Abstract
Background
Cue-induced craving plays an important role in relapse, and the neural correlates of cue-induced craving have been elucidated using fMRI. This study examined the utility of real-time fMRI (rtfMRI) neurofeedback to strengthen self-regulation of craving-related neural activation and cue-reactivity in cigarette smokers.
Methods
Nicotine-dependent smokers were randomized to rtfMRI neurofeedback or to a no-feedback control group. Participants completed 3 neuroimaging visits. Within each visit, an initial run during which smoking-related cues were used to provoke craving, an individualized craving-related region of interest (ROI) in the prefrontal cortex or anterior cingulate cortex was identified. In the rtfMRI group, activity from the ROI was fed back via a visual display during 3 subsequent runs while participants were instructed to reduce craving during cue exposure. The control group had an identical experience with no feedback provided.
Results
Forty-four nicotine-dependent smokers were recruited to participate in our study; data from the 33 participants who completed a 1-week follow-up visit were included in the analysis. Subjective craving ratings and cue-induced brain activation were lower in the rtfMRI group than in the control group.
Limitations
As participants were not seeking treatment, clinical outcomes are lacking.
Conclusion
Nicotine-dependent smokers receiving rtfMRI feedback from an individualized ROI attenuated smoking cue–elicited neural activation and craving, relative to a control group. Further studies are needed in treatment-seeking smokers to determine if this intervention can translate into a clinically meaningful treatment modality.
Introduction
Tobacco use is an important public health concern. Cigarette smoking is the leading preventable cause of morbidity and mortality in the United States, with about 443 000 smoking-related deaths each year.1 The majority of smokers want to quit, and about half have tried to quit in the previous year; however, only about 6% are able to stop smoking for at least 6 months.2 Even with combined medication and cognitive behavioural therapies, the most common outcome at 1 year following a quit attempt is relapse.3 There is a clear need for new and improved treatments for smoking cessation.
Exposure to smoking-related cues elicits robust craving and measurable physiologic reactivity among smokers.4,5 Previous research has found an association between craving and relapse to smoking during a quit attempt;6,7 however, the precise role of craving remains a focus of debate.8 Naturalistic studies in which smokers used hand-held computers to monitor (in real time) their smoking behaviour in their own environments have demonstrated that a 1-point increase on a 10-point measure of craving was associated with a 33% increase in the odds of smoking.9 These findings support a causal relationship between craving, cue-reactivity, continued smoking and relapse. Thus, modifying or reducing craving and the physiologic response to smoking cues has the potential to improve smoking cessation outcomes.
In the past century, modern medicine has developed increasingly sophisticated tools for feedback of biological signals. The initial signals chosen as targets for biofeedback, such as heart rate (HR) and skin conductance (SC), have yielded some positive results for conditions such as migraine headaches10 and anxiety disorders.11 However, these peripheral biological signals may not be robust enough to modulate complex neural processes such as drug craving. The first studies to provide neurofeedback to patients with addictive disorders used electroencephalography (EEG), an indirect measure of distributed neural activity.12 Several small studies suggested that moderation of EEG α–θ brainwave activity via neurofeedback was associated with decreased alcohol craving, improved stress tolerance and sustained abstinence/remission.13,14 Recent developments in real-time fMRI (rtfMRI) feedback now allow for feedback from discretely defined cortical or subcortical brain regions.15,16 In recent years, preliminary studies with mixed results have demonstrated successful rtfMRI feedback modulation of brain activation associated with pain,17 depression18 and affect regulation.19
The application of rtfMRI feedback in the treatment of nicotine dependence is in the early phase of development and testing. Using an imagined movement task, initial studies determined that intermittent rtfMRI feedback was superior to continuous feedback. Additionally, providing no feedback was a better control than false feedback, which was frustrating and produced negative emotions.20 Subsequent studies in nicotine-dependent adults demonstrated that, compared with baseline, smokers were able to significantly reduce both subjective craving and activation in the anterior cingulate cortex (ACC) during rtfMRI neurofeedback. Of note, the reduction in neural activation was significantly correlated with reduction in subjective measures of craving.21 Subsequent studies found that feedback from the ACC was more effective than simultaneous feedback from the medial prefrontal cortex (mPFC) and that smokers could reduce craving-related brain activation and subjective craving with feedback from the ACC across visits.12,23
The present study was designed to replicate and extend these pilot findings through a randomized, controlled study of rtfMRI neurofeedback on craving response to smoking cues in nicotine-dependent smokers.
Methods
Participants
We recruited non–treatment seeking, nicotine-dependent smokers (≥ 10 cigarettes/d) between the ages of 18 and 60 years through flyers, newspaper and Internet advertisements. All study procedures were approved by the Medical University of South Carolina Institutional Review Board and were in accordance with Good Clinical Practice Guidelines and the Declaration of Helsinki. Participants provided informed consent and completed an initial assessment. Recent smoking was confirmed with an exhaled carbon monoxide (CO) level (≥ 10 ppm) measured using a Micro-Smokelyzer (Bedfont Scientific Ltd.). We obtained a detailed tobacco use history and administered the Fagerström Test for Nicotine Dependence (FTND)24,25 and the Questionnaire of Smoking Urges-Brief (QSU-B; Factor 1 subscale reflects anticipation of the pleasurable outcomes of smoking and Factor 2, the relief of nicotine withdrawal).26 The Minnesota International Neuropsychiatric Interview was used to assess current psychiatric and substance use disorders.27 A physical examination assessed current physical health, and participants provided a urine sample, which we screened for illicit drug use. Exclusion criteria were answering “no” when asked if they had any motivation to quit; use of other tobacco products; current use of nicotine replacement therapy, bupropion or varenicline; medical conditions or medications that could affect brain function; current or past DSM-IV Axis I disorders; pregnancy; and non-nicotine substance dependence or abuse. Following enrollment in the study, participants were randomized into the feedback or control group with a 1:1 allocation ratio to stratify the groups by sex and low/high FTND (≥ 5).
Scanning procedures
Scanning was performed using a 3 T MRI Trio (Siemens Medical). Images were acquired using a standard multislice single-shot gradient echo-planar imaging sequence (repetition time 2.2 s, echo time 35 ms, 64 × 64 matrix, parallel imaging factor of 2, 3 mm3 voxels, 271 volumes, 36 slices). Participants completed 3 rtfMRI feedback visits within a 3-week period. Prior to each scan, participants completed the FTND, craving analogue scale (CAS), and QSU-B. The QSU-B and CAS were administered again after each scanning session. Participants were instructed to not smoke for the 2 hours prior to the scanning session, which would allow for a degree of craving and responsiveness to cues without creating the confound of a ceiling effect from longer periods of abstinence.
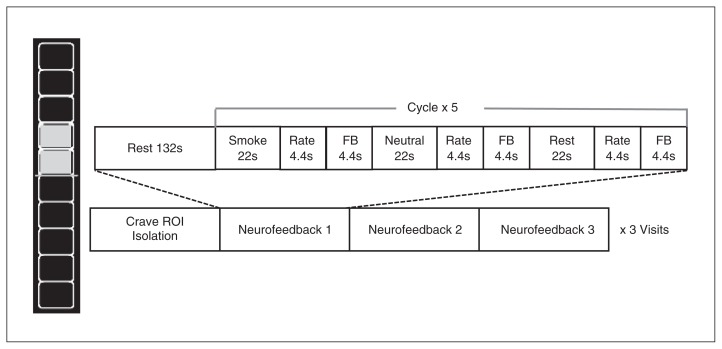
Each rtfMRI scanning visit consisted of 4 10-minute smoking cue exposure runs: an initial craving region of interest (ROI) identification run (run 1) followed by 3 neurofeedback runs (runs 2–4). Images were presented with E-Prime 2.0 software (Psychology Software Tools) and viewed via a mirror attached to the head coil. A handpad was used to record periodic craving measures. Prior to each run, participants handled and smelled a preferred brand cigarette. A verbal craving rating (1 = low, 10 = high) was acquired before and after each run. Each run was composed of a smoking cue exposure task used in previous studies21,28–30 with 3 types of blocks: smoking-related pictures (smoke), non–smoking related pictures (neutral) and a crosshair (rest). Each block consisted of 5 pictures displayed for 4.4 s each. The smoking pictures included packages of cigarettes, lit cigarettes and environments where cigarettes are commonly smoked. Different cigarette brands were used according to participants’ preferences. A matched set of non–smoking related pictures consisted of neutral objects (e.g., pencils, small bowls, individual holding pen in the mount), including images from the International Affective Picture System.31
Runs began with a rest baseline period (132 s) followed by 5 neurofeedback epochs (92.4 s) consisting of smoke, neutral and rest blocks (22 s each). Each block was followed by a (4.4 s) 5-point self-rating of craving and a (4.4 s) neurofeedback thermometer display. During run 1, individuals were instructed to allow themselves to crave during smoke blocks to allow for isolation of the individualized craving-related ROI within the PFC for feedback during that visit. During neurofeedback runs (runs 2–4), participants were instructed to reduce the urge to smoke and decrease the thermometer bar value during smoke blocks (Fig. 1). During the rest and neutral blocks, participants were told that they would see a blank thermometer with the word “INACTIVE” at the bottom, which meant the thermometer was turned off. For participants in the neurofeedback group, the brain activity level from the ROI was translated into a thermometer-shaped visual feedback signal; those in the control group were shown a blank thermometer bar. Participants were given several general suggestions to help reduce their cravings; for example, we instructed them to “try thinking about other things (dinner, what you will be doing later), the benefits of quitting, the negative effects of smoking, or any thoughts you use to reduce your craving normally.” Participants were encouraged to try different methods and to use the feedback to figure out what strategies worked best for them.
Fig. 1.
Neurofeedback task design. Participants completed 3 scanning visits, each containing a crave region of interest (ROI) run followed by 3 neurofeedback runs. Smoke and neutral blocks contained 5 images displayed for 4.4 s each. Feedback (FB) was active only after smoke blocks for the FB group and was displayed as a thermometer bar. The control group was shown a blank thermometer. Each thermometer block indicates a percent signal change within the ROI of 0.3%.
Real-time feedback data processing
The rtfMRI processing was based on the Siemens research mode and is similar to that used in previously published studies.21,23 Data exported in real time were analyzed using Turbo-BrainVoyager (TBV) software (Brain Innovation), which used a fast connection between the MRI scanner and the computer running TBV that incrementally computes statistical maps and conducts real-time preprocessing, including 3-dimensional motion correction, spatial smoothing using an 8 mm3 Gaussian kernel and temporal filtering or drift removal.32 During run 1 on each scanning day, we examined the real-time difference image in the smoke > rest condition and selected an ROI (t = 3.0, a default setting in TBV). The ROIs were targeted in a region approximate to the ACC (including the mPFC and orbitofrontal cortex [OFC]), and were individualized based on the participant’s activation that day. The following settings were used to generate neurofeedback: an intermittent signal from the target area (average values to calculate feedback value = 6 time points) was displayed using a thermometer reflecting the difference between response in the ROI to smoke images relative to the preceding rest block (feedback – rest ÷ rest × 100). Consistent with previous research,20 the mean of the last 6 time points was selected to avoid incorporation of the delayed hemodynamic response, producing a more accurate reflection of changes in blood flow. The scaling of the thermometer was in steps of 0.03%, with a maximum percent signal change (PSC) of 1.5% (feedback bar ± 5). The dynamic ROI option on TBV was used to create an optimized sub-ROI for the feedback signal by selecting the top 33% (defined by the t value for the contrast between the regulation predictor and baseline) of the voxels from the target region. The first thermometer display in each run was inactive to allow for sufficient data for feedback.
Data analysis
Offline rtfMRI data analysis
Off-line fMRI data were analyzed using Statistical Parametric Mapping 8 (Wellcome Department of Cognitive Neurology) in MATLAB version 7.3 (MathWorks). Standard preprocessing steps included realignment, normalization to Montreal Neurological Institute (MNI) space and smoothing with an isotropic 8 mm3 Gaussian kernel. Time series of each individual’s neurofeedback ROI were extracted from the spatially smoothed data using MarsBaR.33 Data were averaged across blocks within the run. The PSC during exposure to smoke blocks was calculated for the crave run (run 1) and the neurofeedback runs (runs 2–4) [(smoke – rest) ÷ rest × 100]. To assess the effect of the neurofeedback condition on attenuation of craving-related activation in the ROI, the activation during the crave run was subtracted from the feedback runs (PSC neurofeedback – PSC crave). The resulting difference scores (3 scores per visit) were entered into a multilevel model analysis with fixed effects and repeated measurements (diagonal residual covariance structure) that included the neurofeedback condition (feedback v. control), feedback run number,3 visit number,3 and their interactions. Participants were included as a repeating factor with random intercepts. A follow-up test examined whether PSC during the crave run differed by condition by entering only PSC from run 1 in the model.
Questionnaire of smoking urges (QSU-B)/subjective data analysis
We analyzed subjective craving data using a multilevel modelling framework with maximum likelihood estimation. All models included a diagonal residual covariance structure and random intercepts; however, random intercepts were removed for models in which intercept variance was nonsignificant. Pre–post scan QSU data (i.e., total, Factor 1, Factor 2) were available at each rtfMRI visit. Post-scan QSU was modelled as the dependent variable, with pre-scan QSU included as a time-varying covariate and condition (neurofeedback v. control), visit1–3 and their interaction included as additional fixed effects.
Results
Baseline characteristics
Forty-four nicotine-dependent smokers were randomized to 1 of 2 conditions: the rtfMRI feedback or the no-feedback control condition. The groups did not differ significantly on any baseline smoking characteristics or demographic variables (Table 1). Both groups smoked approximately 1 pack per day for a similar length of time and were moderately nicotine-dependent, as measured using the FTND. Mean responses to the statement “I want to quit smoking” (1 = not at all, 5 = extremely true) did not differ between the feedback and control groups (3.76 v. 4.05). One participant in the control group was unable to complete the study owing to claustrophobia. Two participants (1 in each group) were excluded following the first scanning visit owing to excess head movement (> 3 mm). Three participants in the control group were lost to follow-up after completing at least 1 scanning visit. An additional 4 participants from the rtfMRI feedback group and 1 participant from the control group were lost to follow-up after the third scanning visit and before the 1-week follow-up visit. There were no significant group differences in demographics or smoking characteristics among participants who completed the 1-week follow-up visit (16 [76%] in the neurofeedback group v. 17 [74%] in the control group). The length of time to complete the 3 scans did not differ significantly between the groups (p = 0.83), with the neurofeedback group completing the 3 scans on average in 11.19 ± 4.04 days and the control group completing on average in 10.88 ± 4.16 days.
Table 1.
Baseline demographic and clinical characteristics of study participants
| Group; mean ± SD* | |||
|---|---|---|---|
|
|
|||
| Characteristic | Feedback (n = 21) | Control (n = 23) | p value |
| Age | 34.1 ± 11.3 | 36.2 ± 10.6 | 0.52 |
| Sex, male:female | 13:8 | 15:8 | 0.82 |
| Race, white:nonwhite | 18:3 | 21:2 | 0.56 |
| Education, yr | 13.75 ± 1.9 | 14.1 ± 1.8 | 0.55 |
| No. of cigarettes/d | 19.1 ± 4.5 | 18.5 ± 7.0 | 0.73 |
| Baseline FTND | 5.3 ± 1.4 | 5.1 ± 1.7 | 0.69 |
| Duration of smoking, yr | 17.1 ± 10.4 | 17.9 ± 10.5 | 0.79 |
| Baseline CO | 17.8 ± 8.0 | 21.1 ± 10.9 | 0.25 |
| Baseline QSU-B | 46.1 ± 13.2 | 46.5 ± 11.8 | 0.93 |
| QSU-B Factor 1 | 27.8 ± 6.9 | 28.7 ± 5.4 | 0.62 |
| QSU-B Factor 2 | 18.4 ± 7.7 | 17.8 ± 7.7 | 0.80 |
CO = carbon monoxide; FTND = Fagerström Test for Nicotine Dependence; QSU-B = Questionnaire of Smoking Urges-Brief; SD = standard deviation.
Unless indicated otherwise.
Region of interests location and response
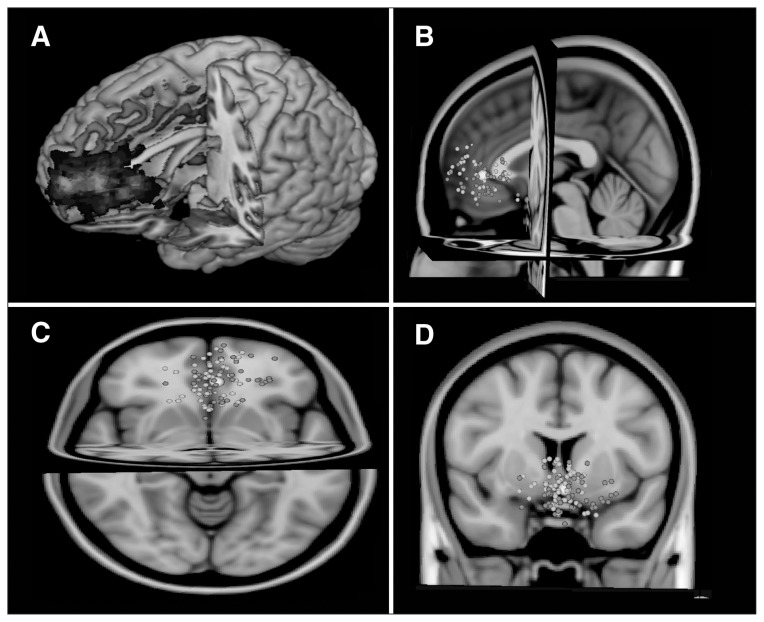
The individualized regions that were isolated for feedback during run 1 were localized in the PFC and are shown in Figure 2; panel A demonstrates the overall group mean, while panels B, C and D show centres of the individual participant ROIs.
Fig. 2.
The individual locations of regions of interest (ROIs; n = 111). Group map from (A) all individual ROIs, (B) sagittal, (C) transverse and (D) coronal. The centre of individual ROIs is displayed in grey for the neurofeedback group and white for the control group. The averaged centre of all ROIs is shown as a large white circle (Montreal Neurological Institute coordinates: x, y, z = −2, 26, 1).
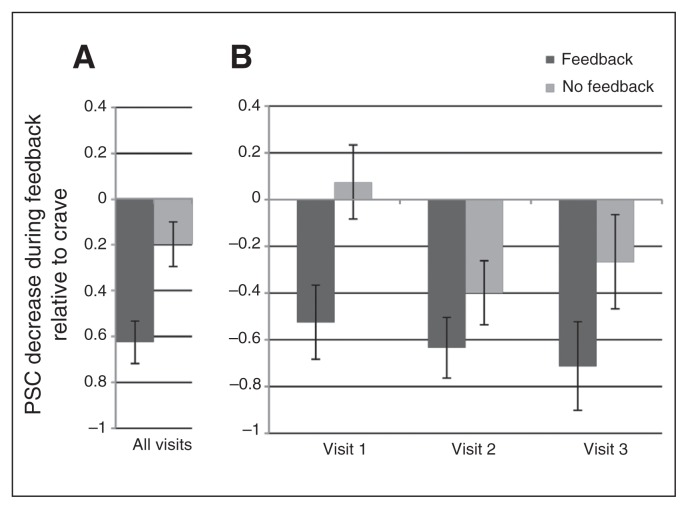
As shown in Figure 3, the PSC analysis of the attenuation of craving-related ROI activation revealed a significant main effect of condition, such that the neurofeedback group had a greater mean reduction in craving-related activation than the control group (−0.62 v. −0.18, F1,248.94 = 10.80, p = 0.001). There were no other significant effects or interactions. Follow-up analysis revealed that the neurofeedback group and the control group did not significantly differ in their response during the crave run across visits (0.83 v. 0.52, p = 0.11).
Fig. 3.
Reduction in craving-related brain activation during feedback by condition. Change in brain response in the individualized feedback region of interest (ROI) was calculated by subtracting percent signal change (PSC) during feedback runs from PSC during the crave run. (A) A linear mixed model showed a significant main effect of feedback condition on PSC reduction during feedback. (B) The PSC decrease at each visit by feedback condition.
Subjective ratings
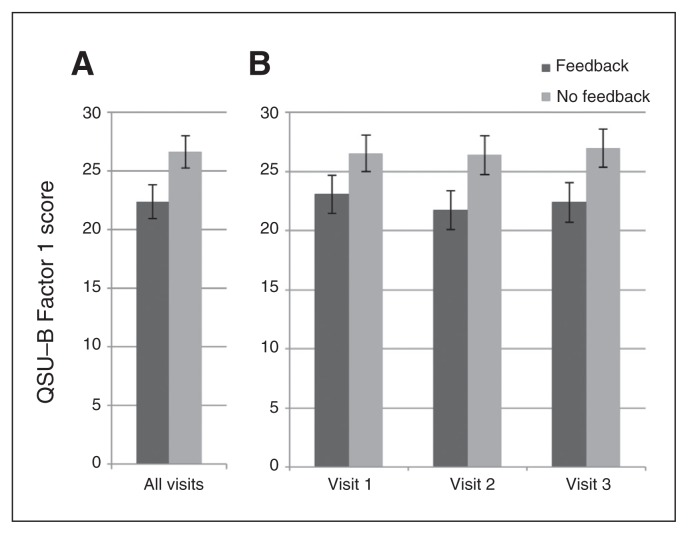
During the scanning visits, we found a group effect (neurofeedback < control), adjusted for prescan repeated baselines, at the trend level for postscan QSU total scores (F1,32.94 = 4.08, p = 0.05). As shown in Figure 4, we found a significant group effect (neurofeedback < control, adjusted for prescan repeated baselines) for postscan QSU Factor 1 scores, a measure of a strong desire to smoke with the anticipation of positive outcomes from smoking (F1,34.52 = 4.52, p = 0.041). In contrast, we found no group difference for postscan QSU Factor 2 scores, a measure of an urgent need to smoke and the expectation of relief of negative affect (F1,33.35 = 2.13, p = 0.15). Across QSU scales, there were no effects of visit or group × visit interactions.
Fig. 4.
Change in postscan QSU-B Factor 1 scores by condition, controlling for pre-scan QSU Factor 1 scores. Scores were adjusted for pre-scan baseline differences. (A) We found a significant effect of feedback condition on Questionnaire of Smoking Urges-Brief (QSU-B) Factor 1 scores. (B) The QSU-B Factor 1 scores at each visit.
Discussion
The present study tested the utility of rtfMRI neurofeedback to reduce craving and activation in an individualized craving-related ROI during exposure to smoking-related cues in nicotine-dependent smokers receiving neurofeedback versus a no-feedback control. The neurofeedback and control groups were well matched, with relatively small dropout rates that did not differ significantly between groups. Compared with the control group, the smokers receiving neurofeedback reduced craving-related activation in the brain across 3 visits. No significant attenuation of craving-related activation across the 3 visits was observed in the no-feedback control group; however, a nonsignificant decrease was noted, suggesting the control group also demonstrated some degree of the ability to downregulate activation in the second and third visit (Fig. 3).
We selected a region approximate to the ACC/mPFC as the ROI based on several prior studies showing that these areas are commonly activated during cue-elicited craving.34–36 However, as described in the Methods section, the individualized craving ROI that was identified for feedback encompassed areas of the mPFC and other PFC regions as well as the ACC. Janes and colleagues36 found increased regional activation in multiple regions, including the ACC and PFC, during pre–quit date exposure to smoking-related cues compared with neutral cues in smokers who slipped compared with abstainers during a quit attempt. In both preclinical37 and human imaging studies,38 the ACC has been reported to be involved in executive functioning, such as decision making, choosing between alternatives and evaluating possible outcomes to optimize results. Additionally, the choice of the ACC in the study design is consistent with previous rtfMRI neurofeedback studies demonstrating that individuals can learn to control both dorsal ACC (dACC)39 and ventral ACC (vACC) activity.40 Though some anatomic specificity is lost, selecting a personalized ROI in the vicinity of the mPFC or ACC maximized the opportunity to provide meaningful feedback, as the selection was based on each individual’s unique pattern of activation (Fig. 2). The group map of all of the individual ROIs encompassed the dACC, vACC and mPFC. Recent advances in neuroimaging have challenged the historical dichotomy between the dACC serving cognitively demanding tasks and the vACC involving emotional processing41 and suggests that both divisions make key contributions to emotional processing42 and decision making.43 The PFC likewise contributes to a network subserving executive functioning, such as general problem-solving, attention, and working memory.44 The mPFC is involved in decision making and memory, and recent evidence suggests that the mPFC is also involved in the emotional response or action evoked by a specific situation or event.45 The challenge of not smoking following exposure to smoking-related cues presents both a cognitive and emotional task for nicotine-dependent smokers, and individual variation in involvement of the ACC and PFC is expected. These findings are in line with the sensitization–homeostasis (SH) model of nicotine dependence,46 which proposes that nicotine’s inhibitory properties are related to its addictive properties. The SH theory suggests that the administration of nicotine inhibits craving, including cue-elicited craving, and the associated neural activation. Homeostatic neuroplastic adaptations oppose nicotine’s action, resulting in a rebound of craving when the nicotine effects wane. As tolerance and withdrawal develop, the duration of the relief from smoking shortens, leading to progressive escalation in cigarette smoking. The utility of rtfMRI neurofeedback may lie in aiding smokers to enhance inhibitory input to reduce craving and decrease the associated activation in the underlying neural circuits. Of note, the mean centre of all the ROIs was located in the pregenual ACC, a subregion found to be preferentially activated by the desire and intention to smoke, with smoking perceived as rewarding.43,47 This finding is consistent with the QSU-B findings that participants in this study smoked more in response to anticipation of the rewarding properties of smoking than from the anticipation of the relief of negative affect.
The neurofeedback group had significantly reduced activation in the individualized ROI compared with the control group during exposure to smoking-related cues while attempting to reduce craving and decrease thermometer bar ratings. Moreover, the groups did not differ in their responses when instructed to crave during the first run, indicating that the reduction in craving-related activation was not driven by differences during the crave runs. While we found a significant main effect of group, we did not find a significant group × visit effect. However, the small, consistent nonsignificant group differences across visits contributed to the overall significant group effect, suggesting that multiple training sessions are needed. Additional paradigm development is needed to determine the optimal timing of the neurofeedback training during a quit attempt as well as the number of visits and number of runs within sessions needed to train smokers to reduce reactivity.
Reactivity to smoking cues is a potentially malleable risk factor for relapse during a quit attempt. Although research on the association between cue-reactivity and relapse is mixed,8,48 cue-induced craving to smoke has been considered one of the driving forces in continued smoking49,50 and relapse during a quit attempt.51,52 Research suggests that in addition to conscious craving induced by smoking-related cues, relapse may occur by the induction of an automatic drug-seeking state coupled with impaired ability to inhibit the urge the smoke.53 As current evidence-based pharmacotherapies have limited impact on the prevention of cue-induced craving and the associated return to smoking,54,55 the development of new therapies with a focus on craving is clearly needed. The results of our study suggest an innovative approach to modulation of smoking cue-induced craving. Future studies are needed to investigate the duration of effects and approaches to generalize the decreased craving in the laboratory setting to real-life exposure to smoking-related cues.
The appropriate control condition for rtfMRI neurofeedback is a fundamental issue. Both no-feedback17 and false-feedback56 control conditions during feedback provided from another individual or nonassociated region have been used in previous research. Both methods have advantages and disadvantages. A false-feedback condition may inadvertently produce frustration and unattended efforts to complete the task, while a no-feedback condition may not completely control for nonessential processes used during the task (e.g., the process of feedback evaluation). In a previous study, our group explored this issue and found widespread activation, including frontal, temporal and parietal areas of the brain,20 with a false-feedback control group. As a result, we elected to use a no-feedback control condition in this study; further research is needed to determine the ideal control condition for rtfMRI neurofeedback research.
The location of the ROI for multiple rtfMRI neurofeedback sessions is also a critical issue for future research. The same ROI from the first scanning session could be used across sessions, or a unique task-driven ROI could be identified for each training session. In this preliminary rtfMRI neurofeedback study, a unique task-driven ROI was selected for each training session. This strategy minimizes the risk of providing neurofeedback from a nonactivated area owing to natural variation in the ROI and possible alterations as a result of previous neurofeedback sessions.
Limitations
Several limitations should be noted in the interpretation and application of our study results. While the smokers reported being moderately motivated to quit, the neurofeedback was not presented as treatment, and they were not required to set a quit date. The study did not include postscan training or other interventions designed to enhance the durability of rtfMRI procedures. Relapse within the first few days of a quit attempt is common, and maintaining abstinence beyond the first few days is the critical issue in the development of more efficacious treatments. Future research in treatment-seeking smokers ready to initiate a cessation attempt is needed, and measurements of actual smoking behaviour are necessary to further investigate the utility of rtfMRI neurofeedback.
The optimal number of training visits is an important area requiring further research. In this study the use of multiple training sessions increased the power and odds of finding a significant group difference. In this early clinical application of rtfMRI neurofeedback, we found a main effect on subjective ratings and PSC with the neurofeedback, with a greater reduction in brain activation compared with the control condition. Participants did not demonstrate a significant progressive improvement with each visit, as a group × visit interaction was not found in either the PSC or subjective ratings; however, a nonsignificant progressive decrease in PSC was noted in the active group. Future studies should systematically compare 1 versus multiple sessions.
Conclusion
Compared with a control group, non–treatment seeking nicotine-dependent smokers receiving rtfMRI feedback from an individualized ROI were able to decrease their neural activity and physiologic and subjective responses to smoking-related cues. The progressive nonsignificant decreases over the 3 scanning visits, suggesting that rtfMRI may be useful in decreasing cue-induced craving, is often associated with relapse in smokers. This innovative approach warrants further exploration and development.
Acknowledgements
The study was supported by grant 5R21DA026085 and 5R33DA026085 from the National Institute of Drug Abuse (co-primary investigators: K. Brady and M. George). The National Institute of Drug Abuse had no role in the study design nor conduct of the study, including data collection, data analysis, interpretation of the results, manuscript preparation, review, and approval or the decision to submit the manuscript for publication. The authors thank Danielle Paquette for her assistance with the laboratory cue-reactivity sessions outside of the scanner and everyone who shared their expertise and assisted in the development of the real-time fMRI neurofeedback paradigm.
Footnotes
Competing interests: None declared.
Contributors: All authors designed the study. K. Hartwell, X. Li and T. LeMatty acquired the data, which C. Hanlon, X. Li, J. Borckardt, M. Canterberry, J. Prisciandaro, M. Moran-Santa Maria, M. George and K. Brady analyzed. K. Hartwell, X. Li, J. Borckardt, M. Canterberry, J. Prisciandaro and T. LeMatty wrote the article, which all authors reviewed and approved for publication.
References
- 1.CDC. Cigarette smoking — United States, 1965–2008. Morbidity and Mortality Weekly Report. 2011;60:109–13. [PubMed] [Google Scholar]
- 2.CDC. Quitting smoking among adults — United States, 2001–2010. [MMWR] Morbidity and Mortality Weekly Report. 2011;60:1513–9. [PubMed] [Google Scholar]
- 3.Piasecki TM. Relapse to smoking. Clin Psychol Rev. 2006;26:196–215. doi: 10.1016/j.cpr.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 4.LaRowe SD, Saladin M, Carpenter M, et al. Reactivity to nicotine cues over repeated cue reactivity sessions. Addict Behav. 2007;32:2888–99. doi: 10.1016/j.addbeh.2007.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saladin ME, Gray KM, Carpenter MJ, et al. Gender differences in craving and cue reactivity to smoking and negative affect/stress cues. Am J Addict. 2012;21:210–20. doi: 10.1111/j.1521-0391.2012.00232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abrams DB, Monti PM, Carey KB, et al. Reactivity to smoking cues and relapse: two studies of discriminant validity. Behav Res Ther. 1988;26:225–33. doi: 10.1016/0005-7967(88)90003-4. [DOI] [PubMed] [Google Scholar]
- 7.Shiffman S, Engberg JB, Paty JA, et al. A day at a time: predicting smoking lapse from daily urge. J Abnorm Psychol. 1997;106:104–16. doi: 10.1037//0021-843x.106.1.104. [DOI] [PubMed] [Google Scholar]
- 8.Wray JM, Gass JC, Tiffany ST. A systematic review of the relationships between craving and smoking cessation. Nicotine Tob Res. 2013;15:1167–82. doi: 10.1093/ntr/nts268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shiffman S, Gwaltney CJ, Balabanis MH, et al. Immediate antecedents of cigarette smoking: an analysis from ecological momentary assessment. J Abnorm Psychol. 2002;111:531–45. doi: 10.1037//0021-843x.111.4.531. [DOI] [PubMed] [Google Scholar]
- 10.Nestoriuc Y, Martin A, Rief W, et al. Biofeedback treatment for headache disorders: a comprehensive efficacy review. Appl Psychophysiol Biofeedback. 2008;33:125–40. doi: 10.1007/s10484-008-9060-3. [DOI] [PubMed] [Google Scholar]
- 11.Domschke K, Dannlowski U. Imaging genetics of anxiety disorders. Neuroimage. 2010;53:822–31. doi: 10.1016/j.neuroimage.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 12.Sokhadze TM, Cannon RL, Trudeau DL. EEG biofeedback as a treatment for substance use disorders: review, rating of efficacy, and recommendations for further research. Appl Psychophysiol Biofeedback. 2008;33:1–28. doi: 10.1007/s10484-007-9047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saxby E, Peniston EG. Alpha-theta brainwave neurofeedback training: an effective treatment for male and female alcoholics with depressive symptoms. J Clin Psychol. 1995;51:685–93. doi: 10.1002/1097-4679(199509)51:5<685::aid-jclp2270510514>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 14.Schneider F, Elbert T, Heimann H, et al. Self-regulation of slow cortical potentials in psychiatric patients: alcohol dependency. Biofeedback Self Regul. 1993;18:23–32. doi: 10.1007/BF00999511. [DOI] [PubMed] [Google Scholar]
- 15.Caria A, Sitaram R, Birbaumer N. Real-time fMRI: a tool for local brain regulation. Neuroscientist. 2012;18:487–501. doi: 10.1177/1073858411407205. [DOI] [PubMed] [Google Scholar]
- 16.Weiskopf N. Real-time fMRI and its application to neurofeedback. Neuroimage. 2012;62:682–92. doi: 10.1016/j.neuroimage.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 17.DeCharms RC, Maeda F, Glover G, et al. Control over brain activation and pain learned by using real-time functional MRI. Proc Natl Acad Sci U S A. 2005;102:18626–31. doi: 10.1073/pnas.0505210102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linden DE, Habes I, Johnston SJ, et al. Real-time self-regulation of emotion networks in patients with depression. PLoS ONE. 2012;7:e38115. doi: 10.1371/journal.pone.0038115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sitaram R, Lee S, Ruiz S, et al. Real-time support vector classification and feedback of multiple emotional brain states. Neuroimage. 2011;56:753–65. doi: 10.1016/j.neuroimage.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Johnson KA, Hartwell K, LeMatty T, et al. Intermittent “real-time” fMRI feedback is superior to continuous presentation for a motor imagery task: a pilot study. J Neuroimaging. 2012;22:58–66. doi: 10.1111/j.1552-6569.2010.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Hartwell KJ, Borckardt J, et al. Volitional reduction of anterior cingulate cortex activity produces decreased cue craving in smoking cessation: a preliminary real-time fMRI study. Addict Biol. 2013;18:739–48. doi: 10.1111/j.1369-1600.2012.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Canterberry M, Hanlon CA, Hartwell KJ, et al. Sustained reduction of nicotine craving with real-time neurofeedback: exploring the role of severity of dependence. Nicotine Tob Res. 2013;15:2120–4. doi: 10.1093/ntr/ntt122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanlon CA, Hartwell KJ, Canterberry M, et al. Reduction of cue-induced craving through realtime neurofeedback in nicotine users: the role of region of interest selection and multiple visits. Psychiatry Res Neuroimaging. 2013;213:79–81. doi: 10.1016/j.pscychresns.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fagerström KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav. 1978;3:235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- 25.Heatherton TF, Kozlowski LT, Frecker RC, et al. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 26.Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- 27.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- 28.Hartwell KJ, Johnson KA, Li X, et al. Neural correlates of craving and resisting craving for tobacco in nicotine dependent smokers. Addict Biol. 2011;16:654–66. doi: 10.1111/j.1369-1600.2011.00340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geier A, Mucha R, Pauli P. Appetitive nature of drug cues confirmed with physiological measures in a model using pictures of smoking. Psychopharmacology (Berl) 2000;150:283–91. doi: 10.1007/s002130000404. [DOI] [PubMed] [Google Scholar]
- 30.Gillbert DRN. International smoking image series with neutral counterparts 1998 [Google Scholar]
- 31.Lang PJ, Bradley MM, Cuthbert BN. Technical report A-8. University of Florida; Gainesville, Fl: 2008. International Affective Picture System (IAPS) affective ratings of pictures and instruction manual. [Google Scholar]
- 32.Goebel R. BrainVoyager — past, present, future. Neuroimage. 2012;62:748–56. doi: 10.1016/j.neuroimage.2012.01.083. [DOI] [PubMed] [Google Scholar]
- 33.Brett M, Johnsrude IS, Owen AM. The problem of functional localization in the human brain. Nat Rev Neurosci. 2002;3:243–9. doi: 10.1038/nrn756. [DOI] [PubMed] [Google Scholar]
- 34.Brody AL, Mandelkern MA, London ED, et al. Brain metabolic changes during cigarette craving. Arch Gen Psychiatry. 2002;59:1162–72. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- 35.McClernon FJ, Hiott FB, Huettel SA, et al. Abstinence-induced changes in self-report craving correlate with event-related fMRI responses to smoking cues. Neuropsychopharmacology. 2005;30:1940–7. doi: 10.1038/sj.npp.1300780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janes AC, Pizzagalli DA, Richardt S, et al. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry. 2010;67:722–9. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kennerley SW, Wallis JD. Encoding of reward and space during a working memory task in the orbitofrontal cortex and anterior cingulate sulcus. J Neurophysiol. 2009;102:3352–64. doi: 10.1152/jn.00273.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allman JM, Hakeem A, Erwin JM, et al. The anterior cingulate cortex. Ann N Y Acad Sci. 2001;935:107–17. [PubMed] [Google Scholar]
- 39.Weiskopf N, Veit R, Erb M, et al. Physiological self-regulation of regional brain activity using real-time functional magnetic resonance imaging (fMRI): methodology and exemplary data. Neuroimage. 2003;19:577–86. doi: 10.1016/s1053-8119(03)00145-9. [DOI] [PubMed] [Google Scholar]
- 40.Hamilton JP, Glover GH, Hsu J-J, et al. Modulation of subgenual anterior cingulate cortex activity with real-time neurofeedback. Hum Brain Mapp. 2011;32:22–31. doi: 10.1002/hbm.20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 42.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu X, Hairston J, Schrier M, et al. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2011;35:1219–36. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Genovesio A, Wise SP, Passingham RE. Prefrontal-parietal function: from foraging to foresight. Trends Cogn Sci. 2014;18:72–81. doi: 10.1016/j.tics.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 45.Euston DR, Gruber AJ, McNaughton BL. The role of medial prefrontal cortex in memory and decision making. Neuron. 2012;76:1057–70. doi: 10.1016/j.neuron.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DiFranza JR, Huang W, King J. Neuroadaptation in nicotine dependence: update on the sensitization-homeostatis model. Brain Sci. 2012;2:523–52. doi: 10.3390/brainsci2040523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rogers RD, Ramnani N, Mackay C, et al. Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biol Psychiatry. 2004;55:594–602. doi: 10.1016/j.biopsych.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 48.Perkins KA. Subjective reactivity to smoking cues as a predictor of quitting success. Nicotine Tob Res. 2012;14:383–7. doi: 10.1093/ntr/ntr229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shiffman S. Refining models of dependence: variations across persons and situations. Br J Addict. 1991;86:611–5. doi: 10.1111/j.1360-0443.1991.tb01817.x. [DOI] [PubMed] [Google Scholar]
- 50.Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychol Rev. 1990;97:147–68. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- 51.Bagot KS, Heishman SJ, Moolchan ET. Tobacco craving predicts lapse to smoking among adolescent smokers in cessation treatment. Nicotine Tob Res. 2007;9:647–52. doi: 10.1080/14622200701365178. [DOI] [PubMed] [Google Scholar]
- 52.Killen JD, Robinson TN, Ammerman S, et al. Randomized clinical trial of the efficacy of bupropion combined with nicotine patch in the treatment of adolescent smokers. J Consult Clin Psychol. 2004;72:729–35. doi: 10.1037/0022-006X.72.4.729. [DOI] [PubMed] [Google Scholar]
- 53.Garavan H, Hester R. The role of cognitive control in cocaine dependence. Neuropsychol Rev. 2007;17:337–45. doi: 10.1007/s11065-007-9034-x. [DOI] [PubMed] [Google Scholar]
- 54.Ferguson SG, Gitchell JG, Shiffman S, et al. Prediction of abstinence at 10 weeks based on smoking status at 2 weeks during a quit attempt: secondary analysis of two parallel, 10-week, randomized, double-blind, placebo-controlled clinical trials of 21-mg nicotine patch in adult smokers. Clin Ther. 2009;31:1957–65. doi: 10.1016/j.clinthera.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 55.Tiffany ST, Cox LS, Elash CA. Effects of transdermal nicotine patches on abstinence-induced and cue-elicited craving in cigarette smokers. J Consult Clin Psychol. 2000;68:233–40. doi: 10.1037//0022-006x.68.2.233. [DOI] [PubMed] [Google Scholar]
- 56.Rota G, Sitaram R, Veit R, et al. Self-regulation of regional cortical activity using real-time fMRI: the right inferior frontal gyrus and linguistic processing. Hum Brain Mapp. 2009;30:1605–14. doi: 10.1002/hbm.20621. [DOI] [PMC free article] [PubMed] [Google Scholar]