Abstract
Neuroinflammation plays a critical role in the regeneration of peripheral nerves following axotomy. An injury to the sciatic nerve leads to significant macrophage accumulation in the L5 DRG, an effect not seen when the dorsal root is injured. We recently demonstrated that this accumulation around axotomized cell bodies is necessary for a peripheral conditioning lesion response to occur. Here we asked whether overexpression of the monocyte chemokine CCL2 specifically in DRG neurons of uninjured mice is sufficient to cause macrophage accumulation and to enhance regeneration or whether other injury-derived signals are required. AAV5-EF1α-CCL2 was injected intrathecally, and this injection led to a time-dependent increase in CCL2 mRNA expression and macrophage accumulation in L5 DRG, with a maximal response at 3 wk post-injection. These changes led to a conditioning-like increase in neurite outgrowth in DRG explant and dissociated cell cultures. This increase in regeneration was dependent upon CCL2 acting through its primary receptor CCR2. When CCL2 was overexpressed in CCR2 −/− mice, macrophage accumulation and enhanced regeneration were not observed. To address the mechanism by which CCL2 overexpression enhances regeneration, we tested for elevated expression of regeneration-associated genes in these animals. Surprisingly, we found that CCL2 overexpression led to a selective increase in LIF mRNA and neuronal phosphorylated STAT3 (pSTAT3) in L5 DRGs, with no change in expression seen in other RAGs such as GAP-43. Blockade of STAT3 phosphorylation by each of two different inhibitors prevented the increase in neurite outgrowth. Thus, CCL2 overexpression is sufficient to induce macrophage accumulation in uninjured L5 DRGs and increase the regenerative capacity of DRG neurons via a STAT3-dependent mechanism.
Keywords: CCL2, MCP-1, DRG, Regeneration, Macrophage, Neuroinflammation, STAT3
Introduction
Injury to peripheral nerves activates an inflammatory response that plays a significant role in axonal regeneration and functional recovery (Bastien and Lacroix, 2014; DeFrancesco-Lisowitz et al., 2014; Popovich and Longbrake, 2008). In response to axotomy, both injured peripheral neuronal cell bodies and Schwann cells distal to the injury site upregulate and release the C-C class chemokine 2 (CCL2), also known as monocyte chemoattractant protein-1 (MCP-1; Schreiber et al., 2001; Subang and Richardson, 2001; Tanaka et al., 2004; Toews et al., 1998). As a result of the expression of this chemokine, monocytes extravasate into both peripheral ganglia and the nerve distal to the injury site, and macrophage accumulation in these areas remains elevated for several weeks (Lu and Richardson, 1993; Perry et al., 1987; Schreiber et al., 1995). Traditionally, investigation of this inflammatory process has focused on actions in the nerve at, or distal to, the site of injury. After axotomy, the distal nerve undergoes Wallerian degeneration, a process that includes fragmentation of the axon and myelin sheath and phagocytosis of debris by macrophages and Schwann cells (Rotshenker, 2011). Clearance of this debris is considered to be a prerequisite for regeneration, as blockade of macrophage accumulation yields incomplete degeneration and suboptimal regeneration and functional recovery (Barrette et al., 2008; Bisby and Chen, 1990; Brown et al., 1994; Brown et al., 1991; Dahlin, 1995; Dailey et al., 1998; Reichert et al., 1994).
It should be noted, however, that in these experiments macrophage accumulation in peripheral ganglia was also almost certainly blocked and this might account for some of the decreased regeneration (Niemi et al., 2013). In fact, recent evidence has shown that the inflammatory response near axotomized neuronal cell bodies is necessary for axonal regeneration following a peripheral nerve injury. Utilizing a global knockout for the C-C class chemokine receptor 2 (CCR2), the primary receptor for CCL2, we showed that if macrophage accumulation in the dorsal root ganglion (DRG) does not occur then axonal regeneration is significantly diminished (Niemi et al., 2013). A related study showed that intrathecal administration of minocycline, which blocked injury-induced ganglionic macrophage accumulation, also reduced regenerative capabilities of DRG neurons (Kwon et al., 2013).
Exogenous activation of inflammation near neuronal cell bodies has also been shown to increase the regenerative capacity of neurons. Injection of C. Parvum into the DRG, creating a local inflammatory response characterized by macrophage accumulation, significantly increased expression of growth-associated protein-43 (GAP-43) and calcitonin gene-related peptide (CGRP), two regeneration-associated genes (RAGs), and axonal regeneration after a dorsal root crush injury, though interestingly not after a sciatic nerve injury (Lu and Richardson, 1991, 1993, 1995).
The most widely studied example of inflammation-induced regeneration is in the optic nerve. Retinal ganglion cells (RGCs) are normally unable to regenerate their axons following optic nerve injury, but are able to do so after inducing an inflammatory reaction in the eye (Leon et al., 2000; Yin et al., 2003). Intraocular injection of zymosan leads to a dramatic increase in the expression of oncomodulin (OCM), a protein that has been proposed to play a key role in inflammation-induced regeneration in the optic nerve (Kurimoto et al., 2010; Yin et al., 2006). Other investigators have shown that lens injury-induced optic nerve is dependent upon the upregulation and actions of two gp130 cytokines, ciliary neurotrophic factor (CNTF) and leukemia inhibitory factor (LIF; Leibinger et al., 2009).
While exogenous activation of local inflammation near neuronal cell bodies is able to greatly increase the regenerative capacity of those neurons, the mechanism by which this occurs and the specific role macrophages play in this response are poorly understood. The inflammatory responses activated by zymosan, and other inflammatory agents, are complex and involve several immune cell types, including CD4-positive cells, neutrophils, and macrophages (Baldwin et al., 2015; Gantner et al., 2003; Kurimoto et al., 2013). We sought to determine if macrophage accumulation alone, without an axonal injury, could increase the regenerative capacity of DRG neurons. We addressed this question by overexpressing the monocyte chemokine CCL2 in uninjured lumbar DRGs and examined whether this singular change was sufficient to cause macrophage accumulation and increased axonal regeneration.
Materials and Methods
Generation and production of an adeno-associated virus (AAV5) CCL2 overexpression vector
Mouse cDNA encoding the open reading frame of CCL2 (Origene; Rockville, MD) was cloned into a pAAV shuttle vector containing the AAV inverted terminal repeats with an expression cassette composed of the human elongation factor-1 alpha (EF1α) promoter, a multiple cloning site, the woodchuck hepatitis virus post-transcriptional regulatory element (WPRE), and a polyadenylation signal. The CCL2 insert was generated by PCR using the following primers: Forward 5′ATA GTC GAC ATG CAG GTC CCT GTC ATG CTT CTG G 3′ and Reverse 5′ ATA AAG CTT CTA GTT CAC TGT CAC ACT GGT CAC TCC TA 3′. PCR products were digested by SalI and EcoRV and subcloned into the MCS of the pAAV shuttle vector to generate the pAAV-EF1α-CCL2 vector. Recombinant AAV5 vectors were generated by transient co-transfection of HEK-293T cells with 37.5 µg of total DNA containing 7.5 µg of pAAV-EF1α-CCL2, 7.5 µg of AAV5 rep-cap plasmid, and 22.5 µg of pAD helper plasmid using the calcium phosphate method. Seventy-two hours after transfection, cells were collected and lysed with 10% chloroform and AAV particles were purified using PEG8000 (Promega; Madison, WI) and benzonase nuclease (Life Technologies; Carlsbad, CA) and concentrated using an Ultra-4 centrifugal filter (EMD/Millipore; Billerica, MA). Titers were determined by quantitative PCR for viral genomic copies extracted from proteinase K-treated viral particles using the following primers: Forward: 5′ATA GTC GAC ATG CAG GTC CCT GTC ATG CTT CTG G 3′ and Reverse 5′ TTG TAG CTC TCC AGC CTA CTC ATT GG 3′. For the present experiments, the following vectors were produced: AAV5-EF1α-CCL2: 2.2×1013 GC/ml and AAV5-EF1α-eYFP: 1.52×1013 GC/ml.
Animals and Sciatic Nerve Transection Injury (SNI)
Eight to twelve-week-old male wild type (WT) mice [C57BL/6J (Jackson Laboratories, Bar Harbor ME)] and mutant mice [C57B6.129S4-Ccr2tm1Ifc/J (CCR2 −/−; Jackson Laboratories)] were utilized for this study. The animals were housed under a 12 h:12 h light:dark cycle with ad libitum access to food and water. SNI was performed on WT mice under isoflurane anesthesia. Briefly, the right sciatic nerve was exposed and transected proximal to the trifurcation and the wound closed with a wound clip. The left sciatic nerve was then exposed but not transected to serve as an internal control. One, two, three, or four weeks after intrathecal injection or 7 d after SNI, the animals were sacrificed by CO2 inhalation, and the lumbar-level DRGs, sciatic nerves, and spinal cords were removed for immunohistochemical or molecular biological analysis. All animal procedures were approved by the Case Western Reserve University’s Institutional Animal Care and Use Committee.
L5 laminectomy and intrathecal injection
The intrathecal injection method was modified from Parikh et al. (2011). Briefly, mice were anesthetized using isoflurane. A small incision was made in the back skin of the mouse and the spinal column was exposed. The site of viral injection was between lumbar levels L5 and L6, where a small laminectomy was performed to expose the dura. The injection was performed with a borosilicate glass capillary (WPI; Sarasota, FL) pulled to a fine point, attached by polyethylene tubing (Thermo Fisher Scientific; Waltham, MA) to a Hamilton syringe. The glass capillary remained at midline and was slowly inserted underneath the dura and further advanced in the subarachnoid space. Ten microliters of AAV5-EF1α-CCL2 or AAV5-EF1α- eYFP were slowly injected using 1% trypan blue added to the AAV particle solution to visualize the injection, and the capillary remained in place for 2 min after the injection. The paraspinal muscles and fascia were repositioned, and the incision closed with wound clips. One, two, three, or four weeks after injection mice were sacrificed and lumbar DRG were harvested for qPCR, IHC, or explant culture.
Immunohistochemistry (IHC)
Generally, lumbar DRGs from CCR2 −/− and WT mice were removed, and the ganglia were desheathed and fixed by immersion in 4% paraformaldehyde. The tissues were cryoprotected in 30% sucrose and embedded in Tissue-Tek O.C.T. compound (Electron Microscopy Sciences; Hatfield, PA). IHC was performed on 10 μm cryostat sections. For quantification of macrophages, a rat monoclonal antibody to CD11b (also known as Mac1, CR3, and integrin αM; 1:100; EMD/Millipore) or CD68 (1:200; AbD Serotec, Oxford, UK) was incubated with tissue sections overnight at 4°C. Given that the same effect was observed with CD11b and CD68, the cells labeled by either of these markers will be referred to as macrophages throughout the paper. For quantification of RAG proteins, a rabbit monoclonal antibody to pSTAT3 (Y705; 1:100; Cell Signaling, Danvers, MA), a rabbit polyclonal antibody to activating transcription factor 3 (ATF3; 1:200; Santa Cruz Biotechnology; Dallas, TX), or a rabbit polyclonal antibody to small proline-rich repeat protein 1a (SPRR1a), generously provided by Dr. Stephen Strittmatter and Dr. William Cafferty (Yale University), was incubated with tissue sections overnight at 4°C. For YFP detection, a rabbit antibody against GFP (1:500; Life Technologies) was incubated with tissue sections for 3 d at 4°C. After washing, the sections were incubated in Cy3 secondary antibody (1:400; Jackson ImmunoResearch Laboratories, Inc.; West Grove, PA) or AlexaFluor 488 secondary antibody (1:500; Life Technologies) for 1 h. In all experiments, sections not exposed to the primary antibody were included for each experimental group. Images were captured at 25x magnification using HCImage software (Hamamatsu Corporation; Bridgewater, NJ) and then quantified using MetaMorph software (Version 7.6.3.0, Molecular Devices; Downingtown, PA). Data are represented as the percentage of the imaged area that was positively immuno-labeled.
Real time PCR (RT-PCR)
The expression of CCL2 mRNA was analyzed by RT- PCR. One, two, three, or four weeks after intrathecal injection both L5 DRGs were removed and stored in RNAlater (Life Technologies) at 4°C. RNA was extracted from pairs of ganglia using the Ambion RNAqueous micro kit. Five samples were included for each time point. Total RNA was quantified and 400 ng were reverse transcribed using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems; Carlsbad, CA). RT-PCR was performed in an ABI Step-One Plus, using prevalidated TaqMan expression assays [CCL2, Mm00441242; glyceraldehyde 3-phosphate dehydrogenase (GAPDH), Mm99999915; ATF3, Mm00476032; GAP-43, Mm00500404; Jun, Mm00495062; Smad1, Mm00484723; Sox11, Mm01281943; galanin, Mm00439056; interleukin (IL)-6, Mm00446190; LIF, Mm00434762; iNos, Mm00440502; CD86, Mm00444543; CD16, Mm00438875; arginase 1, Mm00475988; CD206, Mm00485148; Fizz1, Mm00445109; Ym1, Mm00657889; Applied Biosystems], and samples were assayed in triplicate. Relative expression was determined using the Comparative Ct Model (ΔΔCt) with GAPDH as the housekeeping gene.
DRG explants
To assess the outgrowth of peripheral neurons in response to injury, we evaluated neurite outgrowth in explanted ganglia (Shoemaker et al., 2005). Three weeks after intrathecal injection of the virus, L5 DRGs from uninjured CCR2 −/− and WT mice were removed, desheathed, placed on coverslips, and overlaid with 7.5 μl Matrigel (Becton Dickinson, Franklin Lakes, NJ). Culture plates were placed in an incubator at 37°C for 5 min to allow gelling of the Matrigel before adding 1 ml F12 medium with the additives described in Hyatt Sachs et al. (2010). Phase-contrast images of neurite outgrowth from each DRG were captured at 24 and 48 h after explantation using an Axiovert 405 M microscope at 10x magnification. Neurite outgrowth was assessed using MetaMorph software by measuring the distance between the edge of the ganglion and the leading tip of the longest 20 processes in each explant. The average length of the 20 longest neurites is taken for each ganglion. At 48 h, explants were fixed and labeled with an antibody against βIII tubulin (1:500; Promega) to visualize the outgrowth. Representative images were taken at 10×. In some experiments, 200 ng/ml of recombinant mouse CCL2 (R&D Systems; Minneapolis, MN) was added to the culture medium at the time of plating.
DRG dissociated cell culture
To assess neurite outgrowth from isolated sensory neurons, we dissociated and cultured DRG neurons from uninjured WT mice or mice injected with AAV5-CCL2 or AAV5-YFP (Sachs et al., 2007). L5 DRGs were removed, cleaned and desheathed. Except where noted all reagents used in the DRG dissociations and culture were from Sigma-Aldrich (St. Louis, MO). Ganglia were incubated in 0.125% collagenase A at 37°C for 1.5 h. The cells were then dissociated by gentle trituration using a P200 pipet in Neurobasal A medium containing 2% B-27 serum free supplement, 2 mM glutamine (both from Life Technologies), 10 U/ml penicillin, and 10 μg/ml streptomycin. The dissociated cells were purified following the procedure of Gavazzi et al. (1999), by centrifugation through 15% BSA at 600 rpm for 6 min. Neurons were resuspended in Neurobasal A containing 50 μg/ml DNase (Type I) and centrifuged at 1000 rpm for 2 min. Supernatants were removed, and cells were resuspended in Neurobasal A. Cells were gently plated (1/2 DRG/coverslip) onto 12 mm coverslips coated with 0.01% poly-L-lysine and 10 μg/ml laminin in a 12-well culture plate. Cells were allowed to adhere undisturbed for 20 min. Each coverslip was then overlaid with 1 ml of Neurobasal A and cultured for 24 h at 37°C in 95% air/5% CO2. In some experiments, 200 ng/ml of recombinant mouse CCL2 (R&D Systems) was added to the culture medium at the time of plating.
Inhibitors of signal transducer and activator of transcription 3 (STAT3) phosphorylation: AG490 and STATTIC
Two inhibitors of STAT3 phosphorylation were utilized in DRG dissociated cell culture to assess the role STAT3 plays in CCL2 overexpression-induced neurite outgrowth. AG490 (50 μM; EMD Millipore; Meydan et al., 1996), a Janus kinase (JAK) inhibitor, and STATTIC (10 μM; EMD Millipore; Schust et al., 2006), a STAT3 SH2 domain binding inhibitor, were used. L5 DRGs from mice 3 wk after intrathecal injection with AAV5-YFP or AAV5-CCL2 were removed, dissociated and cultured as described above. At the time of plating, cells were cultured in Neurobasal A medium containing AG490 (50 μM), STATTIC (10 μM), or DMSO (0.17 μl/ml) for 24 h.
Analysis of neurite outgrowth in cultured neurons
Cells were fixed in 4% paraformaldehyde for 20 min at room temperature, washed in PBS, and labeled with a mouse monoclonal antibody to βIII tubulin (1:900 for DRG; Promega), followed by a 45 min incubation in an AlexaFluor 488 labeled secondary antibody (1:400, Life Technologies, Grand Island). Coverslips were placed onto slides with FluoroGel (Electron Microscopy Sciences). Fourteen-bit images were collected on a Leica DMI 6000 B inverted microscope (Leica Microsystems; Wetzlar, Germany) using a Retiga Aqua blue camera (Q-imaging; Vancouver, British Columbia). Briefly, the entire coverslip was imaged at 10x and all resulting images were stitched together using the scan slide function in MetaMorph Imaging Software (Molecular Devices) to generate a full resolution composite image. The composite image was then subjected to neurite outgrowth analysis using the neurite outgrowth module in MetaMorph software. The longest neurite from each βIII tubulin-positive neuron with a process of at least 1.5 times the diameter of the cell body was measured.
Analysis of pixel intensity of pSTAT3 in cultured neurons
Cells were fixed in 4% paraformaldehyde for 20 min at room temperature, washed in PBS, and labeled with a mouse monoclonal antibody to βIII tubulin (1:900; Promega) and a polyclonal antibody against pSTAT3 (Y705; 1:80; Cell Signaling), followed by a 1 h incubation in an AlexaFluor 488 labeled secondary antibody (1:400, Life Technologies) to visualize βIII tubulin and an AlexaFluor 647 labeled secondary antibody (1:200; Jackson Immuno) to visualize pSTAT3. Coverslips were placed onto slides with FluoroGel (Electron Microscopy Sciences) and imaged as described above. The longest neurite from each βIII tubulin-positive neuron with a process of at least 1.5 times the diameter of the cell body was measured using the MetaMorph software. Data for neurite outgrowth was then expressed as the average length of the longest neurite for each group. To assess the average pixel intensity of pSTAT3 in culture, the nucleus of each neuron was outlined and the average pixel intensity within the nucleus was measured using the MetaMorph software. The same neurons were measured for both neurite outgrowth and pSTAT3 nuclear pixel intensity.
Statistics
Data are expressed as the means + S.E.M. and were analyzed by one-way or two-way ANOVA followed by Tukey’s post-hoc test. P values less than 0.05 were considered statistically significant.
Results
Intrathecal injection of AAV5-CCL2 results in a time-dependent overexpression of CCL2 mRNA in uninjured L5 DRGs
To overexpress the chemokine CCL2 in uninjured DRGs, we constructed an AAV5 vector encoding for CCL2 driven by the EF1α promoter (AAV5-CCL2; Fig. 1A). Intrathecal delivery of various AAV serotypes has been shown to allow for infection of DRG neurons (Iwamoto et al., 2009; Parikh et al., 2011; Wang et al., 2005), and a screen of AAV serotypes revealed AAV5 as the most efficient for infection of DRG neurons (Mason et al., 2010). To test the efficiency and localization of intrathecal delivery of AAV5, we first injected an AAV5 vector expressing eYFP driven by the EF1α promoter (AAV5-YFP; Fig. 1B–E′). Three weeks after injection of 10 μl of high-titer virus, lumbar DRGs, lumbar spinal cord, and sciatic nerves were removed and immuno-labeled with an antibody against YFP to assess infection efficiency and localization. Lumbar DRGs showed significant YFP labeling of neuronal cell bodies with minor localization to axons (Fig. 1B–C′), resulting in a DRG neuron labeling efficiency of 37.2% ± 5.7%. Only sparse axonal YFP labeling was found in the sciatic nerve (Fig. 1D). AAV5-YFP also resulted in significant labeling of neurons in the lumbar spinal cord (Fig. 1E), with some specificity to neurons and processes in the dorsal horn (Fig. 1E′).
Fig. 1. Intrathecal injection of AAV5 infects lumbar DRG neurons and leads to a time-dependent overexpression of CCL2.

Schematic of AAV5-EF1α-CCL2 vector. We used AAV5-EF1α-eYFP as a control (A). Representative images of AAV5-YFP localization in L4 (B) and L5 DRG (C) at 10×, visualized by anti-YFP labeling. High magnification image of YFP labeling in L5 DRG at 25× (C′). AAV5-YFP injection led to a 37.2% ± 5.7% DRG neuron infection efficiency. Representative image of sparse YFP axonal labeling in the sciatic nerve (D). A representative image of YFP localization in the lumbar spinal cord at low magnification (E) and high magnification (E′) shows preferential expression in the dorsal horn. Injection of AAV5-CCL2 resulted in a time-dependent overexpression of CCL2 mRNA in L5 DRG quantified using the ΔΔCt method normalized to GAPDH (F). For images: Scale bar=100 μm. For RT-PCR: n=5 per group. *p < 0.05. **p < 0.001.
Intrathecal administration of AAV5-CCL2 resulted in a time-dependent overexpression of CCL2 mRNA in L5 DRGs (Fig. 1F). By 1 wk after delivery, CCL2 mRNA showed a 2.9-fold increase and by 3 wk there was a 9.1-fold overexpression compared to AAV5-YFP controls. This level of overexpression is comparable to the injury-induced increase in CCL2 mRNA in the L5 DRG seen at 1 d after sciatic nerve transection (Niemi et al., 2013). The intrathecal delivery method was chosen specifically because it allows for significant CCL2 overexpression in DRGs, while leaving the ganglia uninjured.
CCL2 overexpression leads to increased CD11b-positive macrophage accumulation
Immuno-labeling with CD11b, a common marker employed for detecting macrophages, was used to assess if CCL2 overexpression was sufficient to increase macrophage accumulation within L5 DRGs. The immune response to injury, and specifically macrophage accumulation near injured neuronal cell bodies, has been shown to be an important mediator of regenerative capacity (Kwon et al., 2013; Lu and Richardson, 1991; Niemi et al., 2013). A time-dependent increase in CD11b-positive macrophages was seen after injection of AAV5-CCL2 compared to AAV5-YFP controls (Fig. 2A–E). AAV5-YFP administration did not alter CD11b-positive macrophage accumulation compared to uninjured WT L5 DRGs (data not shown). Two weeks after viral delivery, there was a 3-fold increase in macrophage accumulation which increased to 4-fold by 3 wk in AAV5-CCL2 mice compared to AAV5-YFP controls (Fig. 2A). This increase in CD11b-positive macrophages following CCL2 overexpression is similar to the injury-induced increase seen in WT mice at 7 d (Niemi et al., 2013). We also utilized a second macrophage marker, CD68, and saw a time-dependent increase in CD68 labeling compared to YFP controls (Fig. 2F–J). CD68 showed considerably more staining than CD11b, which could indicate that some of the CD68-positive macrophages are CD11b-negative.
Fig. 2. CCL2 overexpression causes a time-dependent increase in CD11b- and CD68-positive macrophage accumulation in L5 DRGs.
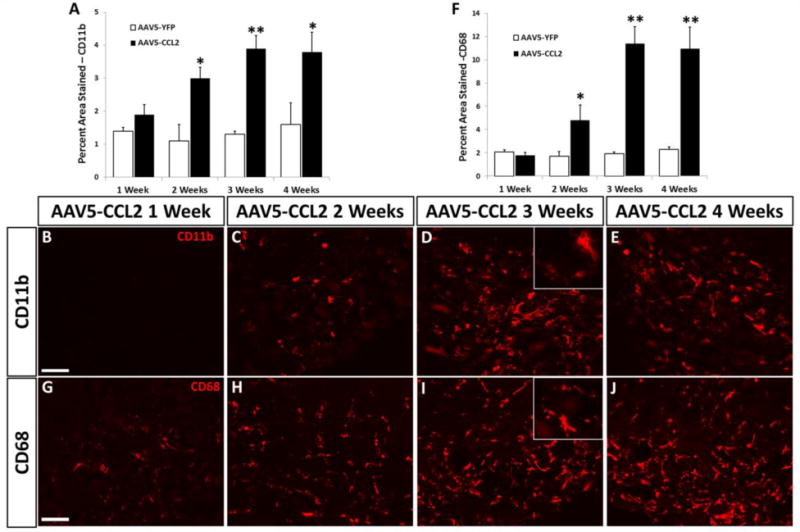
Immunohistochemical labeling of DRG sections with an antibody against CD11b (A) or CD68 (F) shows significant increases at 2, 3, and 4 wk after injection of AAV5-CCL2 compared to AAV5-YFP (A,F). Data were quantified as a percentage of the tissue area positively immuno-labeled. Representative images of CD11b staining in L5 DRG 1, 2, 3, and 4 wk after intrathecal injection of AAV5-CCL2 (B–E). Representative images of CD68 staining in L5 DRG 1, 2, 3, and 4 wk after intrathecal injection of AAV5-CCL2 (G–J). Inset images taken at 63×. Scale bar=100 μm. n=5 per group. *p < 0.05. **p < 0.001.
Peripheral nerve injury has been shown to induce monocyte entry into ganglia through CCL2/CCR2 signaling (Niemi et al., 2013). The present data show that CCL2 overexpression is sufficient to cause macrophage accumulation within the L5 DRG even in the absence of nerve injury.
CCL2 overexpression and macrophage accumulation cause a conditioning-like increase in neurite outgrowth in vitro
To test if CCL2 overexpression and the subsequent increase in macrophage accumulation in the absence of injury enhance the regenerative capacity of DRG neurons, L5 DRGs from AAV5-CCL2 and AAV5-YFP mice were placed in explant culture 3 wk after intrathecal injection. After 48 h in culture, DRG explants overexpressing CCL2 showed significantly longer neurite outgrowth than DRGs from mice receiving the control virus (Fig. 3A–C). We also measured regeneration in DRG dissociated cell cultures. We have found that dissociation of the DRG does not completely eliminate glial cells from the culture (data not shown); however, it does disrupt the proximity of non-neuronal cells and neurons. After 24 h in culture, DRG neurons overexpressing CCL2 grew significantly longer neurites than neurons from mice receiving the control virus (Fig. 3D–F). To examine whether the increased regenerative responses observed from both explant and dissociated cell cultures might be due to a direct effect of CCL2 acting on the DRG neurons at the time of regeneration, recombinant mouse CCL2 protein (200 ng/ml; Bianchi et al., 2005) was added to DRG explant cultures from WT mice. Exogenous CCL2 addition to culture medium did not increase the length of axon regeneration after 48 h in culture when compared to controls (Fig. 3G–I). The lack of an effect of exogenous CCL2 on neurite outgrowth could be due to inefficient penetration of the protein into the explants. To allow for better exposure of DRG neurons to CCL2, exogenous CCL2 was added to DRG dissociated cell cultures. However, exogenous CCL2 addition still had no effect on neurite outgrowth after 24 h in culture (Fig. 3J–L). This suggests that CCL2 does not act directly on neurons to induce increased regeneration. Thus, CCL2 exerts a conditioning-like effect to increase the regenerative capacity of neurons in vivo, prior to placing them in culture.
Fig. 3. A conditioning-like increase in neurite outgrowth from DRG neurons is observed with in vivo CCL2 overexpression but not after exogenous CCL2 addition at the time of culturing.

Quantification of neurite outgrowth in explant culture measured after 24 and 48 h, 3 wk after intrathecal injection of AAV5-CCL2 or AAV5-YFP (A). Data is represented as the mean length, in microns, of the 20 longest neurites. Representative images of neurite outgrowth after 48 h in explant culture, visualized with βIII tubulin, for AAV5-YFP (B) and AAV5-CCL2 (C). Quantification of neurite outgrowth in dissociated cell culture measured at 24 h, 3 wk after intrathecal injection of AAV5-CCL2 or AAV5-YFP (D). Representative images of neurite outgrowth in dissociated cell culture at 24 h for AAV5-YFP (E) and AAV5-CCL2 (F). Quantification of neurite outgrowth in WT DRG explant culture measured after 24 and 48 h, with or without exogenous CCL2 (200 ng/mL) added to the culture medium at the time of plating (G). Representative images of neurite outgrowth after 48 h in culture for control (H) and exogenous CCL2 (I). Quantification of neurite outgrowth in WT DRG dissociated cell culture at 24 h, with or without exogenous CCL2 (200 ng/mL) added to the culture medium at the time of plating (J). Representative images of neurite outgrowth in dissociated cell culture for control (K) and exogenous CCL2 (L). Scale bar=100 μm. For CCL2 overexpression explant cultures: n=4 per group. *p < 0.05. For CCL2 overexpression dissociated cell cultures: n=180-251 neurons per group. **p < 0.001. For exogenous CCL2 explant cultures: n=6 per group. For exogenous CCL2 dissociated cell cultures: n=253–397 neurons per group.
Macrophages have been shown to increase the regenerative response of DRG neurons through releasable factors (Kigerl et al., 2009); however, this ability was dependent upon the activation state of the macrophages. Macrophages stimulated to an anti-inflammatory state were growth-promoting, and pro-inflammatory macrophages were growth-inhibiting (Gordon and Taylor, 2005; Kigerl et al., 2009). Given, the increased regeneration we see with CCL2 overexpression, we sought to determine if macrophages in our system expressed an activation state which would be expected to support regeneration. We assayed the mRNA expression of a subset of anti-inflammatory (arginase 1, CD206, Fizz 1, Ym 1) and pro-inflammatory (iNos, CD86, CD16) macrophage markers in L5 DRG (Fig. 4A; Gordon and Taylor, 2005; Martinez and Gordon, 2014). Expression of the anti-inflammatory markers CD206 and Fizz 1 significantly increased, 3.1 and 11.2-fold, respectively, 3 wk after injection of AAV5-CCL2 compared to AAV5-YFP. mRNA levels for the pro-inflammatory macrophage markers did not change with CCL2 overexpression. This profile of anti-inflammatory macrophage marker expression is similar, though not identical, to the expression observed in WT L5 DRGs 7 d after SNI (Fig. 4B).
Fig. 4. Two anti-inflammatory macrophage markers and no pro-inflammatory markers are significantly increased in uninjured L5 DRGs 3 wk after intrathecal injection of AAV5-CCL2.
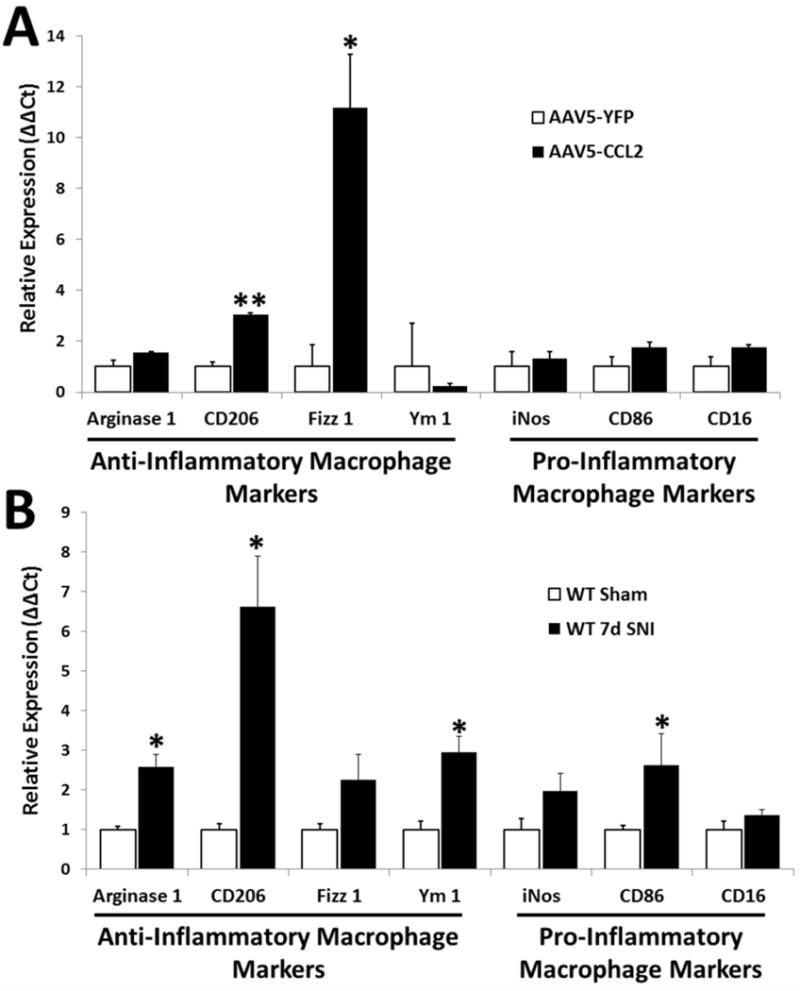
Relative expression of mRNA for 4 anti-inflammatory [arginase 1, CD206, Fizz1, and Ym1] and 3 pro-inflammatory [iNos, CD86, CD16] macrophage markers in L5 DRGs 3 wk after injection of AAV5-CCL2 or AAV5-YFP (A) or 7 d after SNI in WT mice compared to sham controls (B). Data was quantified using the ΔΔCt method normalized to GAPDH. n=5 per group. *p < 0.05. **p < 0.001.
CCL2 acts through CCR2 to elicit changes in macrophage accumulation and regeneration
To determine if the changes mediated by CCL2 overexpression require the chemokine receptor CCR2, AAV5-CCL2 was delivered to CCR2 −/− mice (Boring et al., 1998). CCR2 is localized to a subpopulation of monocytes (Geissmann et al., 2003; Taylor and Gordon, 2003) and is necessary for their chemotactic responses to CCL2. CCR2 also plays an important role in monocyte exit from the bone marrow (Han et al., 1998; Serbina and Pamer, 2006). Three weeks after intrathecal injection of AAV5-CCL2, a significant increase in CCL2 mRNA (>7 fold) was found in CCR2 −/− L5 DRGs compared to that after injection with AAV5-YFP (Fig. 5A). However, CCL2 overexpression did not yield a significant increase in CD11b-positive macrophages in the L5 DRG 3 wk after virus administration in CCR2 −/− mice (Fig. 5B). Furthermore, overexpression of CCL2 in CCR2 −/− mice did not result in increased axonal regeneration, measured at 24 and 48 h in DRG explant culture 3 wk after intrathecal injection (Fig. 5C). These data show that CCL2 must act through its primary receptor CCR2 to elicit increases in macrophage accumulation and regeneration in the DRG.
Fig. 5. Overexpression of CCL2 in CCR2 −/− mice does not cause macrophage accumulation or increased neurite outgrowth in L5 DRGs.

Expression of CCL2 mRNA measured 3 wk after intrathecal injection of AAV5-CCL2 or AAV5-YFP in CCR2 −/− mice, quantified using the ΔΔCt method normalizing to GAPDH (A). Immunohistochemical labeling of DRG sections with an antibody against CD11b shows no difference between AAV5-CCL2 and AAV5-YFP 3 wk after injection (B). Data was quantified as a percentage of the imaged area positively immuno-labeled. Quantification of neurite outgrowth in explant culture measured at 24 and 48 h, 3 wk after intrathecal injection of AAV5-CCL2 or AAV5-YFP in CCR2 −/− mice (C). Data are represented as the mean length, in microns of the 20 longest neurites. For RT-PCR: n=5 per group. **p < 0.001. For IHC: n=5 per group. For explant cultures: n=5 per group.
CCL2 overexpression leads to a selective increase in LIF mRNA and activation of STAT3
To begin to ascertain the mechanism by which CCL2 overexpression in DRG neurons could lead to a conditioning-like increase in neurite outgrowth, the expression of various RAGs were screened in L5 DRGs 3 wk after intrathecal injection of AAV5-CCL2 or AAV5-YFP. The mRNA expression of LIF, IL-6, GAP-43, JUN, activating transcription factor 3 (ATF3), galanin, Smad1, and Sox11 were assayed using RT-PCR and quantified using the comparative Ct method with GAPDH as the housekeeping gene. CCL2 overexpression resulted in a significant increase in LIF expression (4-fold) compared to YFP controls (Fig. 6A). None of the other mRNAs showed a significant change in ganglia overexpressing CCL2. This is in contrast to the RAG expression profile observed 7 d after SNI where all of the RAGs measured, with the exception of LIF, show significant upregulation (Fig. 6B).
Fig. 6. LIF mRNA and pSTAT3 are significantly increased in uninjured L5 DRGs 3 wk after intrathecal injection of AAV5-CCL2.

Relative expression of various RAGs in L5 DRGs 3 wk after intrathecal virus infection of uninjured DRGs quantified using the ΔΔCt method normalizing to GAPDH (A). Relative expression of various RAGs in L5 DRGs 7 d after SNI in WT mice compared to sham controls (B). Representative images of pSTAT3 staining in L5 DRGs 3 wk after injection of AAV5-YFP (C), AAV5-CCL2 with inset image taken at 63× (D), AAV5-CCL2 in CCR2 −/− mice (E), or 7 d after SNI in WT mice (F). Quantification of pSTAT3 staining represented as the percentage of the imaged area positively immuno-labeled (G). Representative images of ATF3 staining in L5 DRGs 3 wk after injection of AAV5-YFP (H), AAV5-CCL2 (I), AAV5-CCL2 in CCR2 −/− mice (J), or 7 d after SNI in WT mice (K). Quantification of ATF3 staining (L). Representative images of SPRR1a staining in L5 DRGs 3 wk after injection of AAV5-YFP (M), AAV5-CCL2 (N), AAV5-CCL2 in CCR2 −/− mice (O), or 7 d after SNI in WT mice (P). Quantification of SPRR1a staining (Q).For RT-PCR: *p < 0.05. **p < 0.001. n=5 per group. For IHC: Scale bar=100 μm. n=8 per group. *p < 0.05. **p < 0.001.
LIF signals through a heterodimeric receptor containing the signaling subunit gp130, and activates the transcription factor STAT3 by inducing its phosphorylation and nuclear translocation (Symes et al., 1994). Therefore, we next tested whether STAT3 phosphorylation was increased in DRG neurons following CCL2 overexpression. Three weeks after intrathecal injection of virus, a 3-fold increase in pSTAT3-positive labeling was seen in AAV5-CCL2 L5 DRGs compared to AAV5-YFP controls and CCR2 −/− mice overexpressing CCL2 (Fig. 6C–E,G). This increased activation of STAT3 in uninjured DRGs following CCL2 overexpression was nevertheless significantly lower than the activation of STAT3 7 d after sciatic nerve transection in WT mice (Fig. 6F,G). ATF3 and SPRR1a, two other well-known RAGs (Bonilla et al., 2002; Seijffers et al., 2007), were also assayed by IHC. Neither ATF3 (Fig. 6H–J,L) nor SPRR1a (Fig. 6M–O,Q) was upregulated following CCL2 overexpression, though both were upregulated 7 d after sciatic nerve transection (Fig. 6K,L,P,Q). Thus, the CCL2 overexpression-induced increase in axonal regeneration may result from activation of STAT3.
pSTAT3 is necessary for the conditioning-like increase in neurite outgrowth observed with CCL2 overexpression
To test whether the CCL2 overexpression-induced increase in neurite outgrowth is dependent upon STAT3 activation, we treated DRG cultured neurons with STAT3 phosphorylation inhibitors. We utilized the Janus kinase (JAK) inhibitor AG490 (Meydan et al., 1996) and the SH2-binding inhibitor STATTIC (Schust et al., 2006). Previous literature has shown that conditioning lesion-induced neurite outgrowth can be abolished through inhibition of STAT3 activation with AG490 in culture (Liu and Snider, 2001) or in vivo (Qiu et al., 2005).
After 24 h in culture, DRG neurons overexpressing CCL2 grew significantly longer neurites and showed significantly higher intensity nuclear pSTAT3 labeling than neurons from mice receiving the control virus (Fig. 7A–H). The addition of AG490 or STATTIC to the culture medium at the time of plating significantly reduced the CCL2 overexpression-induced neurite outgrowth compared to vehicle-treated CCL2 overexpressing neurons (Fig 7A). This reduction in neurite outgrowth, similiar to AAV5-YFP levels, coincided with a significant reduction in the pSTAT3 labeling intensity following treatment with AG490 or STATTIC (Fig. 7B). These data suggest that the CCL2 overexpression induced increase in neurite outgrowth is dependent upon the activation of pSTAT3.
Fig. 7. pSTAT3 is required for the CCL2 overexpression-induced increase in neurite outgrowth.

Quantification of neurite outgrowth in DRG dissociated cell culture in the presence of inhibitors of STAT3 phosphorylation, AG490 (50 nM) or STATTIC (10 nM), or vehicle control, measured after 24 h, 3 wk after intrathecal injection of AAV5-CCL2 or AAV5-YFP (A). Quantification of pSTAT3 nuclear pixel intensity in dissociated cell culture in the presence of the STAT3 phosphorylation inhibitors, AG490 or STATTIC, or vehicle control, measured after 24 h, 3 wk after intrathecal injection of AAV5-CCL2 or AAV5-YFP (B). Representative images of neurite outgrowth and pSTAT3 are shown for AAV5-YFP Vehicle (C–E), AAV5-CCL2 Vehicle (F–H), AAV5-YFP AG490 (I–K), AAV5-CCL2 AG490 (L–N), AAV5-YFP STATTIC (O-Q), and AAV5-CCL2 STATTIC (R–T). Insets are digitally magnifications of the neuronal cell body. Scale bar =100 μm. For neurite outgrowth: n=51–99 neurons per group. **p < 0.001. For pixel intensity: n=51–99 neurons per group. *p < 0.05.
Discussion
In the present study, we have demonstrated that overexpression of CCL2 in uninjured DRG neurons is sufficient to cause macrophage accumulation in the ganglion. We further showed that this overexpression alone results in a conditioning-like increase in neurite outgrowth, increased LIF mRNA, and activation of STAT3 in DRG neurons.
Sciatic nerve injury produces dramatic changes in DRG neurons and in non-neuronal cell populations both distal to the site of injury and in the ganglion (Bastien and Lacroix, 2014; Lieberman, 1971). These include activation and proliferation of satellite glial cells (Hanani et al., 2002), dedifferentiation and proliferation of Schwann cells distal to the injury site (Napoli et al., 2012), and macrophage accumulation distal to the site of injury and in the DRG (Lu and Richardson, 1993; Perry and Brown, 1992). In addition, the expression of hundreds of genes is altered (Costigan et al., 2002; Nagarajan et al., 2002). Therefore, the fact that overexpression of one gene, CCL2, is sufficient to increase the regenerative capacity of DRG neurons is remarkable.
Macrophage stimulation of axonal regeneration
Studies on the immune consequences of nerve injury have focused on actions at or distal to the injury site, in particular the role of macrophages in Wallerian degeneration (for reviews see Bruck, 1997; DeFrancesco-Lisowitz et al., 2014). The actions of multiple immune cells in the distal nerve also may have bearing on the process of regeneration (e.g., Brown et al., 1991; Schmid et al., 2013; Vargas et al., 2010). For example, inhibition of macrophage accumulation in the distal nerve following injury showed significant reductions in myelin clearance and significant delays in functional recovery of hind paw motor control (Barrette et al., 2008). Whether these two effects are causally related is not clear, however, because of the likelihood that in these experiments there were also decreases in macrophage accumulation in the DRG.
While macrophage accumulation in peripheral ganglia following axotomy was described in the 1990s (Lu and Richardson, 1993; Schreiber et al., 1995), their functions have not been clear. Injection of peritoneal macrophages or bacteria (C. parvum) into the DRG to induce a local inflammatory response resulted in increases in RAG expression and increased regeneration after a dorsal root injury though not after sciatic nerve transection (Lu and Richardson, 1993, 1995). Recent evidence has shown that inhibition of the injury-induced macrophage accumulation in lumbar DRGs results in the abolishment of the conditioning-lesion response both in vitro and in vivo (Kwon et al., 2013; Niemi et al., 2013).
What remains unclear is how macrophages influence the regenerative capacity of peripheral neurons. Neuroinflammation is often referred to as a “double-edged sword” as illustrated by the divergent role macrophages play in two CNS injury models. Depletion of hematogeneous macrophages leads to improved functional recovery after spinal cord injury (Popovich et al., 1999). However, stimulation of an immune response in the eye in conjunction with optic nerve injury significantly increases regeneration of retinal ganglion cell axons, though the involvement of both neutrophils and macrophages has been implicated (Kurimoto et al., 2013; Yin et al., 2003; Yin et al., 2006). These differing effects presumably result from the heterogeneity of macrophage activation following nervous system injury. Upon entry into tissue, monocyte-derived macrophages can take on a spectrum of activation states (for review see Gordon and Taylor, 2005; Martinez and Gordon, 2014). Representing the polar opposites on this spectrum, macrophage activation states have been described as pro-inflammatory/M1 or anti-inflammatory/M2 (Gordon and Taylor, 2005; Martinez and Gordon, 2014). Work by Kigerl et al. (2009) demonstrated that conditioned media from bone marrow-derived macrophages stimulated to an anti-inflammatory/M2 activation state with IL-4, induced significant increases in neurite outgrowth from DRG neurons on both growth-permissive and growth-inhibiting substrates. The identity of the factor(s) released from these M2 macrophages to stimulate axonal regeneration remains unknown. Given the increases in regeneration observed as a result of CCL2 overexpression, we hypothesized that the macrophages in our system are anti-inflammatory in nature. The upregulation of CD206 and Fizz 1 mRNA, two anti-inflammatory macrophage markers, and the lack of change in three pro-inflammatory markers are consistent with this hypothesis. CD206, a mannose receptor, is a useful marker of anti-inflammatory/M2 polarization as pro-inflammatory/M1 macrophages do not express this receptor (Alan et al., 1984). Fizz-1, a cysteine-rich secreted protein, has been implicated in the regulation of extracellular matrix and in wound healing (Holcomb et al., 2000), and is specifically expressed by macrophages in response to IL-4 both in vivo and in vitro (Raes et al., 2002).
Activation of a regeneration-associated signaling pathway
In a search for the mechanism by which CCL2 overexpression leads to a conditioning-like increase in neurite outgrowth, we assayed the expression of various RAGs, expecting to observe an increase in a number of mRNAs. Surprisingly of the eight RAGs we examined, we found that only LIF was significantly increased 3 wk after intrathecal delivery of AAV5-CCL2. In addition, the main signaling mechanism by which LIF acts, namely the phosphorylation of STAT3, increased as a result of CCL2 overexpression. This specificity in CCL2 overexpression-mediated RAG expression suggests that LIF and STAT3 may be responsible for the increase in axonal regeneration. LIF has been implicated previously in conditioning lesion-induced sensory and sympathetic neuron regeneration (Cafferty et al., 2001; Hyatt Sachs et al., 2010).
Following sciatic nerve injury, LIF is expressed by Schwann cells in the nerve and is retrogradely transported to the DRG (Banner and Patterson, 1994; Curtis et al., 1994; Thompson et al., 1997). In addition, LIF expression increases in non-neuronal cells in the axotomized ganglia (i.e., superior cervical ganglion), probably in satellite cells (Banner and Patterson, 1994; Sun et al., 1994). In our overexpression system, the cell expressing LIF has yet to be identified. While the 4-fold increase in LIF mRNA seen in DRGs following CCL2 overexpression is impressive, LIF expression in ganglia following axotomy reaches increases of >100-fold (Banner and Patterson, 1994; Habecker et al., 2009; Sun et al., 1996).
Axotomy induces the expression of both IL-6 and LIF in peripheral ganglia (Cafferty et al., 2004; Cafferty et al., 2001; Habecker et al., 2009; Sun et al., 1994). Thus, it was unexpected to see only LIF, and not also IL-6, mRNA increase with CCL2 overexpression. We also assayed the expression of two known gp130 cytokine/STAT3-dependent genes, galanin and GAP-43 (Ogai et al., 2014; Qiu et al., 2005; Rao et al., 1993; Zigmond, 2012) and found that their expression remained unchanged following CCL2 overexpression. Although initially surprising, this may indicate that LIF-induced gene expression requires more than a 4-fold increase in the cytokine. Another common RAG, ATF3, also remained unchanged following CCL2 overexpression. While ATF3 has been shown to play an important role in axon regeneration (Seijffers et al., 2006; Seijffers et al., 2007), its expression is not known to be gp130-dependent (Habecker et al., 2009).
Activation of the gp130 receptor subunit by LIF and other IL-6 family cytokines results in the activation of JAK2 and the phosphorylation of STAT3 (Heinrich et al., 1998). STAT3 activation has been shown to play a major role in axon regeneration following injury. STAT3 is activated in DRG neurons after a sciatic nerve injury, but not after a dorsal column lesion (Qiu et al., 2005). Inhibition of STAT3 phosphorylation, through administration of the JAK inhibitor AG490, results in significantly diminished DRG axonal regeneration in vitro after a conditioning lesion (Liu and Snider, 2001), as well as significantly reduced conditioning lesion-dependent outgrowth after a dorsal column lesion (Qiu et al., 2005). Utilizing in vivo imaging and selective deletion of STAT3 in DRG neurons, STAT3 was shown to play a role in the initiation of axonal regeneration following a sciatic nerve injury (Bareyre et al., 2011). Our finding that CCL2 overexpression-induced increases in neurite outgrowth are abolished through the application of STAT3 phosphorylation inhibitors aligns with this previous work. Thus, the conditioning-like increase in neurite outgrowth caused by CCL2 overexpression occurs via a STAT3-dependent mechanism.
CCL2 action in the ganglia
CCL2 is upregulated and expressed by neurons in peripheral ganglia following nerve injury (Niemi et al., 2013; Schreiber et al., 2001; Tanaka et al., 2004). This upregulation and its action through the chemokine receptor, CCR2, are necessary for the injury-induced increase in macrophage accumulation in the DRG (Niemi et al., 2013). CCL2 primarily acts as a monocyte chemokine by inducing CCR2+ monocyte extravasation into tissue (Geissmann et al., 2003). Yet, CCL2 has also been shown to contribute to the development and maintenance of pain by acting directly on sensory neurons (Abbadie et al., 2009), and it has been demonstrated that CCL2 can depolarize DRG neurons, following pain-causing injuries, through direct action on the neurons (White et al., 2005). While CCL2 has also been shown to cause neurite outgrowth from embryonic avian statoacoustic ganglia, in vitro (Bianchi et al., 2005), in our preparation, CCL2 does not act directly on neurons to stimulate an increase in outgrowth.
Previous studies have also suggested that CCL2 can stimulate IL-4 production, a cytokine implicated in anti-inflammatory macrophage activation (Karpus et al., 1997). Evaluation of the general immune responses mounted in a CCL2 −/− mouse revealed decreased anti-inflammatory cytokine expression in response to bacterial immunization (Gu et al., 2000). Furthermore, macrophages stimulated with M-CSF in vitro in the presence of a CCL2 blocking antibody show significant increases in M1 or pro-inflammatory marker expression, while the presence of CCL2 induced expression of the anti-inflammatory cytokine IL-10 (Sierra-Filardi et al., 2014). It is interesting to note that we have observed increased protein expression of M-CSF in peripheral ganglia following nerve injury (Niemi and Zigmond, unpublished observations). These data suggest that CCL2 can lead to an anti-inflammatory stimulation of macrophages in addition to being chemotactic to CCR2-positive monocytes. This would favor a macrophage activation state which is capable of promoting axonal growth.
Conclusions
In conclusion, CCL2 overexpression induces macrophage accumulation in uninjured lumbar DRG and leads to a conditioning-like increase in axonal outgrowth. CCL2 overexpression leads to activation of STAT3, a regeneration-associated signaling molecule. CCL2 must act through its primary receptor, CCR2, to elicit subsequent changes in macrophage accumulation and neurite outgrowth. Finally, the conditioning-like increase in neurite outgrowth induced by CCL2 overexpression is abolished through inhibition of STAT3 phosphorylation. Taken together, CCL2 overexpression in DRG neurons is sufficient to increase the regenerative capacity of DRG neurons through a STAT3-dependent mechanism.
Highlights.
Intrathecal injection of AAV5-CCL2 leads to overexpression of the chemokine in DRGs
CCL2 overexpression causes macrophage accumulation in uninjured DRGs
A conditioning-like increase in neurite outgrowth in CCL2 overexpression
LIF mRNA and pSTAT3 are significantly increased in DRGs overexpressing CCL2
Overexpression-induced neurite outgrowth occurs via a STAT3-dependent mechanism
Acknowledgments
This work was supported by a National Institutes of Health grant DK097223 to R.E.Z. J.P.N was supported by a training grant NS077888. Breeding and genotyping of animals were carried out by the Case Western Reserve Visual Sciences Specialized Animal Research Core, and imaging of the DRG dissociated cell cultures was conducted with the assistance of Scott Howell of the Visual Sciences Microscopy and Digital Imaging Core (EY11373). The authors thank Yi-Lan Weng (Johns Hopkins University) for invaluable assistance in the design and creation of the AAV5-CCL2. We also thank Dr. Stephen Strittmatter and Dr. William Cafferty (Yale University) for the SPRR1a antibody. Finally we thank Dr. Molly Ingersoll, Jane Lindborg and Dr. Angela Filous for helpful discussions and comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: J.P.N., R.E.Z. designed research; J.C. contributed materials and reagents; J.P.N., A.D-L., M.H. performed research; J.P.N, R.E.Z. analyzed data; J.P.N., R.E.Z. wrote the paper.
Conflicts of Interest: None
References
- Abbadie C, Bhangoo S, De Koninck Y, Malcangio M, Melik-Parsadaniantz S, White FA. Chemokines and pain mechanisms. Brain Res Rev. 2009;60:125–134. doi: 10.1016/j.brainresrev.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alan R, Ezekowitz B, Gordon S. Alterations of surface properties by macrophage activation: expression of receptors for Fc and mannose-terminal glycoproteins and differentiation antigens, Macrophage Activation. Springer. 1984:33–56. doi: 10.1007/978-1-4757-1445-6_2. [DOI] [PubMed] [Google Scholar]
- Baldwin KT, Carbajal KS, Segal BM, Giger RJ. Neuroinflammation triggered by β-glucan/dectin-1 signaling enables CNS axon regeneration. Proc Natl Acd Sci USA. 2015;112:2581–2586. doi: 10.1073/pnas.1423221112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banner LR, Patterson PH. Major changes in the expression of the mRNAs for cholinergic differentiation factor/leukemia inhibitory factor and its receptor after injury to adult peripheral nerves and ganglia. Proc Natl Acad Sci USA. 1994;91:7109–7113. doi: 10.1073/pnas.91.15.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bareyre FM, Garzorz N, Lang C, Misgeld T, Buning H, Kerschensteiner M. In vivo imaging reveals a phase-specific role of STAT3 during central and peripheral nervous system axon regeneration. Proc Natl Acd Sci USA. 2011;108:6282–6287. doi: 10.1073/pnas.1015239108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrette B, Hebert MA, Filali M, Lafortune K, Vallieres N, Gowing G, Julien JP, Lacroix S. Requirement of myeloid cells for axon regeneration. J Neurosci. 2008;28:9363–9376. doi: 10.1523/JNEUROSCI.1447-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastien D, Lacroix S. Cytokine pathways regulating glial and leukocyte function after spinal cord and peripheral nerve injury. Exp Neurol. 2014;258:62–77. doi: 10.1016/j.expneurol.2014.04.006. [DOI] [PubMed] [Google Scholar]
- Bianchi LM, Daruwalla Z, Roth TM, Attia NP, Lukacs NW, Richards AL, White IO, Allen SJ, Barald KF. Immortalized mouse inner ear cell lines demonstrate a role for chemokines in promoting the growth of developing statoacoustic ganglion neurons. J Assoc Res Otolaryngol. 2005;6:355–367. doi: 10.1007/s10162-005-0013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisby MA, Chen S. Delayed wallerian degeneration in sciatic nerves of C57BL/Ola mice is associated with impaired regeneration of sensory axons. Brain Res. 1990;530:117–120. doi: 10.1016/0006-8993(90)90666-y. [DOI] [PubMed] [Google Scholar]
- Bonilla IE, Tanabe K, Strittmatter SM. Small proline-rich repeat protein 1A is expressed by axotomized neurons and promotes axonal outgrowth. J Neurosci. 2002;22:1303–1315. doi: 10.1523/JNEUROSCI.22-04-01303.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–897. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- Brown MC, Perry VH, Hunt SP, Lapper SR. Further studies on motor and sensory nerve regeneration in mice with delayed Wallerian degeneration. Eur J Neurosci. 1994;6:420–428. doi: 10.1111/j.1460-9568.1994.tb00285.x. [DOI] [PubMed] [Google Scholar]
- Brown MC, Perry VH, Lunn ER, Gordon S, Heumann R. Macrophage dependence of peripheral sensory nerve regeneration: possible involvement of nerve growth factor. Neuron. 1991;6:359–370. doi: 10.1016/0896-6273(91)90245-u. [DOI] [PubMed] [Google Scholar]
- Bruck W. The role of macrophages in Wallerian degeneration. Brain Path. 1997;7:741–752. doi: 10.1111/j.1750-3639.1997.tb01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafferty WB, Gardiner NJ, Das P, Qiu J, McMahon SB, Thompson SW. Conditioning injury-induced spinal axon regeneration fails in interleukin-6 knock-out mice. J Neurosci. 2004;24:4432–4443. doi: 10.1523/JNEUROSCI.2245-02.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafferty WB, Gardiner NJ, Gavazzi I, Powell J, McMahon SB, Heath JK, Munson J, Cohen J, Thompson SW. Leukemia inhibitory factor determines the growth status of injured adult sensory neurons. J Neurosci. 2001;21:7161–7170. doi: 10.1523/JNEUROSCI.21-18-07161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costigan M, Befort K, Karchewski L, Griffin RS, D’Urso D, Allchorne A, Sitarski J, Mannion JW, Pratt RE, Woolf CJ. Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci. 2002;3:16. doi: 10.1186/1471-2202-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis R, Scherer SS, Somogyi R, Adryan KM, Ip NY, Zhu Y, Lindsay RM, DiStefano PS. Retrograde axonal transport of LIF is increased by peripheral nerve injury: correlation with increased LIF expression in distal nerve. Neuron. 1994;12:191–204. doi: 10.1016/0896-6273(94)90163-5. [DOI] [PubMed] [Google Scholar]
- Dahlin LB. Prevention of macrophage invasion impairs regeneration in nerve grafts. Brain Res. 1995;679:274–280. doi: 10.1016/0006-8993(95)00249-p. [DOI] [PubMed] [Google Scholar]
- Dailey AT, Avellino AM, Benthem L, Silver J, Kliot M. Complement depletion reduces macrophage infiltration and activation during Wallerian degeneration and axonal regeneration. J Neurosci. 1998;18:6713–6722. doi: 10.1523/JNEUROSCI.18-17-06713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFrancesco-Lisowitz A, Lindborg J, Niemi J, Zigmond R. The neuroimmunology of degeneration and regeneration in the peripheral nervous system. Neuroscience. 2014 doi: 10.1016/j.neuroscience.2014.09.027. Sept. 19, [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med. 2003;197:1107–1117. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavazzi I, Kumar RD, McMahon SB, Cohen J. Growth responses of different subpopulations of adult sensory neurons to neurotrophic factors in vitro. Euro J Neurosci. 1999;11:3405–3414. doi: 10.1046/j.1460-9568.1999.00756.x. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immun. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- Gu L, Tseng S, Horner RM, Tam C, Loda M, Rollins BJ. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature. 2000;404:407–411. doi: 10.1038/35006097. [DOI] [PubMed] [Google Scholar]
- Habecker BA, Sachs HH, Rohrer H, Zigmond RE. The dependence on gp130 cytokines of axotomy induced neuropeptide expression in adult sympathetic neurons. Dev Neurobiol. 2009;69:392–400. doi: 10.1002/dneu.20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han KH, Tangirala RK, Green SR, Quehenberger O. Chemokine Receptor CCR2 Expression and Monocyte Chemoattractant Protein-1–Mediated Chemotaxis in Human Monocytes A Regulatory Role for Plasma LDL. Art Throm Vas Bio. 1998;18:1983–1991. doi: 10.1161/01.atv.18.12.1983. [DOI] [PubMed] [Google Scholar]
- Hanani M, Huang T, Cherkas P, Ledda M, Pannese E. Glial cell plasticity in sensory ganglia induced by nerve damage. Neuroscience. 2002;114:279–283. doi: 10.1016/s0306-4522(02)00279-8. [DOI] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. J Biochem. 1998;334(Pt 2):297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcomb IN, Kabakoff RC, Chan B, Baker TW, Gurney A, Henzel W, Nelson C, Lowman HB, Wright BD, Skelton NJ. FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family. The EMBO journal. 2000;19:4046–4055. doi: 10.1093/emboj/19.15.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt Sachs H, Rohrer H, Zigmond RE. The conditioning lesion effect on sympathetic neurite outgrowth is dependent on gp130 cytokines. Exp Neurol. 2010;223:516–522. doi: 10.1016/j.expneurol.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto N, Watanabe A, Yamamoto M, Miyake N, Kurai T, Teramoto A, Shimada T. Global diffuse distribution in the brain and efficient gene delivery to the dorsal root ganglia by intrathecal injection of adeno-associated viral vector serotype 1. J Gene Med. 2009;11:498–505. doi: 10.1002/jgm.1325. [DOI] [PubMed] [Google Scholar]
- Karpus WJ, Lukacs NW, Kennedy KJ, Smith WS, Hurst SD, Barrett TA. Differential CC chemokine-induced enhancement of Thelper cell cytokine production. J Immunol. 1997;158:4129–4136. [PubMed] [Google Scholar]
- Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurimoto T, Yin Y, Habboub G, Gilbert HY, Li Y, Nakao S, Hafezi-Moghadam A, Benowitz LI. Neutrophils express oncomodulin and promote optic nerve regeneration. J Neurosci. 2013;33:14816–14824. doi: 10.1523/JNEUROSCI.5511-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurimoto T, Yin Y, Omura K, Gilbert HY, Kim D, Cen LP, Moko L, Kugler S, Benowitz LI. Long-distance axon regeneration in the mature optic nerve: contributions of oncomodulin, cAMP, and pten gene deletion. J Neurosci. 2010;30:15654–15663. doi: 10.1523/JNEUROSCI.4340-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon MJ, Kim J, Shin H, Jeong SR, Kang YM, Choi JY, Hwang DH, Kim BG. Contribution of macrophages to enhanced regenerative capacity of dorsal root ganglia sensory neurons by conditioning injury. J Neurosci. 2013;33:15095–15108. doi: 10.1523/JNEUROSCI.0278-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibinger M, Muller A, Andreadaki A, Hauk TG, Kirsch M, Fischer D. Neuroprotective and axon growth-promoting effects following inflammatory stimulation on mature retinal ganglion cells in mice depend on ciliary neurotrophic factor and leukemia inhibitory factor. J Neurosci. 2009;29:14334–14341. doi: 10.1523/JNEUROSCI.2770-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon S, Yin Y, Nguyen J, Irwin N, Benowitz LI. Lens injury stimulates axon regeneration in the mature rat optic nerve. J Neurosci. 2000;20:4615–4626. doi: 10.1523/JNEUROSCI.20-12-04615.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman AR. The axon reaction: a review of the principle features of perikaryal responses to axon injury. In: Pfeiffer CC, Smythies JR, editors. Int Rev Neurobio. Academic Press; New York: 1971. pp. 49–124. [DOI] [PubMed] [Google Scholar]
- Liu RY, Snider WD. Different signaling pathways mediate regenerative versus developmental sensory axon growth. J Neurosci. 2001;21:RC164. doi: 10.1523/JNEUROSCI.21-17-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Richardson PM. Inflammation near the nerve cell body enhances axonal regeneration. J Neurosci. 1991;11:972–978. doi: 10.1523/JNEUROSCI.11-04-00972.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Richardson PM. Responses of macrophages in rat dorsal root ganglia following peripheral nerve injury. J Neurocytol. 1993;22:334–341. doi: 10.1007/BF01195557. [DOI] [PubMed] [Google Scholar]
- Lu X, Richardson PM. Changes in neuronal mRNAs induced by a local inflammatory reaction. J Neurosci Res. 1995;41:8–14. doi: 10.1002/jnr.490410103. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MR, Ehlert EM, Eggers R, Pool CW, Hermening S, Huseinovic A, Timmermans E, Blits B, Verhaagen J. Comparison of AAV serotypes for gene delivery to dorsal root ganglion neurons. Mol Ther. 2010;18:715–724. doi: 10.1038/mt.2010.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meydan N, Grunberger T, Dadi H, Shahar M, Arpaia E, Lapidot Z, Leeder JS, Freedman M, Cohen A, Gazit A, Levitzki A, Roifman CM. Inhibition of acute lymphoblastic leukaemia by a Jak-2 inhibitor. Nature. 1996;379:645–648. doi: 10.1038/379645a0. [DOI] [PubMed] [Google Scholar]
- Nagarajan R, Le N, Mahoney H, Araki T, Milbrandt J. Deciphering peripheral nerve myelination by using Schwann cell expression profiling. Proc Natl Acad Sci USA. 2002;99:8998–9003. doi: 10.1073/pnas.132080999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli I, Noon LA, Ribeiro S, Kerai AP, Parrinello S, Rosenberg LH, Collins MJ, Harrisingh MC, White IJ, Woodhoo A, Lloyd AC. A central role for the ERK-signaling pathway in controlling Schwann cell plasticity and peripheral nerve regeneration in vivo. Neuron. 2012;73:729–742. doi: 10.1016/j.neuron.2011.11.031. [DOI] [PubMed] [Google Scholar]
- Niemi JP, DeFrancesco-Lisowitz A, Roldan-Hernandez L, Lindborg JA, Mandell D, Zigmond RE. A critical role for macrophages near axotomized neuronal cell bodies in stimulating nerve regeneration. J Neurosci. 2013;33:16236–16248. doi: 10.1523/JNEUROSCI.3319-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogai K, Kuwana A, Hisano S, Nagashima M, Koriyama Y, Sugitani K, Mawatari K, Nakashima H, Kato S. Upregulation of leukemia inhibitory factor (LIF) during the early stage of optic nerve regeneration in zebrafish. PLoS One. 2014;9:e106010. doi: 10.1371/journal.pone.0106010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh P, Hao Y, Hosseinkhani M, Patil SB, Huntley GW, Tessier-Lavigne M, Zou H. Regeneration of axons in injured spinal cord by activation of bone morphogenetic protein/Smad1 signaling pathway in adult neurons. Proc Natl Acd Sci USA. 2011;108:E99–E107. doi: 10.1073/pnas.1100426108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Brown MC. Role of macrophages in peripheral nerve degeneration and repair. Bioessays. 1992;14:401–406. doi: 10.1002/bies.950140610. [DOI] [PubMed] [Google Scholar]
- Perry VH, Brown MC, Gordon S. The macrophage response to central and peripheral nerve injury. A possible role for macrophages in regeneration. J Exp Med. 1987;165:1218–1223. doi: 10.1084/jem.165.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovich PG, Guan Z, Wei P, Huitinga I, van Rooijen N, Stokes BT. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp Neurol. 1999;158:351–365. doi: 10.1006/exnr.1999.7118. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Longbrake EE. Can the immune system be harnessed to repair the CNS? Nat Rev Neurosci. 2008;9:481–493. doi: 10.1038/nrn2398. [DOI] [PubMed] [Google Scholar]
- Qiu J, Cafferty WB, McMahon SB, Thompson SW. Conditioning injury-induced spinal axon regeneration requires signal transducer and activator of transcription 3 activation. J Neurosci. 2005;25:1645–1653. doi: 10.1523/JNEUROSCI.3269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raes G, Noël W, Beschin A, Brys L, De Baetselier P, Hassanzadeh GG. FIZZ1 and Ym as tools to discriminate between differentially activated macrophages. Journal of Immunology Research. 2002;9:151–159. doi: 10.1080/1044667031000137629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MS, Sun Y, Escary JL, Perreau J, Tresser S, Patterson PH, Zigmond RE, Brulet P, Landis SC. Leukemia inhibitory factor mediates an injury response but not a targetdirected developmental transmitter switch in sympathetic neurons. Neuron. 1993;11:1175–1185. doi: 10.1016/0896-6273(93)90229-k. [DOI] [PubMed] [Google Scholar]
- Reichert F, Saada A, Rotshenker S. Peripheral nerve injury induces Schwann cells to express two macrophage phenotypes: phagocytosis and the galactose-specific lectin MAC-2. J Neurosci. 1994;14:3231–3245. doi: 10.1523/JNEUROSCI.14-05-03231.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotshenker S. Wallerian degeneration: the innate-immune response to traumatic nerve injury. J Neuroinflammation. 2011;8:109. doi: 10.1186/1742-2094-8-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs HH, Wynick D, Zigmond RE. Galanin plays a role in the conditioning lesion effect in sensory neurons. Neuroreport. 2007;18:1729–1733. doi: 10.1097/WNR.0b013e3282f0d3f4. [DOI] [PubMed] [Google Scholar]
- Schmid AB, Coppieters MW, Ruitenberg MJ, McLachlan EM. Local and remote immune-mediated inflammation after mild peripheral nerve compression in rats. J Neuropathol Exp Neurol. 2013;72:662–680. doi: 10.1097/NEN.0b013e318298de5b. [DOI] [PubMed] [Google Scholar]
- Schreiber RC, Krivacic K, Kirby B, Vaccariello SA, Wei T, Ransohoff RM, Zigmond RE. Monocyte chemoattractant protein (MCP)-1 is rapidly expressed by sympathetic ganglion neurons following axonal injury. Neuroreport. 2001;12:601–606. doi: 10.1097/00001756-200103050-00034. [DOI] [PubMed] [Google Scholar]
- Schreiber RC, Shadiack AM, Bennett TA, Sedwick CE, Zigmond RE. Changes in the macrophage population of the rat superior cervical ganglion after postganglionic nerve injury. J Neurobiol. 1995;27:141–153. doi: 10.1002/neu.480270203. [DOI] [PubMed] [Google Scholar]
- Schust J, Sperl B, Hollis A, Mayer TU, Berg T. Stattic: a small-molecule inhibitor of STAT3 activation and dimerization. Chemistry and Biology. 2006;13:1235–1242. doi: 10.1016/j.chembiol.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Seijffers R, Allchorne AJ, Woolf CJ. The transcription factor ATF-3 promotes neurite outgrowth. Mol Cell Neurosci. 2006;32:143–154. doi: 10.1016/j.mcn.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Seijffers R, Mills CD, Woolf CJ. ATF3 increases the intrinsic growth state of DRG neurons to enhance peripheral nerve regeneration. J Neurosci. 2007;27:7911–7920. doi: 10.1523/JNEUROSCI.5313-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- Shoemaker SE, Sachs HH, Vaccariello SA, Zigmond RE. A conditioning lesion enhances sympathetic neurite outgrowth. Exp Neurol. 2005;194:432–443. doi: 10.1016/j.expneurol.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Sierra-Filardi E, Nieto C, Domínguez-Soto Á, Barroso R, Sánchez-Mateos P, Puig-Kroger A, López-Bravo M, Joven J, Ardavín C, Rodríguez-Fernández JL. CCL2 shapes macrophage polarization by GM-CSF and M-CSF: identification of CCL2/CCR2-dependent gene expression profile. J Immunol. 2014;192:3858–3867. doi: 10.4049/jimmunol.1302821. [DOI] [PubMed] [Google Scholar]
- Subang MC, Richardson PM. Influence of injury and cytokines on synthesis of monocyte chemoattractant protein-1 mRNA in peripheral nervous tissue. Eur J Neurosci. 2001;13:521–528. doi: 10.1046/j.1460-9568.2001.01425.x. [DOI] [PubMed] [Google Scholar]
- Sun Y, Landis SC, Zigmond RE. Signals triggering the induction of leukemia inhibitory factor in sympathetic superior cervical ganglia and their nerve trunks after axonal injury. Mol Cell Neurosci. 1996;7:152–163. doi: 10.1006/mcne.1996.0012. [DOI] [PubMed] [Google Scholar]
- Sun Y, Rao MS, Zigmond RE, Landis SC. Regulation of vasoactive intestinal peptide expression in sympathetic neurons in culture and after axotomy: the role of cholinergic differentiation factor/leukemia inhibitory factor. J Neurobiol. 1994;25:415–430. doi: 10.1002/neu.480250407. [DOI] [PubMed] [Google Scholar]
- Symes A, Lewis S, Corpus L, Rajan P, Hyman SE, Fink JS. STAT proteins participate in the regulation of the vasoactive intestinal peptide gene by the ciliary neurotrophic factor family of cytokines. Mol Endocrinol. 1994;8:1750–1763. doi: 10.1210/mend.8.12.7708062. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Minami M, Nakagawa T, Satoh M. Enhanced production of monocyte chemoattractant protein-1 in the dorsal root ganglia in a rat model of neuropathic pain: possible involvement in the development of neuropathic pain. Neurosci Res. 2004;48:463–469. doi: 10.1016/j.neures.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Taylor PR, Gordon S. Monocyte heterogeneity and innate immunity. Immunity. 2003;19:2–4. doi: 10.1016/s1074-7613(03)00178-x. [DOI] [PubMed] [Google Scholar]
- Thompson SW, Vernallis AB, Heath JK, Priestley JV. Leukaemia inhibitory factor is retrogradely transported by a distinct population of adult rat sensory neurons: co-localization with trkA and other neurochemical markers. Eur J Neurosci. 1997;9:1244–1251. doi: 10.1111/j.1460-9568.1997.tb01479.x. [DOI] [PubMed] [Google Scholar]
- Toews AD, Barrett C, Morell P. Monocyte chemoattractant protein 1 is responsible for macrophage recruitment following injury to sciatic nerve. J Neurosci Res. 1998;53:260–267. doi: 10.1002/(SICI)1097-4547(19980715)53:2<260::AID-JNR15>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Vargas ME, Watanabe J, Singh SJ, Robinson WH, Barres BA. Endogenous antibodies promote rapid myelin clearance and effective axon regeneration after nerve injury. Proc Natl Acd Sci USA. 2010;107:11993–11998. doi: 10.1073/pnas.1001948107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang C, Zeng J, Xu X, Hwang PY, Yee WC, Ng YK, Wang S. Gene transfer to dorsal root ganglia by intrathecal injection: effects on regeneration of peripheral nerves. Mol Ther. 2005;12:314–320. doi: 10.1016/j.ymthe.2005.03.032. [DOI] [PubMed] [Google Scholar]
- White FA, Sun J, Waters SM, Ma C, Ren D, Ripsch M, Steflik J, Cortright DN, LaMotte RH, Miller RJ. Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proc Natl Acd Sci USA. 2005;102:14092–14097. doi: 10.1073/pnas.0503496102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Cui Q, Li Y, Irwin N, Fischer D, Harvey AR, Benowitz LI. Macrophage-derived factors stimulate optic nerve regeneration. J Neurosci. 2003;23:2284–2293. doi: 10.1523/JNEUROSCI.23-06-02284.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Henzl MT, Lorber B, Nakazawa T, Thomas TT, Jiang F, Langer R, Benowitz LI. Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells. Nat Neurosci. 2006;9:843–852. doi: 10.1038/nn1701. [DOI] [PubMed] [Google Scholar]
- Zigmond RE. gp130 cytokines are positive signals triggering changes in gene expression and axon outgrowth in peripheral neurons following injury. Front Mol Neurosci. 2012;4:1–18. doi: 10.3389/fnmol.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]


