Abstract
Background
Healthy first-degree relatives of patients with affective disorders are at increased risk for affective disorders and express discrete structural and functional abnormalities in the brain reward system. However, value-based decision making is not well understood in these at-risk individuals.
Methods
We investigated healthy monozygotic and dizygotic twins with or without a co-twin history of affective disorders (high-risk and low-risk groups, respectively) using functional MRI during a gambling task. We assessed group differences in activity related to gambling risk over the entire brain.
Results
We included 30 monozygotic and 37 dizygotic twins in our analysis. Neural activity in the anterior insula and ventral striatum increased linearly with the amount of gambling risk in the entire cohort. Individual neuroticism scores were positively correlated with the neural response in the ventral striatum to increasing gambling risk and negatively correlated with individual risk-taking behaviour. Compared with low-risk twins, the high-risk twins showed a bilateral reduction of risk-related activity in the middle insula extending into the temporal cortex with increasing gambling risk. Post hoc analyses revealed that this effect was strongest in dizygotic twins.
Limitations
The relatively old average age of the mono- and dizygotic twin cohort (49.2 yr) may indicate an increased resilience to affective disorders. The size of the monozygotic high-risk group was relatively small (n = 13).
Conclusion
The reduced processing of risk magnitude in the middle insula may indicate a deficient integration of exteroceptive information related to risk-related cues with interoceptive states in individuals at familial risk for affective disorders. Impaired risk processing might contribute to increased vulnerability to affective disorders.
Introduction
Heritable genetic traits and environmental components both contribute to the etiology of affective disorders. While the specific contribution of these 2 factors to disorder onset remains elusive, family and twin studies have consistently identified a family history of affective disorders as a major risk factor.1 First-degree relatives of patients with depression have a 2-fold to 4-fold increased risk for depression than individuals with no psychiatric history in first-degree relatives.2 Yet the specific neurobiological correlates mediating this vulnerability to affective disorders, which are transmitted in affected families, remain to be identified.
Recent studies in high-risk individuals have revealed that healthy individuals at familial risk for affective disorders show a range of neurobiological abnormalities. Two studies from our group showed lower serotonin (5-HT) transporter binding in the prefrontal cortex3 and decreased hippocampal volume.4 We recently observed altered neural response in the hippocampus and orbitofrontal cortex to monetary gains and losses in healthy first-degree relatives of patients with depression.5 Accordingly, other groups have shown altered frontal and subcortical reward-related neural processes in healthy individuals at familial risk for affective disorders.6–8
Prospective longitudinal studies may provide valuable insights into the association between risk factors and disorder onset. By following healthy high-risk individuals for 7 years before the onset of any affective disorder, we were able to show that discrete subclinical symptoms predict subsequent disorder onset.9 The personality trait neuroticism, which refers to emotional instability, vulnerability to stress and anxiety disorders,10 was also found to predict subsequent disorder onset.9,11 A better insight into the association between abnormal neural responses in high-risk individuals and state measures of psychopathology may increase our understanding of the neural mechanisms underlying affective disorders with the potential to aid earlier diagnosis and more effective treatment.
Studies comparing healthy twins with and without a co-twin history of affective disorders provide a unique potential to identify subclinical traits and characterize their impact on clinical manifestation due to their high concordance rates12 of about 70% for monozygotic (MZ) twins and 35% for dizygotic (DZ) twins.13 Here we studied a group of healthy, never-depressed MZ and DZ twins with or without a co-twin with a history of affective disorders (high-risk and low-risk groups, respectively). Using functional MRI (fMRI), we assessed the differences in brain response during the performance of a gambling task, which probed brain activity during 2 critical events: making risky choices and receiving monetary rewards. Genetic similarity to the twin proband has been shown to affect the concordance rate12,13 and may have an impact on the strength of the subclinical traits for affective disorders. Therefore, we studied the effect of the zygosity (MZ or DZ) of our twin sample on regional brain activity during the gambling task. Finally, we explored whether subclinical depression symptoms and the personality trait neuroticism may account for differences in neural response between the high-risk and control groups. Compared with the low-risk group, we expected the high-risk group to show an altered neural response in key brain regions involved in risk-related decision making, such as the ventral striatum (VS) and insula, regions previously shown to display functional abnormalities in individuals with depression and in healthy high-risk individuals.7,14
Methods
Participants
We recruited healthy MZ and DZ twins who had never been treated for an affective disorder to participate in the present study. The participants were a subset of a larger twin cohort recruited for a study on demographic and clinical risk markers for depression.15 In that study, twins were identified through record linkage between the nationwide Danish Twin Registry, The Danish Psychiatric Central Research Register and the Danish Civil registration system. The included high-risk twins had a co-twin (proband) who was treated in a psychiatric hospital for an affective episode. Age-, sex- and zygosity-matched control twins (low-risk) were also invited. The low-risk twins had no known co-twin or fist-degree relative history of affective disorders or other severe psychiatric illness. The included high- and low-risk twins had no record in the Danish Psychiatric Central Research Register, and clinical interviews were used to ascertain that all participants were mentally healthy and had never experienced a depression episode. The exclusion criteria for both twin cohorts were a personal history of depression episodes, earlier medical treatment for an affective episode, severe organic brain disease or schizophrenia. In all, 120 high-risk and 114 low-risk individuals met the inclusion criteria and participated in the study.15 Following a 7-year period, the participants were contacted for a personal follow-up interview.9 The twins who agreed to participate in the 7-year follow-up were also invited to participate in the present fMRI study.
Our behavioural data analysis included data from all twins who participaated in the 7-year follow-up. However, we had to exclude some data sets from fMRI analysis owing to either risk-seeking or risk-averse choice bias, which resulted in an insufficient number of events precluding a good estimation of the neural response to all risk choices. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Danish Ministry of Health, The Danish Regional Scientific Ethical Committee and the Data Inspection Agency. All participants provided written informed consent following adequate understanding of all experimental procedures.
The card gambling task
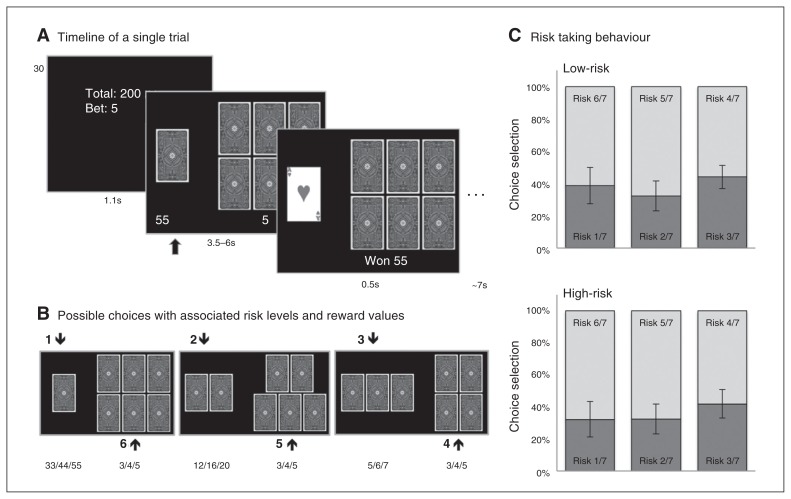
The participants performed a gambling task during fMRI; the task is described in detail in the study by Macoveanu and colleagues.16 During a trial, participants were first shown an information screen displaying the accumulated amount of money in Danish kroner (6 DKK, equivalent to $1 USD) and the bet size, which could be lost. During the choice phase, 7 playing cards, including the ace of hearts, were randomly distributed face down into 2 sets (Fig. 1A). Participants were asked to indicate which of the 2 sets they believed contained the ace. During the outcome phase, the location of the ace was revealed. A correct choice was rewarded with the amount displayed below the set (risk-dependent). An incorrect choice resulted in the loss of the bet value (risk-independent). In a 2-choice design, a low-risk option (with odds of 4 in 7, 5 in 7 and 6 in 7) was associated with a corresponding high-risk option (with odds of 3 in 7, 2 in 7 and 1 in 7). There were 6 possible risk choices and associated rewards, depending on the number of cards in each set (Fig. 1B). To facilitate an even choice distribution, the expected values of the high- and low-risk choices (i.e., the sum of probabilistically weighted wins and losses) were matched. Participants performed the gambling task in 2 fMRI runs with a 1-minute break in between runs. Each of the 2 runs lasted for 11 minutes and included different randomization of 28 choices between 1 and 6 cards, 28 choices between 2 and 5 cards, 28 choices between 3 and 4 cards and 28 null events of the same duration as a real event in which a fixation cross was presented instead of the task screen.
Fig. 1.
The card gambling task. Figure adapted with permission from Macoveanu and colleagues.16 (A) All trials had 3 phases: information, choice and outcome. During the information phase, participants were informed about the sum of money they had accumulated and the bet size (3, 4 or 5 Danish kroner [DKK]), which could be lost. In the choice phase, 2 sets of cards were presented together with the associated monetary reward. Participants chose the set of cards in which they believed the ace of hearts would be hidden. In the outcome phase, the ace of hearts was revealed, providing the participants with feedback on whether they chose the right set and whether they won the associated reward or lost the bet. (B) The 6 possible choices with associated winning amounts in DKK. (C) Risk choice behaviour during the gambling task. The panel shows the distribution of the 6 risk choices across the high-risk twins and low-risk twins. Choices are paired according to the 3 trial types, with the dark shade representing choices with winning odds less than 50% and the light shade representing choices with winning odds greater than 50%.
Evaluation of choice behaviour during gambling
We performed the statistical assessment using SPSS Statistics version 20 (IBM). Group differences in risk-taking behaviour were evaluated using analysis of variance (ANOVA) models with familial risk (high, low) and zygosity (MZ, DZ) as between-subjects factors and gambling risk (odds of 4 in 7, 5 in 7 and 6 in 7) as a within-subjects factor. For significant group interactions, we used independent sample t tests to identify the contributing factors. The significance threshold was set at p < 0.05, using Greenhouse–Geisser correction for nonsphericity when appropriate. We further calculated an individual risk-taking ratio as the sum of risk-weighted high-risk choices over the highest possible risk score as a behavioural index of risk taking during the gambling task. Thus, a risk ratio of 1 indicates that the participant made only high-risk choices during the gambling task, and a risk ratio of 0 indicates that the participant made only low-risk choices.
Neuroticism and depression scales
On the day of the MRI intervention, we asked the participants to complete self-reported questionnaires assessing psychopathological states. Subclinical depression symptoms were evaluated according to the Beck Depression Inventory (BDI),17 and neuroticism scores were assesed using the Eysenck Personality Questionnaire (EPQ).18 Group differences in BDI and neuroticism scores were investigated using ANOVA models with familial risk and zygozity as between-subjects factors. The significance criterion was the same as for the choice behaviour analisys described previously. Group data are shown as means ± standard deviation. We explored possible correlations between neuroticism scores and the individual risk-taking ratio by calculating the Pearson correlation coefficient and performing a 2-tailed significance test.
MRI data acquisition
All MRI measurements were performed on a 3 T scanner (Siemens Trio) using an 8-channel head array coil. The blood oxygen level–dependent (BOLD) fMRI sequence invovled a T2*-weighted gradient echo spiral echo-planar imaging (EPI) sequence with a repetition time (TR) of 2.5 s, echo time (TE) of 26 ms and flip angle of 90°. The measurements were obtained in 2 runs, each with 260 volumes and a duration of 11 minutes. Each volume consisted of 41 slices of 3 mm thickness and a between-slice gap of 25%. The field of view (FOV) was 256 × 256 mm using a 64 × 64 grid. The EPI sequence was optimized for signal recovery from the orbitofrontal cortex by tilting slice orientation from a transverse toward a coronal orientation by about 30° and by using a preparation gradient pulse.19 In addition, high-resolution 3-dimensional (3D) structural T1-weighted spin echo images were obtained after the first session of BOLD fMRI (inversion time 800 ms, TE 3.93 ms, TR 1540 ms, flip angle 9°, FOV 256 × 256, 192 slices).
Functional MRI data analysis
Preprocessing and statistical analysis of the acquired functional images was performed using SPM8 (www.fil.ion.ucl.ac.uk/spm/software/spm8). The images were realigned to the first image in the time series, normalized to a standard template and smoothed using a symmetric 8 mm Gaussian kernel. For the first-level statistical analysis we implemented event-related subject models with 6 regressors for the choice phase (1 for each risk level, from the lowest odds [1 in 7] to the highest odds [6 in 7], as illustrated in Fig. 1B) and 6 regressors for the outcome phase (3 for negative and 3 for positive events). Owing to different interindividual bias in risk preference, we achieved a sufficient number of measurements for all types of outcome events by grouping the outcome events in pairs: outcome events preceded by choices with odds of 1 in 7 and 2 in 7 were modelled together as high-risk events, outcome events preceded by choices with odds of 3 in 7 and 4 in 7 as medium-risk events and outcome events preceded by choices with odds of 5 in 7 and 6 in 7 as low-risk events. In addition to the 6 choice phase regressors and 6 outcome regressors, the model also included 24 nuisance regressors to correct for movement (basic, squared, spin history basic, spin history squared for 3D rotation and translation movements). We constructed 3 first-level contrasts: 1) a gambling risk contrast in which we assigned the 6 risk levels weights corresponding with a linear increase with the risk size, 2) a positive outcome contrast (high-risk wins > low-risk wins) and 3) a negative outcome contrast (low-risk loss > high-risk loss). Because the loss amount was invariable and matched the bet size, we hypothesized that losing following a low-risk choice would be perceived as more aversive than losing following a high-risk choice because low-risk choices were associated with a higher value of the missed reward.16 Using an exploratory approach, we investigated possible interactions between familial risk and zygosity. We included the first-level contrasts in a 2 × 2 second-level ANOVA model with familial risk (high, low) and zygosity (MZ, DZ) as between-subjects factors. For significant findings we used post hoc t tests to identify which of the factors contributed to the observed interaction effect.
When testing for differences in clinical and demographic data we found significant differences in neuroticism scores between the high- and low-risk groups. We therefore set up a post hoc analysis in which we added the neuroticism scores as covariates. This enabled us to examine whether the observed differences in neural response could be explained by differences in neuroticism. In separate analyses, we explored possible correlations between risk-related increase in neural activity and the individual risk-taking ratio and neuroticism scores across all participants.
We considered clusters to be significant at p < 0.05 after family-wise error (FWE) correction for multiple nonindependent comparisons. The extent threshold for each cluster was set at an uncorrected voxel threshold of p < 0.001. For regions that were defined a priori as regions of interest (VS and insula) the correction for multiple comparisons was restricted to a mask including these regions constructed using the Wake Forest University PickAtlas toolbox and the automated anatomical labelling (AAL) atlas.20,21 Significant clusters are reported with Z scores and stereotactic Montreal Neurological Institute (MNI) coordinates of the regional maxima in millimetres.
Results
Participants
Sixty-seven healthy MZ and DZ twins, never treated for an affective disorder, took part in the present study. The demographic and clinical characteristics of participants are shown in Table 1. We had to exclude 5 data sets from fMRI analysis owing to either risk-seeking or risk-averse choice bias (2 high-risk and 3 low-risk individuals). This bias resulted in an insufficient number of events precluding a good estimation of the neural response to all risk choices, leaving 62 participants for the fMRI analysis.
Table 1.
Demographic and clinical characteristics of study participants
| Characteristic | Group; mean ± SD* | p value | |
|---|---|---|---|
|
| |||
| High risk | Low risk | ||
| MZ twins | |||
| Group size | 13 | 17 | |
| Female sex | 8 | 11 | |
| Age, yr | 49.7 ± 14.8 | 43.4 ± 9.9 | 0.17 |
| BDI score | 1.6 ± 2.2 | 1.3 ± 1.8 | 0.68 |
| Neuroticism† | 3.1 ± 3.1 | 4.7 ± 4.2 | 0.29 |
| DZ twins | |||
| Group size | 18 | 19 | |
| Female sex | 12 | 11 | |
| Age, yr | 48.9 ± 10.9 | 50.1 ± 10.3 | 0.57 |
| BDI score | 2.4 ± 3.5 | 1.1 ± 1.2 | 0.16 |
| Neuroticism† | 6.2 ± 4.9 | 3.5 ± 2.8 | 0.05 |
BDI = Beck Depression Inventory; DZ = dizygotic; MZ = monozygotic; SD = standard deviation.
Unless otherwise indicated.
Interaction effect (group × zygosity: F1,62 = 5.0, p = 0.03). High-risk DZ twins had higher neuroticism scores than high-risk MZ twins (p = 0.05).
Gambling behaviour
There was a general preference for low-risk choices across all participants (main effect of risk: F2,95 = 9.7, p = 0.001, Fig. 1C), but no significant difference in risk-taking behaviour between the high-risk and low-risk twins (F2,95 = 0.9, p = 0.39).
Neuroticism and depression scales
We found a significant familial risk × zygosity interaction in neuroticism scores (F1,65 = 5.0, p = 0.030). The neuroticism scores were higher in the high-risk DZ twins than the low-risk DZ twins (t35 = 2.02, p = 0.05) and the high-risk MZ twins (t29 = 2.03, p = 0.05; Table 1). There was no significant difference between the high-risk and low-risk MZ twins in neuroticism scores (p > 0.05). We further found a negative correlation between neuroticism scores and the risk-taking ratio across all participants (Pearson r = −0.25, n = 62, p = 0.047). Subclinical depression symptoms as reflected by BDI scores did not differ between high- and low-risk groups or between MZ and DZ twins, and all participants were within the range of minimal depression.
Neural response to the choice and outcome phases
Risk-related neural activity during choices
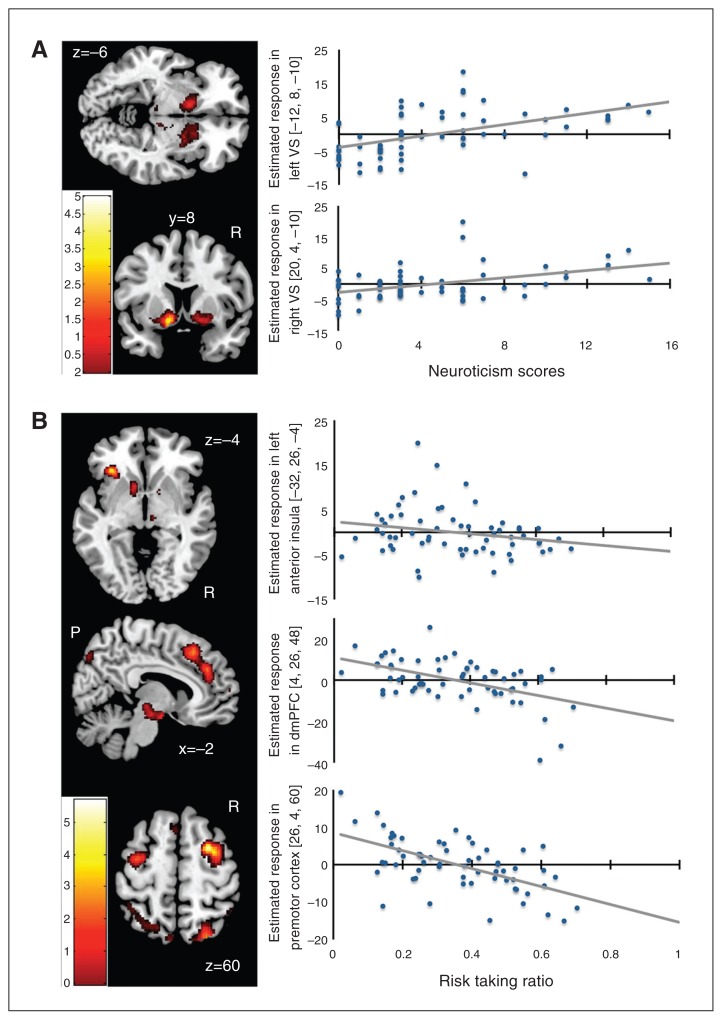
Across all participants, the neural response during the choice phase scaled linearly with the magnitude of the gambling risk in a widespread cortico-subcortical network, comprising the anterior insula, VS, anterior and posterior cingulate cortex (Table 2A). There was a positive correlation between the neuroticism scores and the risk-related increase in neural activity during the choice phase in the VS (Table 2B, Fig. 2A). The individual risk-taking ratio (risk seeking behaviour) correlated positively with the risk-related increase in neural activity in the right superior temporal cortex. Conversely, the individual risk-taking ratio correlated negatively with risk-related increase in neural activity in several brain regions, including the left anterior insula and dorsomedial prefrontal cortex (dmPFC; Table 2C and 2D, Fig. 2B). In these brain regions, individuals preferring risk-avoiding decisions showed stronger increases in choice activity with increasing risk of a given gamble.
Table 2.
Functional MRI results across all participants during the choice phase*
| Phase; region | Side | MNI coordinates | Z score | Cluster size | Cluster p value | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| x | y | z | |||||
| A. Linear increase in risk-related activity during the choice phase (extent threshold p < 0.05 FWE) | |||||||
| Ventral striatum | L | −16 | 6 | −8 | > 8 | 5382 | < 0.001 |
| R | 16 | 10 | −8 | > 8 | |||
| Thalamus | R | 8 | −22 | −4 | > 8 | ||
| Anterior insula | L | −28 | 16 | −6 | 6.68 | ||
| R | 32 | 22 | −10 | 5.45 | |||
| Dorsomedial prefrontal cortex | L | −6 | 34 | 30 | 6.93 | 5254 | < 0.001 |
| Anterior cingulate cortex | R | 10 | 30 | 26 | 6.39 | ||
| Middle occipital cortex | L | −34 | −76 | 36 | 6.93 | 429 | < 0.001 |
| R | 44 | −76 | 18 | 6.16 | 624 | < 0.001 | |
| Cerebellum | R | 8 | −62 | 22 | 6.35 | 233 | < 0.001 |
| L | −8 | −58 | −20 | 4.84 | |||
| Posterior cingulate cortex | L | −6 | −32 | 30 | 6.25 | 472 | < 0.001 |
| R | 10 | −36 | 30 | 5.44 | |||
| Precuneus | L | −12 | −68 | 32 | 6.20 | 1334 | < 0.001 |
| R | 16 | −64 | 48 | 5.88 | |||
| Middle temporal cortex | L | −54 | −50 | 12 | 5.95 | 624 | < 0.001 |
| Middle frontal cortex | L | −22 | 28 | 50 | 5.61 | 61 | < 0.001 |
| Inferior frontal cortex | L | −46 | 8 | 18 | 5.56 | 151 | < 0.001 |
| Lateral orbitofrontal cortex | L | −42 | 48 | 0 | 5.32 | 56 | < 0.001 |
| Precentral gyrus | R | 48 | −8 | 44 | 5.31 | 137 | < 0.001 |
| B. Positive linear association between risk-related increase in neural activity during the choice phase and individual neuroticism scores | |||||||
| Ventral striatum | L | −12 | 8 | −10 | 4.13 | 60 | 0.044 |
| R | 20 | 4 | −10 | 3.53 | 49 | 0.06 | |
| C. Positive linear association between risk-related increase in neural activity during the choice phase and individual risk taking ratio | |||||||
| Superior temporal sulcus | R | 52 | −32 | 22 | 4.11 | 215 | 0.035 |
| D. Negative linear association between risk-related increase in neural activity during the choice phase and individual risk taking ratio | |||||||
| Rostral dorsal premotor cortex | R | 26 | 4 | 60 | 5.06 | 232 | 0.026 |
| Inferior frontal gyrus | R | 48 | 16 | 34 | 4.63 | 244 | 0.021 |
| Midbrain | L | −6 | −6 | −16 | 4.59 | 272 | 0.013 |
| Insula | L | −32 | 26 | −4 | 4.40 | 63 | 0.041 |
| Superior parietal lobule | R | 26 | −74 | 54 | 4.36 | 503 | < 0.001 |
| Dorsomedial prefrontal cortex | R | 8 | 42 | 30 | 4.24 | 511 | 0.001 |
| R | 4 | 26 | 48 | 4.14 | |||
FWE = familiy-wise error; L = left; MNI = Montreal Neurological Institute; R = right.
Regional peaks (cluster p FWE < 0.05; clusters ≥ 40 voxels; extend threshold p ≤ 0.001, uncorrected, unless otherwise stated). Z statistics and cluster size in voxels.
Fig. 2.
Correlation analyses. (A) Regions showing a positive linear association between risk-related increase in neural activity during the choice phase and individual neuroticism scores. (B) Regions showing a negative linear association between risk-related increase in neural activity during the choice phase and the individual risk taking ratio. A low risk-taking ratio indicates risk-averse choices, whereas a high risk taking ratio indicates risky choices during gambling. The colour bar indicates t scores; extent threshold p < 0.005, uncorrected.
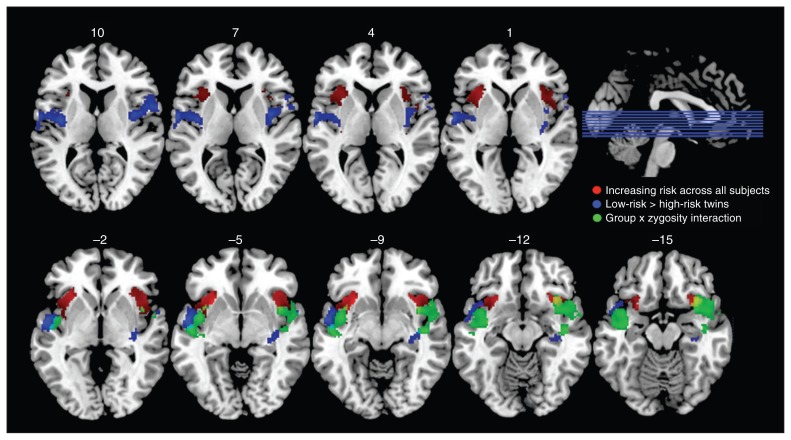
The 2-factor ANOVA (between-group factors: familial risk, zygosity) testing group differences in neural response to increasing gambling risk yielded 2 significant group differences. Independent of zygosity, high-risk twins displayed a reduced risk-related increase in neural activity compared with low-risk twins in the middle part of left (cluster pFWE = 0.001) and right insula (cluster pFWE = 0.044) extending into the temporal cortex (Fig. 3). Peak effect sizes at respective voxels were MNI: x, y, z = −36, −10, 12, Z = 4.1 in the left middle insula; MNI: x, y, z = −56, −2, −4, Z = 4.4 in the superior temporal gyrus; MNI: x, y, z = 40, −2, 8, Z = 3.98 in the right middle insula; and MNI: x, y, z = 58, 6, 12, Z = 3.94 in the superior temporal gyrus. There was a differential response between high-risk and low-risk twins in the right anterior insula (pFWE = 0.005) and left middle insula (pFWE = 0.022) depending on zygosity (Fig. 3). Peak effect sizes at respective voxels were MNI: x, y, z = −40, 2, −18, Z = 3.56 in the left insula and MNI: x, y, z = 34, 14, −18, Z = 4.23 in the right insula. Post hoc t tests revealed a significant reduction in these regions in the high-risk DZ twins compared with the low-risk DZ twins, but no significant change in the MZ group. Peak effect sizes were MNI: x, y, z = −44, 8, −10, Z = 3.57, pFWE = 0.023 in the in left insula and MNI: x, y, z = 36, 10, −12, Z = 4.27, pFWE = 0.001 in the right insula. The group differences in the risk-related increase in neural activity were not explained by the group differences in neuroticism scores.
Fig. 3.
Group differences in brain response during the choice phase. The blue clusters comprise contiguous voxels where high-risk twins displayed an attenuated increase in choice-related activity with higher gambling risk relative to low-risk twins. Reduced risk-related activity was located in the middle part of the insula extending into the superior temporal cortex. The green clusters are regions in the insula showing an interaction effect between familial risk and zygosity. There was a reduced influence of risk on choice-related activity in dizygotic high-risk twins as opposed to dizygotic low-risk twins, but no such difference was present in the monozygotic group. The red clusters show a risk-related increase in the insula’s response during the choice phase across all participants. For all clusters, we applied an extent threshold of p < 0.005, uncorrected.
Gambling outcomes
Across all participants negative gambling outcomes (high v. low missed reward) led to increased neural response in a region encompassing the VS, caudate nucleus and anterior insula (Table 3). Positive outcomes (high v. low reward) resulted in increased neural response in a widespread cortico-subcortical network scaled to reward magnitude, comprising the anterior cingulate cortex, thalamus, anterior insula and hippocampus across all participants (Table 3). The neuroticism scores did not correlate with the neural response to either negative or positive outcomes in any brain regions. Furthermore, there was no effect of risk status or zygosity on the neural response to positive or to negative outcomes.
Table 3.
Functional MRI results across all participants during the outcome phase
| Phase; region | Side | MNI coordinates | Z score | Cluster size | p value | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| x | y | z | |||||
| A. Negative outcomes: large missed monetary reward following low-risk choice > small missed monetary rewards following high-risk choice | |||||||
| Ventral striatum | L | −12 | 8 | −6 | 5.39 | 389 | 0.005 |
| Caudate nucleus | L | −8 | 8 | 6 | 4.73 | ||
| Ventral striatum | R | 10 | 10 | 10 | 4.48 | 188 | 0.09 |
| B. Positive outcomes: high monetary reward following high-risk choice > low monetary reward following low-risk choice (extend threshold p < 0.05, FWE-corrected) | |||||||
| Middle temporal gyrus | R | 58 | −44 | 6 | 6.22 | 272 | < 0.001 |
| Anterior cingulate cortex | L | −10 | 36 | 14 | 6.18 | 544 | < 0.001 |
| R | 12 | 42 | 16 | 6.12 | |||
| Hippocampus | R | 16 | −24 | −10 | 5.89 | 141 | < 0.001 |
| Thalamus | L | −12 | −2 | 4 | 5.89 | 231 | < 0.001 |
| R | 4 | −4 | 4 | 4.92 | |||
| Posterior cerebellum | R | 26 | −76 | 28 | 5.57 | < 0.001 | |
| Inferior frontal gyrus | R | 50 | 34 | 20 | 5.47 | 46 | < 0.001 |
| Anterior insula | R | 28 | 20 | −12 | 5.32 | 118 | < 0.001 |
| L | −30 | 16 | −14 | 5.37 | 46 | < 0.001 | |
| Fusiform gyrus | L | −40 | −58 | −20 | 5.18 | 49 | < 0.001 |
FWE = familiy-wise error; L = left; MNI = Montreal Neurological Institute; R = right.
Regional peaks (cluster pFWE < 0.05; clusters ≥ 40 voxels; extend threshold p ≤ 0.001 uncorrected unless otherwise stated). Z statistics and cluster size in voxels.
Discussion
Healthy never-depressed twins with a co-twin history of depression showed an altered activity profile of the insular cortex when making risky decisions during a gambling task relative to low-risk twins. The attenuated risk-related increase in neural activity was found symmetrically in a bilateral cluster centred on the middle insular cortex, extending into the right anterior insular cortex and the superior temporal gyrus. Since depression scores did not differ significantly between the high- and low-risk twins and were within the normal range, the reduced sensitivity of the insula to the riskiness of gambles cannot be attributed to a subclinical manifestation of affective disorders.
Insula and risk taking
The insula is involved in a wide range of brain functions depending on the anatomic location.22–24 Posterior parts of the insula are densely connected with posterior temporal and parietal areas and have functionally been linked to pain and vestibular and sensorimotor processing.25,26 In contrast, the anterior region is coupled with frontal association areas and contributes to higher cognitive processes.27 Neuroimaging and lesion studies have consistently shown an involvement of the anterior insula in risk-taking during experimental tasks involving monetary reward and punishment.28–32 Specifically, insula activity has been associated with risk-averse financial decisions,29,31,33 and is suggested to be involved in learning the negative value of loss-predicting cues.34 In good agreement with its functional involvement in risk-taking, in both the high-risk and the low-risk twins, the anterior insula scaled its activity during the choice phase to the risk of the gamble, along with other brain regions that were previously shown to be involved in risk-related decision making.35 Interestingly, interindividual variations in choice behaviour during fMRI accounted for differences in risk-related activation of the left anterior insula. This region showed a stronger increase in activity with risk magnitude in those individuals who made more risk-averse choices. This finding further corroborates the notion that the anterior insula mediates risk aversion.
Reduced risk-related activity in the middle insula in high-risk twins
While the risk-related activity increase was similar across groups in the anterior insula, the middle insula displayed a reduced scaling of choice-related activity to the risk of a monetary gamble in high-risk twins compared with low-risk twins. The observed reduction in insula reactivity to risky choices was not mediated by group differences in neuroticism scores. In the middle part of the insular cortex, the chemical sensory, social–emotional, cognitive and sensorimotor areas are spatially overlapped, facilitating the integration of interoceptive and exteroceptive information in support of awareness.36,37 Interestingly, relative to the healthy control participants, unmedicated patients with major depressive disorder (MDD) showed decreased activity bilaterally in the middle insula during a task requiring attention to visceral interoceptive sensations.38 The abnormal interoceptive representation may further lead to a deficient integration between exteroceptive and interoceptive information. Hence, the reduced scaling of the middle insula to the risk magnitude in high-risk twins may reflect a deficient integration of information related to external risk-related cues with interoceptive states.
An abnormal top–down regulation of emotional processing in the insular cortex has been proposed to play a key role in the pathophysiology of MDD, which is associated with deficits in emotional processing with a bias toward stimuli of negative valence.39 A few imaging studies comparing patients with MDD and healthy controls have shown that patients with MDD display increased neural response to emotionally salient stimuli in a widespread network including the insula.40,41 An increased insular sensitivity has also been found in healthy adolescents with 1 depressed parent compared with low-risk controls following administration of an unpleasant taste.42 However, contrary to these findings, a meta-analysis by Fitzgerald and colleagues14 reported consistent insula hypoactivity (among other regions) in resting state paradigms and emotional activation studies and that pharmacological treatment increases activity in these regions. Further, Sliz and Hayley39 reported reduced insula activity in patients with MDD performing cognitive-based tasks that required working memory, retrieval and active processing. Young daughters of mothers with a history of MDD demonstrated reduced activation in the right putamen and left insula and increased activation in the right insula compared with low-risk counterparts while anticipating gains in an incentive delay task.7 The differences in insula activity in patients with MDD has been attributed to differing population samples in relation to comorbid disorders, medication, number of depression episodes and paradigms used in the studies.39
Differences between MZ and DZ twins
Separate post hoc analyses in the MZ and DZ groups revealed that the attenuated insula reactivity to increasing gambling risk as well as the relative increase in neuroticism scores were significant only when comparing high- and low-risk DZ twins but not when comparing high- and low-risk MZ twins. These findings were unexpected owing to the greater genetic resemblance in the MZ twins. The smaller sample size, and therefore statistical power, in the MZ compared with the DZ group (17 low- v. 13 high-risk compared with 19 low- v. 18 high-risk, respectively) may explain why the differences were not significant in the MZ twins. However, the counterintuitive findings are supported by those of a previous imaging study in a larger high-risk twin cohort in which more prominent hippocampal volume reduction was found in the DZ twins than in high-risk MZ twins.4 It is possible that the observed decrease in insula reactivity to increasing gambling risk and increased neuroticism scores may be best explained by environmental rather than genetic influences. Further, since the rate at which the risk for affective disorders decreases with age in high-risk individuals is not well understood, a higher rate for the MZ twins than the DZ twins may result in a higher ratio of resilient MZ twins than DZ twins in the relatively older MZ cohort.
Effect of neuroticism on risk taking
The personality trait neuroticism is linked to the tendency to experience negative emotions and includes traits such as anxiety, anger and depressed mood.43 In a larger group of mixed MZ and DZ twins, we previously observed that self-rated neuroticism scores were predictive of later development of affective disorders.9 In the present study, we were able to confirm this finding in the DZ group, in which high-risk DZ twins scored higher in the neuroticism test than low-risk DZ twins (Table 1). Furthermore, higher neuroticism scores were associated with decreased risk taking behaviour, strengthening the link between neuroticism and risk aversion.44
The interindividual variation in neuroticism scores showed a positive association with the neural response of the VS to increasingly risky gambles in the entire cohort. This finding corroborates previous data linking neuroticism scores with D2 receptor density in the VS in a small sample of healthy volunteers.45 Recent imaging studies in humans have revealed the key involvement of the VS in reward-based learning by specifically coding for salient prediction error signals, such as better than expected outcomes.46,47 We suggest that in individuals with high neuroticism scores, risky decisions might be associated with stronger dopamine signalling and thus may cause more sensitive or excessive reactions to the stress perceived during risk taking. This might contribute to an increased aversion to risky decisions.
Limitations
The average age of our cohort was relatively old at 49.2 years, which is generally considered to be above the average age at onset of the first episode of depression in genetically predisposed individuals. The older age may contribute to an increased resilience to affective disorders. However, we have previously observed that high-risk twins retain an increased risk for affective disorders through middle age.9 Furthermore, the sample size of the MZ twins was relatively small.
Conclusion
To our knowledge, this is the first study to reveal a diminished sensitivity of the middle insular cortex to the risk magnitude associated with gambling behaviour in twins at high risk for affective disorders. Based on current knowledge on the neurophysiology of the insular cortex, this abnormal activity profile may reflect a deficient integration in the insula of exteroceptive information related to risk-related cues with interoceptive states in high-risk twins. This deficit may increase the vulnerability to affective disorders in healthy individuals and constitute a significant component of the pathophysiology of affective disorders.
Acknowledgements
The study was supported by the Danish Council for Independent Research, Lundbeck Foundation and the Foundation of Einar Geert-Joergensen and wife Ellen Geert-Joergensen. J. Macoveanu is funded through a centre grant of the Lundbeck Foundation for the Center for Integrated Molecular Brain Imaging. H.R. Siebner is supported by a Grant of Excellence on the control of actions “ContAct” from Lundbeck Foundation (R59 A5399). The authors acknowledge the Simon Spies Foundation for their donation of the Siemens Trio Scanner.
Footnotes
Competing interests: None declared for J. Macoveanu. K. Miskowiak has received consultancy fees from Lundbeck. L.V. Kessing has within the last 3 years been a consultant for Lundbeck and AstraZeneca. M. Vinberg has been a consultant for Eli Lilly, Lundbeck, Servier and Astra Zeneca. H.R. Siebner over the past 4 years has received honoraria as a reviewing editor for NeuroImage, as a speaker for Biogen Idec Denmark A/S, and as a scientific advisor for Lundbeck A/S, Valby, Denmark.
Contributors: All authors designed the study, wrote and reviewed the article and aproved the final version for publication. K. Miskowiak and M. Vinberg acquired the data, which J. Macoveanu and H.R. Siebner analyzed.
References
- 1.Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157:1552–62. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- 2.Weissman MM, Wickramaratne P, Adams PB, et al. The relationship between panic disorder and major depression. A new family study. Arch Gen Psychiatry. 1993;50:767–80. doi: 10.1001/archpsyc.1993.01820220017003. [DOI] [PubMed] [Google Scholar]
- 3.Frokjaer VG, Vinberg M, Erritzoe D, et al. High familial risk for mood disorder is associated with low dorsolateral prefrontal cortex serotonin transporter binding. Neuroimage. 2009;46:360–6. doi: 10.1016/j.neuroimage.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Baaré WFC, Vinberg M, Knudsen GM, et al. Hippocampal volume changes in healthy subjects at risk of unipolar depression. J Psychiatr Res. 2010;44:655–62. doi: 10.1016/j.jpsychires.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Macoveanu J, Knorr U, Skimminge A, et al. Altered reward processing in the orbitofrontal cortex and hippocampus in healthy first-degree relatives of patients with depression. Psychol Med. 2014;44:1183–95. doi: 10.1017/S0033291713001815. [DOI] [PubMed] [Google Scholar]
- 6.Mannie ZN, Taylor MJ, Harmer CJ, et al. Frontolimbic responses to emotional faces in young people at familial risk of depression. J Affect Disord. 2011;130:127–32. doi: 10.1016/j.jad.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 7.Gotlib IH, Hamilton JP, Cooney RE, et al. Neural processing of reward and loss in girls at risk for major depression. Arch Gen Psychiatry. 2010;67:380–7. doi: 10.1001/archgenpsychiatry.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lisiecka DM, Carballedo A, Fagan AJ, et al. Altered inhibition of negative emotions in subjects at family risk of major depressive disorder. J Psychiatr Res. 2012;46:181–8. doi: 10.1016/j.jpsychires.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Vinberg M, Miskowiak K, Kessing LV. Risk markers for affective disorder, a seven-years follow up study of a twin cohort at low and high risk for affective disorder. J Psychiatr Res. 2013;47:565–71. doi: 10.1016/j.jpsychires.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Eysenck HJ. The structure of human personality. 3rd ed. London: Methuen; 1970. [Google Scholar]
- 11.Christensen MV, Kessing LV. Do personality traits predict first onset in depressive and bipolar disorder? Nord J Psychiatry. 2006;60:79–88. doi: 10.1080/08039480600600300. [DOI] [PubMed] [Google Scholar]
- 12.Berrettini W. Genetics of major mood disorders. Psychiatry (Edgmont) 2004;1:38–48. [PMC free article] [PubMed] [Google Scholar]
- 13.Kendler KS, Pedersen N, Johnson L, et al. A pilot Swedish twin study of affective illness, including hospital- and population-ascertained subsamples. Arch Gen Psychiatry. 1993;50:699–700. doi: 10.1001/archpsyc.1993.01820210033004. [DOI] [PubMed] [Google Scholar]
- 14.Fitzgerald PB, Laird AR, Maller J, et al. A meta-analytic study of changes in brain activation in depression. Hum Brain Mapp. 2008;29:683–95. doi: 10.1002/hbm.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christensen MV, Kyvik KO, Kessing LV. Subclinical psychopathology and socio-economic status in unaffected twins discordant for affective disorder. J Psychiatr Res. 2007;41:229–38. doi: 10.1016/j.jpsychires.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Macoveanu J, Rowe JB, Hornboll B, et al. Playing it safe but losing anyway-Serotonergic signaling of negative outcomes in dorsomedial prefrontal cortex in the context of risk-aversion. Eur Neuropsychopharmacol. 2013;23:919–30. doi: 10.1016/j.euroneuro.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 18.Eysenck HJ, Eysenck SBG. Manual of the Eysenck Personality Questionnaire. London: Hodder & Stoughton; 1975. [Google Scholar]
- 19.Deichmann R, Gottfried JA, Hutton C, et al. Optimized EPI for fMRI studies of the orbitofrontal cortex. Neuroimage. 2003;19:430–41. doi: 10.1016/s1053-8119(03)00073-9. [DOI] [PubMed] [Google Scholar]
- 20.Maldjian JA, Laurienti PJ, Kraft RA, et al. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 21.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 22.Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev. 1996;22:229–44. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- 23.Klein TA, Ullsperger M, Danielmeier C. Error awareness and the insula: links to neurological and psychiatric diseases. Front Hum Neurosci. 2013;7:14. doi: 10.3389/fnhum.2013.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alcauter S, Lin W, Keith Smith J, et al. Consistent anterior-posterior segregation of the insula during the first 2 years of life. Cereb Cortex. 2015;25:1176–87. doi: 10.1093/cercor/bht312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 26.Wager TD, Rilling JK, Smith EE, et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–7. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 27.Eckert MA, Menon V, Walczak A, et al. At the heart of the ventral attention system: the right anterior insula. Hum Brain Mapp. 2009;30:2530–41. doi: 10.1002/hbm.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Critchley HD, Mathias CJ, Dolan RJ. Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron. 2001;29:537–45. doi: 10.1016/s0896-6273(01)00225-2. [DOI] [PubMed] [Google Scholar]
- 29.Preuschoff K, Quartz SR, Bossaerts P. Human insula activation reflects risk prediction errors as well as risk. J Neurosci. 2008;28:2745–52. doi: 10.1523/JNEUROSCI.4286-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Studer B, Apergis-Schoute AM, Robbins TW, et al. What are the odds? The neural correlates of active choice during gambling. Front Neurosci. 2012;6:46. doi: 10.3389/fnins.2012.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuhnen CM, Knutson B. The neural basis of financial risk taking. Neuron. 2005;47:763–70. doi: 10.1016/j.neuron.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 32.Clark L, Bechara A, Damasio H, et al. Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain. 2008;131:1311–22. doi: 10.1093/brain/awn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weller JA, Levin IP, Shiv B, et al. The effects of insula damage on decision-making for risky gains and losses. Soc Neurosci. 2009;4:347–58. doi: 10.1080/17470910902934400. [DOI] [PubMed] [Google Scholar]
- 34.Palminteri S, Justo D, Jauffret C, et al. Critical roles for anterior insula and dorsal striatum in punishment-based avoidance learning. Neuron. 2012;76:998–1009. doi: 10.1016/j.neuron.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 35.Doya K. Modulators of decision making. Nat Neurosci. 2008;11:410–6. doi: 10.1038/nn2077. [DOI] [PubMed] [Google Scholar]
- 36.Kurth F, Zilles K, Fox PT, et al. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 2010;214:519–34. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Craig ADB. Significance of the insula for the evolution of human awareness of feelings from the body. Ann N Y Acad Sci. 2011;1225:72–82. doi: 10.1111/j.1749-6632.2011.05990.x. [DOI] [PubMed] [Google Scholar]
- 38.Avery JA, Drevets WC, Moseman SE, et al. Major depressive disorder is associated with abnormal interoceptive activity and functional connectivity in the insula. Biol Psychiatry. 2014;76:258–66. doi: 10.1016/j.biopsych.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sliz D, Hayley S. Major depressive disorder and alterations in insular cortical activity: a review of current functional magnetic imaging research. Front Hum Neurosci. 2012;6:323. doi: 10.3389/fnhum.2012.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Surguladze SA, El-Hage W, Dalgleish T, et al. Depression is associated with increased sensitivity to signals of disgust: a functional magnetic resonance imaging study. J Psychiatr Res. 2010;44:894–902. doi: 10.1016/j.jpsychires.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herwig U, Brühl AB, Kaffenberger T, et al. Neural correlates of “pessimistic” attitude in depression. Psychol Med. 2010;40:789–800. doi: 10.1017/S0033291709991073. [DOI] [PubMed] [Google Scholar]
- 42.McCabe C, Woffindale C, Harmer CJ, et al. Neural processing of reward and punishment in young people at increased familial risk of depression. Biol Psychiatry. 2012;72:588–94. doi: 10.1016/j.biopsych.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 43.Matthews G, Deary IJ, Whiteman MC. Personality Traits. 3rd ed. Cambridge, UK: Cambridge University Press; 2009. [Google Scholar]
- 44.Kuhnen CM, Samanez-Larkin GR, Knutson B. Serotonergic genotypes, neuroticism, and financial choices. PLoS ONE. 2013;8:e54632. doi: 10.1371/journal.pone.0054632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee IH, Cheng CC, Yang YK, et al. Correlation between striatal dopamine D2 receptor density and neuroticism in community volunteers. Psychiatry Res. 2005;138:259–64. doi: 10.1016/j.pscychresns.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 46.Daniel R, Pollmann S. A universal role of the ventral striatum in reward-based learning: evidence from human studies. Neurobiol Learn Mem. 2014;114:90–100. doi: 10.1016/j.nlm.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kurniawan IT, Guitart-Masip M, Dayan P, et al. Effort and valuation in the brain: the effects of anticipation and execution. J Neurosci. 2013;33:6160–9. doi: 10.1523/JNEUROSCI.4777-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]