Abstract
Polymeric microparticle(MPs)-in-gel formulations for extended delivery of octreotide were developed. We investigated influence of polymer composition on acylation of octreotide and kinetics of release during in vitro release from biodegradable polymeric formulations. Polycaprolactone (PCL), polylactic acid (PLA), polyglycolic acid (PGA) and polyethylene glycol (PEG) based triblock (TB≈PCL10k-PEG2k-PCL10k) and pentablock (PBA≈PLA3k-PCL7k-PEG2k-PCL7k-PLA3k and PBB≈PGA3k-PCL7k-PEG2k-PCL7k-PGA3k) polymers were investigated. Octreotide was encapsulated in MPs using methanol-oil/water emulsion solvent evaporation method. The particles were characterized for size, morphology, encapsulation efficiency, drug loading and in vitro release. Release samples were subjected to HPLC analysis for quantitation and HPLC-MS analysis for identification of native and chemically modified octreotide adducts. Entrapment efficiency of methanol-oil/water method with TB, PBA and PBB polymers were 45%, 60%, and 82%, respectively. A significant fraction of released octreotide was acylated from lactide and glycolide based PBA (53%) and PBB (92%) polymers. Substantial amount of peptide was not released from PBB polymers after 330 days of incubation. Complete release of octreotide was achieved from TB polymer over a period of 3 months with minimal acylation of peptide (13%). PCL based polymers resulted in minimal acylation of peptide and hence may be suitable for extended peptide and protein delivery. Conversely, polymers having PLA and PGA blocks may not be appropriate for peptide delivery due to acylation and incomplete release.
Keywords: polycaprolactone, polyglycolic acid, Polylactic acid, hydrogel, peptide, microparticles, drug delivery, sustained delivery
Graphical Abstract
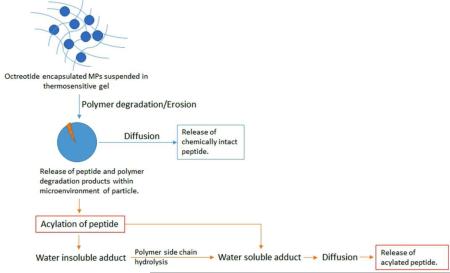
Factors influencing native and acylated peptide release from biodegradable MPs in gel
1. Introduction
Proteins and peptides based biotherapeutics are currently being introduced rapidly into the clinical trials for treatment of a number of diseases. Proteins/peptides suffer from a myriad of delivery related constrains such as poor bioavailability, permeability across biological membranes and in vivo stability. Short in vivo half-life leads to frequent multiple parenteral administrations, which lowers patient compliance. Sustained delivery of biologics may address these issues and improve their potential as therapeutics. Among the approaches investigated for sustained delivery of biologics, nano- and microparticles are in the forefront and clinically available for various ailments, such as Sandostatin LAR® depot containing octreotide (Fogueri and Singh, 2009; Vaishya et al., 2015a; Wang et al., 2013).
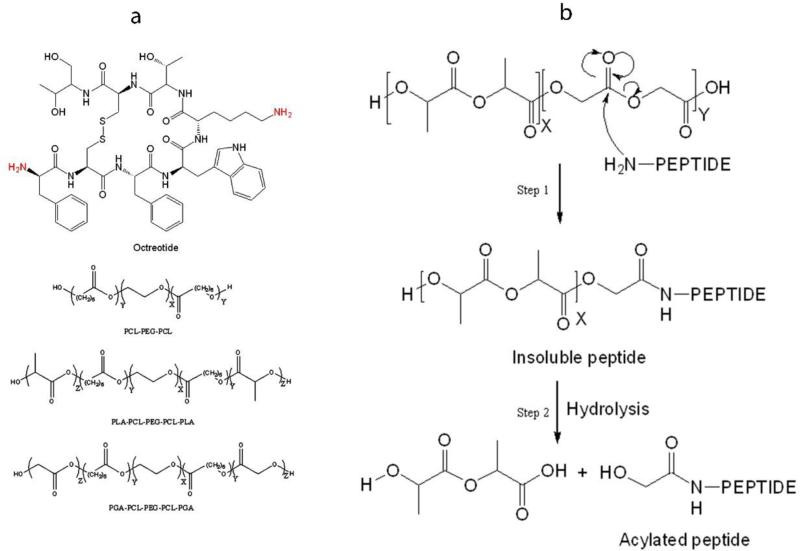
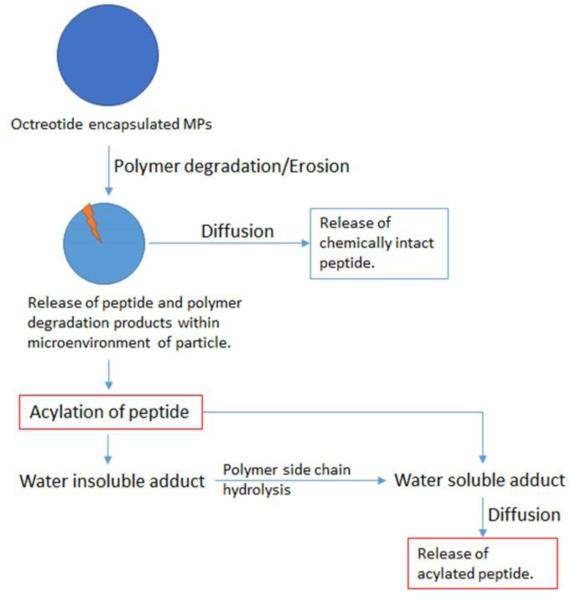
Octreotide is a semisynthetic cyclic octapeptide, a somatostatin analogue, indicated for acromegaly (Fig. 1a) (Feelders et al., 2009). It is also recommended for symptomatic relief by suppressing severe diarrhea and flushing episodes associated with metastatic carcinoid tumors (De Martino et al., 2010; Feelders et al., 2009; Modlin et al., 2006). Half-life of this peptide following S.C. and I.M. administrations is short, about 100 min (Chanson et al., 1993). It is marketed as solution for subcutaneous injection (lactate buffer, pH 4.2) and as a depot form for intramuscular administration. PLGA-glucose star polymer microparticles (MPs) (size ~40 μm) provide delivery over a period of 4 weeks. PLGA has been widely investigated for the delivery of bioactive peptide and proteins (Kapoor et al., 2015; Ma, 2014; Sadat Tabatabaei Mirakabad et al., 2014; Vaishya et al., 2015a). However, it may not be the best polymer for delivering peptide and protein biologics due to acylation of biologics during release (Ghassemi et al., 2012; Ibrahim et al., 2005; Murty et al., 2003). It has been well documented that chemical stability of octreotide is compromised due to acylation during the release (Ghassemi et al., 2012). Less than 20% of native octreotide was released from Sandostatin LAR® depot during in vitro release (Ghassemi et al., 2012). Nearly 60% octreotide was released as acylated adduct over period of 3 months (Ghassemi et al., 2012). Chemical derivatization of octreotide is because of reaction of peptide with degraded PLGA oligomers inside the MP core. Structure of octreotide and mechanism of peptide acylation is illustrated in Fig. 1. Preliminary studies indicates that nucleophilic attack of amine from peptide on partial positive carbonyl carbon is responsible for chemical derivatization of peptide during release (Fig. 1b). The mechanism of acylation reaction is nucleophilic carbonyl substitution. Similar phenomenon has been reported for other protein and peptides such as bovine serum albumin, human atrial natriuretic peptide, human parathyroid hormone, insulin and salmon calcitonin (Ghalanbor et al., 2012; Ibrahim et al., 2005; Lucke et al., 2002; Na et al., 2003; Zhang and Schwendeman, 2012). Acylation of peptide also leads to formation of water insoluble adducts resulting in incomplete release of peptide from MPs (Fig. 1b, step 1) (Murty et al., 2003). As a result, 15% of octreotide was not released, which may be attributed to formation of water insoluble adducts (Ghassemi et al., 2012).
Figure 1.
(a) Structure of octreotide and polymers for MPs and gel. (b) Nucleophilic carbonyl-substitution mechanism of peptide acylation in PLGA microparticles. Nucleophilic attack of amine from peptide on partial positive carbon of degraded PLGA fragment results into formation of insoluble adduct (Step 1). Alcohol (-OH) and thiol (-SH) groups may also act as nucleophile (Murty et al., 2003). Hydrolysis of polymer side chain in insoluble peptide adduct results in release of soluble acylated peptide (Step 2).
Several approaches have been investigated to minimize peptide acylation with some degree of success. Some noteworthy strategies include polymer modifications, chemical modification of octreotide and encapsulation of divalent cations (Ahn et al., 2011; Ghassemi et al., 2012; Na and DeLuca, 2005; Na et al., 2005; Sophocleous et al., 2009; Zhang et al., 2009). Ahn et al. and Na et al. chemically modified octreotide at reactive amines to minimize acylation by maleic anhydride and PEG. Ahn et al. showed that maleic anhydride conjugated octreotide inhibited acylation of peptide and only 10% peptide underwent acylation from PLGA films (Ahn et al., 2011). Na et al. reported that PEGlyation significantly lowered the adsorption of peptide on PLGA surface and may minimize acylation of peptide. However, in all these studies peptide derivatives were physically incubated with PLGA in solution instead of encapsulating derivatives in microparticles (Na and DeLuca, 2005; Na et al., 2005). Zhang et al. and Sophocleous et al. showed that simple encapsulation of divalent cationic salts in PLGA MPs prevents peptide sorption on PLGA and thereby minimize octreotide acylation, nonetheless there was a limited success in preventing peptide acylation (Sophocleous et al., 2009; Zhang et al., 2009). Ghassemi et al. modified polymer Poly(D,L-lactide-co-hydroxymethyl glycolide) to minimize nucleophilic attack of octreotide amine on glycolide by steric hindrance and were able to obtain more than 70% octreotide in native form during in vitro release (Ghassemi et al., 2012). Thus, acylation of peptide in lactide- and glycolide-based polymers is a significant roadblock preventing the development of clinically suitable sustained-release formulations for protein and peptide biologics.
We have previously shown that acylation of octreotide from PGLA MPs-in-gel system can be minimized by using hydrophobic ion-pairing approach (Vaishya et al., 2015b). However, release rate was very high and as a result >80% octreotide was released within 3 weeks. Thus, there is an unmet need to develop an extended release formulation to deliver peptides in active form over period of months. Many researchers have investigated thermosensitive TB copolymers (A-B-A or B-A-B) composed of PLA/PCL/PLGA blocks (A) and PEG block (B) for their applicability in the development of sustained delivery formulation (Ghalanbor et al., 2012; Kapoor et al., 2015; Lucke et al., 2002). PCL polymer is known for their semi-crystalline nature which is responsible for their slow degradation (Dash and Konkimalla, 2012; Vaishya et al., 2014). We hypothesized that PCL based polymer may minimize or prevent the chemical degradation of octreotide. In the present research article, MPs of triblock polymer (PCL10k-PEG2k-PCL10k) and pentablock co-polymers (PLA3k-PCL7k-PEG2k-PCL7k-PLA3k and PGA3k-PCL7k-PEG2k-PCL7k-PGA3k) were evaluated for extended delivery of octreotide. Further, to sustain the release and minimize the possibility of burst effect (Wang et al., 2002), MPs were suspended in thermo-responsive gel solution. Special emphasis was placed on acylation of octreotide during release from caprolactone, glycolide and lactide based polymers.
2. Material and methods
2.1. Materials
Octreotide acetate (PubChem CID: 383414) was procured from ChinaPeptides Co., Ltd (Shanghai, China). D,L-lactide (PubChem CID: 65432), glycolide (PubChem CID: 7272), caprolactone (PubChem CID: 10401), stannous octoate, Poly(ethylene glycol) (Mw 2000 g/mol) and polyvinyl alcohol were obtained from Sigma Aldrich (St Louis, MO). All other solvents/chemicals were of analytical reagent grade.
2.2. Methods
2.2.1. Preparation and characterization of triblock and pentablock co-polymers
TB (PCL10k-PEG2k-PCL10k) and PBA (PLA3k-PCL7k-PEG2k-PCL7k-PLA3k) and PBB (PGA3k-PCL7k-PEG2k-PCL7k-PGA3k) co-polymers were synthesized by ring-opening bulk copolymerization following a published protocol from our laboratory (Patel et al., 2015). General structures of these polymers are shown in Fig. 1a. Subscript represents molecular weight of polymer segment. PEG (Mw 2000 g/mol) with both hydroxyl end was vacuum-dried for 4 h, which acted as initiator to polymerize ε-caprolactone using stannous octoate (0.5 wt %) as catalyst. The reaction was carried out in a closed vessel under nitrogen, at 130°C for 36 h. For synthesis of PB polymers (PBA and PBB), polymer PCL7k-PEG2k-PCL7k was synthesized. Subsequently, PLA or PGA segment was polymerized on the flank of triblock PCL7k-PEG2k-PCL7k polymer to obtain PBA or PBB polymer. The resultant polymer was purified by cold ether precipitation. Purified polymer was vacuum-dried followed by freeze-drying to remove any residual solvent and moisture. Structure and molecular weight of TB copolymers were confirmed by 1H-NMR and gel permeation chromatography (GPC).
Proton-NMR
Following previously published method of 1H-NMR spectroscopy, polymeric material was dissolved in CDCl3 and analyzed by Varian-400 NMR spectrometer (Patel et al., 2015).
Gel permeation chromatography (GPC)
Purity, molecular weights (Mw and Mn) and polydispersity were determined by GPC following our previously published protocol (Patel et al., 2015). Polymeric samples were analyzed via PSS (Polymer Standards Service) WinGPC Unity version 7.5.0 system equipped with light scattering detector coupled to the Tosoh Ecosec system. Mobile phase consisted of tetrahydrofuran (THF), at flow rate of 1 mL/min. Separation was carried out on Styragel HR-3 column. Five mg of polymeric material was dissolved in THF for sample preparation.
2.2.2. Preparation of octreotide encapsulated microparticles
Octreotide-loaded MPs were prepared using TB, PBA or PBB polymer by methanol-oil/water solvent evaporation method. Briefly, methanol phase (Me) consisted of 5 mg octreotide dissolved in 50 μL of methanol containing triethyl amine (TEA) (mole ratio Octreotide:TEA::1:2.5). Polymer (TB or PB) was dissolved in dichloromethane (DCM) (100 mg in 500 μL DCM) constituting oil (O) phase. The methanolic phase containing octreotide was mixed with polymer solution aided by probe sonication for 30 sec at 2 output to make Me-O phase. This Me-O phase was slowly added to the water phase (650 μL, 1% PVA with 5% NaCl) under constant stirring to form a Me-O/W1 emulsion. Emulsion was further diluted with 3 ml of 1% PVA (5% NaCl) (W2) and stirred for 3 h under hood to remove organic solvent. The MPs were separated by centrifugation at 15,000 RPM for 10 min. Particles were washed three times with DDI water. MPs were freeze-dried using mannitol (2% w/v) as a cryoprotectant and stored at −20°C until further use. Ghost MPs were prepared following the same method except no octreotide was added during preparation.
2.2.3. Drug loading (%) and Entrapment Efficiency (%)
Amount of octreotide in MPs was measured by UV spectroscopy. A known amount of freeze dried MPs (5 mg, n=3) were dissolved in 200 μL DMSO. UV absorbance was measured at 280 nm. Ghost MPs served as blank. Entrapment efficiency (%EE) and Drug loading (%DL) were calculated by following equations 1 and 2, respectively.
| (1) |
| (2) |
2.2.4. Powder X-ray diffraction (PXRD)
Physical state of polymers was investigated by PXRD analysis. MiniFlex automated X-ray diffractometer (Rigaku, The Woodlands, Texas) with Ni-filtered Cu-kα radiation (30 kV and 15 mA) was employed to study diffraction patterns at room temperature. The detector was a solid state detector with slit and Ni foil K beta filter. The sample holder was a silicon zero background plate. A few hundred mg of sample was smeared into a thin layer on the Si plate. The sample was rotated around a horizontal axis. The source (tube) was fixed and the detector rotated around the sample axis at twice the speed of the sample rotation.
2.2.5. Scanning electron microscopy
SEM analysis was performed to analyze particle morphology (size and shape). Briefly, freeze-dried MPs were applied to carbon film positioned on an aluminum stub. Surface of particles on carbon film was sputter-coated for one min with (SCD050, Leica Microsystems GmbH, Wetzlar, Germany) Au-Pd alloy under centrifugation followed by sample analysis in Phenom Pro desktop scanning electron microscope.
2.2.6. Release study
Freeze-dried MPs were subjected to in vitro release study in PBS buffer (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4; pH 7.4) at 37°C. Predetermined amount of MPs were suspended in 400 μL of thermosensitive gel solution (20 wt%) maintained at 4°C to prevent accidental gelation. TBMPs were suspended in triblock gel (PCL1000-PEG1000-PCL1000) solution. PBAMPs and PBBMPs were suspended in PB gel (PLA250-PCL1250-PEG1500-PCL1250-PLA250) solution. Preparation and characterization of these gelling polymers has been published earlier (Patel et al., 2014b; Tamboli et al., 2013). Composition of MPs and gel are depicted in Table 1. Resulting MPs in gel suspension was incubated at 37°C for 2 min followed by slow addition of 800 μL of PBS (pH 7.4), preincubated at 37°C. Samples were agitated at 30 RPM. At predefined time intervals, 700 μL of clear supernatant was collected and replaced with fresh PBS. Release samples were evaluated for amount of native and chemically-modified octreotide using HPLC assay. The experiments were carried out in quadruplets and plotted as cumulative octreotide released (%) relative to %EE vs. time. Ghost MPs were kept as control.
Table 1.
Compositions of MPs-in-gel formulation for release study.
| Octreotide-encapsulated MPs-in-gel | MPs polymer composition | Thermoresponsive Gel polymer composition |
|---|---|---|
| TBMPs | PCL10k-PEG2k-PCL10k | PCL1000-PEG1000-PCL1000 |
| PBAMPs | PLA3k-PCL7k-PEG2k-PCL7k-PLA3k | PLA250-PCL1250-PEG1500-PCL1250-PLA250 |
| PBBMPs | PGA3k-PCL7k-PEG2k-PCL7k-PGA3k | PLA250-PCL1250-PEG1500-PCL1250-PLA250 |
2.2.7. High-performance liquid chromatography (HPLC) assay
Native and acylated octreotide concentration in released media was quantified by HPLC assay. A Shimadhu (Shimadzu Scientific Instruments, Columbia, MD, USA) HPLC system coupled with pumps (LC-20AT), degasser (DGU-20A3R), DAD detector (SPD-20AV) and autosampler (SIL-20AHT) was employed. Phenomenax column (Phenomenex C18 kinetex column 100X4.6 mm, 5μm) along with a guard column (Phenomenex SecuritGuard Catridges, C18, 4×2 mm) was used at total flow rate of 0.5 mL/min. A gradient elution method was employed for separation where mobile Phase A (HPCL water (Sigma) with 0.1% formic acid) at 10% and mobile phase B (ACN with 0.1% formic acid) at 90% were ran for first 2 min followed by a linear gradient to reach 100% phase B at 18 min. Concentration for octreotide standards ranged from 100 μg/ml to 3.1 μg/ml prepared in PBS. DAD detector was set at 280 nm for quantification. Injection volume was 50 μl.
2.2.8. HPLC-MS analysis
HPLC-MS was performed using electrospray ionization (ESI) in positive ion mode on a QTrap® API-3200 mass spectrometer, equipped with Shimadzu quaternary pump, vacuum degasser, DAD detector and autosampler (Shimadzu Scientific Instruments, Columbia, MD, USA). Data acquisition and data processing were performed using Analyst 1.4.2 software package (Applied Biosystems, Foster City, CA, USA). LC conditions including column and gradient composition remains same as explained in the earlier HPLC assay. Injection volumes were 30 μl for all samples. UV detector was set on 280 nm and MS was set in range of 200 to 1700 amu. Extracted ion chromatogram for native and acylated peptide was obtained from total ion chromatogram and compared with UV chromatogram to identify native octreotide and chemically acylated adducts.
3. Results and Discussion
Peptide therapeutics typically exhibit short half-lives following systemic administration due to enzymatic degradation. Many of these biologics are indicated for chronic conditions such as octreotide for acromegaly and insulin for diabetes. Hence, these biologics are suitable candidates for sustained release formulations. Biodegradable block polymers offer many advantages over random block polymers such as PLGA. One good example is tunable chemical properties such as hydrophobicity and hydrophilicity of polymer. By tuning these properties, block co-polymers can be tailored for better entrapment efficiency and controlled release.
3.1. Characterization of polymers
Nano- and micro-particles based biodegradable systems have been extensively studied for extended delivery of proteins and peptide. They protect entrapped biomolecules against enzymatic degradation. Nonetheless, when using biodegradable polymers, protein/peptide-polymer interaction must be carefully investigated. Degradation products of polymers must be compatible with biologics. Chemical derivatization i.e. acylation of peptides has been reported from lactide and glycolide based polymers. We hypothesized that PCL based polymer may minimize or prevent the chemical degradation of octreotide. Hence, TB polymer (PCL10k-PEG2k-PCL10k) was designed solely based on caprolactone units to investigate the influence on chemical stability of peptide during the release without interference of lactide or glycolide units. Pentablock co-polymers PBA (PLA3k-PCL7k-PEG2k-PCL7k-PLA3k) and PBB (PGA3k-PCL7k-PEG2k-PCL7k-PGA3k) were synthesized to lower crystallinity of PCL blocks, which may improve loading of peptide in MPs. Block co-polymers were synthesized by well-established ring-opening polymerization method reported from our laboratory (Patel et al., 2015). Weight and number average molecular weights and polydispersity indices (PDI) from NMR and GPC are presented in Table 2. Molecular weight calculated from 1H-NMR was close to molecular weight (Mn) calculated based on feed ratio suggesting nearly all monomer reacted to form polymer chain. Polydispersity for TB, PBA and PBB polymers were 1.59, 1.39 and 1.42, respectively.
Table 2.
Proton-NMR and GPC analysis of polymers.
| Code | Mna (gm/mol) | Mnb (gm/mol) | Mnc (gm/mol) | Mwc (gm/mol) | PDIc |
|---|---|---|---|---|---|
| TB | 22000 | 21300 | 16800 | 26800 | 1.6 |
| PBA | 22000 | 20800 | 17200 | 24000 | 1.4 |
| PBB | 22000 | 20100 | 17000 | 24200 | 1.4 |
Mn-Number average molecular weight, Mw-Weight average molecular weight.
Theoretical value, calculated according to the feed ratio.
Calculated from 1H-NMR results
Determined by GPC analysis
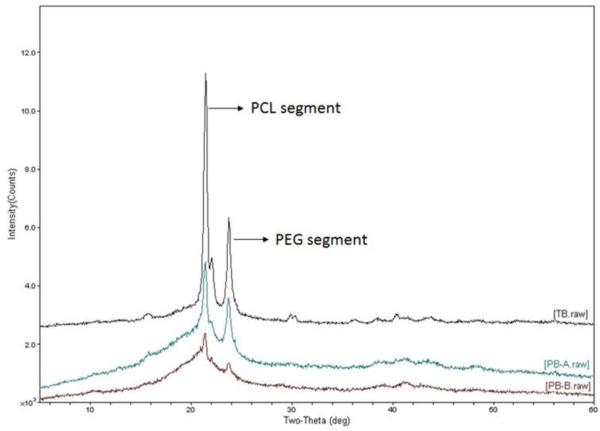
It has been previously shown that PCL possesses semicrystalline structure and the extent of crystallinity depends on the molecular weight of PCL segment and polymer composition i.e., presence of other block polymers such as PLA and PGA in case of PBA and PBB polymers, respectively (Patel et al., 2014a; Patel et al., 2015; Patel et al., 2014b; Peponi et al., 2012; Vaishya et al., 2014). We have also reported that crystallinity of polymer directly influences the release rate (Patel et al., 2015; Tamboli et al., 2013). Hence, x-ray diffraction analysis was performed to determine the physical state of newly synthesized polymers. XRD patterns are depicted in Fig. 2. TB polymer showed presence of very sharp peaks at 2θ 21.9 and 23.8 associated with PCL and PEG segments, respectively (Vaishya et al., 2014). However, incorporation of PLA blocks in triblock polymer diminished the intensity of peaks corresponding to PCL and PEG segments. Addition of PGA segment further diminished the crystallinity of PCL and PEG and the overall polymer was almost amorphous in nature. These observations corroborates with previously published study (Patel et al., 2015). Reduction in crystallinity of PCL could be because of hydrophobic interactions between heterogeneous polymer chains (PCL/PLA and PCL/PGA) which would minimize interaction of PCL chains leading to crystallization.
Figure 2.
Powder X-ray diffraction patterns for TB (PCL10k-PEG2k-PCL10k), PBA (PLA3k-PCL7k-PEG2k-PCL7k-PLA3k) and PBB (PGA3k-PCL7k-PEG2k-PCL7k-PGA3k) polymers. Crystallinity of PCL segment decreased progressively in pentablock PBA and PBB polymers.
3.2. Preparation and characterization of octreotide-loaded MPs
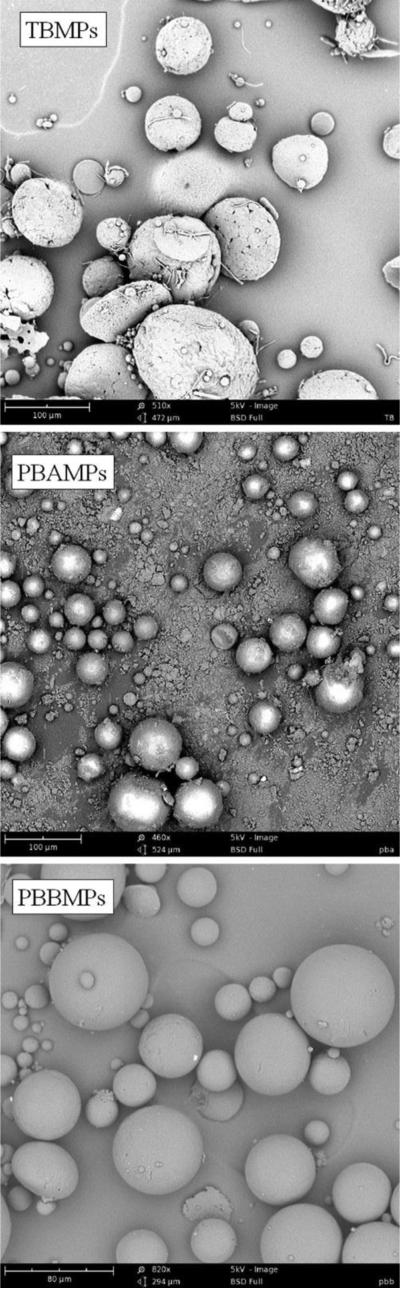
Octreotide was encapsulated in MPs of TB and PB polymers by methanol-oil/water emulsion solvent evaporation method as conventional water/oil/water method resulted in poor entrapment efficiency (unpublished data). Addition of TEA converted ionized amine of octreotide to its more hydrophobic base-form and hence it preferably partitioned into oil phase improving the entrapment efficiency. Particles were characterized for entrapment efficiency (%EE) and drug loading (%DL) by simple UV method. Table 3 represents entrapment efficiency and drug loading for TBMPs, PBAMPs and PBBMPs. Order of %EE and %DL for polymers was PBB>PBA>TB (ANOVA p<0.05). Higher %EE could be attributed to minimal crystallinity of PCL segment in PB copolymers (Fig. 2). Similar results have been observed in previously published studies, where nanoparticles were prepared with TB and PB polymers (Patel et al., 2015; Tamboli et al., 2013). Drug loading with TB and PB-A polymers were found to be low when compared with marketed PLGA Sandostatin LAR® (DL 4.1±0.1%) (Ghassemi et al., 2012). This could be due to a variety of reasons. Low %DL could be due to different polymer compositions or use of different method of MPs preparation. We also performed particle size analysis with DLS, however due to high polydispersity and large size result on particle size were inconclusive (unpublished data). Therefore, SEM analysis was performed to determine surface morphology of MPs (Fig. 3a-3c). MPs prepared using Me-O/W method were spherical in shape. TBMPs appeared relatively more porous than PBAMPs and PBBMPs. MPs also were slightly polydispersed with particle size less than 100 μm based on SEM analysis (Fig. 3a-3c).
Table 3.
%EE and %DL for MPs prepared by emulsion solvent evaporation method.
| Polymer | %DL | %EE |
|---|---|---|
| TB | 2.0±0.3 | 45±9.4 |
| PB-A | 3.1±0.5 | 60±11 |
| PB-B | 4.2±0.2 | 82±3.3 |
Data represented as mean ± SD, n=4.
Figure 3.
Scanning electron micrograph for (a) TBMPs, (b) PBAMPs and (c) PBBMPs.
3.3. Kinetics of octreotide release
Primary objectives of this research were to deliver octreotide over period of months and investigate chemical instability of peptide during release. Hence, octreotide release kinetics from TBMPs, PBAMPs and PBBMPs was investigated by in vitro release studies. Release studies were performed by suspending MPs in the thermosensitive gels (Table 1). Release samples were analyzed by HPLC assay to quantitate native and acylated octreotide. Hydrophobic octreotide adducts eluted following the elution of native octreotide. Similar results have been observed by other investigators (Ghassemi et al., 2012; Lucke and Gopferich, 2003; Murty et al., 2003). Peaks in UV chromatogram were assigned to native and chemically-modified octreotide using HPLC-MS analysis. The phenomenon of peptide acylation has been well studied and it is known that the process occurs during the release, within MPs core, and not while preparation or following the release in media (Ghassemi et al., 2012; Murty et al., 2003). Octreotide possesses excellent stability at pH 7.4 at 37°C, which is the release condition (Murty et al., 2003). In addition, HPLC chromatogram did not show any peak corresponding to degraded peptide, suggesting the peptide possesses excellent stability throughout release period. Further, to distinguish junk peaks from native and acylated peptides in chromatogram, corresponding ghost MPs were kept for release and sampled at same time intervals along with octreotide-loaded MPs.
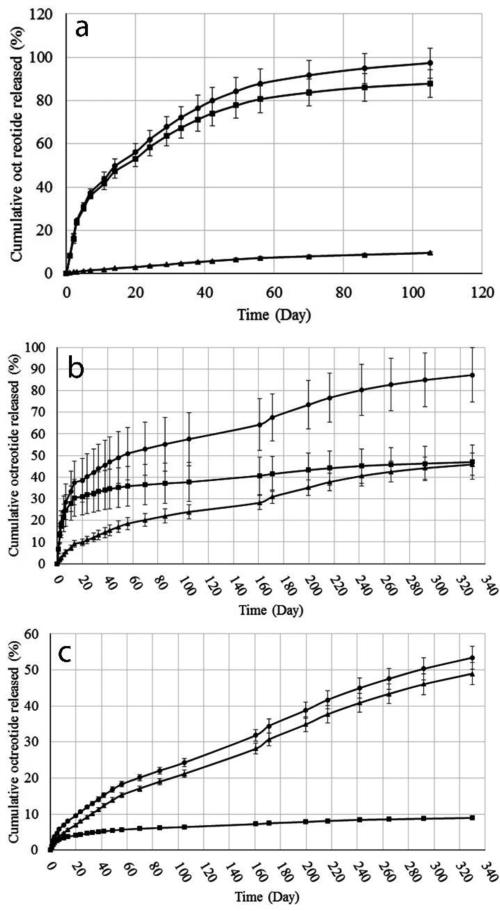
Fig. 4a-4c depict the release profiles of native, acylated and total octreotide from TB, PBA and PBB MPs-in-gel, respectively. A summary of cumulative octreotide release (total, native and acylated) is depicted in Table 4. In case of TBMPs and PBAMPs, near complete release of octreotide was achieved (Fig. 4a and 4b, Table 4). Only 53±3.2% of total octreotide was released over period of 10 months from glycolide-based PBB polymer (Fig. 4c and Table 4). No substantial burst release (<10%) was observed from all MPs compositions owing to thermosensitive gel (Table 4), which is in accordance with previously published reports (Patel et al., 2015; Patel et al., 2014b). Burst release occurs due to quick release of surface bound drug upon hydration of particles in release media. Thermosensitive gel provides an additional diffusion barrier to the drug released from MPs and minimizes burst phase (Patel et al., 2015; Patel et al., 2014b).
Figure 4.
In vitro release of total (●), native (■) and acylated (▲) octreotide from (a) TBMPs-in-gel (b) PBAMPs-in-gel and (c) PBBMPs-in-gel. Data presented as Mean ± SD, n=4.
Table 4.
Summary of in vitro release of octreotide in PBS buffer at 37°C.
| MPs polymer | Total Octreotide released (%) | Native octreotide released (%) | aAcylated octreotide released (%) | Burst release (%) | bAcylated octreotide released (%) | Release duration |
|---|---|---|---|---|---|---|
| TB | 97±6.9 | 88±6.4 | 10±0.6 | 8.4±0.8 | 13±0.4% | 105 Days |
| PBA | 87±12 | 47±7.9 | 46±5.1 | 7.1±2.3 | 53±3.0% | 330 Days |
| PBB | 53±3.2 | 9.0±0.3 | 49±3.0 | 1.4±0.3 | 92±0.5% | 330 Days |
Data represented as mean ± SD, n=4.
Burst release is total octreotide (%) released at 24h.
calculated with respect to %EE.
Calculated with respect to total octreotide released.
MPs with all polymers resulted in sustained release of peptide over a period of months. TB polymer, consisting polycaprolactone blocks, was the most efficient in preserving chemical stability of encapsulated peptide among three polymers used in this study. Compared to marketed PLGA MPs formulation of octreotide (Sandostatin LAR® depot) where ~60% of octreotide is acylated during in vitro release (Ghassemi et al., 2012), only 10% of peptide was released as acylated adduct and 88% of peptide was released in its native form (Fig. 4a and Table 4) from PCL polymer based composite formulation. In contrary, a significant fraction of released peptide was acylated during the release from PBAMPs and PBBMPs. Release profile for acylated octreotide showed a steady release of acylated adducts over a long period for PBAMPs and PBBMPs (Fig. 4b and 4c). Total 87% was released, where 46% of peptide was acylated adducts from PBAMPs over period of 10 months. PBBMPs resulted in release of total 53% peptide, where only 9% was in native form. Fraction of acylated peptide was significantly higher in case of PBBMPs, where 49% was released as derivatized peptides, which was 92% of total released peptide (Table 4). The propensity of protein and peptide to undergo acylation from lactide and glycolide based polymer has been reported (Ghalanbor et al., 2012; Ibrahim et al., 2005; Lucke et al., 2002; Na et al., 2003; Zhang and Schwendeman, 2012). Peptides are more prone to acylation in polymers rich in glycolide compared to lactide (Murty et al., 2003), which explains substantially high acylation from PGA-bases PBBMPs systems. Presence of methyl group close to carbonyl carbon in lactide sterically hinders nucleophilic attack (Ghassemi et al., 2012). While PGA offers no steric hindrance and nucleophilic attack of peptide is relatively facile resulting in significant peptide derivatization. The order for extent of octreotide derivatization was PBBMPs>PBAMPs>TBMPs. It is worth noting that a substantial fraction of peptide was not released from PBB polymers matrix. This phenomenon can be explained by formation of water insoluble adducts upon acylation (Fig. 1b, step 1). Subsequent release of peptide is dependent on hydrolytic degradation of polymer chain (Fig. 1b, step 2).
Rate limiting factors controlling drug release from a biodegradable system may include dissolution, diffusion and/or degradation of polymer. Dissolution may not be a rate limiting factor as octreotide possesses excellent aqueous solubility. We have utilized a composite system consisting of microparticles embedded in hydrogel to achieve long-term delivery. Please refer to table 1 for chemical composition of MPs-in-gel systems. Chemical compositions of triblock and pentablock polymers utilized to prepare MPs are different. However, due to different structures of polymers for MPs individual contribution of gelling polymer in these composite systems cannot be estimated accurately. As a result, further in this section, the composite system i.e., MPs-in-gel were compared to one another rather than individual comparison of MPs or gelling polymers. The mechanism of release may further be complicated due to acylation of peptide. We theorize that the release mechanism could be diffusion and/or degradation controlled. Fig. 5 represents hypothetical steps leading to release of native and acylated peptide from biodegradable MPs-in-gel composite system. Release of native peptide may be controlled by rate of polymer erosion and diffusion through hydrogel matrix (Fig. 5). Release of acylated peptide may be a complicated process influenced by bulk erosion, rate of peptide acylation, polymer side-chain hydrolysis and diffusion through the hydrogel matrix (Fig. 5). Polymer degradation may or may not contribute as rate limiting factor depending on the composition of polymer and rate of polymer degradation.
Figure 5.
Process of native and acylated octreotide release from MPs-in-gel combination. Release of native peptide may be controlled by rate of polymer degradation and diffusion through hydrogel matrix. Release of acylated peptide may be influenced by rate of polymer degradation, rate of peptide acylation, polymer side-chain hydrolysis and diffusion through the hydrogel matrix.
Various mechanistic and kinetic models were explored in order to determine the mechanism and order of octreotide release. Data was fitted to Higuchi, Korsmeyer-Peppas and Hixson-Crowell models to delineate the mechanism of release. Zero and first order models were utilized to determine the order of release process. Zero order rate indicates that the release rate is independent of drug concentration as opposite to first order rate where rate of release is directly proportional to the initial drug concentration. The model explaining most variability was identified by coefficient of determination (R2) value. Table 5 lists calculated R2 values and associated parameters for all models. It is evident that release of octreotide (total, native and acylated) was a first order process suggesting that release rate is dependent on concentration (Table 5).
Table 5.
Summary of goodness of fit (R2) for kinetic and mechanistic models for in vitro release of total, native and acylated octreotide from TB, PBA and PBB MPs-in-gel.
| MPs polymer | Octreotide | Kinetic and Mechanistic Models | ||||||
|---|---|---|---|---|---|---|---|---|
| Higuchi Model | Korsmeyer-Peppas model | Hixson-Crowell model | Zero Order | First order | ||||
| R2 | R2 | n | R2 | R2 | R2 | Rate constant k (Day−1) | ||
| TB | Total | 0.9265 | 0.9559 | 0.6157 | 0.8442 | 0.1569 | 0.9975 | 0.3316 |
| native | 0.8984 | 0.9534 | 0.6089 | 0.6325 | 0.0315 | 0.9400 | 0.0203 | |
| Acylated | 0.9501 | 0.9865 | 0.7700 | 0.8986 | 0.8894 | 0.9571 | 0.0009 | |
| PBA | Total | 0.7886 | 0.9211 | 0.3897 | 0.5746 | 0.2120 | 0.9714 | 0.0053 |
| native | 0.2620 | 0.8472 | 0.2469 | 0.7002 | 0.6466 | 0.7261 | 0.0012 | |
| Acylated | 0.9905 | 0.9817 | 0.6217 | 0.8856 | 0.8167 | 0.9777 | 0.0018 | |
| PBB | Total | 0.9770 | 0.9959 | 0.5882 | 0.9474 | 0.8928 | 0.9946 | 0.0023 |
| native | 0.7329 | 0.9688 | 0.3307 | 0.3570 | 0.4230 | 0.8372 | 0.0002 | |
| Acylated | 0.9406 | 0.9967 | 0.6893 | 0.9722 | 0.9406 | 0.9958 | 0.0021 | |
Higuchi model describes Fickian release from a solid or semisolid matrix type of planar system and the drug release occurs via diffusion through porous matrix (Higuchi, 1963). Korsmeyer-Peppas model is widely applied to distinguish between diffusion-controlled (Fickian) and anomalous (non-Fickian) release mechanisms for polymeric delivery systems (Korsmeyer et al., 1983). The value of release exponent n≤0.45 indicates diffusion-controlled release whereas 0.45<n<0.89 indicates anomalous release mechanism. The Hixson-Crowell cube root model indicates that the drug release from systems undergoing a change in surface area and diameter of particles (Hixson and Crowell, 1931).
According to R2 values, release of total octreotide from TB and PB polymers can be explained by Korsmeyer-Peppas model (Table 5). Values of release exponent (n) for total octreotide release from TBMPs and PBBMPs were 0.62 and 0.59, suggesting anomalous mechanism of release involving degradation of polymer and diffusion of released peptide through the hydrogel. On contrary, n-value for total octreotide was <0.45 from PBAMPs suggesting diffusion controlled release mechanism.
The mechanism for release of native octreotide from TBMPs was non-Fickian according to Korsmeyer-Peppas model. On the other hand, release of native octreotide was diffusion-controlled for PBAMPs and PBBMPs. Release of native octreotide may be dependent on polymer degradation and diffusion of native peptide out of MPs-in-gel depot (Fig. 5). Slower degradation of PCL polymer (Tamboli et al., 2013), in combination with additional diffusion barrier by hydrogel may be responsible for the non-Fickian mechanism of release from TB polymer. However, PBA and PBB polymers are nearly amorphous in comparison to TB polymers. Polymer degradation may not be a rate limiting factor for PBA and PBB polymers due to rapid degradation of polymer. Therefore, diffusion through porous hydrogel is the only rate-limiting factors for PBA and PBB polymers.
Similarly, release mechanism for acylated octreotide from MPs of TB, PBA and PBB polymers can be explained by Korsmeyer-Peppas model. Release exponents (n) were >0.45, suggesting the mechanism of acylated peptide release was non-Fickian for all MPs. The n-values for acylated octreotide were the highest compared to total and native octreotide from all polymers. In case of PBAMPs, best fit for acylated peptide release was observed for Higuchi model (R2=0.9905) suggesting diffusion-controlled release. However, Korsmeyer-Peppas model also had high R2 value for fit (R2=0.9817) with n-value of 0.62 indicating non-Fickian mechanism. We theorize that Korsmeyer-Peppas model could better explain mechanism of acylated octreotide release from PBAMPs because of possibility of additional steps involving polymer degradation. We hypothesized that the release of acylated peptide may be influenced by polymer degradation. As shown in Fig. 5, release of acylated octreotide is a multistep process, where peptide is first released inside microenvironment of particle via polymer degradation followed by acylation. Octreotide acylation may lead to formation of water insoluble adduct, which may require further hydrolysis of polymer side-chain to form water soluble acylated adduct. Formation of insoluble acylated adducts may also be responsible for significantly slow release rate from least hydrophobic PBB polymer. Water soluble acylated octreotide is then diffused out through porous hydrogel. Thus, influence of polymer degradation cannot be neglected and release mechanism for acylated peptide release may be anomalous as predicted by Korsmeyer-Peppas model.
3.4. Identification acylated octreotide adducts
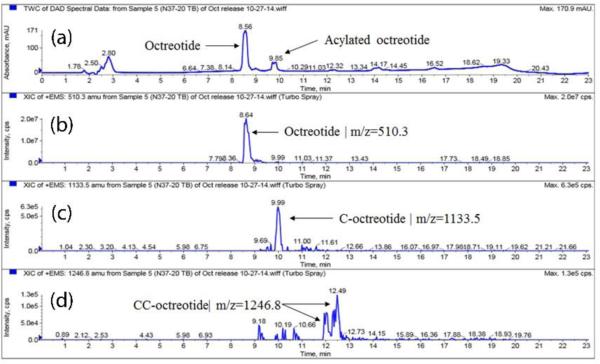
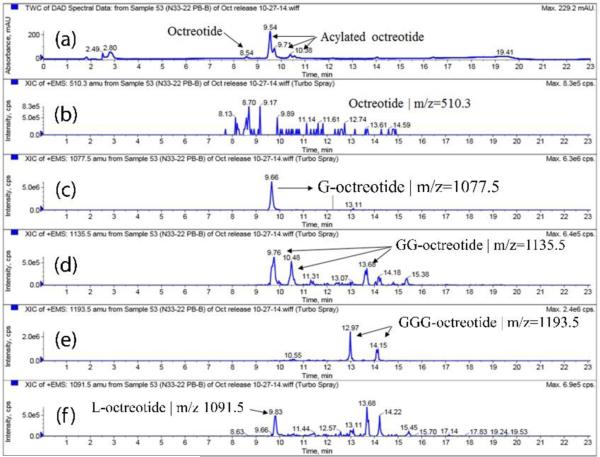
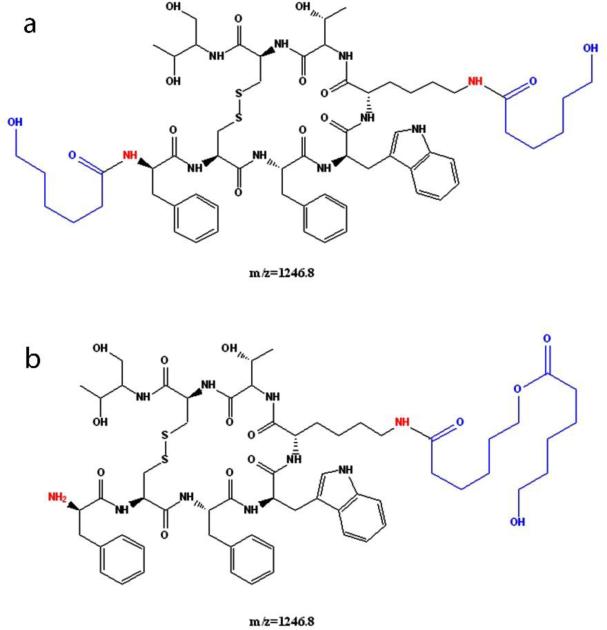
The chemical instability, acylation, of peptide is a major concern with biodegradable particulate delivery systems; hence, the aim of this research was to identify polymer composition that can provide maximal chemical stability to the peptide during release. The peaks in the HPLC chromatogram were assigned to native and acylated adducts by HPLC-MS analysis. Fig. 6, 8 and 9 depicts UV and MS spectrums (extracted ion chromatograms) for TBMPs, PBAMPs and PBBMPs release samples. Native octreotide eluted at 8.5 min (UV, Fig. 6a) and corresponding peak in MS (EIC) appears at 8.6 min (Fig 6b). Octreotide appeared as +1 and +2 charge states (z) resulting in species of 1019.3 m/z and 510.3 m/z. Peak at RT 9.8 min (UV, Fig. 6a) corresponds to C-octreotide (m/z 1133.5, RT 9.99 min) as shown in chromatograms. The MS profile was also extracted for 1246.8 m/z, associated with CC-Octreotide. Two peaks at 12.1 and 12.4 min were only observed in MS chromatogram (Fig. 6d). Multiple peaks could be explained by acylation of peptide at different functional groups such as amines and alcohol. One such example of dicaprolactoyl octreotide with different structures but similar mass is shown in Fig 7. Dicaprolactoyl adducts of m/z 1246.8 could form by acylation of both amines or acylation of single amine giving rise to two adducts with similar molecular weight but different hydrophobicity which would result in multiple peaks in MS chromatogram. Low concentration of CC-octreotide may be due to low aqueous solubility. CC-octreotide following hydrolysis at CC ester linkage would result in release of C-octreotide eventually. Unlike native octreotide, masses with multiple charge states (z>1) were not observed for acylated adduct.
Figure 6.
HPLC-MS spectrum of release sample (Day 105) from TBMPs-in-gel. (a) UV chromatogram. Extracted ion chromatogram (EIC) for (b) octreotide, m/z 510.3 (c) caprolactoyl-octreotide, m/z 1133.5 and (d) di-caprolactoyl-octreotide, m/z 1246.8. EIC for octreotide (m/z 510.3) shows peak at 8.64 RT which corresponds to peak at 8.56 min in UV chromatogram. Acylated adduct caprolactoyl-octreotide elutes at RT 9.85 min (UV chromatogram) and at corresponding retention time in EIC (m/z 1133.5 and RT 9.99 min). Di-caprolactoyl-octreotide adduct (m/z 1246.8) appears at two RT however no corresponding peak in UV chromatogram is observed due to possibility of low concentration.
Figure 8.
HPLC-MS spectrum of release sample (Day 171) from PBAMPs-in-gel. (a) UV chromatogram. Extracted ion chromatogram (EIC) for (b) octreotide, m/z 510.3 (c) Lactoyl-octreotide, m/z 1091.5 and (d) Di-lactoyl-octreotide, m/z 1163.2.
Figure 9.
HPLC-MS spectrum of release sample (Day 171) from PBBMPs-in-gel. (a) UV chromatogram. Extracted ion chromatogram (EIC) for (b) octreotide, m/z 510.3 (c) Glycoyl-octreotide, m/z 1077.5 (d) Di-glycoyl-octreotide, m/z 1135.5 (e) Tri-glycoyl-octreotide, m/z 1193.5 (f) Lactoyl-octreotide, m/z 1091.5.
Figure 7.
Structures of di-caprolactoyl octreotide adducts with different structures and similar mass (m/z 1246.8).
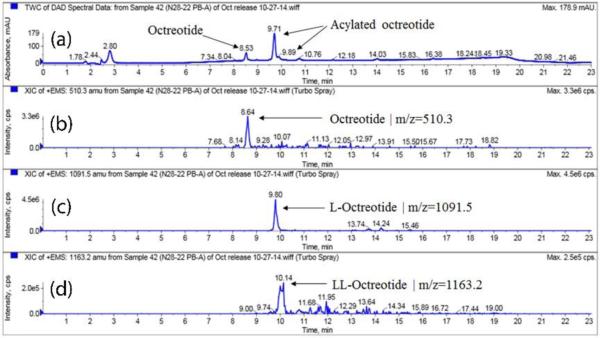
Similarly, release samples for PBAMPs and PBBMPs were analyzed by HPLC-MS. As expected, retention time (RT) for native octreotide remained unchanged (Fig. 8a-8b). PBA polymer consisted of PLA segment; therefore, we observed m/z 1091.5 (UV chromatogram RT 9.7 min and EIC RT 9.8 min) and 1163.2 (UV chromatogram RT 9.8 min and EIC RT 10.1 min) associated with mono and di-lactoyl adducts, respectively (Fig. 8c-8d). In case of PBBMPs, acylated adducts mono-, di- and tri-glycolide derivatives of octreotide were observed (Fig. 9c-9e). Di-glycoyl (GG-octreotide) and Tri-glycoyl (GGG-octreotide) adducts were observed at two different RT, like CC-octreotide adduct. This observation is in corroboration with published literature (Murty et al., 2003). It is possible for di- and tri- adducts to form with different structures with similar m/z due to acylation at amines and/or alcohol functional groups as explained earlier for dicaprolactoyl octreotide. It is worth noting that the thermosensitive gel used for release studies also contained PLA segment (Table 1). Interestingly, mono lactoyl adduct (m/z 1091.5) was observed in the release samples of PBBMPs (EIC RT 9.8 min, Fig. 9f). This observation consistent with L-octreotide elution in release samples of PBAMPs at same RT of 9.8 min (Fig. 8c). This observation indicates that the acylation of peptide may also take place in hydrogels matrix apart from the MPs core. The summary of octreotide adducts identified using HPLC-MS analysis is depicted in Table 6 along with corresponding observed mass (m/z) and RT.
Table 6.
Summary of octreotide adducts identified using UFLC-MS analysis.
| Polymer | Acylated species | M+H/Z | Retention time (min) |
|---|---|---|---|
| TB | C-Octreotide | 1133.5 | 9.9 |
| CC-Octreotide | 1246.8 | 12.1, 12.4 | |
| PBA | L-Octreotide | 1091.5 | 9.8 |
| LL-Octreotide | 1163.2 | 10.1 | |
| C-Octreotide | 1133.5 | 9.9 | |
| CC-Octreotide | 1246.8 | ND | |
| PBB | G-Octreotide | 1077.5 | 9.6 |
| GG-Octreotide | 1135.5 | 9.7, 10.4, 13.6 | |
| GGG-Octreotide | 1193.5 | 12.9, 14.1 | |
| L-Octreotide | 1091.5 | 9.8 | |
| C-Octreotide | 1133.5 | ND | |
| CC-Octreotide | 1246.8 | ND |
C- Caprolactyl, L- Lactoyl, G- Glycoyl, ND- Not detected.
Four samples for TBMPs and nine samples for PBAMPs and PBBMPs, at a month time interval, were selected for analyses.
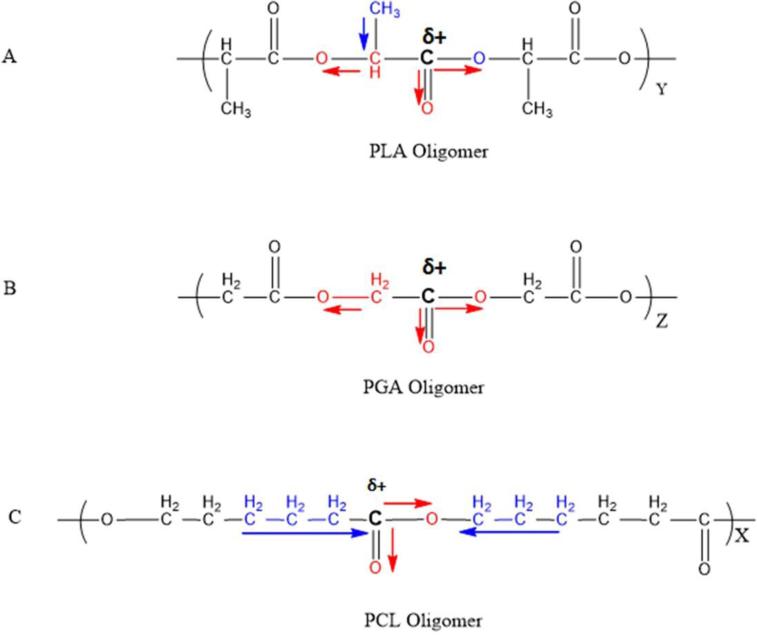
Only 10% octreotide was acylated for TB polymer despite absence of steric hindrance like in case of PBA. There may be two reasons for this observation. First, it may be due to slow degradation of polymer owing to its semicrystalline nature. It is established that the peptide acylation occurs inside the MPs. The process is facilitated because the area is rich in degraded polymer products and released peptide. Hence, less or negligible acylation of peptide in TP polymer may be attributed to slower degradation of the polymer. In case of PBA and PBB polymers, PLA and PGA blocks degrade at rapid rate than PCL segment resulting in formation of corresponding acylated adducts. Secondly, it might be due to relatively lower reactivity of carbonyl carbon of PCL compared to PLA or PGA segment. Low reactivity could be attributed to hydrophobic effects and/or electron donating inductive effect of alkyl chains adjacent to carbonyl carbon. The carbonyl carbon in PCL may carry less partial positive charge because of electron donating effect of alkyl group (-CH2-) as depicted in Fig. 10c. Where as in case of PLA and PGA oligomers, the electron withdrawal effect of electronegative oxygen dominates resulting in stronger partial positive charge on carbonyl carbon making it facile target for nucleophilic attack (Fig 10a-10b).
Figure 10.
Schematic depicting electron withdrawal effect of electronegative atoms and electron donating effect of alkyl group responsible for partial positive charge on carbonyl carbon in PLA, PGA and PCL oligomers.
4. Conclusion
Octreotide encapsulated MPs with excellent entrapment efficiency were successfully prepared and characterized. MPs-in-gel composite formulation sustained release of octreotide over period of months. A large fraction of released peptide was acylated during release from PBA and PBB polymer. In addition, release was incomplete from glycolide based PBB polymers. PB polymers having PLA and PGA segments are less suitable polymer for peptide delivery despite having significantly better entrapment efficiency. TBMPs resulted in minimal chemical derivatization of peptide and sustained release of octreotide over a period of three months suggesting TB polymers could be best suited for sustained peptide delivery.
Acknowledgements
This research was supported by grants R01 EY 09171-14 and R01 EY 10659-12 from the National health Institute. The authors would like to thank Dr. James Murowchick from the School of Arts and Sciences, University of Missouri-Kansas City for XRD analysis and Ashwin Parenky from Department of Pharmaceutical Sciences, Mercer University for SEM analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn JH, Park EJ, Lee HS, Lee KC, Na DH. Reversible blocking of amino groups of octreotide for the inhibition of formation of acylated peptide impurities in poly(lactide-coglycolide) delivery systems. AAPS PharmSciTech. 2011;12:1220–1226. doi: 10.1208/s12249-011-9694-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanson P, Timsit J, Harris AG. Clinical pharmacokinetics of octreotide. Therapeutic applications in patients with pituitary tumours. Clinical pharmacokinetics. 1993;25:375–391. doi: 10.2165/00003088-199325050-00004. [DOI] [PubMed] [Google Scholar]
- Dash TK, Konkimalla VB. Poly-small je, Ukrainian-caprolactone based formulations for drug delivery and tissue engineering: A review. Journal of controlled release : official journal of the Controlled Release Society. 2012;158:15–33. doi: 10.1016/j.jconrel.2011.09.064. [DOI] [PubMed] [Google Scholar]
- De Martino MC, Hofland LJ, Lamberts SW. Somatostatin and somatostatin receptors: from basic concepts to clinical applications. Progress in brain research. 2010;182:255–280. doi: 10.1016/S0079-6123(10)82011-4. [DOI] [PubMed] [Google Scholar]
- Feelders RA, Hofland LJ, van Aken MO, Neggers SJ, Lamberts SW, de Herder WW, van der Lely AJ. Medical therapy of acromegaly: efficacy and safety of somatostatin analogues. Drugs. 2009;69:2207–2226. doi: 10.2165/11318510-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Fogueri LR, Singh S. Smart polymers for controlled delivery of proteins and peptides: a review of patents. Recent patents on drug delivery & formulation. 2009;3:40–48. doi: 10.2174/187221109787158300. [DOI] [PubMed] [Google Scholar]
- Ghalanbor Z, Korber M, Bodmeier R. Protein release from poly(lactide-co-glycolide) implants prepared by hot-melt extrusion: thioester formation as a reason for incomplete release. International journal of pharmaceutics. 2012;438:302–306. doi: 10.1016/j.ijpharm.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Ghassemi AH, van Steenbergen MJ, Barendregt A, Talsma H, Kok RJ, van Nostrum CF, Crommelin DJ, Hennink WE. Controlled release of octreotide and assessment of peptide acylation from poly(D,L-lactide-co-hydroxymethyl glycolide) compared to PLGA microspheres. Pharmaceutical research. 2012;29:110–120. doi: 10.1007/s11095-011-0517-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi T. Mechanism of Sustained-Action Medication. Theoretical Analysis of Rate of Release of Solid Drugs Dispersed in Solid Matrices. Journal of pharmaceutical sciences. 1963;52:1145–1149. doi: 10.1002/jps.2600521210. [DOI] [PubMed] [Google Scholar]
- Hixson AW, Crowell JH. Dependence of Reaction Velocity upon surface and Agitation. Industrial & Engineering Chemistry. 1931;23:923–931. [Google Scholar]
- Ibrahim MA, Ismail A, Fetouh MI, Gopferich A. Stability of insulin during the erosion of poly(lactic acid) and poly(lactic-co-glycolic acid) microspheres. Journal of controlled release : official journal of the Controlled Release Society. 2005;106:241–252. doi: 10.1016/j.jconrel.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Kapoor DN, Bhatia A, Kaur R, Sharma R, Kaur G, Dhawan S. PLGA: a unique polymer for drug delivery. Therapeutic delivery. 2015;6:41–58. doi: 10.4155/tde.14.91. [DOI] [PubMed] [Google Scholar]
- Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA. Mechanisms of solute release from porous hydrophilic polymers. International journal of pharmaceutics. 1983;15:25–35. [Google Scholar]
- Lucke A, Fustella E, Tessmar J, Gazzaniga A, Gopferich A. The effect of poly(ethylene glycol)-poly(D,L-lactic acid) diblock copolymers on peptide acylation. Journal of controlled release : official journal of the Controlled Release Society. 2002;80:157–168. doi: 10.1016/s0168-3659(02)00020-2. [DOI] [PubMed] [Google Scholar]
- Lucke A, Gopferich A. Acylation of peptides by lactic acid solutions. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2003;55:27–33. doi: 10.1016/s0939-6411(02)00138-8. [DOI] [PubMed] [Google Scholar]
- Ma G. Microencapsulation of protein drugs for drug delivery: strategy, preparation, and applications. Journal of controlled release : official journal of the Controlled Release Society. 2014;193:324–340. doi: 10.1016/j.jconrel.2014.09.003. [DOI] [PubMed] [Google Scholar]
- Modlin IM, Latich I, Kidd M, Zikusoka M, Eick G. Therapeutic options for gastrointestinal carcinoids. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2006;4:526–547. doi: 10.1016/j.cgh.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Murty SB, Goodman J, Thanoo BC, DeLuca PP. Identification of chemically modified peptide from poly(D,L-lactide-co-glycolide) microspheres under in vitro release conditions. AAPS PharmSciTech. 2003;4:E50. doi: 10.1208/pt040450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na DH, DeLuca PP. PEGylation of octreotide: I. Separation of positional isomers and stability against acylation by poly(D,L-lactide-co-glycolide). Pharmaceutical research. 2005;22:736–742. doi: 10.1007/s11095-005-2589-4. [DOI] [PubMed] [Google Scholar]
- Na DH, Lee KC, DeLuca PP. PEGylation of octreotide: II. Effect of N-terminal mono-PEGylation on biological activity and pharmacokinetics. Pharmaceutical research. 2005;22:743–749. doi: 10.1007/s11095-005-2590-y. [DOI] [PubMed] [Google Scholar]
- Na DH, Youn YS, Lee SD, Son MW, Kim WB, DeLuca PP, Lee KC. Monitoring of peptide acylation inside degrading PLGA microspheres by capillary electrophoresis and MALDI-TOF mass spectrometry. Journal of controlled release : official journal of the Controlled Release Society. 2003;92:291–299. doi: 10.1016/s0168-3659(03)00366-3. [DOI] [PubMed] [Google Scholar]
- Patel SP, Vaishya R, Mishra GP, Tamboli V, Pal D, Mitra AK. Tailor-made pentablock copolymer based formulation for sustained ocular delivery of protein therapeutics. Journal of drug delivery. 2014a;2014:401747. doi: 10.1155/2014/401747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SP, Vaishya R, Pal D, Mitra AK. Novel pentablock copolymer-based nanoparticulate systems for sustained protein delivery. AAPS PharmSciTech. 2015;16:327–343. doi: 10.1208/s12249-014-0196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SP, Vaishya R, Yang X, Pal D, Mitra AK. Novel thermosensitive pentablock copolymers for sustained delivery of proteins in the treatment of posterior segment diseases. Protein and peptide letters. 2014b;21:1185–1200. doi: 10.2174/092986652111141001122054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peponi L, Navarro-Baena I, Báez JE, Kenny JM, Marcos-Fernández A. Effect of the molecular weight on the crystallinity of PCL-b-PLLA di-block copolymers. Polymer. 2012;53:4561–4568. [Google Scholar]
- Sadat Tabatabaei Mirakabad F, Nejati-Koshki K, Akbarzadeh A, Yamchi MR, Milani M, Zarghami N, Zeighamian V, Rahimzadeh A, Alimohammadi S, Hanifehpour Y, Joo SW. PLGA-based nanoparticles as cancer drug delivery systems. Asian Pacific journal of cancer prevention : APJCP. 2014;15:517–535. doi: 10.7314/apjcp.2014.15.2.517. [DOI] [PubMed] [Google Scholar]
- Sophocleous AM, Zhang Y, Schwendeman SP. A new class of inhibitors of peptide sorption and acylation in PLGA. Journal of controlled release : official journal of the Controlled Release Society. 2009;137:179–184. doi: 10.1016/j.jconrel.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamboli V, Mishra GP, Mitra AK. Novel pentablock copolymer (PLA-PCL-PEGPCL-PLA) based nanoparticles for controlled drug delivery: Effect of copolymer compositions on the crystallinity of copolymers and in vitro drug release profile from nanoparticles. Colloid and polymer science. 2013;291:1235–1245. doi: 10.1007/s00396-012-2854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishya R, Khurana V, Patel S, Mitra AK. Long-term delivery of protein therapeutics. Expert opinion on drug delivery. 2015a;12:415–440. doi: 10.1517/17425247.2015.961420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishya RD, Gokulgandhi M, Patel S, Minocha M, Mitra AK. Novel dexamethasone-loaded nanomicelles for the intermediate and posterior segment uveitis. AAPS PharmSciTech. 2014;15:1238–1251. doi: 10.1208/s12249-014-0100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishya RD, Mandal A, Gokulgandhi M, Patel S, Mitra AK. Reversible hydrophobic ion-paring complex strategy to minimize acylation of octreotide during long-term delivery from PLGA microparticles. International journal of pharmaceutics. 2015b;489:237–245. doi: 10.1016/j.ijpharm.2015.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang BM, Schwendeman SP. Characterization of the initial burst release of a model peptide from poly(D,L-lactide-co-glycolide) microspheres. Journal of controlled release : official journal of the Controlled Release Society. 2002;82:289–307. doi: 10.1016/s0168-3659(02)00137-2. [DOI] [PubMed] [Google Scholar]
- Wang L, Liu Y, Zhang W, Chen X, Yang T, Ma G. Microspheres and microcapsules for protein delivery: strategies of drug activity retention. Current pharmaceutical design. 2013;19:6340–6352. doi: 10.2174/1381612811319350010. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Schwendeman SP. Minimizing acylation of peptides in PLGA microspheres. Journal of controlled release : official journal of the Controlled Release Society. 2012;162:119–126. doi: 10.1016/j.jconrel.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Sophocleous AM, Schwendeman SP. Inhibition of peptide acylation in PLGA microspheres with water-soluble divalent cationic salts. Pharmaceutical research. 2009;26:1986–1994. doi: 10.1007/s11095-009-9914-2. [DOI] [PMC free article] [PubMed] [Google Scholar]