Abstract
The sphingosine 1-phosphate receptor 1 (S1PR1) is one of five G protein–coupled receptors activated by the lipid sphingosine 1-phosphate (S1P). Stimulation of S1PR1 by binding S1P or the synthetic agonist FTY720P results in rapid desensitization, associated in part with depletion of receptor from the cell surface. We report here combining spinning disc confocal fluorescence microscopy and flow cytometry to show that rapid internalization of activated S1PR1 relies on a functional clathrin-mediated endocytic pathway. Uptake of activated S1PR1 was strongly inhibited in cells disrupted in their clathrin-mediated endocytosis by depleting clathrin or AP-2 or by treating cells with dynasore-OH. The uptake of activated S1P1R was strongly inhibited in cells lacking both β-arrestin1 and β-arrestin2, indicating that activated S1PR1 follows the canonical route of endocytosis for GPCR's.
Keywords: G-protein coupled receptors, vesicular traffic, down regulation, clathrin
INTRODUCTION
Sphingosine-1-phosphate (S1P) is the natural activating ligand for the five-member family of lipid sphingosine 1-phosphate receptors (S1PR) [1,2]. The best studied is S1PR1, also known as the endothelial differentiation (EDG-1) receptor, a seven-transmembrane-spanning domain G-protein coupled receptor (GPCR) [2]. Activation of S1PR1 is required for maintaining vascular tone and angiogenesis; it is also involved in regulating lymphocyte traffic [3,4]. Synthetic agonists of S1PR1 such as the recently approved Gilenya (fingolimod, FTY720P) block lymphocyte egress from lymph nodes and are presently under clinical evaluation for the control of autoimmune diseases and treatment of multiple sclerosis [5]. As with other GPCRs, exposure of S1PR1 to S1P or to its synthetic agonists results in rapid desensitization [6], a key regulatory step that helps turn off the signaling pathway. S1PR1 desensitization requires phosphorylation of a stretch of 5 serine residues located at its cytosolic C-terminal portion [7]. Efficient endocytosis of activated S1P1R also depends on these 5 serine residues [3,8,9]. S1P1R activated with natural or synthetic agonists strongly associated with the non-visual βarrestin1 (arrestin-2) and βarrestin2 (arrestin-3) [10,11]; the role of this b-arrestin recruitment in S1P1R internalization was not directly demonstrated, however. Although required, S1PR1 endocytosis is not sufficient for its ubiquitinylation and β1- and β2-arrestin proteasome-mediated degradation [8]. While T-cells from mice expressing an internalization deficient S1PR1 showed normal T cell trafficking under homeostatic conditions, their egress from lymph nodes was diminished in animals treated with FTY270P [6]; this observation highlights the importance of regulated endocytosis and associated surface downregulation of S1PR1 for its physiological function.
The inhibitory effect of the endocytic inhibitors concanavalin and cadaverine on internalization of a PDGF-actived, PDGFβ-receptor/S1PR1 complex in airway smooth muscle cells has been cited as evidence for clathrin-dependent uptake [2]. The internalization route used by activated S1PR1 remains to be determined since these compounds do not interfere directly with the formation of endocytic clathrin coated pits and vesicles. More recently it was shown that activation of S1PR1 with S1P in CD4+T cells resulted in translocation of clathrin from the plasma membrane and its accumulation as intracellular punctate colocalizing with S1PR1 [12]. This was taken as evidence that a clathrin-mediated uptake pathway mediates the internalization of activated S1PR1 and its intracellular accumulation in coated vesicles. This interpretation, however, is at odds with the generally accepted view that the clathrin coat surrounding internalized coated vesicles dissociates within seconds of coated pit budding from the plasma membrane [2,13,14]. Incubation with pitstop, an inhibitor of clathrin-mediated endocytosis also reduced the association of activated S1P1R with the intracellular clathrin punctate [12]. It has recently been shown, however, that pitstop also prevents clathrin-independent endocytosis [3,13,15] and that its inhibitory effect on the clathrin-mediated pathway is likely to be non-specific [16]. While this paper was under revision, the role of dynamin2 in the ligand-mediated uptake of S1P1R and the traffic of CD4+T cells was demonstrated using an inducible dynamin2 knock-out mouse model [17].
Here we combine use of spinning disc confocal fluorescence microscopy and flow cytometry to show in Hela, HEK293A and MEF cells that S1PR1 activated by S1P or by FTY720P is rapidly internalized by a process dependent on clathrin and its endocytic adaptor AP-2, key proteins required to form endocytic coated pits and vesicles. We also show that uptake of activated S1PR1 requires dynamin and the non-visual βarrestin1 (arrestin-2) and βarrestin2 (arrestin-3).
RESULTS AND DISCUSSION
Internalization assay
We followed uptake of S1PR1 activated with its natural lipid ligand S1P or with its agonist FTY720P using two complementary internalization assays. The first assay, based on single cell spinning disc confocal fluorescence microscopy, visualized the intracellular localization of fluorescently tagged S1PR1. The second, based on flow cytometry of a cell population, measured the surface downregulation of fluorescently tagged S1PR1.
The experiments were carried out with cells ectopically expressing S1PR1 fused at its N-terminus at, in the lumen, to a tandem repeat of three hemagglutinin epitope tags (3xHA) and at its C-terminus, in the cytosol, with eGFP or mCherry (3xHA-S1PR1-eGFP and 3xHA-S1PR1-mCherry, respectively). The cellular location of S1PR1 and the extent of its internalization in the absence or presence of ligand were determined with the visualization and flow cytometry approaches by monitoring the signal of fluorescently-tagged mouse monoclonal antibody specific for the HA epitope. We used the HA-specific antibody to label the surface population of receptor and as a surrogate for an internalization probe in lieu of the natural or synthetic ligands for the following reasons. (1) The hydrophobic character of S1P would have resulted in a high membrane background due to the non-specific membrane binding of fluorescently tagged S1P. (2) Fluorescently tagged FTY720P was not readily available. (3) We could not identify a source of antibodies specific for the ectodomain of non-denatured S1PR1 (required for in vivo tracking experiments). The fluorescence signal elicited by eGFP or by transferrin were used to outline the cell volume in the imaging experiments while the fluorescence signals elicited by eGFP or mCherry in the flow cytometry-based internalization assays were used to determine the total cellular content of all S1PR1 molecules and also to normalize for the total amount of receptor expressed.
All internalization assays described here required using cells first incubated overnight (16 −18 hrs) with DMEM containing 2% delipidated serum (charcoal/dextran stripped) and then for 1 −1.5 hr with serum deprived medium before the actual experiment. This procedure was necessary to increase the amount of S1PR1 at the cell surface presumably by preventing receptor activation and subsequent internalization mediated by presence in the serum of its ligand (Figure S1 and related references [3,8,18-20]).
We first used the fluorescence microscopy assay to establish that addition of the HA-antibody to the medium for 15 min at 37 °C recognized the 3xHA epitope tag in S1PR1 at the cell surface of HeLa cells transiently expressing 3xHA-S1PR1-eGFP. After this incubation, the cells were acid wash to remove the surface-bound antibody, fixed in the presence of detergent and further incubated with fluorescently labeled secondary antibody specific for the mouse antibody. Representative images showing staining specificity and minimal surface staining are shown in Figs. 1A and B; they correspond to approximately middle views through the cells obtained from a 3D stack acquired by spinning disc confocal microscopy in the absence and presence of ectopically expressed 3xHA-S1PR1-eGFP. These images confirmed the labeling specificity by showing absence of any staining in cells not expressing 3xHA-S1PR1-eGFP (Fig. 1A) and minimal intracellular staining in cell expressing non-activated 3xHA-S1PR1-eGFP (Fig. 1B). The image absence of intracellular labeling with the HA-antibody in Fig. 1B is an important control demonstrating that incubation with the HA-antibody alone did not stimulate 3xHA-S1PR1-eGFP uptake (see below).
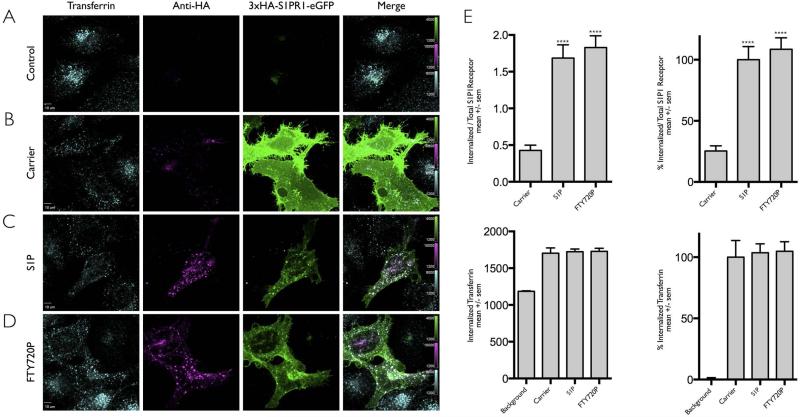
Figure 1. Ligand induced internalization of S1PR1 assayed by fluorescence microscopy.
(A-D) HeLa cells not expressing (A) or transiently expressing 3xHA-S1PR1-eGFP (B-D) were incubated with anti-HA antibody to label receptor at the cell surface and then follow their internalization upon incubation with DMSO only (Carrier) or the S1PR1 ligands (S1P or FTY720P). Fluorescent transferrin-Alexa Fluor 647 was used to monitor the clathrin-based receptor mediated endocytosis of transferrin. HA antibody was used to follow the uptake of S1P1R. Images for each condition correspond to a center plane obtained using spinning disc confocal microscopy from which quantification data was obtained. An acid wash step at the end of the experiment was used to remove the surface bound transferrin or HA antibodies. Scale bar 10 μm.
(E) Quantification of the internalization data obtained in panels A-D. The bar graphs show the integrated fluorescence signal of internalized transferrin or S1PR1 contained within the mask from the center plane of each cell; the amount of internalized S1P1R (HA fluorescence signal) was normalized by the eGFP fluorescence corresponding to the total cellular content of 3xHA-S1PR1-eGFP. The right panels presents the data normalized to the values obtained for S1PR1 in the presence of its S1P ligand and for transferrin in the presence of DMSO only (Carrier). Data from cells expressed as mean +/− sem (standard deviation of the mean). The following number cells were analyzed per each condition: Carrier, 25; S1P, 30; FTY720P, 28. The symbol **** highlights the statistical significance of the difference between carrier and ligand (p < 0.0001).
The fluorescence microscopy assay was then used to follow the stimulated internalization of S1PR1 by S1P in HeLa cells transiently expressing 3xHA-S1PR1-eGFP (Fig. 1C). In agreement with previous results [1,21] we observed a characteristic punctate pattern of colocalizing HA- and eGFP-containing spots corresponding to internalized HA-antibody bound to 3xHA-S1PR1-eGFP obtained upon sequential incubation at 37°C of the HA-antibody for 15 min followed by addition of S1P for another 30 min (Fig. 1C). This incubation period agreed with earlier observations [9] showing a robust uptake of activated S1PR1 starting approximately 15 min after ligand addition and lasting for at least another 45 min.[2-4] Similar uptake of activated 3xHA-S1PR1-eGFP was obtained with cells incubated with S1P alone (not shown), suggesting that presence of the HA-antibody did not interfere with the ligand-induced internalization of S1PR1. Extensive quantitative analysis of data (Fig. 1E) from fluorescence images obtained from the same experiments as those shown in Fig. 1 (see also similar analysis for the images in Figs. 3-5) fully agreed with the qualitative conclusions obtained by visual inspection of these images.
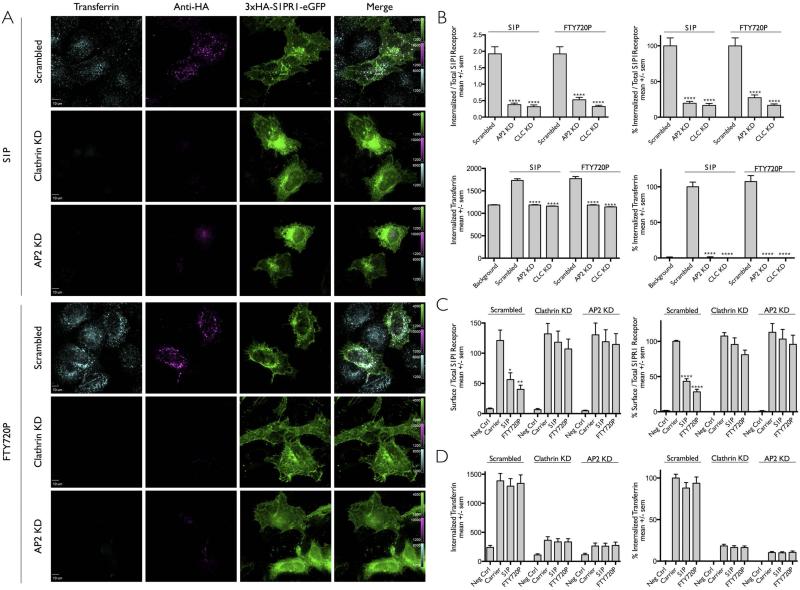
Figure 3. Ligand induced internalization of S1PR1 requires a functional clathrin/AP2 mediated endocytosis pathway.
(A) Effect of clathrin or AP2 depletion in the uptake of S1PR1 in Hela cells transiently expressing 3xHA-S1P1-eGFP. Cells were depleted of clathrin or of its endocytic adaptor AP2 by transduction with lentiviruses encoding shRNA specific for clathrin heavy chain (CLC) or the μ2 subunit of AP2 (AP2); scrambled shRNA was used as a negative control. The cells were incubated with anti-HA antibody to follow the uptake of S1PR1 upon addition of DMSO only (Carrier) or the ligands (S1P or FTY720P). Transferrin-Alexa Fluor 647 was used to monitor its clathrin-based receptor mediated endocytosis. The representative images correspond to a center plane from which quantification data was obtained. An acid wash step at the end of the experiment was used to remove surface bound transferrin and anti-HA antibodies. Scale bar 10 μm.
(B) Quantification of the internalization data obtained in panel (A). Data in the left panels processed as in Fig. 1E. The normalized data presented in the right panels were normalized to the values obtained with scrambled shRNA and in the presence of S1P. Data expressed as mean +/− sem obtained from the following number of cells: 35, 29 and 36 in the presence of S1P of cells treated with scrambled shRNA or shRNA for μ2 of AP2 or CLC; 29, 22 and 31 in the presence of FTY270P of cells treated with scrambled shRNA or shRNA for AP2 or CLC. The symbol (****) highlights that the difference between carrier and ligand were statistically significant with p < 0.0001.
(C) Effect of clathrin or AP2 depletion in the uptake of S1PR1 in HEK 293A cells stably expressing 3xHA-S1PR1-mCherry. Cells were depleted of clathrin or of its endocytic adaptor AP2 with corresponding shRNA's; scrambled shRNA was used as a negative control. The bar graphs show mean +/− sem of internalization data obtained by flow cytometry normalized to values obtained in the absence of ligand. The bar graphs in the right panels were normalized to the values obtained using scrambled shRNA in the absence of ligand (Carrier). The symbols (*, ** and ****) highlight that the difference between carrier and ligand were statistically significant with a p < 0.02, 0.001 and 0.0001 respectively.
(D) Amount of fluorescent transferrin-Alexa Fluor 647 internalized by the cells analyzed in panel (C) analyzed as Fig. 2B.
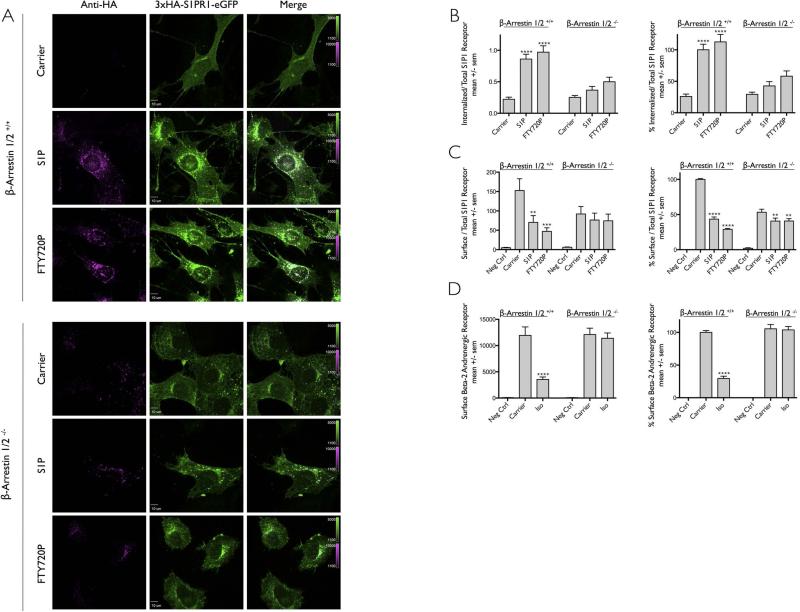
Figure 5. Role of β-arrestins during ligand induced internalization of S1PR1.
(A) MEF cells from mice expressing (β-Arrestin1/2+/+) or not (β-Arrestin1/2−/−) β-arrestin1 and β-arrestin2 and stably expressing 3xHA-S1PR1-eGFP were incubated with anti-HA antibody in the presence of DMSO only (Carrier) or the ligands S1P or FTY720P. Images for each condition correspond to a center plane obtained using spinning disc confocal microscopy. An acid wash step at the end of the experiment was used to remove most of the surface bound anti-HA antibodies. Scale bar 10 μm.
(B) Quantification of the internalization data obtained in panel (A) expressed as mean +/− sem. Normalized data correspond to the values obtained using β-arrestin 1/2 +/+ cells in the presence of S1P. Number of β-arrestin 1/2 +/+ cells analyzed were 27, 37 and 38 for Carrier, S1P, and FTY720P, respectively; number of corresponding β-arrestin 1/2 −/− cells were 37, 33 and 34. The symbol **** highlights that the difference between carrier and ligands were statistically significant with p values of < 0.0001.
(C) Flow cytometry analysis of S1PR1 uptake in the presence (β-arrestin 1/2 +/+) or simultaneous absence (β-arrestin 1/2 −/−) of both β-arrestins. The data is from 5 independent experiments. The symbols ** and *** highlight that the difference between carrier and ligand were statistically significant with p value < 0.0001 and 0.004 respectively. Data on the right panel were normalized to the values obtained using β-arrestin 1/2 +/+ cells in the absence of ligand (Carrier). The symbols **** and ** indicate statistical significance difference with p values of <0.0001 and 0.008 respectively.
(D) Flow cytometry analysis of β2AR uptake in the presence (β-arrestin 1/2 +/+) or simultaneous absence (β-arrestin 1/2 −/−) of both β-arrestins. Cells stably expressing Flag-β2-AR were treated with (Iso) or without (Carrier) 25μM Isoproterenol at 37°C for 25 minutes. The amount of surface receptor was determined by antibody labeling followed by flow cytometry. Data on the right panel normalized to the values obtained using β-arrestin 1/2 +/+ cells in the absence of ligand (Carrier). The data is from 6 independent experiments, 3 of which were conducted simultaneous to those in panel C. The symbol **** highlights that the difference between carrier and ligand were statistically significant with a p value < 0.0001.
To compare the intracellular endocytic traffic of activated S1PR1 with the constitutive clathrin-mediated endocytic traffic of transferrin, we incubated the cells with fluorescently labeled transferrin-Alexa Fluor647, added during the last 8 min of the S1PR1 internalization fluorescence microscopy assay. The images showed the expected intracellular fluorescent spots corresponding to endosomes containing internalized transferrin-Alexa Fluor647, and in agreement with earlier results [3,5], they also showed partial colocalization with the HA-antibody fluorescent signal corresponding to the ligand-activated internalized S1PR1-eGFP (Fig. 1C), suggesting that a fraction of the internalized S1PR1 and transferrin receptors populated different endosomal compartments (Fig. 1C). Similar results were obtained with HeLa cells incubated with the agonist FTY720P transiently expressing S1PR1 (Fig. 1D); we observed intracellular accumulation of S1P1R that partially colocalized with internalized transferrin. Others have previously shown that when cells expressing S1P1R where incubated with its agonists for significantly longer incubations periods (> 4hr), then S1P1R continued its endocytic traffic away from endosomal compartments containing internalized Tf and accumulated within the perinuclear region partially colocalizing with Golgi-markers [10,11].
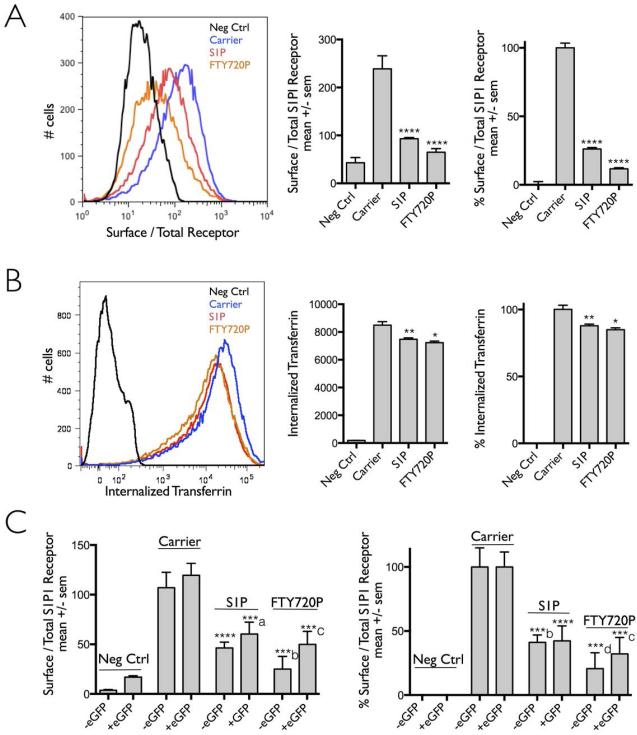
We also used a flow cytometry-based internalization assay to measure in a population of cells the extent of S1PR1 uptake stimulated by S1P or FTY720P (Fig. 2), by comparing the amount of HA-antibody at the cell surface normalized by the total amount of 3xHAS1PR1-mcherry stably expressed in HEK293A cells before and after incubation for 30 min at 37 °C with S1P or FTY720P. Surface staining of the cells with the HA-antibody was carried at the end of the experiment after shifting the cells to 4°C. Representative flow cytometry plots (Fig. 2A) showed that incubation with S1P or FTY720P induced a ~ 75% and ~ 85% reduction in the surface signal of S1PR1 when compared to cells not exposed to these ligands. The same cells were assayed for the intracellular accumulation of transferrin-Alexa Fluor647 added to the medium for 8 min at the end of the period when the cells were incubated with the S1PR1 ligands (Fig 2B). These data demonstrate that the constitutive clathrin-based endocytic uptake of transferrin was minimally affected by presence of the S1PR1 ligands or by activation of the receptor.
Figure 2. Ligand induced internalization of S1PR1 assayed by flow cytometry.
HEK 293A cells stably expressing 3xHA-S1PR1-mCherry were incubated or not with anti-HA antibody to label receptor at the cell surface and then follow their internalization upon incubation with DMSO only (carrier) or the S1PR1 ligands (S1P or FTY720P). Fluorescent transferrin-Alexa Fluor 647 was used to monitor the clathrin-based receptor mediated endocytosis of transferrin. Data obtained using flow cytometry was from more than 10,000 cells analyzed per condition.
(A) Plot distribution of a representative experiment comparing the amount of receptor remaining on the cell surface following receptor with or without activation with its ligands. The bar graphs correspond to mean +/− sem from 5 independent experiments; the right panel presents the data normalized to the values obtained in the absence of ligand (Carrier). The symbol (****) highlights the statistical significance of the difference between carrier and ligand (p < 0.0001).
(B) Plot distribution of the same experiment analyzed in panel (A) showing the amount of fluorescent transferrin-Alexa Fluor 647 internalized in the presence and absence of S1PR1 ligands. Bar graphs represent the amount of fluorescent transferrin-Alexa Fluor 647 internalized by the cells analyzed in panel (A). The values are mean +/− sem (left panel) and are normalized to the uptake signal obtained in the absence of ligand (Carrier, right panel). The symbols (**, *) highlight the statistical significance of the difference between carrier and S1P (p < 0.03) or between carrier and FTY720P (p < 0.01), respectively.
(C) Bar graph representation of the amount of S1PR1 receptor remaining on the cell surface determined by flow cytometry following receptor activation by its ligands without or with eGFP added to the c-terminus of S1PR1. The experimental data (left panel) and the normalized data (right panel) represent the total amount of receptor present in the same cell and corresponds to the mean +/− sem from 2 independent experiments. Data in the right panel was normalized to the uptake in the absence of ligand (Carrier). The symbols ****, ***a, ***b, ***c, ***d highlight the statistical significance of the difference between carrier and ligand (p < 0.0001, 0.0008, 0.0006, 0.0002, 0.0007). For each ligand condition, between the presence and absence of eGFP added to the c-terminus of S1P1R was no statistically different.
The flow-cytometry based internalization assay was also used to confirm that C-terminal addition of eGFP to the cytosolic domain of S1P1R did not affect in a significant way the uptake of S1PR1 activated by S1P or FTY720 (Fig 2C).
The clathrin endocytic machinery is required for the stimulated uptake of S1PR1
Since clathrin/AP2 coated pits and vesicles are often the main internalization route utilized by activated GPCRs, we investigated their possible role in the internalization of S1PR1 using the imaging visualization assay with Hela cells transiently expressing 3xHA-S1PR1-eGFP (Fig. 3A) or the flow cytometry internalization assay with the HEK 293Acells stably expressing 3xHA-S1PR1-mCherry (Fig. 3B). We tested the effect of loss of clathrin triskelions or AP2 adaptors, by depletion of clathrin heavy chain or the μ2 subunit of AP2. We used lentivirus vectors expressing specific shRNAs for targeted mRNA interference and a lentivirus expressing scrambled shRNA as a negative control [6,22,23]. The cells were first exposed to the appropriate viruses for 24 hrs, then to puromycin for 4 days to select for transduced cells and finally analyzed for mRNA depletion by qPCR and for transferrin or S1PR1 uptake using the imaging or flow cytometry internalization assays. This protocol led to mRNA depletion of ~ 97% and ~ 89% for clathrin heavy chain and AP2 μ2 respectively. The decreased expression of clathrin heavy chain and AP2 μ2 mRNA agreed with the dramatic reduction of internalized transferrin assayed by imaging (Fig. 3A, B) and by flow cytometry (Fig. 3C) [7,24,25]. As also shown in Fig. 3, we found a pronounced block in the uptake of S1P or FTY270P activated S1PR1 in cells depleted of clathrin or AP2. These results indicate that the clathrin endocytic machinery is required for the ligand-dependent internalization of S1PR1.
Role of β-arrestins in the stimulated uptake of S1PR1
We explored the role of non-visual arrestins in the uptake of S1PR1 using mouse embryo fibroblast cells (MEFs) derived from wild type mice (Arr1/2+/+) or mice lacking both β-arrestin1 and β-arrestin2 (Arr1/2−/−) [3,8,9,26] stably expressing 3xHA-S1PR1-eGFP. Data obtained from the fluorescence microscopy internalization assay showed the appearance of intracellular punctate spots in wild type Arr1/2+/+ MEFs exposed to S1P or FTY720P (Fig 5A). Visualization of Arr1/2−/− MEFs lacking β-arrestin1 and β-arrestin2 showed decreased expression of 3xHA-S1PR1-eGFP. It remains to be determined whether this decrease results from a deficiency of protein expression, traffic, or protein stability. Imaging of Arr1/2−/− MEFs also demonstrated appearance of a few intracellular HA-containing spots even though the cells were not exposed to S1P or FTY720P (Fig. 5A and 5B); this observation suggests that S1P1R undergoes a very small amount of ligand-independent internalization in the absence of β-arrestins. Addition of S1P or FTY720P ligands further induced the internalization of also a small amount of S1P1R in cells lacking β-arrestins (Fig. 5A and 5B). These observations were confirmed with the flow cytometry-based assay (Fig. 5C). From these data we conclude that, β-arrestin 1 and 2 are required for the majority of the ligand-induced endocytosis. As control for the endocytic assays, we generated MEFs expressing or not β1/2-arrestins and stably expressing the β2 adrenergic receptor (B2AR) known to require β-arrestins for its ligand-dependent internalization. Using the flow cytometry assay, we confirmed essentially full inhibition of B2AR ligand-dependent uptake in the Arr1/2−/− MEFs (Fig. 5D). We conclude that as with many other G-coupled receptors, presence of β-arrestin 1 and 2 plays a key role for the ligand-stimulated endocytosis of S1PR1.
CONCLUSION
We show here that the internalization of S1PR1 activated by its natural ligand S1P or the agonist FTY720P follows the clathrin-endocytic pathway, an entry route shared with many other activated G-protein coupled receptor family members. We find also found that the presence of β-arrestins enables efficient internalization. The small level of internalization observed in the simultaneous absence of both non-visual β-arrestins suggest it is possible that in extreme conditions another class of adaptor might also link S1PR1 to the endocytic machinery, like the recently uncovered role of disheveled during the Wnt-activated uptake of the G-coupled receptor Frizzled [8,27,28].
MATERIALS AND METHODS
Reagents
Sphingosine-1-phosphate (S1P) and FTY720-phosphate (FTY720P) were from Echelon Bioscience (Salt Lake City, UT), poly-d-lysine (Sigma) and collagen (BD Biosciences). Rabbit polyclonal anti Edg1 (ab11424) was from Abcam. Monoclonal mouse anti-HA antibody F-7 was from Santa Cruz Biotechnology. Secondary donkey anti-mouse antibodies (Invitrogen) fluorescently tagged with Alexa Fluor 568 were used for all fluorescence microscopy experiments. Alexa Fluor 647 for flow cytometry experiments in Figs. 2 and 5; goat anti-mouse antibodies (Invitrogen) fluorescently tagged with Alexa Fluor 488 were used for flow cytometry experiments in Figs. 2-4. Rabbit-anti Edg1 (ab11424) was from Abcam). Anti-FLAG mouse monoclonal antibody (F1804; Sigma-Aldrich) was used in Fig. 5. Human transferrin conjugated to Alexa Fluor 647 was from Invitrogen. BD Perm/Wash™ buffer (554723) containing saponin and fetal bovine serum was from BD Biosciences and was used according to the manufacturer's protocol. DMEM and Opti-MEMR were from Invitrogen and Life Technologies, respectively. Charcoal treated FBS was from Atlanta Biologicals. Isopreternol (I6504) and Polybrene purchased from Sigma-Aldrich.
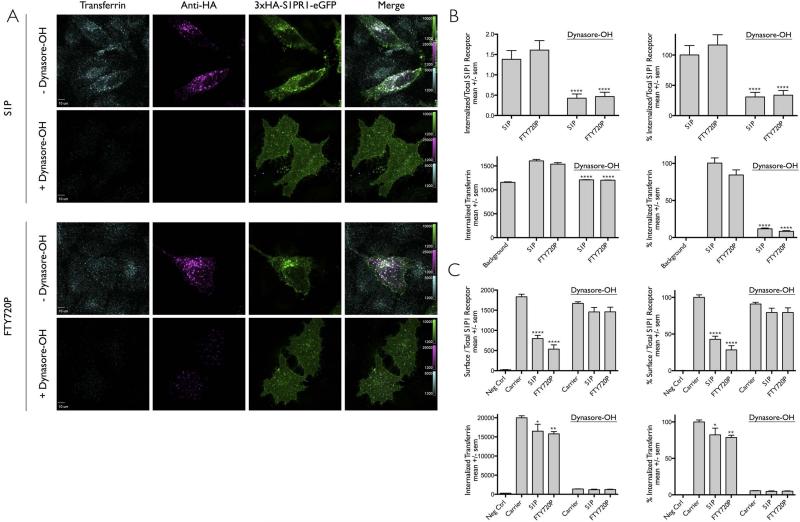
Figure 4. Ligand induced internalization of S1PR1 is prevented by interference with dynamin function.
(A) HeLa cells transiently expressing 3xHA-S1PR1-eGFP were incubated with anti-HA antibody in the absence (− dynasore-OH) or presence (+ dynasore-OH) of the modified more potent cell permeable inhibitor of dynamin, dynasore-OH. Following brief 15 min incubation with dynasore-OH, the media was replaced for 30 min with a new solution only containing DMSO, S1P or FTY720P. Fluorescent transferrin-Alexa Fluor 647 was used to follow the efficiency of the clathrin-based endocytic pathway. Images obtained using spinning disc confocal microscopy correspond to a center plane from which quantification data was obtained. An acid wash step at the end of the experiment was used to remove the surface bound transferrin and anti-HA antibodies. Scale bar, 10 μm.
(B) Quantification of the data in panel (A) processed as in Fig. 1E. The data in the right panels were normalized for the uptake of S1P1R and for transferrin in the presence of S1P. The following number of cells were analyzed: 21 and 22 without Dynasore-OH and treated with S1P and FTY720P; respectively. 20 and 21 with dynasore-OH and treated with S1P and FTY720P. The symbol (****) highlights that the differences between carrier and ligand were statistically significant with p < 0.0001.
(C) HEK 293A cells stably expressing 3xHA-S1PR1-mCherry used to follow by flow cytometry the effect of dynasore-OH on the ligand-induced uptake of S1PR1. The cells were incubated or not for 15 min with anti-HA antibody in the presence of carrier (DMSO) or dynasore-OH. Following this incubation, cells were incubated for 30 min with media containing carrier, S1P or FTY720P but without dynasore-OH. Transferrin-Alexa Fluor 647 uptake was used to follow its clathrin-based receptor mediated endocytosis. Data (mean +/− sem) from 3 independent experiments per condition showing the effect of dynasore-OH in the amount of S1PR1 remaining on the surface corrected by the total amount of receptor in the same cell normalized to data obtained in the absence of ligand and the internalization of transferrin-Alexa Fluor 647. The data in the right panels were normalized to the values obtained in the absence of ligand (Carrier) and dynasore-OH. The symbols (****, **, *) highlight that the difference between carrier and ligand were statistically significant with p values of < 0.0001, 0.004 and 0.03, respectively.
Dynasore-OH was custom-synthesized by our laboratory and used to prepare a 200 mM stock solution dissolved in DMSO and kept at −80°C [6,29,30]. Immediately before starting an experiment and following a procedure completed in less than 5 minutes, an aliquot of the stock solution was thawed and diluted to 80 mM with DMSO followed by a rapid sequential dilution first with Opti-MEMR containing 2% DMSO and no serum to 400 μM and then to 30 μM with Opti-MEM containing 0.15% DMSO and no serum. Cells were immediately incubated with this solution for 15 minutes followed by exchange with a fresh solution containing the ligand of interest but with out dynasore-OH (to ensure that free dynasore-OH would not bind to the relatively hydrophobic ligands and potentially interfere with their activity). Control experiments (not shown) indicated full inhibition of transferrin uptake after a washout period of 30 min, similar in extent as observed in the continuous presence of dynasore-OH.
Plasmids
The plasmid encoding 3xHA-S1PR1 (Cat # EDG010TN00) was from the Missouri S&T cDNA Resource Center (www.cdna.org). A PCR-based mutagenesis reaction was used to generate 3xHA-S1P1-mCherry and 3xHA-S1P1-eGFP by replacing the sequence encoding the stop codon with a BamHI site at the C-terminus of S1P1. The resulting product encoding the open reading frame of 3xHA-S1PR1 was inserted in-frame into the KpnI and BamHI restrictions sites of pmCherry N-1 or pEGFP N-1 (Clontech). The lentivirus encoding 3xHA-S1PR1-eGFP was generated by PCR amplification of 3xHA-S1PR1-eGFP to include a NotI and BglII restriction sites then used for ligation into the backbone pHage vector digested with NotI and BamHI. The lentivirus encoding Flag-β2-AR was generated by PCR amplification of Flag-β2-AR (Addgene #14697) subcloned into the pHage-EF1alpha-IRES-Puromycin vector.
Cells
HeLa, MEFs (Wild type β-arrestin clone 6P67 and cell line double knockout for β-arrestin 1/2 clone D2P30) and HEK 293A cells were maintained in DMEM (Invitrogen) supplemented with 10% FBS (Atlanta Biologicals). Transfection of HeLa (1:3 μg DNA: μl reagent) or HEK293A (1:3) cells was performed using FugeneHD (Roche/Promega) following the manufacturer's instructions. Transient expression of 3xHA-S1PR1 in HeLa cells was achieved by transfection with a peGFP N-1 plasmid containing 3xHA-S1PR1. HEK293A cells stably expressing 3xHA-S1P1-mCherry were generated by transfection with pmCherry N-1 3xHA-S1P1 and selected using 800 μg/ml of G418. The surviving population was sorted on the basis of mCherry expression using flow cytometry on a BD Aria II equipped with a 594 nm laser. The cells expressing mCherry were subject to a second round of G418 selection with 800 μg/ml and cell sorting. The resulting population of cells was maintained with medium containing 200 μg/ml G418 and frozen stocks kept in liquid nitrogen. MEF cells stably expressing β1 and β2-arrestins (Arr1/2 +/+) or not (Arr1/2 −/−) were cultured to ~70% confluency in 6-well dishes, media was exchanged with 1ml DMEM containing 10% FBS and 8 μg/ml polybrene prior to transduction. Undiluted supernatant containing lentivirus carrying 3xHA-S1PR1-GFP IRES Puromycin (200 μl) or Flag-β2-AR-IRES Puromycin (300 μl) were added to cells. After 48 hours, cells transduced with 3xHA-S1PR1-eGFP were selected by replacing the medium with DMEM containing 10% FBS and 4 μg/ml puromycin; cells transduced with Flag-β2-AR were selected 24 hours post infection by replacing the medium with DMEM containing 10% FBS and 6ug/ml or 2 ug/ml puromycin for Arr1/2 +/+ or Arr1/2 −/−, respectively. The resulting cell populations were stored in liquid nitrogen.
Fluorescence microscopy
Cells grown in DMEM supplemented with 10% FBS were trypsinized, diluted with medium without trypsin and plated for 3 hrs onto # 1.5 12 mm in diameter glass coverslips coated with poly-d-lysine (100 μg/ml) and collagen (20 μg/ml). When needed, non-transduced MEFs cells were included to serve as internal controls. The adhered cells were cultured overnight in DMEM supplemented with 2% charcoal treated FBS. Prior to the experiment, cells were cultured for 1.5 hours in serum free Opti-MEMR and then for 15 minutes with mouse ~ 0.8 μg/ml anti-HA F-7 antibody diluted in serum free Opti-MEMR. S1P or FTY720P were then added for 30 min; during the final 8 min of incubation the media with DMSO or ligand contained 25ug/ml transferrin-Alexa Fluor 647. All the previous steps were carried out at 37°C in the presence of 5% CO2. The surface bound antibodies and transferrin were removed with an acid wash step after the internalization period and before fixation as follows: cells were washed 3 times for 1 min in ice cold PBS, followed by 3 washes for 30 s with ice cold acid buffer (150 mM NaCl, 1 mM MgCl2, 0.125 mM CaCl2, 100 mM Glycine pH 2.5), followed by two rinses with ice cold PBS and then fixed for 15 min with ice cold 4% formaldehyde in PBS. Samples were then incubated at ~24°C with PBS for 30 min in the presence of 0.1% Triton-X100 3% BSA. Cells were then incubated with donkey anti-mouse Alexa Fluor 568 in PBS 0.1% Triton X-100 for 1 hr and washed 3 times for 5 min in PBS 0.1% Triton X-100. Coverslips were finally mounted in 90% glycerol/10% PBS and kept at 4 °C for not more than 24 hr before imaging.
Spinning disc confocal fluorescence microscopy was done using a Marianas System™ (Intelligent Imaging Innovations; Denver, CO). The system consisted of a 200M fully automated inverted microscope (Carl Zeiss, Inc.; Thornwood, NY) attached to a CSU-22 spinning disc confocal head (Yokogawa Electric Corporation; Tokyo Japan) with a Borealis modification (Spectral Applied Research; Ontario Canada) to enhanced the excitation light throughput. Cobolt (491 nm/50 mw and 561 nm/50 mw) and Crystal (660nm/40mw) lasers provided the excitation. Emission was collected through 525/50, 620/60 and 680/long-pass filters (Semrock, Inc.; Rochester NY) and images obtained using a 63 × 1.4 NA Plan Apochromatic oil immersion objective lens (Carl Zeiss, Inc.; Thornwood, NY) and a QuantEM electron multiplying back-thinned CCD camera (Photometrics; Tucson AZ). 3D acquisition of sequential images spaced 0.2 μ apart was achieved using a piezocontrolled stage (PZ-2000, ASI Imaging; Eugene OR). Images were collected using Slidebook 5™ (Intelligent Imaging Innovations; Denver, CO).
Image quantification and analysis
A central optical section along the z-axis of cells expressing or not 3xHA-S1PR1-eGFP was used to generate a mask within the cell to determine the amount of internalized fluorescent S1P1R or transferrin. The amounts of HA-antibody (Alexa-568 or Alexa-647) and total S1PR1 receptor (eGFP) internalized within a single cell were obtained by determining the mean fluorescence intensity in its mask corrected for the corresponding values from a cell not expressing 3xHA-S1PR1-eGFP. The amount of internalized S1PR1 is presented as the ratio between the antibody signal and the eGFP signal. The amount of internalized transferrin is expressed as the mean value of fluorescence transferrin in the mask within the cell. The transferrin signal was normalized to the mean value from regions devoid of cells. Graphpad Prism 6.0 was used for plotting; Statistical analysis was performed using a one-way ANOVA analysis and Bonferroni Post-Test (Graphpad Prism 6.0).
Flow cytometry analysis
HEK 293A cells stably expressing 3xHA-S1P1-mCherry or MEF cells stably expressing 3xHA-S1P1-eGFP were plated in a 12 well dish at a density of 6× 104 cells/well. The next day cells were washed twice with PBS and then grown overnight in DMEM supplemented with 2% charcoal treated FBS. Immediately before incubation with ligand, the cells were serum starved for 1.5 h by incubation in Opti-MEMR. To initiate receptor internalization, media was exchanged for Opti-MEMR containing 0.1% DMSO alone (carrier), 0.1% DMSO with 1 μM S1P, or 0.1 % DMSO with 100 nM FTY720P at 37°C for 30 min. Ligands were diluted sequentially into 100% DMSO, then Opti-MEMR 3% DMSO and to the final concentration by dilution into Opti-MEMR (final DMSO, 0.1%). During the last 8 min of incubation 5 μg/ml final concentration of transferrin Alexa Fluor 647 was added to the medium containing carrier or ligand. Cells were then washed three times with ice-cold PBS and incubated for 1h at 4°C in 400 μl ice-cold PBS supplemented with 1% BSA and ~ 0.8 μg/ml anti-HA F-7 antibody, followed by two washes with ice-cold PBS for 1 min each. Cells were then incubated in ice-cold PBS supplemented with 1% BSA including 2 μg/mL goat anti-mouse secondary antibody Alexa Fluor 488 for HEK293Acells or Alexa Fluor 647 for MEF cells for 1 h on ice. Following two one min washes with ice-cold PBS, the cells were detached from the plates by incubation for 3 min in PBS containing 5 mM EDTA pre-warmed to 37°C. Detached cells were resuspended in ice-cold PBS 1% BSA, transferred to microfuge tubes and pelleted at 700g (2500 rpm) for 3 min at 4°C in an Eppendorf refrigerated centrifuge. Following two further rinses with ice-cold PBS supplemented with 1% BSA, cells were fixed for 15 min at 4°C with 2% formaldehyde dissolved in PBS, washed twice with PBS 1% BSA, resuspended in PBS containing 0.1% BSA and kept for analysis at 4°. At least 20,000 cells per condition were analyzed within 12 hours of fixation using a BD FACS AriaII or BD Fortessa sorters. Due to the heterogeneity in ectopic expression of 3xHA-S1P1-mCherry and 3xHA-S1P1-eGFP, we normalized the fluorescence HA signal of each cell by the fluorescence signal elicited by mCherry or eGFP in the same cell, then multiplied by 100. Changes in the amount of surface S1PR1 are expressed as the fluorescence HA signal relative to the signal from non-stimulated cells. MEF cells (Arr1/2 +/+) and (Arr1/2 −/−) stably expressing Flag-β2-AR were treated with or without 25 μM Isoproterenol at 37°C for 25 minutes. Ligand activation was immediately terminated by 4°C incubation. The amount of surface Flag-β2-AR was determined by labeling with mouse anti-Flag (1:200) and goat-anti-mouse AF647 antibodies at 4°C. Fluorescent intensity was then quantified by flow cytometery as described above. Statistical analysis was performed using a one-way ANOVA analysis and Bonferroni Post-Test (Graphpad Prism 6.0).
HEK 293A cells stably expressing 3xHA-S1PR1 or 3xHA-S1PR1-eGFP were used in the experiments designed to test of the potential effect of including EGFP at the C-terminus of S1P1R. Cells were prepared for flow cytometry as described above with the following modifications: after incubation with the antibody specific for HA, the cells were incubated with donkey anti-mouse Alexa Fluor 647 (to quantify the receptor at the cell surface), then briefly fixed for 15 min with 4% formaldehyde dissolved in ice cold PBS, permeabilized with BD Perm/Wash™ buffer for 15 min and then incubated with rabbit-anti Edg1 for 1 hour (to quantify total receptor). Following two washes with ice cold PBS, cells were incubated at 4 °C with donkey anti-rabbit Alexa Fluor 594 antibody for 30 min, washed twice with ice cold PBS and finally subjected to flow cytometry analysis. The ratio of receptor at the surface with respect to total receptor was determined as the ratio of the signals between HA-tag (Alexa Fluor 647) and S1PR1 (Alexa Fluor 594).
shRNAs mediated depletion of clathrin and AP2
The lentivirus backbone shRNA vectors were obtained from the RNAi consortium shRNA library from the Broad Institute and virus produced according to the procedures described in http://www.broadinstitute.org/rnai/trc/lib. Briefly, lentiviruses were produced in low-passage HEK 293A cells plated on 6 well dishes containing 2 ml of DMEM and 10% FBS (obtained from the Broad Institute) by co-transfection of the lentivirus backbone and the packing system (gag/pol, tat, rev, VSV-G). Supernatants were collected 72, 96, and 120 hours post-transfection, and pooled into a volume of 6 ml.
HEK 293A cells stably expressing 3xHA-S1P1-mCherry or the parental HeLa cells were cultured in 6-well dishes to ~70% confluency. The cells were then incubated for 15 min at 37°C with 1ml DMEM supplemented with 10% FBS and 8 μg/ml polybrene followed by addition of 100 μl of undiluted supernatant containing the appropriate lentivirus. Cells were incubated with virus containing medium for 24 hours, followed by an exchange of the medium with DMEM supplemented with 10% FBS and 4ug/ml of puromycin. Transduced HEK 293A cells were selected during 5 days for puromycin resistance and then subjected to analysis. Transduced HeLa cells were selected with puromycin for two days, followed by transfection to express 3xHA-S1P1-eGFP and selection for three additional days with puromycin and finally subjected to analysis. The sequences targeting clathrin heavy chain and the μ2 subunit of AP2-were CGGTTGCTCTTGTTACGGATA and GCTGGATGAGATTCTAGACT, respectively. The scrambled sequence was CCTAAGGTTAAGTCGCCCTCG.
Supplementary Material
SYNOPSIS.
We show here that the G protein–coupled receptor sphingosine 1-phosphate receptor 1 (S1PR1) activated by its natural lipid ligand S1P or the synthetic agonist FTY720P is rapidly internalized by the clathrin-dependent endocytic pathway. Depletion of activated S1PR1 from the cell surface relies on clathrin, its endocytic adaptor AP-2 dynamin and the non-visual βarrestin1 and βarrestin2, suggesting that activated S1PR1 is down-regulated from the cell surface using the canonical GPCR entry route.
ACKNOWLEDGMENTS
The experiments associated with Fig. 5D were carried out by YLK. We thank Eric Marino for maintaining the Imaging Resource used in this study, Natasha Barteneva, Ken Ketman and Isabel Beerman for their assistance with flow cytometry and Greg Findlay for technical assistance. Steeve Boulant and Weibo Li for help setting up the lentivirus technology and members of our laboratory for helpful discussions. We also thank Robbie Henderson for additional support and discussions. The β-arrestin deficient and control MEFs were kindly provided by Dr. Lefkowitz. This work was supported in part by grants to T.K. from GSK and NIH GM-075252.
REFERENCES
- 1.Lee MJ, Van Brocklyn JR, Thangada S, Liu CH, Hand AR, Menzeleev R, Spiegel S, Hla T. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 1998;279:1552–1555. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- 2.Waters C, Sambi B, Kong K-C, Thompson D, Pitson SM, Pyne S, Pyne NJ. Sphingosine 1-phosphate and platelet-derived growth factor (PDGF) act via PDGF beta receptor-sphingosine 1-phosphate receptor complexes in airway smooth muscle cells. J Biol Chem. 2003;278:6282–6290. doi: 10.1074/jbc.M208560200. [DOI] [PubMed] [Google Scholar]
- 3.Liu CH, Thangada S, Lee MJ, Van Brocklyn JR, Spiegel S, Hla T. Ligand-induced trafficking of the sphingosine-1-phosphate receptor EDG-1. Mol Biol Cell. 1999;10:1179–1190. doi: 10.1091/mbc.10.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang F, Van Brocklyn JR, Hobson JP, Movafagh S, Zukowska-Grojec Z, Milstien S, Spiegel S. Sphingosine 1-phosphate stimulates cell migration through a G(i)-coupled cell surface receptor. Potential involvement in angiogenesis. J Biol Chem. 1999;274:35343–35350. doi: 10.1074/jbc.274.50.35343. [DOI] [PubMed] [Google Scholar]
- 5.Albert R, Hinterding K, Brinkmann V, Guerini D, Müller-Hartwieg C, Knecht H, Simeon C, Streiff M, Wagner T, Welzenbach K, Zecri F, Zollinger M, Cooke N, Francotte E. Novel immunomodulator FTY720 is phosphorylated in rats and humans to form a single stereoisomer. Identification, chemical proof, and biological characterization of the biologically active species and its enantiomer. J Med Chem. 2005;48:5373–5377. doi: 10.1021/jm050242f. [DOI] [PubMed] [Google Scholar]
- 6.Thangada S, Khanna KM, Blaho VA, Oo ML, Im D-S, Guo C, Lefrancois L, Hla T. Cell-surface residence of sphingosine 1-phosphate receptor 1 on lymphocytes determines lymphocyte egress kinetics. J Exp Med. 2010;207:1475–1483. doi: 10.1084/jem.20091343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watterson KR, Johnston E, Chalmers C, Pronin A, Cook SJ, Benovic JL, Palmer TM. Dual regulation of EDG1/S1P(1) receptor phosphorylation and internalization by protein kinase C and G-protein-coupled receptor kinase 2. J Biol Chem. 2002;277:5767–5777. doi: 10.1074/jbc.M110647200. [DOI] [PubMed] [Google Scholar]
- 8.Oo ML, Thangada S, Wu M-T, Liu CH, Macdonald TL, Lynch KR, Lin C-Y, Hla T. Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J Biol Chem. 2007;282:9082–9089. doi: 10.1074/jbc.M610318200. [DOI] [PubMed] [Google Scholar]
- 9.Bankovich AJ, Shiow LR, Cyster JG. CD69 suppresses sphingosine 1-phosophate receptor-1 (S1P1) function through interaction with membrane helix 4. Journal of Biological Chemistry. 2010;285:22328–22337. doi: 10.1074/jbc.M110.123299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gatfield J, Monnier L, Studer R, Bolli MH, Steiner B, Nayler O. Sphingosine-1-phosphate (S1P) displays sustained S1P1 receptor agonism and signaling through S1P lyase-dependent receptor recycling. Cell Signal. 2014;26:1576–1588. doi: 10.1016/j.cellsig.2014.03.029. [DOI] [PubMed] [Google Scholar]
- 11.Sykes DA, Riddy DM, Stamp C, Bradley ME, McGuiness N, Sattikar A, Guerini D, Rodrigues I, Glaenzel A, Dowling MR, Mullershausen F, Charlton SJ. Investigating the molecular mechanisms through which FTY720-P causes persistent S1P1 receptor internalization. Br J Pharmacol. 2014;171:4797–4807. doi: 10.1111/bph.12620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nomachi A, Yoshinaga M, Liu J, Kanchanawong P, Tohyama K, Thumkeo D, Watanabe T, Narumiya S, Hirata T. Moesin Controls Clathrin-Mediated S1PR1 Internalization in T Cells. PLoS ONE. 2013;8:e82590. doi: 10.1371/journal.pone.0082590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor MJ, Perrais D, Merrifield CJ. A high precision survey of the molecular dynamics of mammalian clathrin-mediated endocytosis. PLoS Biol. 2011;9:e1000604. doi: 10.1371/journal.pbio.1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehrlich M, Boll W, van Oijen A, Hariharan R, Chandran K, Nibert ML, Kirchhausen T. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell. 2004;118:591–605. doi: 10.1016/j.cell.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 15.Dutta D, Williamson CD, Cole NB, Donaldson JG. Pitstop 2 Is a Potent Inhibitor of Clathrin-Independent Endocytosis. PLoS ONE. 2012;7:e45799. doi: 10.1371/journal.pone.0045799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Willox AK, Sahraoui YME, Royle SJ. Non-specificity of Pitstop 2 in clathrin-mediated endocytosis. Biology Open. 2014;3:326–331. doi: 10.1242/bio.20147955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willinger T, Ferguson SM, Pereira JP, De Camilli P, Flavell RA. Dynamin 2-dependent endocytosis is required for sustained S1PR1 signaling. J Exp Med. 2014;211:685–700. doi: 10.1084/jem.20131343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oo ML, Chang S-H, Thangada S, Wu M-T, Rezaul K, Blaho V, Hwang S-I, Han DK, Hla T. Engagement of S1P1-degradative mechanisms leads to vascular leak in mice. J Clin Invest. 121:2290–2300. doi: 10.1172/JCI45403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jo E, Sanna MG, Gonzalez-Cabrera PJ, Thangada S, Tigyi G, Osborne DA, Hla T, Parrill AL, Rosen H. S1P1-selective in vivo-active agonists from high-throughput screening: off-the-shelf chemical probes of receptor interactions, signaling, and fate. Chem Biol. 2005;12:703–715. doi: 10.1016/j.chembiol.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez-Cabrera PJ, Hla T, Rosen H. Mapping pathways downstream of sphingosine 1-phosphate subtype 1 by differential chemical perturbation and proteomics. J Biol Chem. 2007;282:7254–7264. doi: 10.1074/jbc.M610581200. [DOI] [PubMed] [Google Scholar]
- 21.Ledezma-Sánchez BA, García-Regalado A, Guzmán-Hernández ML, Vázquez-Prado J. Sphingosine-1-phosphate receptor S1P1 is regulated by direct interactions with P-Rex1, a Rac guanine nucleotide exchange factor. Biochem Biophys Res Commun. 2010;391:1647–1652. doi: 10.1016/j.bbrc.2009.12.108. [DOI] [PubMed] [Google Scholar]
- 22.Ilinskaya A, Heidecker G, Derse D. Opposing effects of a tyrosine-based sorting motif and a PDZ-binding motif regulate human T-lymphotropic virus type 1 envelope trafficking. J Virol. 2010;84:6995–7004. doi: 10.1128/JVI.01853-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Popova JS. Clathrin-mediated Endocytosis of m3 Muscarinic Receptors: ROLES FOR G AND TUBULIN. Journal of Biological Chemistry. 2004;279:30410–30418. doi: 10.1074/jbc.M402871200. [DOI] [PubMed] [Google Scholar]
- 24.Goodman OB, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature. 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- 25.Lefkowitz RJ, Whalen EJ. beta-arrestins: traffic cops of cell signaling. Curr Opin Cell Biol. 2004;16:162–168. doi: 10.1016/j.ceb.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Kohout TA, Lin FS, Perry SJ, Conner DA, Lefkowitz RJ. beta-Arrestin 1 and 2 differentially regulate heptahelical receptor signaling and trafficking. Proc Natl Acad Sci USA. 2001;98:1601–1606. doi: 10.1073/pnas.041608198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu A, Rual J-F, Tamai K, Harada Y, Vidal M, He X, Kirchhausen T. Association of Dishevelled with the clathrin AP-2 adaptor is required for Frizzled endocytosis and planar cell polarity signaling. Dev Cell. 2007;12:129–141. doi: 10.1016/j.devcel.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu A, Xing Y, Harrison SC, Kirchhausen T. Structural analysis of the interaction between Dishevelled2 and clathrin AP-2 adaptor, a critical step in noncanonical Wnt signaling. Structure. 2010;18:1311–1320. doi: 10.1016/j.str.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tacheva-Grigorova SK, Santos AJM, Boucrot E, Kirchhausen T. Clathrin-mediated endocytosis persists during unperturbed mitosis. Cell Rep. 2013;4:659–668. doi: 10.1016/j.celrep.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCluskey A, Daniel JA, Hadzic G, Chau N, Clayton EL, Mariana A, Whiting A, Gorgani N, Lloyd J, Quan A, Moshkanbaryans L, Krishnan S, Perera S, Chircop M, Kleist von L, McGeachie AB, Howes MT, Parton RG, Campbell M, Sakoff JA, Wang X, Sun J-Y, Robertson MJ, Deane FM, Nguyen TH, Meunier FA, Cousin MA, Robinson PJ. Building a Better Dynasore: The Dyngo Compounds Potently Inhibit Dynamin and Endocytosis. Traffic. 2013 doi: 10.1111/tra.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.