Abstract
Objective
Brown adipose tissue (BAT) is a highly metabolic tissue that generates heat and is negatively associated with obesity. BAT has been proposed to mediate both cold-induced thermogenesis (CIT) and diet-induced thermogenesis (DIT). We therefore investigated whether there is a relationship between CIT and DIT in humans.
Methods
Nine healthy men (23±3 years old, 23.0±1.8 kg/m2) completed 20 minutes of cold exposure (4°C) five days per week for four weeks. Before and after the intervention, CIT (the increase in RMR at 16°C relative to 22°C) was measured by a ventilated hood indirect calorimeter, whereas DIT was measured as the 24-hour thermic response to one day of 50% overfeeding (TEF150%) in a respiratory chamber.
Results
After the cold intervention, CIT more than doubled from 5.2±14.2% at baseline to 12.0±11.1% (p=0.05), in parallel with increased SNS activity. However, twenty-four-hour energy expenditure (2166±206 vs. 2118±188 kcal/day; p=0.15) and TEF150% (7.4±2.7% vs. 7.7±1.6%; p=0.78) were unchanged. Moreover, there was no association between CIT and TEF150% at baseline or post-intervention, nor in their changes (p≥0.47).
Conclusions
Cold acclimation resulted in increased CIT but not TEF150%. Therefore, it is likely that CIT and DIT are mediated by distinct regulatory mechanisms.
Keywords: diet-induced thermogenesis (DIT), cold-induced thermogenesis (CIT), brown adipose tissue (BAT), overfeeding, energy expenditure
INTRODUCTION
Negative energy balance is required to reduce body weight and is achieved by reducing energy intake, increasing energy expenditure (EE), or both. The recent identification of active brown adipose tissue (BAT) in adults (1–5) has revived interest in targeting BAT to increase the facultative components of EE and potentially promote weight loss (6). In response to cold exposure, BAT generates heat to help maintain core body temperature, a phenomena termed cold-induced thermogenesis (CIT) (6). The heat induced by BAT is generated via mitochondrial uncoupling through mitochondrial uncoupling protein 1 (UCP1) and is enabled by BAT’s multiple lipid droplets, abundant mitochondria, high vascularity, and rich innervation by the sympathetic nervous system (SNS). These unique characteristics serve to fuel an intense metabolic activity, as demonstrated by a two-fold increase in blood flow (7–9), a several-fold increase in glucose and free fatty acid uptake (3, 8, 10–12), and an increase in oxidative metabolism (10, 13) upon cold stimulation.
Since the recent finding that active BAT is present in a majority of adults (2, 4, 8, 10, 14–17), several studies have reported that BAT activity is inversely associated with BMI (1, 2, 4, 5, 14–16), suggesting a role for BAT in the regulation of energy balance. Acute cold exposure typically increases EE by 5–17% (2, 9, 11, 12, 15, 17–27), in parallel with increases in BAT activity (12, 14, 28). However, it has been reported that CIT is significantly lower in overweight and BAT-negative individuals (2, 9, 11, 15, 17, 21, 25, 26, 28). Interestingly, longer-term cold exposure upregulates BAT activity (and possibly mass) (1, 4, 13, 14, 19, 28, 29) and increases CIT (14, 24, 28, 30), resulting in modest body weight reduction (19, 28) and slightly improved glucose metabolism (11, 19, 27, 29). Thus, habitual exposure to cold may provide a simple way to enhance EE and to improve metabolic health through BAT-mediated CIT.
In addition to CIT, BAT has also been hypothesized to regulate diet-induced thermogenesis (DIT). Rothwell and Stock initially observed that overfeeding resulted in less weight gained than anticipated but that the effect was inhibited by propranolol (31), suggesting that SNS stimulation of BAT may be involved; these changes occurred in parallel with an increase in BAT mass Later studies showed that even at thermoneutrality, UCP1 knockout mice are heavier than their wild-type counterparts (32) and that BAT transplantation reduces body weight (33). Since then, several studies in rodents (20, 31, 34) and humans (18, 19, 34) have reported associations between postprandial EE and BAT or CIT; however, many other studies refute this association (20, 27, 35–38).
Since the overall relationships among BAT activity, CIT, and DIT are unclear, we designed a study to determine whether the magnitude of changes in EE in response to cold (CIT) and overfeeding in humans are correlated. To assess the potential for DIT (which requires long-term overfeeding studies), we measured the thermic response during one day of 50% overfeeding (TEF150%) in a respiratory chamber. TEF150% represents the increase in postprandial EE due to the consumption of 150 % of the daily energy requirement divided by the ingested calories. This measure can be viewed as halfway between the thermic effect of food (TEF; excess energy expended in response to a single meal) and DIT (adaptive dietary thermogenesis in response to long-term overfeeding). We hypothesized that cold acclimation would increase both CIT and TEF150% and that their changes would be correlated, thus suggesting that CIT and DIT are regulated by similar mechanisms.
METHODS
Participants
Eleven participants enrolled in this clinical trial (NCT01898949) at Pennington Biomedical Research Center (Baton Rouge, LA). Participants were recruited via electronic advertising targeting healthy, lean men (BMI between 18.5–25 kg/m2) between the ages of 18 and 35. Potential participants were excluded for smoking, chronic alcohol consumption (>3 drinks/day), current use of medication, recent changes in body weight (>2 kg in the prior 6 months), impaired fasting glucose (>100 mg/dL), regular intense exercise (>3 times/week), or chronic disease. The study was approved by the Pennington Biomedical Institutional Review Board and was conducted in accord with the Declaration of Helsinki. All participants provided written informed consent prior to participation.
Study Design
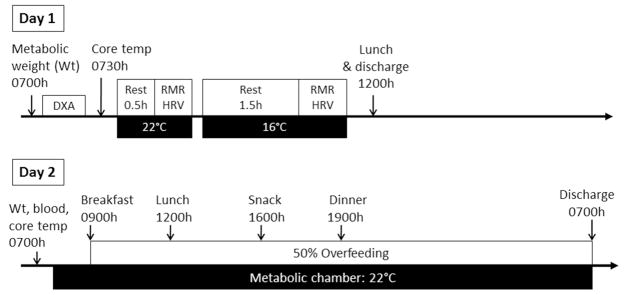
To increase the capacity for non-shivering thermogenesis and likely BAT activity, participants spent 20 minutes per day, five consecutive days per week, for four weeks in a cold room (4°C). This protocol was piloted to see whether intense cold exposure for short periods of time could be used as an effective cold exposure intervention, thus reducing participant time burden. In the cold room, participants wore light clothing (T-shirt, shorts and light shoes, e.g., flip flops) and a wrist watch to monitor heart rate variability (HRV) to track SNS activity. Participants stood for the duration of the cold exposure, and compliance to the protocol was closely monitored. Before and after the cold exposure intervention, EE in response to acute cold exposure (CIT) and overfeeding (TEF150%) were measured according to a 2-day testing protocol (Figure 1). Body composition was measured by DXA (Hologics, Bedford, MA), and EE was measured by indirect calorimetry using a ventilated hood system, with concomitant HRV assessment, in both thermoneutral (22°C) and cold conditions (16°C). Core temperature was monitored throughout the EE measurements using an ingestible telemetric temperature capsule (CorTemp, HQInc, Palmetto, FL). The following day, vital signs (blood pressure, pulse, temperature) were measured, and a fasting blood sample was collected to measure glucose (Beckman Coulter DXC600) and insulin (Siemen Immulite 2000). Participants were then admitted to the respiratory chamber for 24 hours and fed an overfeeding diet (50% above energy requirements) at thermoneutrality (22°C).
FIGURE 1. Two-day Testing Protocol.
On Day 1, RMR and HRV were measured at thermoneutrality (22°C) and in moderate cold (16°C) conditions with a ventilated hood indirect calorimeter. On Day 2, TEE, DIT, SPA, and SMR were measured in a respiratory chamber in response to overfeeding at thermoneutrality.
Cold-Induced Thermogenesis (CIT)
Resting metabolic rate (RMR) was measured at thermoneutrality and in response to acute cold by indirect calorimetry using a ventilated hood system (Max II Metabolic Cart; AEI Technologies, Naperville, IL). Participants refrained from performing vigorous physical activity and from consuming caffeine-containing drinks for 24 hours prior to testing. In the morning after an overnight fast, participants rested supine at thermoneutrality (22°C) for 30 minutes, and then RMR was measured for 30 minutes. Participants were then rolled on a wheelchair into a cold room (16°C). After 90 minutes of acute cold exposure, the 30-minute RMR measurement was repeated. CIT was calculated as the percent increase in RMR following acute cold exposure relative to RMR in the thermoneutral condition. In addition, HRV was measured during the 8 minutes prior to each RMR measurement using a heart rate chest strap and monitor (Polar S810, Polar Electro Oy, Kempele, Finland).
Thermic Effect of Food for One Day of 50% Overfeeding (TEF150%)
EE was measured over 24 hours in a respiratory chamber (39) at thermoneutrality (22°C) while participants consumed 150% of their weight maintenance energy requirements. Energy requirements were estimated using the Dietary Reference Intakes equation (40) for resting EE (REE), adjusted with an activity factor of 1.3. At 0730 h, participants swallowed a core temperature capsule, and fasting blood was drawn for measurement of glucose and insulin. Participants were dressed in medical scrubs and then entered the chamber and were instructed to limit motion and lay supine on the bed for the first hour of measurement to assess REE. Thereafter, participants were allowed to move freely in the chamber, but exercise was not permitted. Breakfast, lunch, an afternoon snack, and dinner were served at 0900 h, 1330 h, 1600 h, and 1900 h, respectively, and provided an overall macronutrient composition of 15% protein, 50% carbohydrate, and 35% fat. Participants were required to eat their meals within 30 minutes. Lights were turned off at 2230 h, and sleeping metabolic rate (SMR) was calculated between 0200 h and 0500 h for all minutes with radar activity <1%. Participants were awoken at 0630 h the following morning, and they exited the chamber at 0715 h. Participants collected all their urine during their chamber stay for assessment of urinary nitrogen and subsequent determination of protein oxidation. Physical activity within the chamber was quantified as percent of time in motion as measured by radar, and EE attributed to this spontaneous physical activity (SPA) was calculated as total daily EE minus REE and TEF150%. TEF150% was determined by subtracting REE from the y-intercept of the regression of EE versus % activity using data averaged in 15-minute intervals, as previously described (39).
Heart Rate Variability (HRV)
Autonomic function was assessed by spectral analysis of HRV using a heart-rate belt and a wrist recorder (Polar S810, Polar Inc, USA). Breathing frequency was controlled at rate of 15 breaths per minute using a metronome. Spectral analysis of heart rate data was performed using the Kubios HRV software (Kubios HRV, v. 2.1; Department of Applied Physics, University of Eastern Finland, Kuopio, Finland). For frequency domain data, a discrete Fourier transformation algorithm was used, and the spectral power was quantified by integrating the areas under the curve for the following frequency bands: very low frequency (VLO; 0.007 to 0.035 Hz), low frequency (LO; 0.035 to 0.15 Hz), high frequency (HI; 0.15 to 0.5 Hz), and total power (0.007 to 0.5 Hz). The spectral power in the VLO and LO were taken to be indices of SNS activity, while the power in the HI band was taken as an index of parasympathetic activity.
Statistical Analysis
Since this was a pilot study with a novel cold exposure intervention, no power analysis was performed. Data are expressed as mean ± SD. The effect of the cold exposure intervention was assessed using a two-tailed Student’s paired T-test, except where otherwise noted. To determine both the separate effects of and the interaction between the cold intervention and ambient temperature condition, 2 × 2 repeated measures ANOVAs were also performed on HRV, CIT, and EE data. The correlation between TEF150% and CIT was considered to be the primary endpoint. Correlations were performed using parametric least squares linear regression. The Type I error rate was set at α = 0.05.
RESULTS
Participant Characteristics
Nine out of 11 healthy, male participants recruited from the Greater Baton Rouge area completed the study; two participants were dropped from the study for non-compliance with the study intervention. The nine completers (8 Caucasian, 1 Hispanic) had a mean age of 23 ± 3 years and a mean BMI of 23.0 ± 1.8 kg/m2 (Table 1).
TABLE 1. Participant Characteristics and Cardiometabolic Indices.
Values are mean ± SD.
| Baseline | Change | P Value | |
|---|---|---|---|
| Age (yrs) | 23 ± 3 | - | - |
| Weight (kg) | 71.5 ± 6.5 | −0.5 ± 0.5 | 0.03* |
| BMI (kg/m2) | 23.0 ± 1.8 | −0.2 ± 0.2 | 0.03* |
| % Body Fat | 22.2 ± 5.7 | - | - |
| Systolic Blood Pressure (mm Hg) | 114 ± 6 | −3.5 ± 4.4 | 0.05* |
| Diastolic Blood Pressure (mm Hg) | 70 ± 6 | −2.6 ± 3.0 | 0.04* |
| Heart Rate (bpm) | 64 ± 5 | −3 ± 5 | 0.09‡ |
| Core Temperature (°C) | 36.7 ± 0.1 | 0.0 ± 0.1 | 0.88 |
| Fasting Glucose (mg/dL) | 90 ± 4 | 2 ± 4 | 0.15 |
| Insulin (mU/L) | 4.7 ± 1.7 | 0.3 ± 2.2 | 0.83 |
| HOMA-IR | 0.84 ± 0.42 | 0.09 ± 0.50 | 0.62 |
| Total Cholesterol (mg/dL) | 167 ± 26 | - | - |
| LDL (mg/dL) | 94.9 ± 16.6 | - | - |
| HDL (mg/dL) | 53.4 ± 11.1 | - | - |
| Triglycerides (mg/dL) | 92 ± 38 | - | - |
P ≤ 0.10,
P ≤ 0.05, according to a two-tailed paired T-test
Effect of the cold intervention on cardiometabolic health
After the four-week cold intervention, participants lost an average of 0.5 ± 0.5 kg (from 71.5 ± 6.5 to 71.0 ± 6.6 kg; p=0.03), which is consistent with weight loss reported in other studies (19, 28). Repeated cold exposure decreased systolic blood pressure from 114 ± 6 to 110 ± 6 mm Hg (p=0.05), while diastolic blood pressure declined from 70 ± 6 to 67 ± 3 mm Hg (p=0.04). Fasting glucose and insulin (Table 1) were unaffected by the cold intervention (p≥0.15) with no change in insulin resistance as estimated by HOMA-IR (p=0.62). Baseline heart rate was 64 ± 5 beats per minute (bpm), and the cold intervention tended to modestly decrease it by 3 ± 5 beats per minute (p=0.09). Core temperature at thermoneutrality was unchanged (p=0.88).
Effect of the cold intervention on cold-induced thermogenesis (CIT)
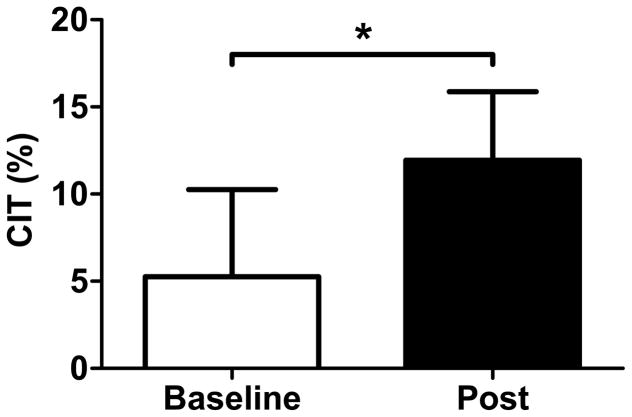
At baseline under thermoneutral conditions, RMR was 1702 ± 124 kcal/24 hours and remained unchanged following the four-week cold intervention (1636 ± 184 kcal/24 hours; p=0.14). Under acute mild cold conditions (16°C), RMR increased from 1782 ± 198 kcal/24 hours during baseline testing to 1824 ± 214 kcal/24 hours during post-intervention testing, and there was no significant temperature by time interaction (p=0.36). CIT was 5.2 ± 14.2% at baseline and increased to 12.0 ± 10.6% after the 20 sessions of cold exposure (p=0.05), indicating that cold acclimation did indeed occur (Figure 2). Shivering was not observed by research staff or reported by the participants at any time during the RMR testing in the mild cold conditions (nor during the respiratory chamber testing), indicating that the increase in EE was due to non-shivering thermogenesis. Lastly, there was no statistically significant change in the respiratory quotient (RQ) in response to either acute cold exposure or the cold intervention (p≥0.10; data not shown).
FIGURE 2. Cold-Induced Thermogenesis (CIT).
Four weeks of repeated cold exposure increased CIT from 5.2 ± 14.2% to 12.0 ± 10.6% (p=0.045), according to a one-tailed paired T-test. CIT was measured as the increase in RMR at 16°C, relative to RMR at 22°C. * P ≤ 0.05
Effect of the cold intervention on EE in response to overfeeding
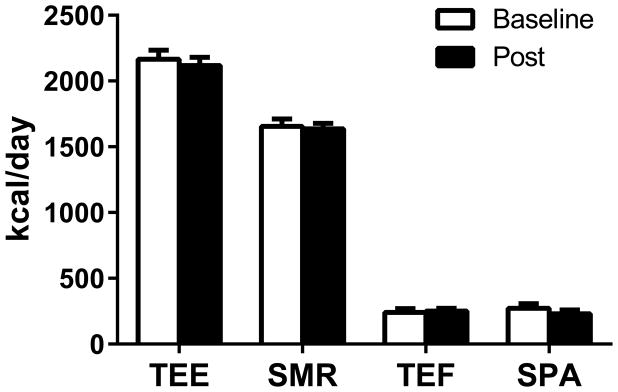
Participants were overfed by 50.2 ± 4.6% at baseline versus 53.1 ± 3.4% post-cold acclimation (p=0.24). Twenty-four-hour EE in response to overfeeding in thermoneutral conditions (Figure 3) was similar at baseline (2166 ± 206 kcal/day) and following the four-week cold intervention (2118 ± 188 kcal/day; p=0.15). The 50% overfeeding resulted in a robust value of 7.4 ± 2.7% for TEF150% before the cold intervention, and this value was unchanged at 7.7 ± 1.6% after the intervention period (p=0.78). The other subcomponents of EE—SMR and EE from SPA—were unchanged following the cold intervention (Figure 3; p ≥ 0.15). We also observed no differences in fat, protein, or carbohydrate oxidation (p ≥ 0.15; data not shown). Similarly, there was no difference in 24-hour core temperature during overfeeding (36.90 ± 0.35 vs. 36.90 ± 0.29°C; p=1.00) before and after the cold intervention.
FIGURE 3. Energy Expenditure in Response to Overfeeding.
Total energy expenditure (TEE), sleeping metabolic rate (SMR), thermic effect of food (TEF), and spontaneous physical activity (SPA) were measured at thermoneutrality (22°C) in response to 50% overfeeding at baseline (open bars) and following four weeks of cold acclimation (closed bars). Cold acclimation did not induce changes in energy expenditure at thermoneutrality, as measured by respiratory chamber (p ≥ 0.15).
Effect of the cold intervention on SNS Activity
Both acute cold exposure and the cold intervention increased HRV as measured by the standard deviation of RR intervals, relative to baseline at thermoneutrality (p=0.02; Table 2). Similarly, SNS activity as indicated by VLO and LO spectral power was significantly increased in response to both acute cold exposure and after cold acclimation, relative to baseline testing in thermoneutrality. At baseline, acute cold exposure stimulated VLO (p=0.04) and total power (p=0.05) by 2.3- and 1.7-fold, respectively. Interestingly, the cold intervention increased VLO power by three-fold (p=0.03) and increased total power by two-fold (p=0.02) under thermoneutral conditions. Under mild cold conditions (16°C), however, the cold intervention did not significantly affect spectral power (p≥0.24); however, the large inter-individual responses to the cold intervention may obscure any modest changes.
TABLE 2. Heart Rate Variability in (A) Thermoneutral (22°C) and (B) Cold Conditions (16°C).
Four weeks of cold acclimation increased very low frequency (VLO), low frequency (LO), and total power, confirming that the cold acclimation upregulated SNS activity. However, the increases only reached statistical significance under thermoneutral conditions. Values are mean ± SD.
| A. Thermoneutral Conditions (22°C)
| |||
|---|---|---|---|
| Baseline | Post | P Value | |
| Mean RR Interval (ms) | 1,045 ± 185 | 1,106 ± 147 | 0.20 |
| SD of RR Intervals (ms) | 96 ± 16 | 122 ± 38 | 0.02* |
| VLO Power (ms2) | 3,658 ± 2,818 | 10,887 ± 8,438 | 0.03* |
| LO Power (ms2) | 2,660 ± 2,217 | 3,547 ± 1,710 | 0.08‡ |
| HI Power (ms2) | 1,843 ± 1,269 | 1,878 ± 1,156 | 0.94 |
| Total Power (ms2) | 8,160 ± 2,544 | 16,313 ± 9,263 | 0.02* |
| B. Cold Conditions (16°C)
| |||
|---|---|---|---|
| Baseline | Post | P Value | |
| Mean RR Interval (ms) | 1,042 ± 198 | 1,037 ± 159 | 0.91 |
| SD of RR Intervals (ms) | 119 ± 36 | 138 ± 44 | 0.28 |
| VLO Power (ms2) | 8,225 ± 5,837 | 13,506 ± 11,298 | 0.24 |
| LO Power (ms2) | 3,121 ± 2,758 | 3,450 ± 2,505 | 0.58 |
| HI Power (ms2) | 3,021 ± 2,989 | 2,005 ± 1,356 | 0.19 |
| Total Power (ms2) | 14,366 ± 9,186 | 18,961 ± 12,849 | 0.38 |
P ≤ 0.10,
P ≤ 0.05, according to a two-tailed paired T-test
Correlations between CIT and TEF150%
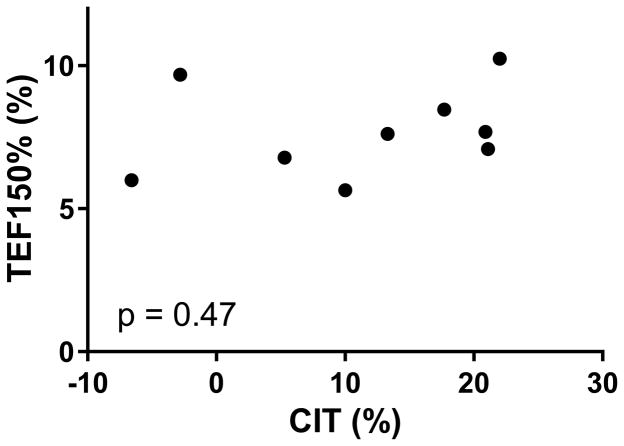
Despite cold acclimation-induced increases in CIT and SNS activity, TEF150% did not increase (p=0.78). Contrary to our hypothesis, no correlations were observed between CIT and TEF150% at baseline or following the cold intervention, nor did the changes in CIT and TEF150% values correlate with each other (p≥0.47; Figure 4). However, baseline TEF150% did negatively correlate with the change in TEF150% (r=−0.83; p=0.006), which likely reflects reversion to the mean.
FIGURE 4. Relationship Between TEF150% and CIT.
There was no correlation between baseline or post-intervention values (shown above), or in the changes in TEF150% and CIT values (p≥0.47).
DISCUSSION
Since the recent discovery of functional BAT in adults (1–5) and its negative correlation with BMI (1, 2, 4, 5, 14–16), there has been a flurry of research to determine whether targeting BAT activity can be used to treat obesity. While much focus is on enhancing CIT or SNS activity, BAT may mediate other facultative components of EE, such as DIT as first proposed in 1979 by Rothwell and Stock (31).
In this study, we investigated whether the thermic response to one day of 50% overfeeding and CIT are mediated by similar mechanisms in humans. To maximize CIT and BAT activity, we exposed nine healthy men to a four-week cold acclimation protocol; cold acclimation is known to enhance BAT activity (13, 14, 19, 28, 29), as well as CIT (14, 28, 30). Following the four-week cold intervention, CIT more than doubled from 5.2 ± 14.2 to 12.0 ± 11.1%, a finding consistent with other studies (14, 28, 30). For instance, Van der Lans and colleagues demonstrated a 7% absolute increase in CIT following exposure to mild cold (16°C) for 6 hours/day for 10 days (14), while Yoneshiro and colleagues reported comparable results after 2 hours of daily exposure to mild cold (19°C) for 6 weeks (28). Our results suggest that enhancing the metabolic response to cold may also be achieved by exposing participants to colder temperatures (4°C) but for shorter durations (e.g., 20 minutes/day over four weeks), thereby significantly reducing participant time burden. Interestingly, in response to our cold intervention, blood pressure slightly but significantly improved following the cold intervention, but HOMA-IR did not. In addition, our HRV results are consistent with those of other studies (e.g., (27)) and demonstrate that the four-week cold intervention increased SNS activity. Both BAT activity and the facultative part of DIT are known to be mediated by SNS activity. Therefore, if the same SNS mechanism mediates both CIT and DIT, we expected that the cold acclimation (which enhances SNS activity even in thermoneutral conditions) would also enhance TEF150%.
However, despite the increases in CIT and SNS activity following four weeks of cold acclimation, the thermic response to 50% overfeeding was unchanged (7.4 ± 2.7% before versus 7.7 ± 1.6% after; p=0.78). In addition, core body temperature, 24-hour EE, and its subcomponents were not altered by the intervention (p≥0.15). Furthermore, CIT and TEF150% were not associated at baseline or post-intervention, nor did the changes in CIT and TEF150% correlate with each other (p≥0.47). In sum, this suggests that DIT and CIT are mediated through different pathways.
Prior rodent and human studies have provided conflicting results on whether BAT and/or CIT are related to DIT (18–20, 31, 32, 34–38). For instance, Rothwell and Stock (31) originally reported enhanced oxygen consumption in response to overfeeding in rats, in parallel with increases in BAT mass, but a later study showed that BAT activity accounted for only 2–3% of DIT in the same rodent model (37). Similarly, the finding that UCP1 knockout mice had reduced DIT (32) was later refuted (36, 38). The knockout animals were hyperphagic, and DIT was erroneously defined as the ability of animals to respond to adrenergic stimulation (36).
Six prior studies in humans have attempted to measure postprandial responses to a meal in the context of BAT, CIT, or cold exposure. One study measured BAT activity by PET/CT in response to 100% overfeeding and found that none of the participants had increased BAT activity with overfeeding, despite 75% of participants having increased BAT activity in response to cold (35). Two studies with EE measurements in respiratory chambers have reported that TEF was associated with CIT and/or BAT activity (19, 34); however, in both studies, TEF was not measured directly, but rather either 24-hour EE or post-prandial EE (both include EE due to physical activity) was used instead. In one of the studies (19), impossibly high values of TEF in excess of 30% were reported. Notably, a third study measuring postprandial EE using the same method came to the opposite conclusion and reported data suggesting that TEF is somewhat blunted upon mild cold exposure (27). Overall, these mixed results underscore that measuring postprandial EE without accounting for activity levels should not be used to estimate TEF.
Only two human studies have directly measured TEF in the context of cold exposure or BAT activity, although neither incorporated both overfeeding and measurement of CIT. One study by Vosselman and colleagues (20) observed no direct relation between either CIT or postprandial BAT activity and TEF in response to a single meal. The second study, by van Marken Litchenbelt and colleagues (18), found no difference in TEF in response to ad libitum food intake measured at 16°C in comparison to thermoneutrality (22°C). We therefore performed the first bona fide investigation of the relationship between CIT and TEF in response to a structured 24-hour overfeeding paradigm, and we report that TEF values before and after the cold intervention were not associated with CIT. Our study does have some limitations, such as the small number of participants, not measuring BAT activity or muscle shivering activity, and measuring TEF by a respiratory chamber method, which is less reproducible than by ventilated hood system. Nonetheless, our results and evidence from other studies indicate that CIT and DIT are probably mediated by different regulatory mechanisms.
The hypothesis that CIT and DIT are mediated by different mechanisms is supported by suggestive but preliminary evidence from mechanistic investigations. First, insulin infusion increases BAT glucose uptake but not tissue perfusion (unlike with cold exposure), suggesting that BAT thermogenesis is not triggered by feeding (8). Second, unlike in rodents, Muzik et al. (9) showed that most of the increase in BAT EE during cold exposure in humans is mediated by changes in blood flow, and thus BAT can account for only up to 15–25 kcal/day.
In conclusion, our observations indicate that as little as 20 minutes of daily exposure to cold (4°C) enhances CIT, in parallel with increased SNS activity. Combined with evidence from previous reports, our results suggest that the mechanisms underlying CIT are probably different from those mediating the thermogenic response to acute overfeeding.
Study Importance Questions.
What is already known about this subject?
Several rodent studies suggest that brown adipose tissue (BAT) mediates diet-induced thermogenesis (DIT) and weight gain in response to positive energy balance, although reports are mixed.
In humans, two studies found that postprandial responses during a single meal do not correlate with BAT activity, and a third study suggests that DIT is not elevated under mild cold conditions. However, three other studies with indirect measurements of DIT report conflicting results on whether cold exposure and/or BAT activity is associated with DIT.
What does this study add?
This study is the first to directly measure CIT and the 24-hour thermic response to one day of 50% overfeeding (without physical activity as a confounding factor) in humans over 24 hours with a surplus of energy (50% overfeeding), rather than in response to a single meal. Measurements were performed before and after a 4-week cold acclimation intervention to increase SNS and BAT activity.
Our data suggests that the 24-hour thermic response to one day of 50% overfeeding and CIT are not associated and are therefore probably mediated by distinct regulatory mechanisms in humans.
Acknowledgments
We thank our study participants and our staff of nurses, technicians, and support staff at Pennington Biomedical Research Center. This work was supported by a NORC Center Grant P30 DK072476 entitled “Nutritional Programming: Environmental and Molecular Interactions” and an anonymous donor to the Pennington Biomedical Foundation. CMP is supported in part by 1 U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center. VL was supported by fellowships from the Swiss National Science Foundation #PBLAP3-133026 and PBLAP3-136942. LMR is supported by R00 HD060762. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding Source: This work was supported by a NORC Center Grant P30 DK072476 entitled “Nutritional Programming: Environmental and Molecular Interactions” and by an anonymous donor to the Pennington Biomedical Foundation.
Footnotes
Disclosure: CMP, VL, EAF, JS, and LMR have nothing to disclosure. ER reports personal fees from Energesis as a consultant, outside the submitted work.
References
- 1.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360(15):1509–17. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360(15):1500–8. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 3.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360(15):1518–25. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 4.Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58(7):1526–31. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zingaretti MC, Crosta F, Vitali A, Guerrieri M, Frontini A, Cannon B, et al. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 2009;23(9):3113–20. doi: 10.1096/fj.09-133546. [DOI] [PubMed] [Google Scholar]
- 6.van Marken Lichtenbelt WD, Schrauwen P. Implications of nonshivering thermogenesis for energy balance regulation in humans. Am J Physiol Regul Integr Comp Physiol. 2011;301(2):R285–96. doi: 10.1152/ajpregu.00652.2010. [DOI] [PubMed] [Google Scholar]
- 7.Foster DO, Frydman ML. Tissue distribution of cold-induced thermogenesis in conscious warm-or cold-acclimated rats reevaluated from changes in tissue blood flow: the dominant role of brown adipose tissue in the replacement of shivering by nonshivering thermogenesis. Can J Physiol Pharmacol. 1979;57(3):257–70. doi: 10.1139/y79-039. [DOI] [PubMed] [Google Scholar]
- 8.Orava J, Nuutila P, Lidell ME, Oikonen V, Noponen T, Viljanen T, et al. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab. 2011;14(2):272–9. doi: 10.1016/j.cmet.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Muzik O, Mangner TJ, Leonard WR, Kumar A, Janisse J, Granneman JG. 15O PET measurement of blood flow and oxygen consumption in cold-activated human brown fat. J Nucl Med. 2013;54(4):523–31. doi: 10.2967/jnumed.112.111336. Epub 2013/01/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ouellet V, Labbe SM, Blondin DP, Phoenix S, Guerin B, Haman F, et al. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest. 2012;122(2):545–52. doi: 10.1172/JCI60433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chondronikola M, Volpi E, Borsheim E, Porter C, Annamalai P, Enerback S, et al. Brown Adipose Tissue Improves Whole Body Glucose Homeostasis and Insulin Sensitivity in Humans. Diabetes. 2014 doi: 10.2337/db14-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen KY, Brychta RJ, Linderman JD, Smith S, Courville A, Dieckmann W, et al. Brown fat activation mediates cold-induced thermogenesis in adult humans in response to a mild decrease in ambient temperature. J Clin Endocrinol Metab. 2013;98(7):E1218–23. doi: 10.1210/jc.2012-4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blondin DP, Labbe SM, Phoenix S, Guerin B, Turcotte EE, Richard D, et al. Contributions of white and brown adipose tissues and skeletal muscles to acute cold-induced metabolic responses in healthy men. J Physiol. 2015;593(3):701–14. doi: 10.1113/jphysiol.2014.283598. Epub 2014/11/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Lans AA, Hoeks J, Brans B, Vijgen GH, Visser MG, Vosselman MJ, et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J Clin Invest. 2013;123(8):3395–403. doi: 10.1172/JCI68993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vijgen GH, Bouvy ND, Teule GJ, Brans B, Schrauwen P, van Marken Lichtenbelt WD. Brown adipose tissue in morbidly obese subjects. PLoS One. 2011;6(2):e17247. doi: 10.1371/journal.pone.0017247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoneshiro T, Aita S, Matsushita M, Okamatsu-Ogura Y, Kameya T, Kawai Y, et al. Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans. Obesity (Silver Spring) 2011;19(9):1755–60. doi: 10.1038/oby.2011.125. Epub 2011/05/14. [DOI] [PubMed] [Google Scholar]
- 17.Yoneshiro T, Aita S, Matsushita M, Kameya T, Nakada K, Kawai Y, et al. Brown adipose tissue, whole-body energy expenditure, and thermogenesis in healthy adult men. Obesity (Silver Spring) 2011;19(1):13–6. doi: 10.1038/oby.2010.105. Epub 2010/05/08. [DOI] [PubMed] [Google Scholar]
- 18.van Marken Lichtenbelt WD, Schrauwen P, van De Kerckhove S, Westerterp-Plantenga MS. Individual variation in body temperature and energy expenditure in response to mild cold. Am J Physiol Endocrinol Metab. 2002;282(5):E1077–83. doi: 10.1152/ajpendo.00020.2001. Epub 2002/04/06. [DOI] [PubMed] [Google Scholar]
- 19.Lee P, Smith S, Linderman J, Courville AB, Brychta RJ, Dieckmann W, et al. Temperature-acclimated brown adipose tissue modulates insulin sensitivity in humans. Diabetes. 2014 doi: 10.2337/db14-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vosselman MJ, Brans B, van der Lans AA, Wierts R, van Baak MA, Mottaghy FM, et al. Brown adipose tissue activity after a high-calorie meal in humans. Am J Clin Nutr. 2013;98(1):57–64. doi: 10.3945/ajcn.113.059022. [DOI] [PubMed] [Google Scholar]
- 21.Muzik O, Mangner TJ, Granneman JG. Assessment of oxidative metabolism in brown fat using PET imaging. Front Endocrinol (Lausanne) 2012;3:15. doi: 10.3389/fendo.2012.00015. Epub 2012/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wijers SL, Schrauwen P, Saris WH, van Marken Lichtenbelt WD. Human skeletal muscle mitochondrial uncoupling is associated with cold induced adaptive thermogenesis. PLoS One. 2008;3(3):e1777. doi: 10.1371/journal.pone.0001777. Epub 2008/03/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wijers SL, Schrauwen P, van Baak MA, Saris WH, van Marken Lichtenbelt WD. Beta-adrenergic receptor blockade does not inhibit cold-induced thermogenesis in humans: possible involvement of brown adipose tissue. J Clin Endocrinol Metab. 2011;96(4):E598–605. doi: 10.1210/jc.2010-1957. Epub 2011/01/29. [DOI] [PubMed] [Google Scholar]
- 24.van Ooijen AM, van Marken Lichtenbelt WD, van Steenhoven AA, Westerterp KR. Seasonal changes in metabolic and temperature responses to cold air in humans. Physiol Behav. 2004;82(2–3):545–53. doi: 10.1016/j.physbeh.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Claessens-van Ooijen AM, Westerterp KR, Wouters L, Schoffelen PF, van Steenhoven AA, van Marken Lichtenbelt WD. Heat production and body temperature during cooling and rewarming in overweight and lean men. Obesity (Silver Spring) 2006;14(11):1914–20. doi: 10.1038/oby.2006.223. Epub 2006/12/01. [DOI] [PubMed] [Google Scholar]
- 26.Wijers SL, Saris WH, van Marken Lichtenbelt WD. Cold-induced adaptive thermogenesis in lean and obese. Obesity (Silver Spring) 2010;18(6):1092–9. doi: 10.1038/oby.2010.74. Epub 2010/04/03. [DOI] [PubMed] [Google Scholar]
- 27.Celi FS, Brychta RJ, Linderman JD, Butler PW, Alberobello AT, Smith S, et al. Minimal changes in environmental temperature result in a significant increase in energy expenditure and changes in the hormonal homeostasis in healthy adults. Eur J Endocrinol. 2010;163(6):863–72. doi: 10.1530/EJE-10-0627. Epub 2010/09/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoneshiro T, Aita S, Matsushita M, Kayahara T, Kameya T, Kawai Y, et al. Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest. 2013;123(8):3404–8. doi: 10.1172/JCI67803. Epub 2013/07/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blondin DP, Labbe SM, Tingelstad HC, Noll C, Kunach M, Phoenix S, et al. Increased brown adipose tissue oxidative capacity in cold-acclimated humans. J Clin Endocrinol Metab. 2014;99(3):E438–46. doi: 10.1210/jc.2013-3901. Epub 2014/01/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis TR. Chamber cold acclimatization in man. Rep US Army Med Res Lab. 1961;475:1–8. [PubMed] [Google Scholar]
- 31.Rothwell NJ, Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Nature. 1979;281(5726):31–5. doi: 10.1038/281031a0. [DOI] [PubMed] [Google Scholar]
- 32.Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009;9(2):203–9. doi: 10.1016/j.cmet.2008.12.014. Epub 2009/02/04. [DOI] [PubMed] [Google Scholar]
- 33.Liu X, Zheng Z, Zhu X, Meng M, Li L, Shen Y, et al. Brown adipose tissue transplantation improves whole-body energy metabolism. Cell Res. 2013;23(6):851–4. doi: 10.1038/cr.2013.64. Epub 2013/05/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wijers SL, Saris WH, van Marken Lichtenbelt WD. Individual thermogenic responses to mild cold and overfeeding are closely related. J Clin Endocrinol Metab. 2007;92(11):4299–305. doi: 10.1210/jc.2007-1065. [DOI] [PubMed] [Google Scholar]
- 35.Schlogl M, Piaggi P, Thiyyagura P, Reiman EM, Chen K, Lutrin C, et al. Overfeeding over 24 hours does not activate brown adipose tissue in humans. J Clin Endocrinol Metab. 2013;98(12):E1956–60. doi: 10.1210/jc.2013-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kozak LP. Brown fat and the myth of diet-induced thermogenesis. Cell Metab. 2010;11(4):263–7. doi: 10.1016/j.cmet.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma SW, Foster DO. Brown adipose tissue, liver, and diet-induced thermogenesis in cafeteria diet-fed rats. Can J Physiol Pharmacol. 1989;67(4):376–81. doi: 10.1139/y89-061. Epub 1989/04/01. [DOI] [PubMed] [Google Scholar]
- 38.Anunciado-Koza R, Ukropec J, Koza RA, Kozak LP. Inactivation of UCP1 and the glycerol phosphate cycle synergistically increases energy expenditure to resist diet-induced obesity. J Biol Chem. 2008;283(41):27688–97. doi: 10.1074/jbc.M804268200. Epub 2008/08/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest. 1986;78(6):1568–78. doi: 10.1172/JCI112749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients) The National Academies Press; 2005. [DOI] [PubMed] [Google Scholar]