Abstract
Two-thirds of all bacterial genomes sequenced to-date possess an organelle for locomotion, referred to as flagella, periplasmic flagella or type IV pili. These genomes may also contain a chemotaxis-signaling system which governs flagellar rotation, thus leading a coordinated function for motility. Motility and chemotaxis are often crucial for infection or disease process caused by pathogenic bacteria. Although motility-associated genes are well-characterized in some organisms, the highly-orchestrated synthesis, regulation, and assembly of periplasmic flagella in spirochetes are just being delineated. Recent advances were fostered by development of unique genetic manipulations in spirochetes coupled with cutting-edge imaging techniques. These contemporary advances in understanding the role of spirochetal motility and chemotaxis in host persistence and disease development are highlighted in this review.
Introduction
Spirochetes are a group of bacteria with distinctive morphology and motility [1–4]. Their morphology is so unique that, upon discovery, Antoine von Leeuwenhoek diagramed spirochetal bacteria as a separate group (Figure 1) [5]. More than 300 years after the discovery of those spirochetes from the human mouth, numerous spirochetal organisms have been identified, many of which are medically significant. Borrelia burgdorferi sensu lato are the causative agents of Lyme disease (i.e. Lyme borreliosis) [6–8], which is the most commonly reported vector-borne disease in the United States and Europe [9;10]. Borrelia hermsii and Borrelia miyamotoi species cause tick-borne relapsing fever, while B. recurrentis causes louse-borne relapsing fever. Many Leptospira species cause leptospirosis, which is a serious health concern for >65% of the world population, particularly in China, India and Brazil [11–15]. Treponema pallidum subspecies pallidum causes syphilis, which is sexually transmitted and is a major public health problem worldwide [16]. Other closely-related treponemes cause yaws, bejel, and pinta. Some Treponema spp. are also associated with digital dermatitis in cattle. Treponema denticola and other oral treponemes are associated with periodontal disease. Brachyspira hyodysenteriae causes swine dysentery, and Brachyspira pilosicoli and Brachyspira aalborgi are associated with human intestinal infections in developing countries. Together, the spirochetes constitute a major global disease burden and there are tremendous interests in identifying better therapeutic targets for these unique bacterial pathogens.
Figure 1.
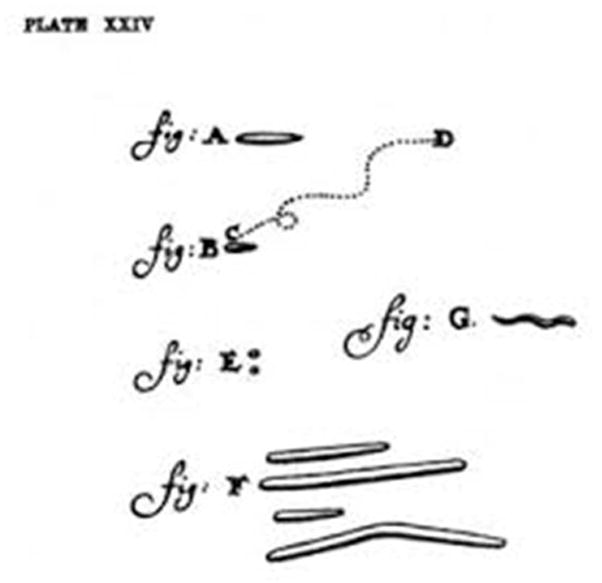
Antonie Van Leeuwenhoek’s illustrations of various bacteria isolated from a human mouth that was published in September 1683, which he referred to as “animalcules.” Bacteria shown are (A) a rod-shaped bacterium, (B) a motile bacterium moving from points (C) to (D), (E) micrococci, (F) fusiform bacteria and (G) a spirochete illustrating characteristic wave-like shape. Adapted from [5].
Notably, recent global signature-tagged mutagenesis studies, as well as infection studies assessing directed mutants, suggested that many genes related to motility and chemotaxis functions are crucial for persistent infection by all pathogenic spirochetes tested to-date [2;17–22]. While there are several excellent review articles on these topics [2;23–26], the focus of this review is to summarize the most recent research findings and describe how they contribute to the current paradigms on the role of spirochetal motility (and chemotaxis) products in the natural enzootic cycle of these bacteria.
Spirochete morphology and motility
Spirochetes are characterized as motile bacteria with distinctive helical or planar flat-wave morphology [2;24;27]. The outer membrane of most spirochetes is a lipid bilayer that lacks the lipopolysaccharide molecules present in most gram-negative bacteria; the Leptospira spp. are the only known exceptions [28]. The inner membrane is typical for prokaryotic cells and is surrounded by a thin peptidoglycan layer that provides strength while being sufficiently flexible for spirochetal motility. Spirochetes flagella are attached at both ends of the cells, but are not located externally as in most gram-negative bacteria, but rather reside in the periplasmic space i.e., between the peptidoglycan layer and outer membrane (Figures 2 and 3) [2]. Each periplasmic flagellum is attached at one pole of the cell, then extends toward the opposite pole of the cell. The flagella from both poles may overlap in the middle of the cell. Spirochete species vary with respect to the size and number of periplasmic flagella they possess. For example, Cristispira spp. are 0.5–3 μm wide, 30–180 μm long, and have over 100 periplasmic flagella attached at each pole. In contrast, the Leptospiraceae (which include Leptospira and Leptonema spp.) are approximately 0.1 μm in diameter, 10–20 μm long, and have only one periplasmic flagellum at each end (total of 2 flagella per cell) [2;24;27;29;30].
Figure 2.
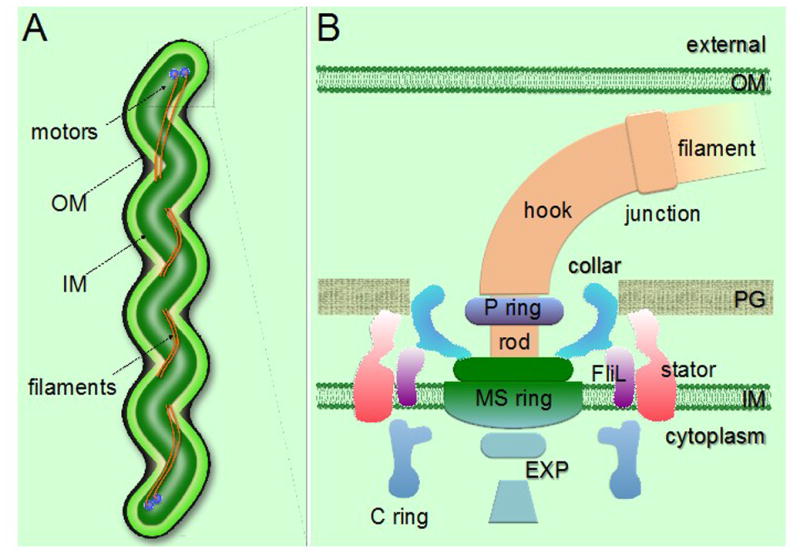
General morphology and periplasmic flagellar structures in spirochetes. (A) Schematic model of a spirochete cell showing the periplasmic flagellar filaments located between the outer membrane (OM) and the inner membrane (IM), causing the characteristic flat-wave morphology. (B) Schematic model of the periplasmic flagellar motor illustrating various flagellar motor components. PG, peptidoglycan layer; EXP, export apparatus.
Figure 3.
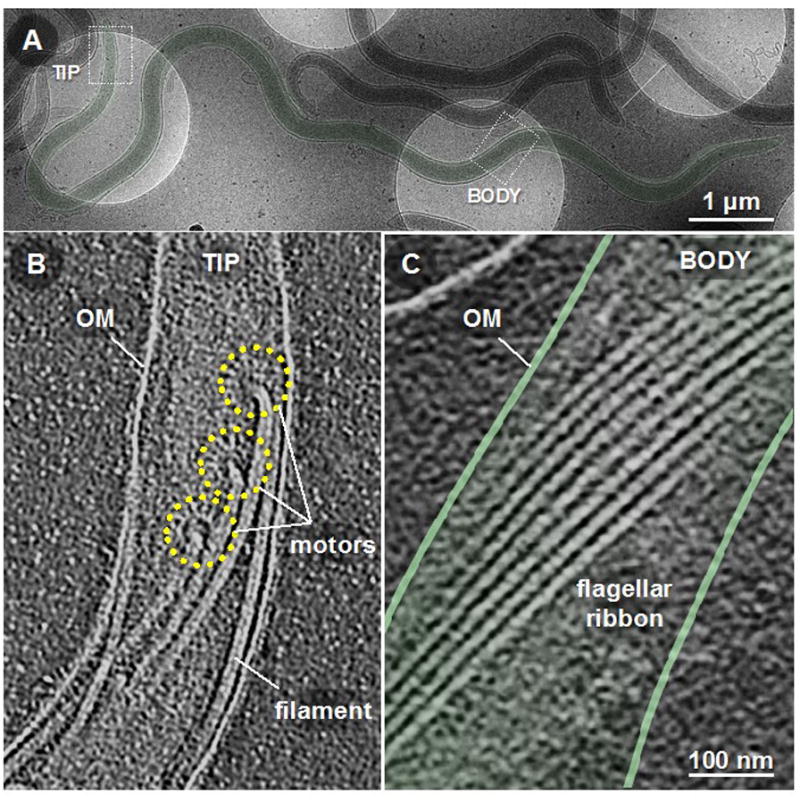
Cellular structures of B. burgdorferi. A B. burgdorferi cell (A) was imaged by cryo-electron tomography microscopy near a cell tip/pole (B) and the middle of the cell body (C). Periplasmic flagellar motors are observed to be attached in an ordered fashion at each cellular pole (B), and regions exhibiting characteristic spirochetal morphology present endoflagella in an ordered flagellar ribbon-like structure. Motor structures are outlined with yellow broken lines. Adapted from [38].
B. burgdorferi is the best-studied organism among the spirochetes, and will constitute the major focus of this review. B. burgdorferi cells are 5–20 μm long, ~0.3 μm in diameter, and possess 7–11 periplasmic flagella (endoflagella) attached to each pole [3;31–33]. During swimming, these flagella located at the poles of the cell must coordinate in order to run, reverse, or flex/tumble. B. burgdorferi asymmetrically rotate their flagella during these swimming patterns [24;34]. Prototypical gram-negative bacterial flagella and periplasmic flagella share substantial amino acid sequence and functional homology, but do possess some unique characteristics. For example, periplasmic flagella bear collar proteins that are unique to the spirochetes (Figure 2) [35;36]. Another unique aspect is that, while external flagella only provide motility for most bacteria, endoflagellar activity (i.e. motor rotation) also produces the spirochetal morphology that is characteristic for these bacteria [30;37;38].
The periplasmic flagellum can be subdivided into three main portions: basal body, hook, and filament (Figure 2) [2;32;39–41]. The basal body is comprised primarily of the export apparatus (EXP), MS ring, C-ring switch complex, collar structure, FliL, and stator (MotA, MotB) (Figure 2) [36;41;42]. The hook, which assembles on rod proteins, is primarily comprised of FlgE. The filament is composed of the minor protein FlaA and major protein FlaB (Figure 2) [43–45]. B. burgdorferi genome sequence analysis suggests that there are more than 50 genes annotated as motility- and chemotaxis-related [2;40]. Recently, four additional genes encoding cyclic-di-GMP metabolizing proteins have also been discovered to be involved in modulating motility/chemotaxis in B. burgdorferi [2;46–49]. Interestingly, the motility and chemotaxis genes are largely controlled by the σ70 subunit of RNA polymerase in B. burgdorferi, whereas those genes in most gram-negative bacteria are regulated in a hierarchical manner [2;50;51].
B. burgdorferi is a parasite that primarily resides in tick and mammalian hosts. Thus, any regulation of motility and chemotaxis should become apparent within those host environments at a particular stage of the bacterial life cycle. Moreover, some flagellar genes (e.g. FlaB) are regulated by the carbon storage regulator CsrA at the post-transcriptional level in B. burgdorferi [52–54]. We will discuss the spatiotemporal regulation of motility during the enzootic life cycle of B. burgdorferi in the following sections.
Motor rotation is essential for the characteristic spirochetal wave-like morphology
For B. burgdorferi strains, 7–11 flagellar motors are inserted in an ordered fashion at each cell pole (Figure 3) [2;32;33;39;41;52;55]. As the flagella wrap around the protoplasmic cell cylinder, they form an ordered ribbon-like structure which is believed to be important for maintaining the characteristic flat-wave morphology of the spirochetes (Figure 3) [27;55]. To support this, mutant cells that only lack the FlaB filaments display a rod-shaped morphology in vitro and are non-motile [30;37]. To address whether possession of endoflagella alone is sufficient for the flat-wave morphology, Sultan et al assessed a motB-deficient (ΔmotB) mutant that possess periplasmic flagella, but lacked the stator protein that is responsible for producing the torque needed to drive flagellar rotation, i.e. should be paralyzed. When assessed in vitro, these cells displayed a normal flat-wave morphology at their poles, but appeared to be more rod-like in the center [38]. When directly visualized in the intact skin of infected mice, the ΔmotB cells displayed no characteristic spirochetal morphology and were non-motile [38]. Cryo-electron tomography analyses of these mutants showed that the endoflagella displayed an ordered flat-ribbon conformation at the poles demonstrating the characteristic spirochetal morphology, but possessed a disordered flagellar ribbon conformation in the center region that lacked the flat-wave morphology [38]. Thus, possession of both endoflagella and flagellar rotation are required together with the activities of the cell cylinder to produce spirochetal morphology, regardless if they are actively motile or stationary [27;37;38;55]. It is important to note that the cell cylinders and flagella are proposed to be elastic materials [27;55]. Thus, applied forces cause these structures to deform and subsequently revert back to their original shape when the force is removed. Several studies have demonstrated that the B. burgdorferi cell cylinder is rod-shaped in the absence of periplasmic flagella, whereas purified periplasmic flagella are shown to be helical [2;25;27]. Thus, the interaction between the cell cylinder and flagella produces the distinct spirochetal flat-wave morphology, which has previously been mathematically modeled [27]. Based on these findings, we predict that the flagellar ribbon interacts with the cell cylinder, and this physical interaction is enhanced by forces provided by flagellar rotation to produce the ordered ribbon structure, which in turn produce the flat-wave morphology [38]. Conversely, spirochete cells that are deficient in any chemotaxis-related genes should also display flat-wave morphology (e.g., ΔcheA2, ΔcheY3, ΔfliG1, ΔcheX mutant cells) [34;56–58].
Regulation of motility/chemotaxis genes and their in vivo activities
B. burgdorferi are highly mobile and invasive organisms that disseminate widely throughout their arthropod and vertebrate hosts [59–61]. When an infected tick begins feeding on a host, the spirochetes in the tick midgut actively traverse the hemocoel into the salivary glands and are subsequently deposited in the skin, where they proliferate and can persist within the dermal tissues for years. Shortly after deposition into the skin, some of the spirochetes disseminate through the extracellular matrices and/or via the bloodstream, can cross the vasculature, and ultimately colonize many distant mammalian tissues (e.g. tibiotarsal joints, heart, and the brain), which can elicit various acute and chronic clinical manifestations [60;62–65]. Because of the integral nature of these transmigrations to the natural enzootic cycle, it would appear that motility and chemotaxis are critical for multiple stages of infection, though little is known regarding the particular motility characteristics that are important for these different migratory stages. Sze et al have demonstrated that chemotaxis mediated by a histidine kinase CheA2 is important for transmission of spirochetes, as well as persistence in mice [66]. In studies using intravital microscopy, Chaconas and colleagues observed that B. burgdorferi within the bloodstream can attach to the vascular endothelium and actively migrate between these cells to disseminate into other tissues. Their studies using different adhesin mutants indicate that wild-type B. burgdorferi cells likely use back-and-forth motility patterns together with binding certain host tissues to allow this endothelial transmigration. Notably, adhesins involved in tethering, dragging and stationary events were observed in greatly reduced numbers in an avirulent B. burgdorferi strain, suggesting that motility alone is insufficient for these activities [64;67–69]. At this time, no non-motile B. burgdorferi clone (e.g. ΔflaB or ΔmotB mutants) has ever been investigated in these assays, but it is highly unlikely such cells could escape from the vasculature since all non-motile strains tested so far are cleared from the murine host within 24–48 hours of intradermal inoculation. This is also supported by the recent observation that paralyzed ΔmotB cells were unable to migrate from their injection site within mouse ear skin [37;38]. Interestingly, long-term intravital microscopy studies assessing B. burgdorferi numbers and motility within skin demonstrate that all persisting wild-type bacteria continuously exhibit back-and-forth motility patterns for ≥720 days post-infection in immunocompetent mice (R. Mark Wooten and M. Motaleb, unpublished data) [70]. This constant motility is in agreement with the findings that flagellar genes are transcribed by constitutive σ70 promoters in B. burgdorferi. These motility characteristics suggest that nutrition is sufficient within a competent vertebrate host to allow B. burgdorferi to constitutively synthesize, as well as rotate the macromolecular flagellar motor structures without regulation, and that constant motility is important for immune evasion. However, the scenario in the tick vector is different (see below).
Motility in the tick vector and transmission between the arthropod and vertebrate host
Unlike in the vertebrate host, constant motility is not observed for spirochetes persisting within the invertebrate tick vector. During tick-feeding, B. burgdorferi are acquired from the vertebrate host, replicate exponentially, and colonize the tick midgut epithelium, where they remain throughout the molt [60;62]. During the subsequent tick blood-meal, replicating spirochetes traverse the midgut, penetrate the salivary glands and are conveyed via the saliva into the dermis of the mammalian host. Dunham et al observed that dissemination of wild-type spirochetes within feeding ticks proceeds in two distinct phases. Initially, non-motile replicating spirochetes appear to be passively transported from the midgut towards the basement membrane by forming confluent networks that surround and/or attach to the differentiating epithelial cells, a process designated as ‘adherence-mediated migration.’ In the second phase, the non-motile B. burgdorferi positioned at or near the basolateral poles of epithelial cells convert into motile organisms that actively traverse the midgut, invade the salivary glands, and subsequently transit into a new vertebrate host [62]. These findings indicate that motility is highly-regulated within the tick host, is likely regulated by factors released from host tissues, and that motility is necessary for efficient transmission to a vertebrate host [71]. It will be interesting to directly test this in the future using B. burgdorferi motility mutants (e.g. ΔflaB or ΔmotB).
Until recently, there was no consensus on whether feeding ticks acquire B. burgdorferi passively from adjacent vascular or other tissues of an infected host, or whether the bacteria sense the feeding tick and actively migrate towards the blood-pit. Bockenstedt et al recently performed studies using two-photon intravital microscopy to actively track GFP-expressing B. burgdorferi migration from an infected mouse to the feeding ticks [65]. These mice had been injected two weeks previously and a persistent disseminated infection had developed within the skin tissues. When naïve ticks were allowed to feed on these mice, a subset of the skin-resident spirochetes demonstrated a directed migration towards the feeding tick within 6 hour of attachment. Since these migrating bacteria were not initially within the vasculature or the subsequently developing feeding pit, the transmission event does not appear to be a passive diffusion event, but rather an active event where the spirochetes migrate through the skin to the bite site. Although the signals perceived by the skin-resident bacteria that elicit this directed migration are currently unknown, it is plausible that components of tick saliva may be foremost in governing such movement, and that the B. burgdorferi chemotactic machinery would be essential for this directed migration. This is supported by in vitro analyses demonstrating that tick saliva, as well as other components that may be associated with tick biology, can elicit directed motility using capillary assays [72;73]. The advancement of intravital microscopy techniques to assess spirochetal motility activities within intact mouse tissues may allow visual studies to directly identify the chemoattractant(s) and the chemotactic pathways that elicit such directed motility by skin-resident B. burgdorferi.
Events that are important for the spatiotemporal regulation of motility (or chemotaxis) during the life cycle of B. burgdorferi is currently not well-understood. The contributions of the two two-component systems, Hk1-Rrp1 and Hk2-Rrp2, during the mammalian- and tick-phase of the spirochetes life cycle is well-documented [60;61;74;75]. One could speculate that motility and the requirement for motor rotation may not be vital for B. burgdorferi survival within the unfed tick, where nutrients are depleted and B. burgdorferi could only acquire the energy for diminished motility [37;38]. Although not studied, some known (e.g. CsrA) or unknown negative regulator(s) may inhibit flagellin (FlaB) synthesis or periplasmic flagellar motor rotation at this stage in order to inhibit motility [52–54]. However, studies do suggest that diffusible components are released from the midgut of feeding ticks that promote the non-motile state demonstrated by B. burgdorferi during the early stages of transmission from the tick to the new vertebrate host [60;62;71]. Regardless, the current literature indicates that B. burgdorferi must migrate from the tick midgut in a directed fashion into a new vertebrate host, and subsequently disseminate from the site of a tick-bite to the distant colonization sites (e.g. heart, joint, brain), causing disease. Furthermore, during a tick blood-meal or within the mammalian dermis after the tick-bite, motility must be activated (by releasing the inhibitory effect of CsrA or by unknown regulators), enabling B. burgdorferi to transmit from the tick to the mammalian host and establish infection [37;38;65].
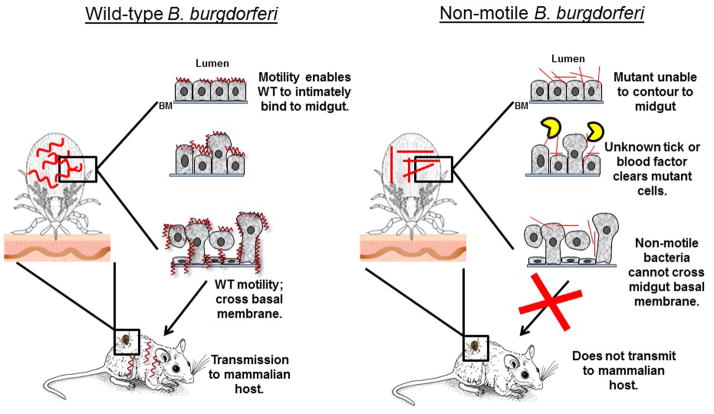
Spirochetal motility does appear to be critical for the long-term survival of B. burgdorferi in the tick. Sultan et al reported that flagellated but paralyzed ΔmotB cells failed to effectively survive in ticks for ≥7 days after the blood meal; the mutant cells were significantly less viable in ticks. This defect could be related to the in vivo growth defect demonstrated by the non-motile B. burgdorferi mutants. However, even if the ΔmotB burden per tick was significantly lower due to an in vivo growth defect, the rate/percentage of the spirochete-positive ticks would be expected to remain unchanged after the blood meal; that did not occur. Instead, the percentage of ΔmotB-infected ticks dropped sharply from 100% to 9% after feeding, while the rate remained unchanged in nymphs colonized with wild-type spirochetes [38]. This clearance of non-motile ΔmotB mutants suggest that spirochetal motility promotes an intimate bacterial interaction in the tick midgut (e.g. B. burgdorferi outer surface protein OspA with tick midgut protein TROSPA) [76], and this interaction protects the organism from potential harm from the blood-induced factors or tick immune responses [63;77] (Figure 4). These conclusions are supported by studies where spirochetes that exhibit even limited modes of motility, including running in one direction (ΔcheA2) or only flex-type of motility (ΔcheX), are able to survive normally in fed ticks [66]; our unpublished results). Thus, the most likely explanation for the reduced persistence in ticks is ΔmotB cells being unable to replicate at the same rate as the motile strains, and/or the non-motile organisms were subsequently cleared more efficiently from the fed-tick midgut by some combination of host blood-components, blood-induced tick factors present in the midgut, or the tick immune system [77–79]. Future studies using directed mutants for different chemotaxis-related genes may help delineate the specific interactions that promote B. burgdorferi persistence within tick hosts.
Figure 4.
Model for the roles of B. burgdorferi motility in bacterial persistence and dissemination within and between hosts. During tick feeding, wild-type (WT) bacteria replicate and use spirochetal back-and-forth motility to cross the tight junction of the midgut basement membrane, and subsequently traverse through the salivary glands into the skin of the mammalian host (left diagram). Dissemination from the host skin tissues to the distant colonization sites also requires bacterial motility. During tick feeding, WT motility likely enables the bacteria to intimately bind to the midgut (e.g. OspA-TROSPA), which is believed to be important for bacterial viability in the tick. WT B. burgdorferi then undergoes a “biphasic mode of dissemination” during tick feeding [62], which we intentionally omitted in this model as we are comparing WT with our genetically modified non-motile mutants, and it has not been investigated if the paralyzed cells also undergo a similar biphasic mode. Red curves and rod-shapes represent WT and non-motile B. burgdorferi, respectively. Half-moon yellow shapes (
 ) represent tick or host blood-borne noxious factors that clear non-motile spirochetes from the tick midgut. BM, basement membrane.
) represent tick or host blood-borne noxious factors that clear non-motile spirochetes from the tick midgut. BM, basement membrane.
Concluding remarks
Spirochetes are unique in many aspects, including how the cell shape is determined, how the periplasmic flagellum is assembled, how the periplasmic flagella at two poles of the bacteria coordinate during swimming, possession of unique flagellar components in the motor, and the absolute requirement of motility and chemotaxis during the enzootic life cycle of B. burgdorferi. Since the development of genetic manipulation tools within the last 15 years, the roles of several B. burgdorferi motility and chemotaxis genes have been reported, allowing certain trends in spirochete pathogenesis to begin coming into focus. However, many important questions remain to be investigated regarding periplasmic flagellar assembly, regulation, motility behavior, and spatial regulation of motility and chemotaxis during the colonization of different hosts.
Highlights.
. While B. burgdorferi organisms were found to be constantly moving within mouse skin tissues, this does not likely occur in the tick vector, especially in the nutritionally-depleted midgut (i.e. during unfed/molting condition). How and when is motility activated?
. Non-motile spirochete mutants demonstrated a decreased ability to survive in the fed-ticks. What is the factor(s) responsible for the diminished viability in the midgut?
. Sultan et al proposed that wild-type motility enables the spirochete to intimately interact with the midgut whereas non-motile mutants are unable due to their lack of motility. What are these interactions that occur in the tick midgut?
. While the shape of most bacterial cells are determined by their peptidoglycan layer, periplasmic flagella and subsequent motor rotation generates the characteristic flat-wave morphology in B. burgdorferi. Does motor rotation guide periplasmic flagella to interact with the cell cylinder to produce the characteristic wave-like morphology?
. In most other motile bacteria, flagellar/chemotaxis genes are regulated in a hierarchical manner. However, this does not appear to occur in B. burgdorferi, where almost all flagellar/chemotaxis-related genes are regulated by σ70. If so, how does the organism sequentially assemble their periplasmic flagella?
Acknowledgments
We apologize to the investigators whose papers were not cited due to page limitations. Our research programs are supported by National Institutes of Health grants 1R01AR060834, 1R56AI105128, 1R21AI113014, 2R01AI087946, and American Heart Association Pre-doctoral Fellowship PRE20490177. We thank M. Caimano for providing some of the diagrams in this article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
* of special interest
- 1.Paster BJ. Phylum XV. Spirochaetes Garrity and Holt 2001. Bergey’s Manual® of Systematic Bacteriology. 2010;4:471–566. [Google Scholar]
- 2*.Charon NW, Cockburn A, Li C, Liu J, Miller KA, Miller MR, Motaleb MA, Wolgemuth CW. The unique paradigm of spirochete motility and chemotaxis. Annu Rev Microbiol. 2012;66:349–370. doi: 10.1146/annurev-micro-092611-150145. An excellent review on motility and chemotaxis of spirochetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstein SF, Buttle KF, Charon NW. Structural analysis of Leptospiraceae and Borrelia burgdorferi by high-voltage electron microscopy. J Bacteriol. 1996;178:6539–6545. doi: 10.1128/jb.178.22.6539-6545.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstein SF, Charon NW, Kreiling JA. Borrelia burgdorferi swims with a planar waveform similar to that of eukaryotic flagella. Proc Natl Acad Sci USA. 1994;91:3433–3437. doi: 10.1073/pnas.91.8.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5*.Leewenhoeck A. An abstract of a letter from Mr. Anthony Leewenhoeck at Delft, dated Sep. 17. 1683 containing some microscopical observations, about animals in the scurf of the teeth, the substance call’d worms in the nose, the cuticula consisting of scales. Philosophical Transactions: The Royal Society Publishing. 1684;14:568–574. This reference documents and illustrates the first spirochetal organism isolated from a human mouth, and shows the distinctive wave-like morphology. [Google Scholar]
- 6*.Bockenstedt LK, Wormser GP. Review: unraveling Lyme disease. Arthritis Rheumatol. 2014;66:2313–2323. doi: 10.1002/art.38756. This study contains the first intravital microscopy images of a feeding Ixodes tick in intact living mouse skin, and demonstrates that skin-resident Borrelia burgdorferi actively migrate to the tick feeding site. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet. 2012;379:461–473. doi: 10.1016/S0140-6736(11)60103-7. [DOI] [PubMed] [Google Scholar]
- 8*.Rosa PA, Tilly K, Stewart PE. The burgeoning molecular genetics of the Lyme disease spirochaete. Nat Rev Microbiol. 2005;3:129–143. doi: 10.1038/nrmicro1086. An excellent review on recent development of genetic manipulation and infection assay tools for Borrelia burgdorferi, allowing significant advances in our understanding of Lyme disease development. [DOI] [PubMed] [Google Scholar]
- 9.Kuehn BM. CDC estimates 300,000 US cases of Lyme disease annually. JAMA. 2013;310:1110. doi: 10.1001/jama.2013.278331. [DOI] [PubMed] [Google Scholar]
- 10.Mead PS. Epidemiology of Lyme disease. Infect Dis Clin North Am. 2015;29:187–210. doi: 10.1016/j.idc.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Haake DA, Levett PN. Leptospirosis in humans. Curr Top Microbiol Immunol. 2015;387:65–97. doi: 10.1007/978-3-662-45059-8_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adler B, de la Pena MA. Leptospira and leptospirosis. Vet Microbiol. 2010;140:287–296. doi: 10.1016/j.vetmic.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Ko AI, Goarant C, Picardeau M. Leptospira: the dawn of the molecular genetics era for an emerging zoonotic pathogen. Nat Rev Microbiol. 2009;7:736–747. doi: 10.1038/nrmicro2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xue F, Yan J, Picardeau M. Evolution and pathogenesis of Leptospira spp.: lessons learned from the genomes. Microbes Infect. 2009;11:328–333. doi: 10.1016/j.micinf.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 15.McBride AJ, Athanazio DA, Reis MG, Ko AI. Leptospirosis. Curr Opin Infect Dis. 2005;18:376–386. doi: 10.1097/01.qco.0000178824.05715.2c. [DOI] [PubMed] [Google Scholar]
- 16.Giacani L, Lukehart SA. The endemic treponematoses. Clin Microbiol Rev. 2014;27:89–115. doi: 10.1128/CMR.00070-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17*.Lin T, Gao L, Zhang C, Odeh E, Jacobs MB, Coutte L, Chaconas G, Philipp MT, Norris SJ. Analysis of an ordered, comprehensive STM mutant library in infectious Borrelia burgdorferi. insights into the genes required for mouse infectivity. PLoS One. 2012;7:e47532. doi: 10.1371/journal.pone.0047532. First signature-tagged mutagenesis and in vivo studies in Borrelia burgdorferi; broadly demonstrates the importance of motility and chemotaxis genes for infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambert A, Picardeau M, Haake DA, Sermswan RW, Srikram A, Adler B, Murray GA. FlaA proteins in Leptospira interrogans are essential for motility and virulence but are not required for formation of the flagellum sheath. Infect Immun. 2012;80:2019–2025. doi: 10.1128/IAI.00131-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao S, Sun A, Ojcius DM, Wu S, Zhao J, Yan J. Inactivation of the fliY gene encoding a flagellar motor switch protein attenuates mobility and virulence of Leptospira interrogans strain Lai. BMC Microbiol. 2009;9:253. doi: 10.1186/1471-2180-9-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guyard C, Raffel SJ, Schrumpf ME, Dahlstrom E, Sturdevant D, Ricklefs SM, Martens C, Hayes SF, Fischer ER, Hansen BT, Porcella SF, Schwan TG. Periplasmic Flagellar Export Apparatus Protein, FliH, Is Involved in Post-Transcriptional Regulation of FlaB, Motility and Virulence of the Relapsing Fever Spirochete Borrelia hermsii. PLoS One. 2013;8:e72550. doi: 10.1371/journal.pone.0072550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosey EL, Kennedy MJ, Yancey RJ., Jr Dual flaA1 flaB1 mutant of Serpulina hyodysenteriae expressing periplasmic flagella is severely attenuated in a murine model of swine dysentery. Infect Immun. 1996;64:4154–4162. doi: 10.1128/iai.64.10.4154-4162.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lux R, Miller JN, Park NH, Shi W. Motility and chemotaxis in tissue penetration of oral epithelial cell layers by Treponema denticola. Infect Immun. 2001;69:6276–6283. doi: 10.1128/IAI.69.10.6276-6283.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldstein SF, Li C, Liu J, Miller MR, Motaleb MA, Norris SJ, Silversmith RE, Wolgemuth CW, Charon NW. The chic motility and chemotaxis of Borrelia burgdorferi. In: Samuels DS, Radolf JD, editors. BORRELIA: Molecular Biology, Host Interactions and Pathogenesis. Caister Academic Press; 2010. pp. 161–181. [Google Scholar]
- 24.Charon NW, Goldstein SF. Genetics of motility and chemotaxis of a fascinating group of bacteria: The Spirochetes. Annu Rev Genet. 2002;36:47–73. doi: 10.1146/annurev.genet.36.041602.134359. [DOI] [PubMed] [Google Scholar]
- 25.Wolgemuth CW, Charon NW, Goldstein SF, Goldstein RE. The flagellar cytoskeleton of the spirochetes. J Mol Microbiol Biotechnol. 2006;11:221–227. doi: 10.1159/000094056. [DOI] [PubMed] [Google Scholar]
- 26.Limberger RJ. The periplasmic flagellum of spirochetes. J Mol Microbiol Biotechnol. 2004;7:30–40. doi: 10.1159/000077867. [DOI] [PubMed] [Google Scholar]
- 27.Dombrowski C, Kan W, Motaleb MA, Charon NW, Goldstein RE, Wolgemuth CW. The elastic basis for the shape of Borrelia burgdorferi. Biophys J. 2009;96:4409–4417. doi: 10.1016/j.bpj.2009.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ben-Menachem G, Kubler-Kielb J, Coxon B, Yergey A, Schneerson R. A newly discovered cholesteryl galactoside from Borrelia burgdorferi. Proc Natl Acad Sci USA. 2003;100:7913–7918. doi: 10.1073/pnas.1232451100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldstein SF, Buttle KF, Charon NW. Structural analysis of Leptospiraceae and Borrelia burgdorferi by high-voltage electron microscopy. J Bacteriol. 1996;178:6539–6545. doi: 10.1128/jb.178.22.6539-6545.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30*.Motaleb MA, Corum L, Bono JL, Elias AF, Rosa P, Samuels DS, Charon NW. Borrelia burgdorferi periplasmic flagella have both skeletal and motility functions. Proc Natl Acad Sci USA. 2000;97:10899–10904. doi: 10.1073/pnas.200221797. This study provides the first experimental evidence that periplasmic flagella encoded by FlaB is essential for the flat-wave morphology and motility of B. burgdorferi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hovind Hougen K. Ultrastructure of spirochetes isolated from Ixodes ricinus and Ixodes dammini. Yale J Biol Med. 1984;57:543–548. [PMC free article] [PubMed] [Google Scholar]
- 32.Kudryashev M, Cyrklaff M, Baumeister W, Simon MM, Wallich R, Frischknecht F. Comparative cryo-electron tomography of pathogenic Lyme disease spirochetes. Mol Microbiol. 2009;71:1415–1434. doi: 10.1111/j.1365-2958.2009.06613.x. [DOI] [PubMed] [Google Scholar]
- 33.Xu H, Raddi G, Liu J, Charon NW, Li C. Chemoreceptors and flagellar motors are subterminally located in close proximity at the two cell poles in spirochetes. J Bacteriol. 2011;193:2652–2656. doi: 10.1128/JB.01530-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34*.Li C, Bakker RG, Motaleb MA, Sartakova ML, Cabello FC, Charon NW. Asymmetrical flagellar rotation in Borrelia burgdorferi nonchemotactic mutants. Proc Natl Acad Sci USA. 2002;99:6169–6174. doi: 10.1073/pnas.092010499. This study illustrates the asymmetric flagellar rotation in B. burgdorferi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen S, Beeby M, Murphy GE, Leadbetter JR, Hendrixson DR, Briegel A, Li Z, Shi J, Tocheva EI, Muller A, Dobro MJ, Jensen GJ. Structural diversity of bacterial flagellar motors. EMBO J. 2011;30:2972–2981. doi: 10.1038/emboj.2011.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao X, Norris SJ, Liu J. Molecular Architecture of Bacterial Flagellar Motor in Cells. Biochem. 2014;53:4323–4333. doi: 10.1021/bi500059y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37*.Sultan SZ, Manne A, Stewart PE, Bestor A, Rosa PA, Charon NW, Motaleb MA. Motility is crucial for the infectious life cycle of Borrelia burgdorferi. Infect Immun. 2013;81:2012–2021. doi: 10.1128/IAI.01228-12. This paper illustrates that motility is crucial for the enzootic life cycle of B. burgdorferi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38*.Sultan SZ, Sekar P, Zhao X, Manne A, Liu J, Wooten RM, Motaleb MA. Motor rotation is essential for the formation of the periplasmic flagellar ribbon, cellular morphology, and Borrelia burgdorferi persistence within Ixodes scapularis tick and murine hosts. Infect Immun. 2015;83:1765–1777. doi: 10.1128/IAI.03097-14. This study demonstrates that motor rotation, together with the possession of periplasmic flagella, is necessary for the flat-wave morphology of B. burgdorferi. It also demonstrates that motility is crucial for bacterial survival in the tick vector. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39*.Zhao X, Zhang K, Boquoi T, Hu B, Motaleb MA, Miller KA, James ME, Charon NW, Manson MD, Norris SJ, Li C, Liu J. Cryoelectron tomography reveals the sequential assembly of bacterial flagella in Borrelia burgdorferi. Proc Natl Acad Sci USA. 2013;110:14390–14395. doi: 10.1073/pnas.1308306110. This study demonstrates the sequential assembly of B. burgdorferi periplasmic flagella using cryo-ET coupled with targeted mutagenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, Lathigra R, White O, Ketchum KA, Dodson R, Hickey EK, Gwinn M, Dougherty B, Tomb JF, Fleischmann RD, Richardson D, Peterson J, Kerlavage AR, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams MD, Gocayne J. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 41*.Liu J, Lin T, Botkin DJ, McCrum E, Winkler H, Norris SJ. Intact flagellar motor of Borrelia burgdorferi revealed by cryo-electron tomography. evidence for stator ring curvature and rotor/C-ring assembly flexion. J Bacteriol. 2009;191:5026–5036. doi: 10.1128/JB.00340-09. Illustrates B. burgdorferi motors using high-throughput cryo-ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Motaleb MA, Pitzer JE, Sultan SZ, Liu J. A novel gene inactivation system reveals an altered periplasmic flagellar orientation in a Borrelia burgdorferi fliL mutant. J Bacteriol. 2011;193:3324–3331. doi: 10.1128/JB.00202-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sal MS, Li C, Motaleb MA, Shibata S, Aizawa S, Charon NW. Borrelia burgdorferi uniquely regulates its motility genes and has an intricate flagellar hook-basal body structure. J Bacteriol. 2008;190:1912–1921. doi: 10.1128/JB.01421-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ge Y, Charon NW. FlaA, a putative flagellar outer sheath protein, is not an immunodominant antigen associated with Lyme disease. Infect Immun. 1997;65:2992–2995. doi: 10.1128/iai.65.7.2992-2995.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Motaleb MA, Sal MS, Charon NW. The decrease in FlaA observed in a flaB mutant of Borrelia burgdorferi occurs posttranscriptionally. J Bacteriol. 2004;186:3703–3711. doi: 10.1128/JB.186.12.3703-3711.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Novak EA, Sultan SZ, Motaleb MA. The cyclic-di-GMP signaling pathway in the Lyme disease spirochete. Front Cell Infect Microbiol. 2014;4:56. doi: 10.3389/fcimb.2014.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pitzer JE, Sultan SZ, Hayakawa Y, Hobbs G, Miller MR, Motaleb MA. Analysis of the Borrelia burgdorferi cyclic-di-GMP binding protein PlzA reveals a role in motility and virulence. Infect Immun. 2011;79:1815–1825. doi: 10.1128/IAI.00075-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48*.Sultan SZ, Pitzer JE, Boquoi T, Hobbs G, Miller MR, Motaleb MA. Analysis of the HD-GYP domain cyclic-di-GMP phosphodiesterase reveals a role in motility and enzootic life cycle of Borrelia burgdorferi. Infect Immun. 2011;79:3273–3283. doi: 10.1128/IAI.05153-11. First study demonstrating that the HD-GYP type of cyclic-di-GMP phosphodiesterases contributes to B. burgdorferi motility. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sultan SZ, Pitzer JE, Miller MR, Motaleb MA. Analysis of a Borrelia burgdorferi phosphodiesterase demonstrates a role for cyclic-di-guanosine monophosphate in motility and virulence. Mol Microbiol. 2010;77:128–142. doi: 10.1111/j.1365-2958.2010.07191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aldridge P, Hughes KT. Regulation of flagellar assembly. Curr Opin Microbiol. 2002;5:160–165. doi: 10.1016/s1369-5274(02)00302-8. [DOI] [PubMed] [Google Scholar]
- 51*.Chevance FF, Hughes KT. Coordinating assembly of a bacterial macromolecular machine. Nat Rev Microbiol. 2008;6:455–465. doi: 10.1038/nrmicro1887. Reviews the cascade control of flagellar assembly in E. coli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52*.Sze CW, Morado DR, Liu J, Charon NW, Xu H, Li C. Carbon storage regulator A (CsrA(Bb)) is a repressor of Borrelia burgdorferi flagellin protein FlaB. Mol Microbiol. 2011;82:851–864. doi: 10.1111/j.1365-2958.2011.07853.x. First direct evidence that B. burgdorferi flagellar (FlaB) synthesis is controlled by CsrA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karna SLR, Sanjuan E, Esteve-Gassent MD, Miller CL, Maruskova M, Seshu J. CsrABb modulates levels of lipoproteins and key regulators of gene expression (RpoS and BosR) critical for pathogenic mechanisms of Borrelia burgdorferi. Infect Immun. 2011;79:732–744. doi: 10.1128/IAI.00882-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sanjuan E, Esteve-Gassent MD, Maruskova M, Seshu J. Overexpression of CsrA (BB0184) alters the morphology and antigen profiles of Borrelia burgdorferi. Infect Immun. 2009;77:5149–5162. doi: 10.1128/IAI.00673-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55*.Charon NW, Goldstein SF, Marko M, Hsieh C, Gebhardt LL, Motaleb MA, Wolgemuth CW, Limberger RJ, Rowe N. The flat ribbon configuration of the periplasmic flagella of Borrelia burgdorferi and its relationship to motility and morphology. J Bacteriol. 2009;191:600–607. doi: 10.1128/JB.01288-08. Cryo-electron tomography microscopy study demonstrates that periplasmic flagella form ribbon-like structure in B. burgdorferi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Motaleb MA, Sultan SZ, Miller MR, Li C, Charon NW. CheY3 of Borrelia burgdorferi is the key response regulator essential for chemotaxis and forms a long-lived phosphorylated intermediate. J Bacteriol. 2011;193:3332–3341. doi: 10.1128/JB.00362-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li C, Xu H, Zhang K, Liang FT. Inactivation of a putative flagellar motor switch protein FliG1 prevents Borrelia burgdorferi from swimming in highly viscous media and blocks its infectivity. Mol Microbiol. 2010;75:1563–1576. doi: 10.1111/j.1365-2958.2010.07078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Motaleb MA, Miller MR, Li C, Bakker RG, Goldstein SF, Silversmith RE, Bourret RB, Charon NW. CheX is a phosphorylated CheY phosphatase essential for Borrelia burgdorferi chemotaxis. J Bacteriol. 2005;187:7963–7969. doi: 10.1128/JB.187.23.7963-7969.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brisson D, Drecktrah D, Eggers CH, Samuels DS. Genetics of Borrelia burgdorferi. Annu Rev Genet. 2012;416:513–534. doi: 10.1146/annurev-genet-011112-112140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol. 2012;10:87–99. doi: 10.1038/nrmicro2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Groshong AM, Blevins JS. Insights into the biology of Borrelia burgdorferi gained through the application of molecular genetics. Adv Appl Microbiol. 2014;86:41–143. doi: 10.1016/B978-0-12-800262-9.00002-0. [DOI] [PubMed] [Google Scholar]
- 62*.Dunham-Ems SM, Caimano MJ, Pal U, Wolgemuth CW, Eggers CH, Balic A, Radolf JD. Live imaging reveals a biphasic mode of dissemination of Borrelia burgdorferi within ticks. J Clin Invest. 2009;119:3652–3665. doi: 10.1172/JCI39401. First direct evidence for a biphasic mode of B. burgdorferi dissemination within ticks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hovius JW, Van Dam AP, Fikrig E. Tick-host-pathogen interactions in Lyme borreliosis. Trends Parasitol. 2007;23:434–438. doi: 10.1016/j.pt.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 64.Coburn J, Leong J, Chaconas G. Illuminating the roles of the Borrelia burgdorferi adhesins. Trends Microbiol. 2013;21:372–379. doi: 10.1016/j.tim.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bockenstedt LK, Gonzalez D, Mao J, Li M, Belperron AA, Haberman A. What ticks do under your skin: two-photon intravital imaging of Ixodes scapularis feeding in the presence of the lyme disease spirochete. Yale J Biol Med. 2014;87:3–13. [PMC free article] [PubMed] [Google Scholar]
- 66.Sze CW, Zhang K, Kariu T, Pal U, Li C. Borrelia burgdorferi needs chemotaxis to establish infection in mammals and to accomplish its enzootic cycle. Infect Immun. 2012;80:2485–2492. doi: 10.1128/IAI.00145-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moriarty TJ, Norman MU, Colarusso P, Bankhead T, Kubes P, Chaconas G. Real-time high resolution 3D imaging of the Lyme disease spirochete adhering to and escaping from the vasculature of a living host. PLoS Pathog. 2008;4:e1000090. doi: 10.1371/journal.ppat.1000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Norman MU, Moriarty TJ, Dresser AR, Millen B, Kubes P, Chaconas G. Molecular mechanisms involved in vascular interactions of the Lyme disease pathogen in a living host. PLoS Pathog. 2008;4:e1000169. doi: 10.1371/journal.ppat.1000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moriarty TJ, Shi M, Lin YP, Ebady R, Zhou H, Odisho T, Hardy PO, Salman-Dilgimen A, Wu J, Weening EH, Skare JT, Kubes P, Leong J, Chaconas G. Vascular binding of a pathogen under shear force through mechanistically distinct sequential interactions with host macromolecules. Mol Microbiol. 2012;86:1116–1131. doi: 10.1111/mmi.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harman MW, Dunham-Ems SM, Caimano MJ, Belperron AA, Bockenstedt LK, Fu HC, Radolf JD, Wolgemuth CW. The heterogeneous motility of the Lyme disease spirochete in gelatin mimics dissemination through tissue. Proc Natl Acad Sci USA. 2012;109:3059–3064. doi: 10.1073/pnas.1114362109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dunham-Ems SM, Caimano MJ, Eggers CH, Radolf JD. Borrelia burgdorferi requires the alternative sigma factor RpoS for dissemination within the vector during tick-to-mammal transmission. PLoS Pathog. 2012;8:e1002532. doi: 10.1371/journal.ppat.1002532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shih CM, Chao LL, Yu CP. Chemotactic migration of the Lyme disease spirochete (Borrelia burgdorferi) to salivary gland extracts of vector ticks. Am J Trop Med Hyg. 2002;66:616–621. doi: 10.4269/ajtmh.2002.66.616. [DOI] [PubMed] [Google Scholar]
- 73.Bakker RG, Li C, Miller MR, Cunningham C, Charon NW. Identification of specific chemoattractants and genetic complementation of a Borrelia burgdorferi chemotaxis mutant. flow cytometry-based capillary tube chemotaxis assay. Appl Environ Microbiol. 2007;73:1180–1188. doi: 10.1128/AEM.01913-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Caimano MJ, Kenedy MR, Kairu T, Desrosiers DD, Harman M, Dunham-Ems S, Akins D, Pal U, Radolf JD. The hybrid sensory histidine kinase Hk1 (BB0420) of Borrelia burgdorferi is part of a two-component system essential for survival in feeding larval and nymphal Ixodes scapularis ticks. Infect Immun. 2011;79:3117–3130. doi: 10.1128/IAI.05136-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Caimano MJ, Dunham-Ems S, Allard AM, Cassera MB, Kenedy M, Radolf JD. Cyclic di-GMP Modulates Gene Expression in Lyme Disease Spirochetes at the Tick-Mammal Interface To Promote Spirochete Survival during the Blood Meal and Tick-to-Mammal Transmission. Infect Immun. 2015;83:3043–3060. doi: 10.1128/IAI.00315-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76*.Pal U, Li X, Wang T, Montgomery RR, Ramamoorthi N, Desilva AM, Bao F, Yang X, Pypaert M, Pradhan D, Kantor FS, Telford S, Anderson JF, Fikrig E. TROSPA, an Ixodes scapularis receptor for Borrelia burgdorferi. Cell. 2004;119:457–468. doi: 10.1016/j.cell.2004.10.027. Demonstrates that the TROPSA tick protein interacts with B. burgdorferi outer surface protein OspA and allows attachment to the tick midgut. [DOI] [PubMed] [Google Scholar]
- 77.Fikrig E, Narasimhan S. Borrelia burgdorferi--traveling incognito? Microbes Infect. 2006;8:1390–1399. doi: 10.1016/j.micinf.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 78.Sonenshine DE, Hynes WL. Molecular characterization and related aspects of the innate immune response in ticks. Front Biosci. 2008;13:7046–7063. doi: 10.2741/3209. [DOI] [PubMed] [Google Scholar]
- 79.Hajdusek O, Sima R, Ayllon N, Jalovecka M, Perner J, de la FJ, Kopacek P. Interaction of the tick immune system with transmitted pathogens. Front Cell Infect Microbiol. 2013;3:26. doi: 10.3389/fcimb.2013.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]