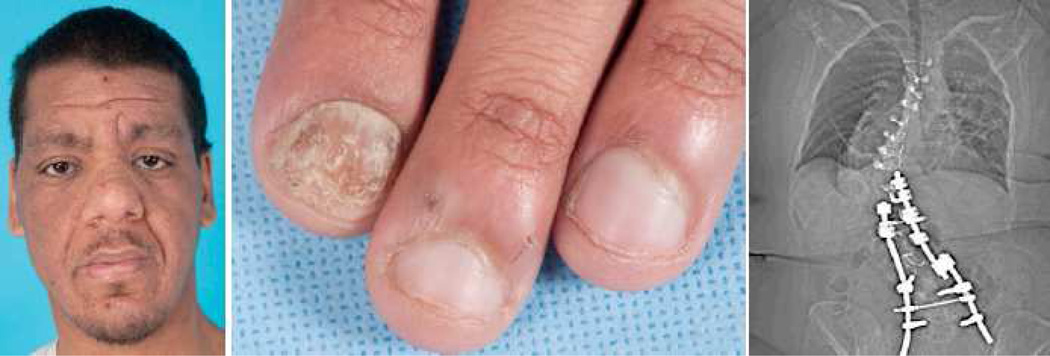
Figure 3.
Characteristic clinical features found in AD-HIES. From left to right: characteristic facies, chronic mucocutaneous candidiasis, and severe scoliosis.
Table 1.
Comparison of the clinical features among the key syndromes of elevated IgE: STAT3, DOCK8, and PGM3
| STAT3 | DOCK8 | PGM3 | |
|---|---|---|---|
| Elevated serum IgE level | +++ | +++ | +++ |
| Peripheral eosinophilia | +++ | +++ | + |
| Low CD4+ Th17 cells | +++ | ++ | − |
| Newborn rash | +++ | + | + |
| Eczematous dermatitis | +++ | +++ | +++ |
| Recurrent skin abscesses | +++ | ++ | ++ |
| Recurrent pneumonias | +++ | ++ | +++ |
| Mucocutaneous candidiasis | +++ | ++ | − |
| Cutaneous viral infections | + | +++ | + |
| Parenchymal lung changes | +++ | + | ++ |
| Asthma | − | ++ | ++ |
| Food allergies | + | +++ | ++ |
| Eosinophilic GI disease | ++ | ++ | + |
| Characteristic facies | +++ | − | + |
| Retained primary teeth | +++ | − | − |
| Scoliosis | +++ | − | ++ |
| Minimal trauma fractures | +++ | − | − |
| Hyperextensibility | +++ | − | + |
| Vascular abnormalities | ++ | − | − |
| Autoimmunity | + | ++ | ++ |
| Malignancy | ++ | +++ | ++ |
Despite the very elevated levels of serum IgE characteristic of these patients, allergy and asthma are not typically severe or difficult to manage in AD-HIES. Siegel et al. reported a diminished allergic phenotype in patients with AD-HIES compared to other patients with a comparably elevated IgE and atopic dermatitis, although allergies are more frequent than those with normal IgE levels [15].
AD-HIES is a multi-system disease with many non-immunologic abnormalities. A characteristic facial appearance usually emerges during adolescence with porous skin, a prominent forehead, deep-set eyes, and a bulbous and broad nose (Figure 3). Most patients fail to shed their primary teeth, requiring medical removal to allow the secondary teeth to emerge normally. Patients also have a high-arched hard palate and palatal and lingual ridges or grooves [8, 10, 16].
Extensive musculoskeletal abnormalities include scoliosis, osteopenia or osteoporosis, minimal trauma fractures, craniosynostosis, and joint hyperextensibility [8, 17] (Figure 3). Joint hyperextensibility occurs in more than two-thirds of AD-HIES patient and may contribute to the high frequency of degenerative bone disease [12].
Recently, manifestations of gastrointestinal disease in AD-HIES patients have been described. In a cohort of 70 individuals, nearly two-thirds of those with AD-HIES who underwent gastrointestinal endoscopy had eosinophilic esophagitis as defined by the updated consensus guidelines [18, 19]. Food allergy was not as common as in DOCK8 deficiency [18].
As with several other primary immunodeficiency diseases, patients with AD-HIES are at increased risk of developing malignancy, particularly non-Hodgkin’s lymphoma [13, 20].
Because the clinical impact of recurrent infections can be profound, the primary focus in management is treatment of infections and prophylaxis to prevent future infections, especially with S. aureus. Suppression of skin colonization with S. aureus through antiseptics, such as dilute bleach baths and chlorhexidine washes, frequently leads to minimal dermatitis. Patients with chronic mucocutaneous candidiasis, or in areas endemic for Coccidioides or histoplasmosis, may also benefit from antifungal prophylaxis.
Parenchymal lung disease and subsequent chronic infection with molds, such as Aspergillus, and Gram-negative bacteria, such as Pseudomonas, contribute to most cases of death in AD-HIES [11]. Because of this, pyogenic pneumonias should be aggressively diagnosed and treated to minimize parenchymal damage.
Immunoglobulin replacement should be considered in those patients with impaired specific antibody responses, bronchiectasis and breakthrough infections while on prophylaxis. Hematopoietic stem cell transplant (HSCT) has been performed infrequently as a possible curative treatment for AD-HIES with varying clinical results [21–23].
2.1.2 Clinical laboratory findings
In addition to the hallmark laboratory finding of elevated serum IgE levels, most patients with AD-HIES have peripheral eosinophilia. IgE levels are often greater than 2000 IU/mL, although may decrease and even normalize as adults, and absolute eosinophil counts are quite variable but frequently greater than 700 cells/µL. Despite this, the underlying etiology of these elevations is still unclear, and the serum eosinophil levels have not correlated with serum IgE level or clinical phenotype. In addition to the peripheral eosinophilia, increased eosinophils have been identified in sputum and abscesses.
Generally, patients with AD-HIES have normal white blood cell counts, although absolute mild neutropenia and leukopenia are fairly common. Serum IgG and IgM levels are often normal, whereas serum IgA may be normal or low. Specific antibody responses to vaccines are variable among patients with AD-HIES, with some patients producing little or no response [12, 24, 25].
2.1.3 Genetics and pathogenesis
Dominant-negative mutations in STAT3 cause AD-HIES. These mutations are primarily missense or single-codon in-frame deletions that result in single amino acid changes [24, 26].
Identifying the genetic mutation in STAT3 has offered important insight on the clinical phenotype. STAT3 is a transcription factor that plays an essential role in the signal transduction of many cytokines, including IL-6, IL-10, IL-21, IL-22, and IL-23. STAT3 is activated by Janus kinase 2 and phosphorylated in order to dimerize and translocate into the nucleus to initiate targeted gene transcription [27, 28]. Because of its involvement with many cytokines, STAT3 plays a crucial role in immunity, inflammation, wound healing, cell survival, embryogenesis, and oncogenesis, with disruption leading to the multisystem nature of AD-HIES.
The most consistent immunologic finding in these patients is a lack of CD4+ Th17 cell differentiation [29–32]. The importance of IL-17 in the clearance of Candida, Klebsiella, and Staphylococcus aureus has been established in mice, and in humans disruption of the IL-17 and IL-22 pathway leading to mucoctuaneous candida susceptibility is evident through several PIDD [33–38].
AD-HIES is also associated with diminished memory T and B lymphocytes. Decreased central memory CD4+ and CD8+ T lymphocytes are clinically evident by the reactivation of latent viral infections, resulting in an increased incidence of zoster and asymptomatic EBV viremia. Decreased memory and class switched B cell is frequently seen as well [3, 25, 39].
2.2 DOCK8 deficiency
2.2.1 Clinical features, infections, and management
DOCK8 mutations were described in 2009 in a subset of patients with an autosomal recessive inheritance pattern of many features of AD-HIES, although lacking most of the skeletal and connective tissue abnormalities [40]. The many clinical characteristics of DOCK8 deficiency include atopic dermatitis, food or environmental allergies, marked IgE and eosinophil elevations, recurrent sinopulmonary infections, recurrent Staphylococcal skin infections or abscesses, mucocutaneous candidiasis, and, distinctly, a breadth of disseminated cutaneous viral infections. Additionally, a significant portion of patients with DOCK8 deficiency went on to develop malignancies, some fatal, likely resulting from the oncogenic properties of the cutaneous viruses and a dysfunction in tumor surveillance [41, 42].
Many patients with DOCK8 deficiency have an exaggerated atopic phenotype when compared to those with AD-HIES, and anaphylaxis and food allergy are more common [15, 41, 43, 44]. Nearly all patients with DOCK8 deficiency (99%) have an eczematous dermatitis that begins in infancy, but less commonly a newborn rash [13, 45, 46]. The immunologic mechanism driving their atopy and eosinophilia is unclear, but is associated with a predominance of Th2 cytokine production and regulatory T lymphocyte deficiency [45, 47].
Nearly 90% of affected patients have recurrent and even concurrent cutaneous viral infections, namely with human papillomavirus, herpes simplex virus, molluscum contagiosum virus, and varicella zoster virus (Figure 4) [41, 43, 44, 48]. Severe systemic viral infections are less frequent [44, 49].
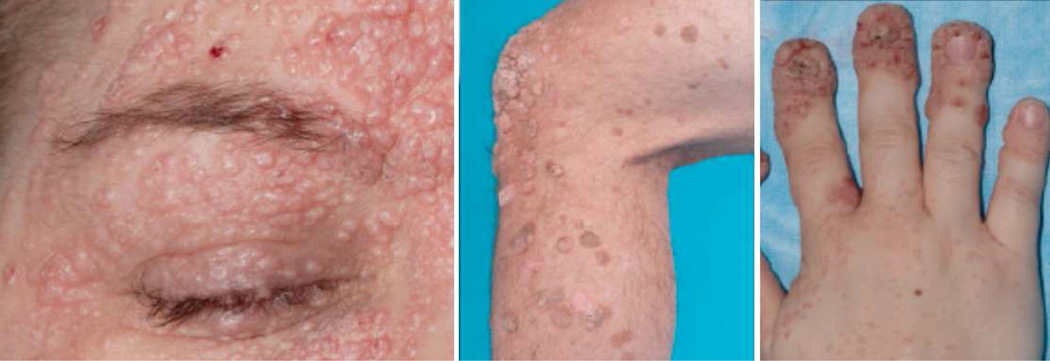
Figure 4.
Cutaneous viral infections seen in DOCK8 deficiency. From left to right: disseminated Molluscum contagiosum due to molluscum contagiosum virus and verrucous and flat warts due to human papillomavirus.
The majority of patients with DOCK8 deficiency (>90%) have recurrent upper respiratory tract infections, pneumonias, sinusitis, and otitis media. Pulmonary pathogens include the more common Streptococcus pneumoniae and Haemophilus influenzae, but the spectrum broadens when bronchiectasis is present [13, 41, 43, 44]. Fungal infections include mucocutaneous candidiasis, cryptococcal meningitis, disseminated histoplasmosis, and Pneumocystis jirovecii [41, 44, 49]. Eosinophilic pneumonias have also been seen in DOCK8 deficiency. Autoimmunity in DOCK8 deficiency has included difficult to treat autoimmune hemolytic anemia, hypothyroidism, and vasculitis [49].
DOCK8 deficiency has a worse prognosis than AD-HIES, with most patients dying in the 2nd and 3rd decade of life. To date, the only curative treatment for DOCK8 deficiency is HSCT, and this should be strongly considered for these patients. Those who have been transplanted had near or complete resolution of their cutaneous viral infections and improvement in their T lymphocyte populations and function, eczema, and recurrent sinopulmonary within a year of transplantation [43, 50–58].
Management of DOCK8 deficiency focuses on treating and preventing infections in a manner similar to those with AD-HIES. Immunoglobulin replacement therapy is frequently indicated due to poor specific antibody production [41, 59]. Acyclovir or valacyclovir should be considered for prophylaxis to prevent recurrent HSV and VZV infections. Warts and molluscum are difficult to treat in this population, and standard therapies tend to be ineffective; interferon-α has had variable benefit [6, 13, 60].
2.2.2 Clinical laboratory findings
Peripheral eosinophilia, typically greater than 1000 cells/µL is common, as is elevated serum IgE. The etiology of the eosinophilia and elevated IgE levels is not well understood. Serum levels of IgM are classically very low or even undetectable [41, 43, 44, 49]. Serum IgG levels are often normal or slightly elevated, whereas serum IgA levels are variable with typically poor specific antibody responses [41].
The combined immune deficiency in DOCK8 patients is apparent with CD4+ and CD8+ T cell lymphopenia that often worsens with age, and variability in NK cell and B lymphocyte deficiency [41, 42]. Additionally, T lymphocyte activation and proliferation are diminished, particularly in the CD8+ population, thus contributing their increased viral susceptibility [41, 44]. Diagnosis is confirmed with DOCK8 sequencing, and can be strongly suggested by absence of expression on flow cytometry [41, 61].
2.2.3 Genetics and pathogenesis
Mutations in DOCK8 render most patients protein negative [41, 44, 49]. Recently revertant mutations have been shown, but the clinical significance remains unknown [62]. DOCK8 belongs to the DOCK180 superfamily of guanine nucleotide exchange factors [63]. These guanine nucleotide exchange factors are known to interact with the Rho family of GTPases, which can be found in all eukaryotic cells and have an important role in organelle and cytoskeletal development, cell migration, phagocytosis, and wound healing [64, 65]. We now know that DOCK8 plays a critical immunologic role, specifically in T, B lymphocyte, and natural killer T cell survival, migration, and synapse formation [42, 66, 67].
2.3 PGM3 deficiency
2.3.1 Clinical features, infections, and management
Mutations in Phosphoglucomutase 3 (PGM3) were recently defined leading to a multisystem disease sharing some clinical features with AD-HIES and DOCK8 deficiency. The initial clinical description of patients with this disease was the report of an autosomal recessive vasculitis-myoclonus syndrome associated with infection and severe atopy in a family with five affected children [68, 69]. The clinical phenotype of PGM3 deficiency is still being defined, but cutaneous leukocytoclastic vasculitis, eczema-like rash, cognitive impairment and myoclonus, and delayed visual and sensory evoked potentials have all been present, as well as severe bronchiectasis [69, 70].
Poor control of viruses has presented as widespread molluscum contagiosum in one affected individual and poor control of EBV, with persistent EBV viremia and EBV associated Hodgkin’s lymphoma in two individuals [69, 70]. Clinical variability is evident by the cohort with B and T lymphopenia similar to that seen in severe combined immunodeficiency (SCID), neutropenia and progression to bone marrow failure [71]. Autoimmune and immune-mediated diseases have included membranoproliferative glomerulonephritis, autoimmune neutropenia, psoriasis and vasculitis. Skeletal abnormalities have manifested as scoliosis, facial dysmorphism, and hyperextensibility. Cognitive impairment appears common and demyelination has been observed on brain MRI for several patients [69–71].
Treatment of PGM3 deficiency is currently supportive. Prophylactic or suppressive antimicrobials should be considered based on clinical presentation. Immunoglobulin replacement may be considered if poor specific antibodies are present, however anecdotal reports of improvement have been mixed. Transplant improved the bone marrow failure and immunologic phenotype in two patients, but the impact on the neurologic and skeletal phenotype is unclear [71]. Dietary supplementation has been used in other congenital disorders of glycosylation, but has not yet been established for this defect [72].
2.3.2 Clinical laboratory findings
Most individuals identified with PGM3 deficiency have had significantly elevated serum IgE, variable eosinophilia and immune abnormalities. Leukopenia appears common with both lymphopenia and neutropenia. The lymphopenia is predominantly from decreased CD8+ T cells and low memory B cells. IgG, IgE and IgA have all been increased, and specific antibodies have been present but less robust than frequently seen in healthy controls. Consistent with the atopic clinical phenotype, the T cell cytokine responses have a Th2 skew with increased secretion ex vivo of IL-4, IL-5 and IL-13. Compared to DOCK8 deficiency and AD-HIES, the T lymphocyte production of IL-17 appears increased, which may explain some of the autoimmunity features and the lack of candidiasis [63, 70].
PGM3 deficiency should be suspected in an individual with an exaggerated allergic phenotype, recurrent infections and neurologic deficits. Glycosylation defects can also be suggested by glycan profiling of urine and serum. In PGM3 deficiency, both O and N-linked glycans are abnormal. Sequencing of PGM3 is key to the diagnosis.
2.3.3 Genetics and pathogenesis
PGM3 is an enzyme responsible for the conversion of GlcNAc-6-phosphate to GlcNAc-1-phosphate, which then converts to UDP-GlcNAc. This is a key early step in multiple glycosylation pathways, and thus essential to allow normal activity of many diverse proteins. As the majority of proteins require glycosylation, absence of activity would not be compatible with life and is embryonic lethal in mice. The disease is autosomal recessive, and all mutations found have been hypomorphic, allowing diminished but present activity. A mouse model of PGM3 deficiency caused by hypomorphic point mutations led to lymphopenia with T lymphocytes predominantly affected, anemia and thrombocytopenia [73].
2.4 Wiskott-Aldrich syndrome
2.4.1 Clinical features, infections, and management
Peripheral and tissue eosinophilia (e.g. in lymph nodes and spleen) are characteristic of Wiskott-Aldrich syndrome (WAS) [74, 75]. WAS was first described in 1954 with its X-linked inheritance pattern of severe eczema, thrombocytopenia with small platelets, and recurrent infections [76]. A wide variety of infections have been reported in WAS and include Pneumocystis jirovecii pneumonia, severe and/or disseminated HSV and varicella, and invasive fungal infections [77]. Because of the significant thrombocytopenia, patients can present with bleeding, petechiae or ecchymoses. Autoimmunity (e.g. autoimmune cytopenias, inflammatory bowel disease, renal disease) and malignancy (notably lymphoma) are seen as well [78]. Similar to those with DOCK8 deficiency, vasculitis and aortic aneurysm have also been described [79, 80].
Morbidity and mortality related to WAS are high if not properly treated. Death is usually secondary to infection, bleeding or malignancy [77]. Because of the poor antibody responses, immunoglobulin replacement therapy is often helpful in reducing serious infections. The immunoglobulin therapy has not shown appreciable impact on the thrombocytopenia, although splenectomy has led to normalization of platelet counts [81, 82]. Those who are status-post splenectomy should receive appropriate prophylaxis with vaccination and penicillin. HSCT or gene therapy should be considered [83, 84].
2.4.2 Clinical laboratory findings
Complete blood counts almost always reveal thrombocytopenia with microthrombocytes on peripheral smear. Peripheral eosinophilia and anemia are often observed, and as with other PIDD, the mechanism driving the eosinophilia is unclear. Typically, serum IgM is decreased, serum IgG and IgA are variable, and serum IgE is elevated. Poor polysaccharide vaccine responses are characteristic [74, 77, 83, 85].
Flow cytometry typically reveals mild T cell lymphopenia, normal circulating B lymphocytes, and normal or increased NK cells. Poor lymphocyte proliferation and NK cytotoxicity is characteristic as WASp (WAS protein) is essential for colocalizing with actin to NK cell activating synapses [83, 85]. In addition to sequencing, flow cytometry is also used to evaluate WASp expression in suspected cases of WAS [83].
2.4.3 Genetics and pathogenesis
Mutations in the gene WAS, located on the X-chromosome, have been identified as causing deficiency in WASp. WASp is a crucial regulator of platelet and lymphocyte development and is critical for cytoskeletal and immunologic synapse formation and regulatory T-cell function [86–88]. Genotype-phenotype correlations have been described with those having X-linked thrombocytopenia and X-linked neutropenia having distinct and often milder phenotypes [89].
3 Severe combined immunodeficiency and related disorders associated with eosinophilia
3.1 ADA-SCID
3.1.1 Clinical features, infections, and management
While eosinophilia is not a prominent feature in all variants of SCID, peripheral eosinophilia is a commonly encountered clinical manifestation seen in ADA-SCID. Because of the profound T, B, and NK cell lymphopenia seen in ADA-SCID, affected individuals typically present in infancy with severe opportunistic infections, such as with Pneumocystis jirovecii pneumonia. Recurrent and/or severe respiratory infections typically occur within the first few months of life, along with failure to tSCIDe, frequent thrush, and chronic diarrhea [85, 90, 91]. Partial ADA deficiency has been described in a subset of patients who typically present later in life, such as in late infancy or early childhood, with a milder phenotype that may include autoimmune manifestations [92].
Atopy is a common feature found in ADA-SCID, found in about half of those with early onset disease. Of the atopic manifestations, allergic rhinitis, asthma, food allergy, mild atopic dermatitis, and urticaria were the most common identified in this population [93].
Given the ubiquitous nature of ADA expression and the profound impacts of deoxydenosine, S-adenosylhomocysteine, and deoxyadenosinetriphosphate accumulation within the tissues, patients with ADA deficiency often present with other systemic manifestations, such as hepatic degeneration and dysfunction, costochondral abnormalities, and skeletal dysplasia [90, 94]. Neurologic motor and hearing impairments (i.e. bilateral sensorineuronal deafness) have also been associated with ADA deficiency, and patients may have cognitive and behavioral deficits [95, 96].
Without early recognition and treatment, ADA-SCID is often fatal within the first year of life [90, 97]. To date, the treatment options for ADA deficiency include allogeneic hematopoietic stem cell therapy, enzyme replacement therapy with pegylated bovine ADA, and gene therapy [98].
3.1.2 Clinical laboratory findings
Patients with ADA-SCID have a severe combined immunodeficiency with a profound deficiency of circulating T lymphocytes, B lymphocytes, and NK cells, more so than any other form of SCID [90, 97]. Serum IgG is usually normal at presentation due to maternal transfer of IgG, but decreases soon after. Serum IgA and IgM levels are variable, although more commonly IgM is low in this population [97]. Serum eosinophilia and IgE levels are also variable but frequently elevated among both those with early and delayed onset, presumably due to their increased CD4+ Th2 cytokine production and their clinical features of atopy [91, 93, 99].
When ADA-SCID is suspected, ADA activity should be assessed and will be low or absent. Additionally, levels of S-adenosylhomocysteine and deoxyadenosinetriphosphate will be elevated in ADA-SCID. Newborn screening for SCID through a quantitative evaluation of T-cell receptor excision circles (TREC) is helpful in identifying infants with SCID that can be further screened for ADA and other etiologies [100, 101].
3.1.3 Genetics and pathogenesis
Accounting for 10–20% of all SCID cases, ADA-SCID results from autosomal recessive mutations in the ADA gene [97, 102]. ADA is an enzyme that is crucial in the purine salvage pathway, catalyzing the deamination of deoxydenosine and adenosine to deoxyinosine and inosine. In an ADA-deficient state, there is build up of deoxydenosine, S-adenosylhomocysteine, and deoxyadenosinetriphosphate in the intra- and extracellular compartments, ultimately leading to impaired T and B lymphocyte development. In addition to inducing thymocyte apoptosis, deoxyadenosinetriphosphate interferes with terminal deoxynucleotidyl transferase activity and restricts V(D)J recombination [97, 103, 104].
3.2 Omenn syndrome
3.2.1 Clinical features, infections, and management
Similar to early-onset ADA-SCID, Omenn syndrome presents in infancy with peripheral eosinophilia, high serum IgE and rash. The rash is typically a generalized and often exfoliative erythroderma in contrast to the pustular or erythema toxicum neonatorum rash seen in AD-HIES [105]. Impairment of V(D)J recombination leads to abnormally expanded T lymphocytes. Affected infants commonly present with recurrent and severe infection, chronic diarrhea, lymphadenopathy, hepatosplenomegaly, and failure to thrive [47].
HSCT can be curative in Omenn syndrome, and without early transplant the disease is often fatal; however, in addition to pre-transplant supportive care with immunoglobulin replacement therapy and prophylactic antimicrobials, these patients also require systemic immunosuppression for their marked lymphoproliferation and inflammation [84, 100, 106–108].
3.2.2 Clinical laboratory findings
Patients with Omenn syndrome have elevated serum IgE and profound eosinophilia, likely resulting from Th2 skewing, despite the low or absent circulating B lymphocytes, decreased IgG, decreased IgM, and decreased IgA [105]. In contrast to classical SCID, patients have normal or even markedly increased circulating activated T lymphocytes; however they are oligoclonal and associated with poor antigenic responses [109–111].
3.2.3 Genetics and pathogenesis
Omenn Syndrome appears to be caused by hypomorphic mutations in genes associated with SCID. These have included include RAG1/2, Artemis, IL-7Rα, DNA ligase IV, RNA-processing endoribonuclease, ADA, and γc [85, 111]. Patients with atypical DiGeorge syndrome (due to a microdeletion at chromosome 22q11.2) may also present with a clinical phenotype that resembles Omenn syndrome [112–114].
4 Immune dysregulatory syndromes associated with eosinophilia
4.1 Autoimmune lymphoproliferative syndrome
4.1.1 Clinical features, infections, and management
While eosinophilia is often not the clinical feature driving presentation of patients with autoimmune lymphoproliferative syndrome (ALPS), eosinophilia is a common manifestation. ALPS typically presents in childhood with marked splenomegaly, generalized lymphadenopathy, and chronic cytopenias. Autoimmune hemolytic anemia, neutropenia and thrombocytopenia are typical. Patients with ALPS are at increased risk for developing B and T cell lymphomas due to the marked lymphoproliferation. In a recent natural history of ALPS study done at the NIH, 12% of the cohort developed either Hodgkin’s or non-Hodgkin’s lymphoma [115, 116].
The management of ALPS focuses on three main aspects of the syndrome: controlling the cytopenias, preventing infection, and malignancy surveillance. Additionally, spleen guards should be worn to protect against rupture and splenectomy should be avoided [117].
4.1.2 Clinical laboratory findings
The unique laboratory finding, which is required for and ALPS diagnosis, is elevated CD3+ αβ CD4-CD8- double negative T lymphocytes (>1.5% of total lymphocytes or >2.5% of CD3+ lymphocytes). Total lymphocyte counts will also be normal or elevated [115, 116]. Serum vitamin B12 levels are elevated in ALPS and have proven to be a reliable biomarker for lymphoproliferation. Patients with ALPS often have elevated serum immunoglobulins (namely IgG, IgA, and IgM) [115]. Anemia, neutropenia or thrombocytopenia are common due to the autoimmunity associated with the syndrome. Autoantibodies are frequently positive, including rheumatoid factor, direct antiglobulin test, anticardiolipin antibody, antithyroid antibodies, and antinuclear antibodies.
Approximately 25% of ALPS patients will have peripheral eosinophila (>750 cells/µL) [115, 116]. The cause of the eosinophilia is likely a result of Th2 skewing that produces an increase in IL-5. Fas-mediated apoptosis of eosinophils (as assessed in vitro) is impaired in patients with ALPS, with and without eosinophilia, suggesting this is not the cause of the observed eosinophilia [118]. Patients with eosinophilic ALPS also have more profound cytopenias and are at significantly increased risk for death due to infectious complications from their immunosuppression [118].
4.1.3 Genetics and pathogenesis
The majority of ALPS cases are due to heterozygous mutations in the FAS gene, which encodes the tumor necrosis factor receptor superfamily-6 protein (TNFRSF6; Fas). Somatic or germline mutations in FAS-ligand, FAS-associated death domain, caspase 8, and caspase 10 have also been identified to cause ALPS. These mutations lead to defective lymphocyte apoptosis and thus lymphoproliferation [115, 119]. An ALPS-like phenotype can also be caused by germline gain-of-function STAT3 mutations [120].
4.2 Immunodysregulation, polyendocrinopathy, enteropathy, X-linked syndrome
4.2.1 Clinical features, infections, and management
Patients with immunodysregulation, polyendocrinopathy, enteropathy, X-linked syndrome frequently present with eosinophilia and severe atopy [121]. In addition, the classic triad of enteropathy, endocrinopathy, and dermatitis is observed [122]. Most commonly these patients present with early onset severe and watery diarrhea, type 1 diabetes mellitus, and failure to thrive [123]. Endoscopic biopsies of the gastrointestinal tract reveal villous blunting and lymphocytic infiltration, similar to that described in celiac disease and graft versus host disease [124]. The dermatitis resembles that of eczema and may vary in severity. IPEX patients have frequent autoimmunity, recurrent infections largely from immunosuppression, and may have food allergy [123, 125].
4.2.2 Clinical laboratory findings
Many of these patients have elevated serum IgE, elevated serum IgA, peripheral eosinophilia, and normal T and B lymphocyte subsets; however, they lack the essential CD4+CD25+ FOXP3+ regulatory T lymphocytes. Regulatory T lymphocytes have been shown to secrete IL-10 and TGF-β, and lead to suppression of serum IgE [126]. Presumably the deficiency in regulatory T lymphocytes contributes to the elevated IgE levels.
4.2.3 Genetics and pathogenesis
Mutations in both the coding and non-coding regions of FOXP3 have been identified in the majority of patients with IPEX. FOXP3 encodes the protein scurfin, a transcription factor which is the master regulator in the development and functioning of regulatory T lymphocytes [127, 128]. Mice lacking FOXP3 (scurfy mice) share many characteristics of patients with IPEX [127]. The lack of regulatory T lymphocytes leads to the loss of peripheral tolerance and marked cytokine overproduction in effector T lymphocytes.
In addition to sequencing, flow cytometry is often used as a clinical tool to evaluate FOXP3 protein expression in suspected cases of IPEX [123]. IPEX-like phenotypes including eosinophilia can also be seen in patients with CD25 deficiency and gain-of-function mutations in STAT1 or STAT3 [120, 129, 130].
4.3 Loeys-Dietz syndrome
4.3.1 Clinical features, infections, and management
Loeys-Dietz syndrome (LDS) is a connective tissue disorder with multisystem involvement, a large segment of which develops significant atopy, and eosinophilia. There is a great deal of clinical overlap between AD-HIES and LDS. Musculoskeletal abnormalities are variable and can include craniosynostosis, retained primary dentition, facial asymmetry, pectus deformity, scoliosis, flat feet, and joint hyperextensibility. Additionally, patients may have a high arched or cleft palate, an abnormal uvula, and hypertelorism. Their skin is classically thin and translucent, and easy bruising and poor wound healing are not uncommon [131, 132]. Vascular anomalies are a prominent feature of LDS. Diffuse arterial abnormalities, such as aneurysms and tortuosity, put patients at great risk for dissection and/or hemorrhages [131, 133].
LDS patients have an exaggerated allergic phenotype, which can include asthma, food allergy, atopic dermatitis, and allergic rhinitis. Gastrointestinal complaints, such as chronic abdominal pain, poor growth, constipation, and vomiting, are common among patients with LDS. Eosinophilic gastrointestinal disease (e.g. eosinophilic esophagitis, colitis, and/or gastritis) is often diagnosed pathologically. Targeted elimination diets appear to be beneficial in reducing clinical symptoms [132, 134].
4.3.2 Clinical laboratory findings
Elevated serum IgE and absolute eosinophil counts are prevalent laboratory findings in patients with LDS. These findings may be in part due to the Th2 skewing that is apparent in this population. The Th2 cytokine production is associated with an increased CD4+CD25+ FOXP3+ regulatory T lymphocyte population, which abnormally produces Th2 cytokines [134].
4.3.3 Genetics and pathogenesis
To date, four genetic mutations have been identified in LDS. These include heterozygous mutations in TGFBR1, TGFBR2, SMAD3, and TGFB2. Each mutation results in altered TGF-β signaling, yielding abnormal collagen and connective tissue growth, and effects on lymphocyte differentiation [131–134]. Despite the genetic and phenotypic variation seen in the LDS types, medical management is similar [132].
5 Dermatologic syndromes with immunodysregulation associated with eosinophilia
5.1 Comel-Netherton syndrome
5.1.1 Clinical features, infections, and management
Comel-Netherton syndrome is a dermatologic condition associated with an exaggerated allergic phenotype including severe atopic dermatitis, allergic rhinitis, peripheral eosinophilia and elevated serum IgE. The hallmark clinical features of Comel-Netherton syndrome are severe congenital ichthyosis and the pathognomonic bamboo hairs, known as trichorrexis invaginata. The extent of skin involvement varies and may include an erythroderma or migrating and scaly plaques [135–137]. The skin lesions are usually quite pruritic secondary to the profound skin barrier defect and enhanced inflammation. S. aureus skin infections are frequent. Additional findings associated with Comel-Netherton syndrome include failure to thrive, sparse or absent hair, eyebrows and eye lashes at birth, and chronic diarrhea [136, 137].
Mortality is highest in the neonatal period from sepsis, dehydration or malnutrition [136]. Management is aimed at systemic and cutaneous symptom management. Emollients are essential for their skin, and adequate nutrition and hydration is critical. Intravenous immunoglobulin and anti-TNF-α monoclonal antibodies have been effective in reducing skin inflammation [137, 138].
5.1.2 Clinical laboratory findings
Elevated serum IgE and absolute eosinophil counts are common and lymphocyte phenotyping may reveal increased NK cells and decreased switched and unswitched memory B cells found [136, 137, 139]. Poor NK cell cytotoxicity has also been identified [137]. Histopathology of skin biopsy reveals epidermal hyperplasia, minimal granular layer, and typically stratum corneum detachment [136].
5.1.3 Genetics and pathogenesis
Comel-Netherton syndrome stems from loss-of-function mutations in SPINK5, which encodes the serine protease inhibitor LEKTI [136, 140, 141]. LEKTI is expressed in both the epithelia and the thymus. Murine models have shown that LEKTI deficiency leads to unopposed kallikrein-related peptidase activity, ultimately causing impaired epidermal differentiation, defective cornification and poor skin barrier formation [136, 142, 143].
5.2 Severe dermatitis, multiple allergies, and metabolic wasting syndrome
5.2.1 Clinical features, infections, and management
SAM syndrome closely resembles that of Comel-Netherton syndrome with congenital ichthyosis, erythroderma, severe atopic dermatitis, peripheral eosinophilia and elevated serum IgE. The dermatitis has been described as papular, scaly and with plaque formation. Like Comel-Netherton syndrome, hair is absent or sparse [143, 144]. Unique to SAM syndrome is the early and severe development of food allergies and prominent metabolic wasting. Malabsorption and failure to thrive are also common, and eosinophilic esophagitis was identified in one patient. Recurrent infections, developmental delay, and minor cardiac defects, such as ventricular septal defects, have also been described [143, 144].
5.2.2 Clinical laboratory findings
Elevated serum IgE and absolute eosinophil counts are found in SAM syndrome. Keratinocytes from those with SAM syndrome showed upregulation of proinflammatory cytokine genes, such as IL5, which likely contributes to the eosinophilia. Other laboratory immune defects have not been described to date. Skin biopsy pathology reveals abnormally formed desmosomes and loss of cell-cell adhesion [144].
5.2.3 Genetics and pathogenesis
Homozygous loss-of-function mutations in DSG-1 were identified in two consanguineous families with SAM syndrome [144]. As with the other desmogleins, DSG-1 plays a crucial role in cell-cell adhesion in keratinocytes, as well as myocardial cells [145]. DSG-1 deficiency leads to absent cell-cell adhesion and abnormally formed epidermal desmosomes [144]. Without adequate cell-cell adhesion, the epidermal barrier dysfunction results.
6 Conclusions
The differential diagnosis of eosinophilia is broad and includes disorders of immune deficiency or dysfunction, especially those with prominent atopy as a clinical manifestation. There is a great deal of overlap in many of these disorders, thus a thorough clinical and laboratory evaluation is warranted.
Key Points.
Eosinophilia can be seen in many disorders of immune deficiency or immune dysregulation; however, there are a few key syndromes that have eosinophilia as a consistent clinical feature.
In these monogenic diseases of immune deficiency or immune dysregulation, peripheral and tissue eosinophil counts are variable and do not correlate with severity of disease.
Ultimately, a marginal number of patients with eosinophilia have an underlying immune defect, but given the profound impact these diseases can have on morbidity and mortality, all cases of eosinophilia warrant a thorough clinical evaluation.
Abbreviations
- AEC
Absolute eosinophil count
- AD-HIES
Autosomal dominant Hyper IgE syndrome
- ADA
Adenosine deaminase
- ALPS
Autoimmune lymphoproliferative syndrome
- AR-HIES
Autosomal recessive Hyper IgE syndrome
- DOCK8
Dedicator of cytokinesis 8
- DSG-1
Desmoglein-1
- FOXP3
Forkhead box protein P3
- G-CSF
Granulocyte-colony stimulating factor
- HIES
Hyper IgE syndrome
- HPV
Human papillomavirus
- HSCT
Hematopoietic stem cell therapy
- HSV
Herpes simplex virus
- IPEX
Immunodysregulation, polyendocrinopathy, enteropathy, X-linked
- LDS
Loeys-Dietz syndrome
- LEKTI
Lymphoepithelial Kazal-type related inhibitor
- MRI
Magnetic resonance imaging
- NIH
National Institutes of Health
- PGM3
Phosphoglucomutase 3
- PIDD
Primary immunodeficiency diseases
- SAM
Severe dermatitis, multiple allergies, and metabolic wasting
- SCID
Severe combined immunodeficiency
- SMAD3
Decapentaplegic homolog 3
- SPINK5
Serine protease inhibitor Kazal-type 5
- STAT3
Signal transducer and activator of transcription 3
- TGFB2
Transforming growth factor-β2 ligand
- TGFBR
Transforming growth factor-β receptor
- TNFRSF6
Tumor necrosis factor receptor superfamily member 6
- TREC
T-cell receptor excision circles
- VZV
Varicella zoster virus
- WAS
Wiskott-Aldrich syndrome
- WASp
Wiskott-Aldrich syndrome protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have nothing to disclose.
References
- 1.Fulkerson PC, et al. IL-5 triggers a cooperative cytokine network that promotes eosinophil precursor maturation. J Immunol. 2014;193(8):4043–4052. doi: 10.4049/jimmunol.1400732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis SD, Schaller J, Wedgwood RJ. Job’s Syndrome. Recurrent, “cold”, staphylococcal abscesses. Lancet. 1966;1(7445):1013–1015. doi: 10.1016/s0140-6736(66)90119-x. [DOI] [PubMed] [Google Scholar]
- 3.Buckley RW. Extreme hyperimmunoglobulinemia E and undue susceptibility to infection. Pediatrics. 1971;49:59–70. [PubMed] [Google Scholar]
- 4.Chamlin SL, et al. Cutaneous manifestations of hyper-IgE syndrome in infants and children. J Pediatr. 2002;141(4):572–575. doi: 10.1067/mpd.2002.127503. [DOI] [PubMed] [Google Scholar]
- 5.Eberting CL, et al. Dermatitis and the newborn rash of hyper-IgE syndrome. Arch Dermatol. 2004;140(9):1119–1125. doi: 10.1001/archderm.140.9.1119. [DOI] [PubMed] [Google Scholar]
- 6.Chu EY, et al. Cutaneous manifestations of DOCK8 deficiency syndrome. Arch Dermatol. 2012;148(1):79–84. doi: 10.1001/archdermatol.2011.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kong HH, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012;22(5):850–859. doi: 10.1101/gr.131029.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grimbacher B, et al. Hyper-IgE syndrome with recurrent infections--an autosomal dominant multisystem disorder. N Engl J Med. 1999;340(9):692–702. doi: 10.1056/NEJM199903043400904. [DOI] [PubMed] [Google Scholar]
- 9.Freeman AF, et al. Pneumocystis jiroveci infection in patients with hyper-immunoglobulin E syndrome. Pediatrics. 2006;118(4):e1271–e1275. doi: 10.1542/peds.2006-0311. [DOI] [PubMed] [Google Scholar]
- 10.Freeman AF, Domingo DL, Holland SM. Hyper IgE (Job’s) syndrome: a primary immune deficiency with oral manifestations. Oral Dis. 2009;15(1):2–7. doi: 10.1111/j.1601-0825.2008.01463.x. [DOI] [PubMed] [Google Scholar]
- 11.Freeman AF, et al. Causes of death in hyper-IgE syndrome. J Allergy Clin Immunol. 2007;119(5):1234–1240. doi: 10.1016/j.jaci.2006.12.666. [DOI] [PubMed] [Google Scholar]
- 12.Sowerwine KJ, Holland SM, Freeman AF. Hyper-IgE syndrome update. Ann N Y Acad Sci. 2012;1250:25–32. doi: 10.1111/j.1749-6632.2011.06387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freeman AF, Holland SM. Clinical manifestations of hyper IgE syndromes. Dis Markers. 2010;29(3–4):123–130. doi: 10.3233/DMA-2010-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siegel AM, et al. A critical role for STAT3 transcription factor signaling in the development and maintenance of human T cell memory. Immunity. 2011;35(5):806–818. doi: 10.1016/j.immuni.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siegel AM, et al. Diminished allergic disease in patients with STAT3 mutations reveals a role for STAT3 signaling in mast cell degranulation. J Allergy Clin Immunol. 2013;132(6):1388–1396. doi: 10.1016/j.jaci.2013.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borges WG, et al. The face of Job. J Pediatr. 1998;133(2):303–305. doi: 10.1016/s0022-3476(98)70243-4. [DOI] [PubMed] [Google Scholar]
- 17.Smithwick EM, et al. Cranial synostosis in Job’s syndrome. Lancet. 1978;1(8068):826. doi: 10.1016/s0140-6736(78)93028-3. [DOI] [PubMed] [Google Scholar]
- 18.Arora M, et al. Gastrointestinal Manifestations of Autosomal Dominant Hyper-IgE (AD-HIES or Job’s) Syndrome. J Allergy Clin Immunol. 2015 In press. [Google Scholar]
- 19.Liacouras CA, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128(1):3–20. e6. doi: 10.1016/j.jaci.2011.02.040. quiz 21-2. [DOI] [PubMed] [Google Scholar]
- 20.Leonard GD, et al. Non-Hodgkin’s lymphoma in Job’s syndrome: a case report and literature review. Leuk Lymphoma. 2004;45(12):2521–2525. doi: 10.1080/10428190400004463. [DOI] [PubMed] [Google Scholar]
- 21.Gennery AR, et al. Bone marrow transplantation does not correct the hyper IgE syndrome. Bone Marrow Transplant. 2000;25(12):1303–1305. doi: 10.1038/sj.bmt.1702446. [DOI] [PubMed] [Google Scholar]
- 22.Goussetis E, et al. Successful long-term immunologic reconstitution by allogeneic hematopoietic stem cell transplantation cures patients with autosomal dominant hyper-IgE syndrome. J Allergy Clin Immunol. 2010;126(2):392–394. doi: 10.1016/j.jaci.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Nester TA, et al. Effects of allogeneic peripheral stem cell transplantation in a patient with job syndrome of hyperimmunoglobulinemia E and recurrent infections. Am J Med. 1998;105(2):162–164. doi: 10.1016/s0002-9343(98)00200-9. [DOI] [PubMed] [Google Scholar]
- 24.Holland SM, et al. STAT3 mutations in the hyper-IgE syndrome. N Engl J Med. 2007;357(16):1608–1619. doi: 10.1056/NEJMoa073687. [DOI] [PubMed] [Google Scholar]
- 25.Speckmann C, et al. Reduced memory B cells in patients with hyper IgE syndrome. Clin Immunol. 2008;129(3):448–454. doi: 10.1016/j.clim.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Minegishi Y, et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448(7157):1058–1062. doi: 10.1038/nature06096. [DOI] [PubMed] [Google Scholar]
- 27.Ihle JN, Kerr IM. Jaks and Stats in signaling by the cytokine receptor superfamily. Trends Genet. 1995;11(2):69–74. doi: 10.1016/s0168-9525(00)89000-9. [DOI] [PubMed] [Google Scholar]
- 28.Schindler C, Darnell JE., Jr Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu Rev Biochem. 1995;64:621–651. doi: 10.1146/annurev.bi.64.070195.003201. [DOI] [PubMed] [Google Scholar]
- 29.Hsu AP, et al. Intermediate phenotypes in patients with autosomal dominant hyper-IgE syndrome caused by somatic mosaicism. J Allergy Clin Immunol. 2013;131(6):1586–1593. doi: 10.1016/j.jaci.2013.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milner JD, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452(7188):773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Renner ED, et al. Novel signal transducer and activator of transcription 3 (STAT3) mutations, reduced T(H)17 cell numbers, and variably defective STAT3 phosphorylation in hyper-IgE syndrome. J Allergy Clin Immunol. 2008;122(1):181–187. doi: 10.1016/j.jaci.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schimke LF, et al. Diagnostic approach to the hyper-IgE syndromes: immunologic and clinical key findings to differentiate hyper-IgE syndromes from atopic dermatitis. J Allergy Clin Immunol. 2010;126(3):611–617. e1. doi: 10.1016/j.jaci.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 33.Cho JS, et al. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J Clin Invest. 2010;120(5):1762–1773. doi: 10.1172/JCI40891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Happel KI, et al. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202(6):761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang W, et al. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190(3):624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 36.Kisand K, et al. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med. 2010;207(2):299–308. doi: 10.1084/jem.20091669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Puel A, et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med. 2010;207(2):291–297. doi: 10.1084/jem.20091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Puel A, et al. Inborn errors of mucocutaneous immunity to Candida albicans in humans: a role for IL-17 cytokines? Curr Opin Immunol. 2010;22(4):467–474. doi: 10.1016/j.coi.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heimall J, et al. Paucity of genotype-phenotype correlations in STAT3 mutation positive Hyper IgE Syndrome (HIES) Clin Immunol. 2011;139(1):75–84. doi: 10.1016/j.clim.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Renner ED, et al. Autosomal recessive hyperimmunoglobulin E syndrome: a distinct disease entity. J Pediatr. 2004;144(1):93–99. doi: 10.1016/S0022-3476(03)00449-9. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Q, et al. Combined immunodeficiency associated with DOCK8 mutations. N Engl J Med. 2009;361(21):2046–2055. doi: 10.1056/NEJMoa0905506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su HC. Dedicator of cytokinesis 8 (DOCK8) deficiency. Curr Opin Allergy Clin Immunol. 2010;10(6):515–520. doi: 10.1097/ACI.0b013e32833fd718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Al-Herz W, et al. Clinical, immunologic and genetic profiles of DOCK8-deficient patients in Kuwait. Clin Immunol. 2012;143(3):266–272. doi: 10.1016/j.clim.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Engelhardt KR, et al. Large deletions and point mutations involving the dedicator of cytokinesis 8 (DOCK8) in the autosomal-recessive form of hyper-IgE syndrome. J Allergy Clin Immunol. 2009;124(6):1289–1302. e4. doi: 10.1016/j.jaci.2009.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Q, et al. Genetic, clinical, and laboratory markers for DOCK8 immunodeficiency syndrome. Dis Markers. 2010;29(3–4):131–139. doi: 10.3233/DMA-2010-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aydin SE, et al. DOCK8 Deficiency: Clinical and Immunological Phenotype and Treatment Options - a Review of 136 Patients. J Clin Immunol. 2015 doi: 10.1007/s10875-014-0126-0. [DOI] [PubMed] [Google Scholar]
- 47.Ozcan E, Notarangelo LD, Geha RS. Primary immune deficiencies with aberrant IgE production. J Allergy Clin Immunol. 2008;122(6):1054–1062. doi: 10.1016/j.jaci.2008.10.023. quiz 1063-4. [DOI] [PubMed] [Google Scholar]
- 48.Leiding JW, Holland SM. Warts and all: human papillomavirus in primary immunodeficiencies. J Allergy Clin Immunol. 2012;130(5):1030–1048. doi: 10.1016/j.jaci.2012.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alsum Z, et al. Clinical, immunological and molecular characterization of DOCK8 and DOCK8-like deficient patients: single center experience of twenty-five patients. J Clin Immunol. 2013;33(1):55–67. doi: 10.1007/s10875-012-9769-x. [DOI] [PubMed] [Google Scholar]
- 50.Al-Mousa H, Hawwari A, Alsum Z. In DOCK8 deficiency donor cell engraftment post-genoidentical hematopoietic stem cell transplantation is possible without conditioning. J Allergy Clin Immunol. 2013;131(4):1244–1245. doi: 10.1016/j.jaci.2012.12.663. [DOI] [PubMed] [Google Scholar]
- 51.Barlogis V, et al. Successful allogeneic hematopoietic stem cell transplantation for DOCK8 deficiency. J Allergy Clin Immunol. 2011;128(2):420–422. e2. doi: 10.1016/j.jaci.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 52.Bittner TC, et al. Successful long-term correction of autosomal recessive hyper-IgE syndrome due to DOCK8 deficiency by hematopoietic stem cell transplantation. Klin Padiatr. 2010;222(6):351–355. doi: 10.1055/s-0030-1265135. [DOI] [PubMed] [Google Scholar]
- 53.Boztug H, et al. Clinical and immunological correction of DOCK8 deficiency by allogeneic hematopoietic stem cell transplantation following a reduced toxicity conditioning regimen. Pediatr Hematol Oncol. 2012;29(7):585–594. doi: 10.3109/08880018.2012.714844. [DOI] [PubMed] [Google Scholar]
- 54.Gatz SA, et al. Curative treatment of autosomal-recessive hyper-IgE syndrome by hematopoietic cell transplantation. Bone Marrow Transplant. 2011;46(4):552–556. doi: 10.1038/bmt.2010.169. [DOI] [PubMed] [Google Scholar]
- 55.Ghosh S, et al. Treosulfan-based conditioning in DOCK8 deficiency: complete lympho-hematopoietic reconstitution with minimal toxicity. Clin Immunol. 2012;145(3):259–261. doi: 10.1016/j.clim.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 56.McDonald DR, et al. Successful engraftment of donor marrow after allogeneic hematopoietic cell transplantation in autosomal-recessive hyper-IgE syndrome caused by dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol. 2010;126(6):1304–1305. e3. doi: 10.1016/j.jaci.2010.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Metin A, et al. Successful bone marrow transplantation for DOCK8 deficient hyper IgE syndrome. Pediatr Transplant. 2012;16(4):398–399. doi: 10.1111/j.1399-3046.2011.01641.x. [DOI] [PubMed] [Google Scholar]
- 58.Cuellar-Rodriguez J, et al. Matched Related and Unrelated Donor Hematopoietic Stem Cell Transplantation for DOCK8 Deficiency. Biol Blood Marrow Transplant. 2015 doi: 10.1016/j.bbmt.2015.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yong PF, et al. An update on the hyper-IgE syndromes. Arthritis Res Ther. 2012;14(6):228. doi: 10.1186/ar4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ramirez-Fort MK, et al. Management of cutaneous human papillomavirus infection: pharmacotherapies. Curr Probl Dermatol. 2014;45:175–185. doi: 10.1159/000356069. [DOI] [PubMed] [Google Scholar]
- 61.Pai SY, et al. Flow cytometry diagnosis of dedicator of cytokinesis 8 (DOCK8) deficiency. J Allergy Clin Immunol. 2014;134(1):221–223. doi: 10.1016/j.jaci.2014.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jing H, et al. Somatic reversion in dedicator of cytokinesis 8 immunodeficiency modulates disease phenotype. J Allergy Clin Immunol. 2014;133(6):1667–1675. doi: 10.1016/j.jaci.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang J, et al. Activation of Rho GTPases by DOCK exchange factors is mediated by a nucleotide sensor. Science. 2009;325(5946):1398–1402. doi: 10.1126/science.1174468. [DOI] [PubMed] [Google Scholar]
- 64.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420(6916):629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 65.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279(5350):509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 66.Harada Y, et al. DOCK8 is a Cdc42 activator critical for interstitial dendritic cell migration during immune responses. Blood. 2012;119(19):4451–4461. doi: 10.1182/blood-2012-01-407098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lambe T, et al. DOCK8 is essential for T-cell survival and the maintenance of CD8+ T-cell memory. Eur J Immunol. 2011;41(12):3423–3435. doi: 10.1002/eji.201141759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hay BN, et al. Familial immunodeficiency with cutaneous vasculitis, myoclonus, and cognitive impairment. Am J Med Genet A. 2004;125A(2):145–151. doi: 10.1002/ajmg.a.20595. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y, et al. Autosomal recessive phosphoglucomutase 3 (PGM3) mutations link glycosylation defects to atopy, immune deficiency, autoimmunity, and neurocognitive impairment. J Allergy Clin Immunol. 2014;133(5):1400–1409. e5. doi: 10.1016/j.jaci.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sassi A, et al. Hypomorphic homozygous mutations in phosphoglucomutase 3 (PGM3) impair immunity and increase serum IgE levels. J Allergy Clin Immunol. 2014;133(5):1410–1419. e13. doi: 10.1016/j.jaci.2014.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stray-Pedersen A, et al. PGM3 mutations cause a congenital disorder of glycosylation with severe immunodeficiency and skeletal dysplasia. Am J Hum Genet. 2014;95(1):96–107. doi: 10.1016/j.ajhg.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tegtmeyer LC, et al. Multiple phenotypes in phosphoglucomutase 1 deficiency. N Engl J Med. 2014;370(6):533–542. doi: 10.1056/NEJMoa1206605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Greig KT, et al. Agm1/Pgm3-mediated sugar nucleotide synthesis is essential for hematopoiesis and development. Mol Cell Biol. 2007;27(16):5849–5859. doi: 10.1128/MCB.00802-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Berglund G, et al. Wiskott-Aldrich syndrome. A study of 6 cases with determination of the immunoglobulins A, D, G, M and ND. Acta Paediatr Scand. 1968;57(2):89–97. doi: 10.1111/j.1651-2227.1968.tb04658.x. [DOI] [PubMed] [Google Scholar]
- 75.Snover DC, et al. Wiskott-Aldrich syndrome: histopathologic findings in the lymph nodes and spleens of 15 patients. Hum Pathol. 1981;12(9):821–831. doi: 10.1016/s0046-8177(81)80085-8. [DOI] [PubMed] [Google Scholar]
- 76.Aldrich RA, Steinberg AG, Campbell DC. Pedigree demonstrating a sex-linked recessive condition characterized by draining ears, eczematoid dermatitis and bloody diarrhea. Pediatrics. 1954;13(2):133–139. [PubMed] [Google Scholar]
- 77.Sullivan KE, et al. A multiinstitutional survey of the Wiskott-Aldrich syndrome. J Pediatr. 1994;125(6 Pt 1):876–885. doi: 10.1016/s0022-3476(05)82002-5. [DOI] [PubMed] [Google Scholar]
- 78.Ochs HD, et al. Wiskott-Aldrich syndrome: diagnosis, clinical and laboratory manifestations, and treatment. Biol Blood Marrow Transplant. 2009;15(1 Suppl):84–90. doi: 10.1016/j.bbmt.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 79.Mahlaoui N, et al. Characteristics and outcome of early-onset, severe forms of Wiskott-Aldrich syndrome. Blood. 2013;121(9):1510–1516. doi: 10.1182/blood-2012-08-448118. [DOI] [PubMed] [Google Scholar]
- 80.Pellier I, et al. Occurrence of aortic aneurysms in 5 cases of Wiskott-Aldrich syndrome. Pediatrics. 2011;127(2):e498–e504. doi: 10.1542/peds.2009-2987. [DOI] [PubMed] [Google Scholar]
- 81.Litzman J, et al. Intravenous immunoglobulin, splenectomy, and antibiotic prophylaxis in Wiskott-Aldrich syndrome. Arch Dis Child. 1996;75(5):436–439. doi: 10.1136/adc.75.5.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mathew P, Conley ME. Effect of intravenous gammaglobulin (IVIG) on the platelet count in patients with Wiskott-Aldrich syndrome. Pediatr Allergy Immunol. 1995;6(2):91–94. doi: 10.1111/j.1399-3038.1995.tb00265.x. [DOI] [PubMed] [Google Scholar]
- 83.Buchbinder D, Nugent DJ, Fillipovich AH. Wiskott-Aldrich syndrome: diagnosis, current management, and emerging treatments. Appl Clin Genet. 2014;7:55–66. doi: 10.2147/TACG.S58444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Worth AJ, Booth C, Veys P. Stem cell transplantation for primary immune deficiency. Curr Opin Hematol. 2013;20(6):501–508. doi: 10.1097/MOH.0b013e328365a13b. [DOI] [PubMed] [Google Scholar]
- 85.Al-Herz W, et al. Primary immunodeficiency diseases: an update on the classification from the international union of immunological societies expert committee for primary immunodeficiency. Front Immunol. 2014;5:162. doi: 10.3389/fimmu.2014.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Notarangelo LD, Ochs HD. Wiskott-Aldrich Syndrome: a model for defective actin reorganization, cell trafficking and synapse formation. Curr Opin Immunol. 2003;15(5):585–591. doi: 10.1016/s0952-7915(03)00112-2. [DOI] [PubMed] [Google Scholar]
- 87.Orange JS, et al. Wiskott-Aldrich syndrome protein is required for NK cell cytotoxicity and colocalizes with actin to NK cell-activating immunologic synapses. Proc Natl Acad Sci U S A. 2002;99(17):11351–11356. doi: 10.1073/pnas.162376099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Matalon O, Reicher B, Barda-Saad M. Wiskott-Aldrich syndrome protein--dynamic regulation of actin homeostasis: from activation through function and signal termination in T lymphocytes. Immunol Rev. 2013;256(1):10–29. doi: 10.1111/imr.12112. [DOI] [PubMed] [Google Scholar]
- 89.Massaad MJ, Ramesh N, Geha RS. Wiskott-Aldrich syndrome: a comprehensive review. Ann N Y Acad Sci. 2013;1285:26–43. doi: 10.1111/nyas.12049. [DOI] [PubMed] [Google Scholar]
- 90.Hirschhorn R, Candotti F. Immunodeficiency due to defects of purine metabolism. In: Ochs HD, Smith CIE, Puck JM, editors. Primary Immunodeficiency Diseases. Oxford, England: Oxford University Press; 2006. pp. 169–196. [Google Scholar]
- 91.Felgentreff K, et al. Clinical and immunological manifestations of patients with atypical severe combined immunodeficiency. Clin Immunol. 2011;141(1):73–82. doi: 10.1016/j.clim.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 92.Sauer AV, et al. Autoimmune dysregulation and purine metabolism in adenosine deaminase deficiency. Front Immunol. 2012;3:265. doi: 10.3389/fimmu.2012.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lawrence MG, et al. Elevated IgE and atopy in patients treated for early-onset ADA-SCID. J Allergy Clin Immunol. 2013;132(6):1444–1446. doi: 10.1016/j.jaci.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cederbaum SD, et al. The chondro-osseous dysplasia of adenosine deaminase deficiency with severe combined immunodeficiency. J Pediatr. 1976;89(5):737–742. doi: 10.1016/s0022-3476(76)80793-7. [DOI] [PubMed] [Google Scholar]
- 95.Hirschhorn R, et al. Amerioration of neurologic abnormalities after “enzyme replacement” in adenosine deaminase deficiency. N Engl J Med. 1980;303(7):377–380. doi: 10.1056/NEJM198008143030706. [DOI] [PubMed] [Google Scholar]
- 96.Rogers MH, et al. Cognitive and behavioral abnormalities in adenosine deaminase deficient severe combined immunodeficiency. J Pediatr. 2001;139(1):44–50. doi: 10.1067/mpd.2001.115023. [DOI] [PubMed] [Google Scholar]
- 97.Buckley RH, et al. Human severe combined immunodeficiency: genetic, phenotypic, and functional diversity in one hundred eight infants. J Pediatr. 1997;130(3):378–387. doi: 10.1016/s0022-3476(97)70199-9. [DOI] [PubMed] [Google Scholar]
- 98.Gaspar HB, et al. How I treat ADA deficiency. Blood. 2009;114(17):3524–3532. doi: 10.1182/blood-2009-06-189209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Santisteban I, et al. Novel splicing, missense, and deletion mutations in seven adenosine deaminase-deficient patients with late/delayed onset of combined immunodeficiency disease. Contribution of genotype to phenotype. J Clin Invest. 1993;92(5):2291–2302. doi: 10.1172/JCI116833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kwan A, et al. Newborn screening for severe combined immunodeficiency in 11 screening programs in the United States. JAMA. 2014;312(7):729–738. doi: 10.1001/jama.2014.9132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Puck JM. Laboratory technology for population-based screening for severe combined immunodeficiency in neonates: the winner is T-cell receptor excision circles. J Allergy Clin Immunol. 2012;129(3):607–616. doi: 10.1016/j.jaci.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hershfield MS. Genotype is an important determinant of phenotype in adenosine deaminase deficiency. Curr Opin Immunol. 2003;15(5):571–577. doi: 10.1016/s0952-7915(03)00104-3. [DOI] [PubMed] [Google Scholar]
- 103.Gangi-Peterson L, et al. Nucleotide pool imbalance and adenosine deaminase deficiency induce alterations of N-region insertions during V(D)J recombination. J Clin Invest. 1999;103(6):833–841. doi: 10.1172/JCI4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hershfield MS, Mitchell BS. Immunodeficiency diseases caused by adenosine deaminase deficiency and purine nucleoside phosphorylase deficiency. In: Scriver CR, et al., editors. The Metabolic and Molecular Basis of Inherited Disease. 7th Edition. New York, NY: McGraw-Hill; 1995. pp. 1725–1768. [Google Scholar]
- 105.Villa A, Notarangelo LD, Roifman CM. Omenn syndrome: inflammation in leaky severe combined immunodeficiency. J Allergy Clin Immunol. 2008;122(6):1082–1086. doi: 10.1016/j.jaci.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 106.Aleman K, et al. Reviewing Omenn syndrome. Eur J Pediatr. 2001;160(12):718–725. doi: 10.1007/s004310100816. [DOI] [PubMed] [Google Scholar]
- 107.Pai SY, et al. Transplantation outcomes for severe combined immunodeficiency, 2000–2009. N Engl J Med. 2014;371(5):434–446. doi: 10.1056/NEJMoa1401177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Siala N, et al. Omenn syndrome: two case reports. Acta Dermatovenerol Croat. 2013;21(4):259–262. [PubMed] [Google Scholar]
- 109.Gennery AR, et al. Omenn’s syndrome occurring in patients without mutations in recombination activating genes. Clin Immunol. 2005;116(3):246–256. doi: 10.1016/j.clim.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 110.Shearer WT, et al. Establishing diagnostic criteria for severe combined immunodeficiency disease (SCID), leaky SCID, and Omenn syndrome: the Primary Immune Deficiency Treatment Consortium experience. J Allergy Clin Immunol. 2014;133(4):1092–1098. doi: 10.1016/j.jaci.2013.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tasher D, Dalal I. The genetic basis of severe combined immunodeficiency and its variants. Appl Clin Genet. 2012;5:67–80. doi: 10.2147/TACG.S18693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Markert ML, et al. Review of 54 patients with complete DiGeorge anomaly enrolled in protocols for thymus transplantation: outcome of 44 consecutive transplants. Blood. 2007;109(10):4539–4547. doi: 10.1182/blood-2006-10-048652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pierdominici M, et al. Biased T-cell receptor repertoires in patients with chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome) Clin Exp Immunol. 2003;132(2):323–331. doi: 10.1046/j.1365-2249.2003.02134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vu QV, et al. Clinical and immunophenotypic features of atypical complete DiGeorge syndrome. Pediatr Int. 2013;55(1):2–6. doi: 10.1111/j.1442-200X.2012.03722.x. [DOI] [PubMed] [Google Scholar]
- 115.Price S, et al. Natural history of autoimmune lymphoproliferative syndrome associated with FAS gene mutations. Blood. 2014;123(13):1989–1999. doi: 10.1182/blood-2013-10-535393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sneller MC, et al. Clincal, immunologic, and genetic features of an autoimmune lymphoproliferative syndrome associated with abnormal lymphocyte apoptosis. Blood. 1997;89(4):1341–1348. [PubMed] [Google Scholar]
- 117.Rao VK, Oliveira JB. How I treat autoimmune lymphoproliferative syndrome. Blood. 2011;118(22):5741–5751. doi: 10.1182/blood-2011-07-325217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kim YJ, et al. Eosinophilia is associated with a higher mortality rate among patients with autoimmune lymphoproliferative syndrome. Am J Hematol. 2007;82(7):615–624. doi: 10.1002/ajh.20851. [DOI] [PubMed] [Google Scholar]
- 119.Neven B, et al. A survey of 90 patients with autoimmune lymphoproliferative syndrome related to TNFRSF6 mutation. Blood. 2011;118(18):4798–4807. doi: 10.1182/blood-2011-04-347641. [DOI] [PubMed] [Google Scholar]
- 120.Milner JD, et al. Early-onset lymphoproliferation and autoimmunity caused by germline STAT3 gain-of-function mutations. Blood. 2015;125(4):591–599. doi: 10.1182/blood-2014-09-602763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ochs HD, Ziegler SF, Torgerson TR. FOXP3 acts as a rheostat of the immune response. Immunol Rev. 2005;203:156–164. doi: 10.1111/j.0105-2896.2005.00231.x. [DOI] [PubMed] [Google Scholar]
- 122.Powell BR, Buist NR, Stenzel P. An X-linked syndrome of diarrhea, polyendocrinopathy, and fatal infection in infancy. J Pediatr. 1982;100(5):731–737. doi: 10.1016/s0022-3476(82)80573-8. [DOI] [PubMed] [Google Scholar]
- 123.d’Hennezel E, et al. The immunogenetics of immune dysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J Med Genet. 2012;49(5):291–302. doi: 10.1136/jmedgenet-2012-100759. [DOI] [PubMed] [Google Scholar]
- 124.Patey-Mariaud de Serre N, et al. Digestive histopathological presentation of IPEX syndrome. Mod Pathol. 2009;22(1):95–102. doi: 10.1038/modpathol.2008.161. [DOI] [PubMed] [Google Scholar]
- 125.Gambineri E, et al. Clinical and molecular profile of a new series of patients with immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome: inconsistent correlation between forkhead box protein 3 expression and disease severity. J Allergy Clin Immunol. 2008;122(6):1105–1112. e1. doi: 10.1016/j.jaci.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 126.Akdis M, Blaser K, Akdis CA. T regulatory cells in allergy: novel concepts in the pathogenesis, prevention, and treatment of allergic diseases. J Allergy Clin Immunol. 2005;116(5):961–968. doi: 10.1016/j.jaci.2005.09.004. quiz 969. [DOI] [PubMed] [Google Scholar]
- 127.Bennett CL, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27(1):20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 128.Wildin RS, Smyk-Pearson S, Filipovich AH. Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J Med Genet. 2002;39(8):537–545. doi: 10.1136/jmg.39.8.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Caudy AA, et al. CD25 deficiency causes an immune dysregulation, polyendocrinopathy, enteropathy, X-linked-like syndrome, and defective IL-10 expression from CD4 lymphocytes. J Allergy Clin Immunol. 2007;119(2):482–487. doi: 10.1016/j.jaci.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 130.Uzel G, et al. Dominant gain-of-function STAT1 mutations in FOXP3 wild-type immune dysregulation-polyendocrinopathy-enteropathy-X-linked-like syndrome. J Allergy Clin Immunol. 2013;131(6):1611–1623. doi: 10.1016/j.jaci.2012.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Loeys BL, et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet. 2005;37(3):275–281. doi: 10.1038/ng1511. [DOI] [PubMed] [Google Scholar]
- 132.MacCarrick G, et al. Loeys-Dietz syndrome: a primer for diagnosis and management. Genet Med. 2014;16(8):576–587. doi: 10.1038/gim.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Loeys BL, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med. 2006;355(8):788–798. doi: 10.1056/NEJMoa055695. [DOI] [PubMed] [Google Scholar]
- 134.Frischmeyer-Guerrerio PA, et al. TGFbeta receptor mutations impose a strong predisposition for human allergic disease. Sci Transl Med. 2013;5(195):195ra94. doi: 10.1126/scitranslmed.3006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Burk C, et al. Netherton syndrome and trichorrhexis invaginata--a novel diagnostic approach. Pediatr Dermatol. 2008;25(2):287–288. doi: 10.1111/j.1525-1470.2008.00663.x. [DOI] [PubMed] [Google Scholar]
- 136.Hovnanian A. Netherton syndrome: skin inflammation and allergy by loss of protease inhibition. Cell Tissue Res. 2013;351(2):289–300. doi: 10.1007/s00441-013-1558-1. [DOI] [PubMed] [Google Scholar]
- 137.Renner ED, et al. Comel-Netherton syndrome defined as primary immunodeficiency. J Allergy Clin Immunol. 2009;124(3):536–543. doi: 10.1016/j.jaci.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fontao L, et al. Infliximab infusions for Netherton syndrome: sustained clinical improvement correlates with a reduction of thymic stromal lymphopoietin levels in the skin. J Invest Dermatol. 2011;131(9):1947–1950. doi: 10.1038/jid.2011.124. [DOI] [PubMed] [Google Scholar]
- 139.Sun JD, Linden KG. Netherton syndrome: a case report and review of the literature. Int J Dermatol. 2006;45(6):693–697. doi: 10.1111/j.1365-4632.2005.02637.x. [DOI] [PubMed] [Google Scholar]
- 140.Chavanas S, et al. Mutations in SPINK5, encoding a serine protease inhibitor, cause Netherton syndrome. Nat Genet. 2000;25(2):141–142. doi: 10.1038/75977. [DOI] [PubMed] [Google Scholar]
- 141.Raghunath M, et al. SPINK5 and Netherton syndrome: novel mutations, demonstration of missing LEKTI, and differential expression of transglutaminases. J Invest Dermatol. 2004;123(3):474–483. doi: 10.1111/j.0022-202X.2004.23220.x. [DOI] [PubMed] [Google Scholar]
- 142.Furio L, Hovnanian A. Netherton syndrome: defective kallikrein inhibition in the skin leads to skin inflammation and allergy. Biol Chem. 2014;395(9):945–958. doi: 10.1515/hsz-2014-0137. [DOI] [PubMed] [Google Scholar]
- 143.Samuelov L, Sprecher E. Peeling off the genetics of atopic dermatitis-like congenital disorders. J Allergy Clin Immunol. 2014;134(4):808–815. doi: 10.1016/j.jaci.2014.07.061. [DOI] [PubMed] [Google Scholar]
- 144.Samuelov L, et al. Desmoglein 1 deficiency results in severe dermatitis, multiple allergies and metabolic wasting. Nat Genet. 2013;45(10):1244–1248. doi: 10.1038/ng.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Amagai M, Stanley JR. Desmoglein as a target in skin disease and beyond. J Invest Dermatol. 2012;132(3 Pt 2):776–784. doi: 10.1038/jid.2011.390. [DOI] [PMC free article] [PubMed] [Google Scholar]