Abstract
General strategies for the chemical synthesis of organic compounds, especially of architecturally complex natural products, are not easily identified. Here, we present a method to establish a strategy for such syntheses, which begins with a process termed ‘network analysis’. This exercise, along with other considerations, has been used to identify a versatile synthetic intermediate that facilitated syntheses of the diterpenoid alkaloids weisaconitine D and liljestrandinine, as well as the core of gomandonine. The diterpenoid alkaloids comprise some of the most architecturally complex and functional group dense secondary metabolites ever isolated. For these reasons, they present a significant challenge for chemical synthesis. The synthesis approach described herein is a notable departure from other strategies adopted for the syntheses of related structures and affords not only the targeted natural products, but also intermediates and derivatives in the three subfamilies of diterpenoid alkaloids (i.e., C-18, C-19, and C-20), providing the first unified synthetic strategy to these natural products. This work validates the utility of network analysis as a starting point for identifying strategies for the syntheses of architecturally complex secondary metabolites. An easily accessible web-based graphing program has been developed for this purpose.
Introduction
Chemical synthesis remains a cornerstone of the enterprise of preparing small molecule active pharmaceutical ingredients (APIs).1,2,3,4 Advances in the field of chemical synthesis continue to be benchmarked by the methods and strategies for the preparation of complex natural products, which, more effectively than any other exercise, expose challenges that still exist in the field.5,6 Over the last half century, natural product synthesis has continued to be driven by three general motivations: 1) to achieve the practical synthesis of highly complex structures for which a synthesis plan is not readily apparent, 2) to highlight the power, as well as identify the scope and limitations of a newly developed synthesis method, and 3) to facilitate exploration of biological function of the synthetically prepared molecules (and their derivatives). While the latter two motivations have received considerable attention (especially over the last two decades), the former motivation, which has historically served to advance the field, has waned as the notion that any desired molecule can be prepared given enough resources and time, has prevailed.7,8,9 Yet, efficient, and versatile syntheses of many complex molecules still have not been realized. This is especially true for molecules that feature polycyclic, highly caged, frameworks for which effective strategic solutions are not immediately obvious. For these architecturally complex skeletons (e.g., aconitine, 1, Figure 1A), the biosynthetic transformations that lead to these secondary metabolites in Nature are often not fully vetted, are low yielding, or cannot be efficiently reproduced in the laboratory.10,11 Therefore, de novo strategic approaches for their chemical syntheses are required.12
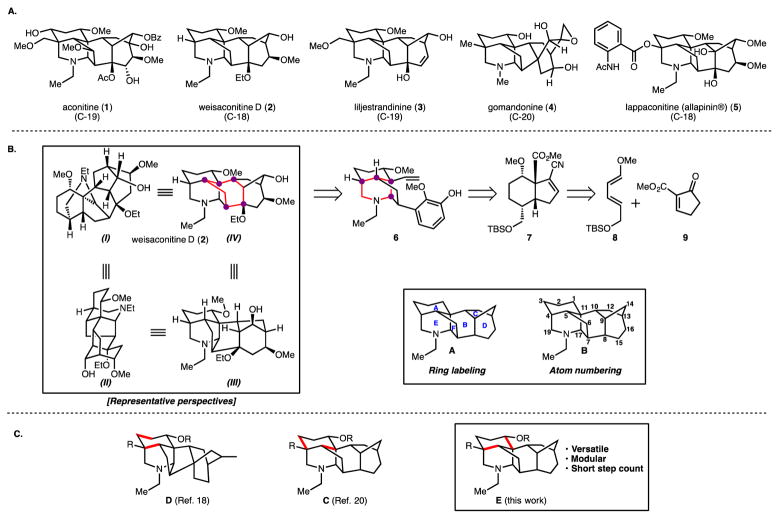
Figure 1. Molecules references in this work and design strategy.
Figure 1A: Selected C-18, C-19, and C-20 aconitine type and denudatine type diterpenoid alkaloids. Figure 1B: Perspective drawings of weisaconitine D (in box), retrosynthetic analysis highlighting maximally bridging rings (in red) and the corresponding bridgehead atoms (in purple) as well as the labeling of rings and atom numbering for the aconitine-type skeleton (in box). Figure 1C: Highlighted bonds that are forged in three different Diels-Alder approaches to the A ring of diterpenoid alkaloids.
Here, we demonstrate that for a subset of topologically complex and functional group dense secondary metabolites in the diterpenoid alkaloid family (representative of the aconitine type; >700 members), the serial application of a concept termed ‘network analysis’ at the initial stages of synthetic planning has unveiled a unified strategy for their synthesis. This type of analysis has proved unexpectedly enabling by identifying a strategy that is a notable departure from previously established synthesis strategies for related alkaloids. The network analysis approach, first introduced by Corey in 1975,13 involves ‘strategic bond disconnections’ of bridged polycycles. Despite the emergence of other philosophies, guidelines, and methods for synthesis in the interim four decades, network analysis remains immutable. Total syntheses of weisaconitine D (2; a C-18 alkaloid) and liljestrandinine (3; C-19), as well as the preparation of the skeleton of natural products in the denudatine family (e.g., gomandonine, 4; C-20) reported herein illustrate the power of this type of analysis.
Beyond their imposing architectures, the diterpenoid alkaloids (including weisaconitine D and liljestrandinine) have also gained in prominence as small molecule ligands for voltage-gated Na+ and K+ ion channels.14 In some cases, these small molecules may be isoform specific in their interactions with ion channels (presumably binding at the aconitine binding site) and therefore hold potential as the basis for novel therapeutics to address myriad channelopathies.15,16 For example, the Na+ channel blocker lappaconitine (allapinin®; 5) is already administered as a non-narcotic analgesic drug.17 However, to better identify the salient features of these molecules that lead to desirable medicinal properties, versatile de novo syntheses are required as they facilitate the synthesis of analogs featuring deep-seated skeletal changes that may not be otherwise efficiently accessed (e.g., by a biomimetic pathway or semi-synthesis).
Results and Discussion
Network analysis as a starting point in retrosynthesis
The application of network analysis to the diterpenoid alkaloids is illustrated in our retrosynthesis of the C-18 diterpenoid alkaloid weisaconitine D (Figure 1B). At the heart of this analysis is to minimize, in the retrosynthetic direction, the number of bridged rings, which, in addition to the density of stereochemically disposed functional groups, heightens the complexity of these molecules. Targeting the maximally bridged ring (highlighted in red for perspective IV of 2; see box in Figure 1B) possessing five bridgehead atoms (highlighted in purple), for disconnection leads back to 6, where in the forward sense, a bicyclization/cycloaddition could be applied to forge the bicyclo[3.2.1]framework. In turn, identification of the piperidine ring in 6 as the maximally bridged ring for this compound triggered a retrosynthetic simplification by disconnection of the C19–N bond (see B for atom numbering) leading back to a bicycle that could be derived from 7. Bicycle 7 was anticipated to be available from diene 8 and dienophile 9 using a Diels–Alder cycloaddition. Of note, while alternative Diels-Alder cycloadditions (compare C, D and E in Figure 1C) have been deployed in related elegant total syntheses,18,20,21 the serial application of network analysis, along with other considerations, led us to an alternative bond construction. Dehydro-hydrindane 7 possesses a variety of strategic synthetic handles that facilitate divergence in the synthetic scheme.
Similar retrosynthetic analyses can be proffered for the C-19 diterpenoid alkaloid liljestrandinine and for the C-20 alkaloid gomandonine (see the Supporting Information, SI, for more details). However, in these cases, the C4 bridgehead carbon would need to be quaternized, and 7 is suited for this purpose. From our analysis, 7 may also be employed in the syntheses of other diterpenoid alkaloids of the hetidine, hetisine, denudatine, and aconitine type (>900 members). Prior reported syntheses of diterpenoid alkaloids have mainly focused on specific targets (e.g., creative syntheses by Fukuyama,18 Baran,19 Gin,20 and Wiesner),21 whereas our synthetic plan targets the range of C-18, C-19 and C-20 diterpenoid alkaloids.
Syntheses of weisaconitine D and liljestrandinine
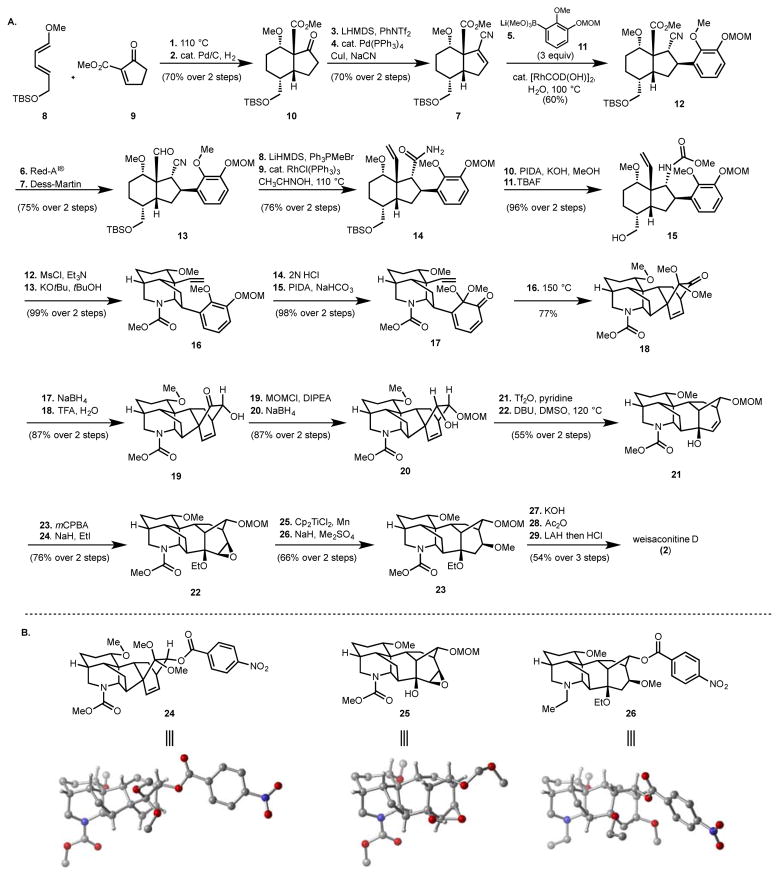
Our synthesis of weisaconitine D (Figure 2A) commenced with the cycloaddition of known diene 822 and cyclopentenone derivative 9,23 yielding a cycloadduct that upon hydrogenation gives bicyclic ketone 10 (70%; 2 steps). Vinyl triflate formation and Pd(0)-catalyzed cross-coupling with cyanide24 yields α,β-unsaturated nitrile 7 (70%; 2 steps), which served as a substrate for a Rh-catalyzed conjugate addition with in situ generated lithium boronate 11, to afford 12 in 60% yield. This conjugate addition step, which required careful optimization, provides a modular way to introduce the guaiacol derivative with high diastereocontrol and sets the stage to access various oxidation patterns on the C/D bicycle of the diterpenoid alkaloids by using other differently substituted arenes. Selective reduction of the ester group of 12 (in the presence of the cyano group) with Red-Al25 and reoxidation of the resulting alcohol group to the aldehyde using the Dess-Martin periodinane reagent gives 13. At this stage, Wittig olefination of the aldehyde group and hydration of the nitrile group using the conditions of Chang26 provides carboxamide 14. Hofmann rearrangement of the amide group and attendant trapping of the intermediate isocyanate with methanol, followed by fluoride-mediated cleavage of the TBS group gives 15. Activation of the primary hydroxyl as the mesylate and exposure to KOtBu effects alkylation to forge the C19–N bond and fashion the piperidine ring of 16 to complete the AEF rings (see A, Figure 1B, for ring labeling) of the C-18 diterpenoid alkaloids. In preparation for the installation of the BCD rings, the MOM group of 16 was removed and the resulting phenol subjected to oxidative dearomatization27 to afford 17. Dienone 17 smoothly undergoes intramolecular Diels–Alder cycloaddition upon heating to 150 °C to provide 18, which is the core framework of the C-20 denudatine type diterpenoid alkaloids (e.g., gomandonine, 4), bearing a bicyclo[2.2.2] moiety. The structure of this polycycle was secured by X-ray crystallographic analysis of benzoylated derivative 24 (Figure 2B). In preparation for the transformation of the bicyclo[2.2.2] structural motif to the bicyclo[3.2.1] framework characteristic of the aconitine-type C-18 and C-19 alkaloids, the carbonyl group of 18 was reduced stereoselectively (presumably steered away from torsional strain with the β-disposed methoxy group of the dimethylketal), and the ketal hydrolyzed to unveil α-ketol 19. Protection (MOM) of the secondary hydroxyl of 19 and diastereoselective reduction of the ketone group provides alcohol 20. At this juncture, in preparation for a Wagner-Meerwein type rearrangement in accordance with the precedent of Wiesner21 and Wang,28 the alcohol group of 20 was activated by triflation and upon subjection of the triflate to DBU and DMSO, hexacycle 21 was isolated in 55% yield over the two steps. In principle, while two isomeric allylic alcohols could result from the Wagner-Meerwein rearrangement, 21 is computed to be the more stable of the two (by 8.7 kcal/mol (gas phase) and 8.4 kcal/mol (DMSO) using DFT/M06-L/6-311G(d,p); see the SI for more details) presumably because it does not possess a strained bridgehead double bond. Several tactics were then explored to achieve a formal hydro-methoxylation of the C15–C16 double bond. These included the use of methanol in the presence of various protic and π-acids to activate the double bond,29 hydroboration (both inter and intramolecular – directed by the secondary hydroxyl at C14 of 21 following MOM cleavage),30 and variants of the hydration method of Mukaiyama31 and Isayama.32 In the end, the successful route to install the requisite methoxy group at C16 of 21 required the use of an epoxide intermediate. Thus, hydroxyl-directed epoxidation of the C15–C16 olefin group of 21 from the β-face using m-CPBA (see CYLview of 25, Figure 2B) and ethylation of the tertiary hydroxyl yielded 22 (76% over 2 steps). Regioselective reductive opening of the epoxide using the conditions of Cuerva and Oltra33 gave a β-disposed secondary alcohol group that was methylated to furnish 23 (66% over 2 steps). With the oxygenation of the D-ring of weisaconitine D secured, all that remained was to install the ethyl group on the piperidine nitrogen and to remove the MOM group to complete the synthesis. These tasks were accomplished in three steps entailing removal of the methoxycarbonyl (MOC) group of 23 (using KOH), acylation of the resulting secondary amine group (using Ac2O), reduction of the acetamide (using LiAlH4) and finally treatment with acid (in the same pot) to remove the MOM group. The total synthesis of weisaconitine D was achieved in 29 total steps from diene 8 and dienophile 9.
Figure 2.
Figure 2A: Reaction sequence for the total synthesis of weisaconitine D. Reagents and conditions: 1. 9 (1.0 equiv.), 8 (2.0 equiv.), toluene, 110 °C, 64 h. 2. Pd/C (10 wt%), H2 gas (1 atm), EtOAc, room temperature (r.t.), 3 h, 70% over two steps. 3. LiHMDS (1.3 equiv.), PhNTf2 (1.4 equiv.), THF, −78 °C to r.t., 12 h. 4. NaCN (2.2 equiv.), Pd(PPh3)4 (0.06 equiv.), CuI (0.12 equiv.), MeCN, reflux, 2 h, 70% over two steps. 5. Lithium boronate 11 (3.0 equiv.), [RhCOD(OH)]2 (0.05 equiv.), dioxane/water, 16 h, 60%. 6. Red-Al® (10 equiv.), CH2Cl2, −78 °C to r.t., 1 h, 82%. 7. Dess-Martin periodinane (2.0 equiv.), NaHCO3 (5.0 equiv.), CH2Cl2, 0 °C, 1.5 h, 91%. 8. PPh3MeBr (3.0 equiv.), LiHMDS (2.5 equiv.), THF, 0 °C to r.t., 1 h, 94%. 9. RhCl(PPh3)3 (0.3 equiv.), CH3CHNOH/PhMe, reflux, 15 h, 81%. 10. KOH (3.4 equiv.), Phenyliodonium diacetate (1.3 equiv.), MeOH, 0 °C to r.t., 3 h. 11. TBAF (3.0 equiv.), THF, r.t., 5 h, 96% over 2 steps. 12. MsCl (1.5 equiv.), CH2Cl2/Et3N, 0 °C, 3 h, 96%. 13. KOtBu (3.0 equiv.), THF, 0 °C to r.t., 2 h, 76%. 14. 2N HCl/iPrOH, 0 °C to r.t., 3.5 h, 99%. 15. Phenyliodonium diacetate (1.5 equiv.), NaHCO3 (5.0 equiv.), MeOH, 0 °C, 1 h, 99%. 16. p-xylene, 150 °C, 17.5 h, 77%. 17. NaBH4 (3.0 equiv.), MeOH, 0 °C to r.t., 3 h. 18. CHCl3/TFA/water, 4 °C, 2 h, 99% over 2 steps. 19. MOMCl (4.9 equiv.), DIPEA (10 equiv.), 4 °C to r.t., 16 h, 92%. 20. NaBH4 (3.3 equiv.), MeOH, 4 °C, 2 h, 95%. 21. Tf2O (10 equiv.), pyridine, CH2Cl2, −78 °C to r.t., 16 h. 22. DBU (3.3 equiv.), DMSO, 120 °C, 1 h, 55% over 2 steps. 23. mCPBA (5.2 equiv.), CH2Cl2, 0 °C to r.t., 16 h, 83%. 24. NaH (15 equiv.), EtI (15 equiv.), THF, 40 °C, 16 h, 95%. 25. Cp2TiCl2 (2.2 equiv.), Mn (7.6 equiv.), H2O (38 equiv.), THF, r.t., 16 h. 26. NaH (12 equiv.), Me2SO4 (7 equiv.), THF, 60 °C, 2 h, 66% over 2 steps. 27. 4M KOH, ethylene glycol, 100 °C, 120 h. 28. Ac2O (9.4 equiv.), pyridine (28 equiv.), CH2Cl2, 0 °C to r.t., 16 h. 29. LAH (10 equiv.), Et2O, 40 °C, 2 h; 2N HCl, THF, 16 h, 54% over 3 steps.
Figure 2B: CYLview images of various intermediates (24, 25) and of derivatized weisaconitine D (26). Most hydrogens (except stereocenters) have been removed for clarity.
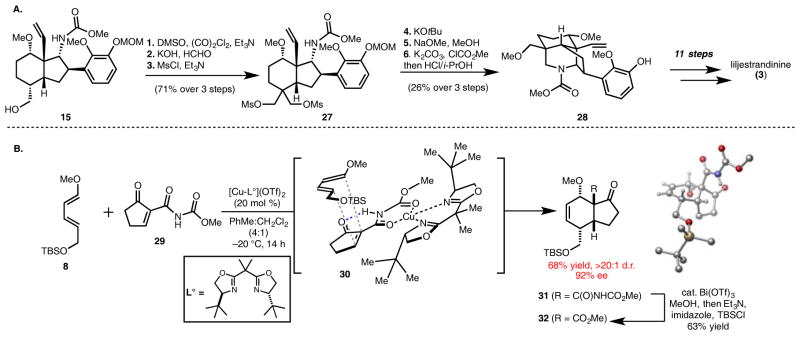
One key challenge that remains unmet in the previous syntheses of C-18, C-19, and C-20 diterpenoid alkaloids is how to achieve modular functionalization of the C4 position of the shared carbon framework. Here, we demonstrate that alcohol 15, a derivative of hydrindene 7, can be utilized in the synthesis of the C-19 diterpenoid alkaloid liljestrandinine, which possesses a methoxymethylene group at C4 (Figure 3A). The primary hydroxyl of 15 was first oxidized using Swern conditions to the corresponding aldehyde (not shown). Various attempts to alkylate the aldehyde enolate (as well as the enolates of related 6,5-bicycles) proved unfruitful and resulted in either non-specific decomposition or the addition of the electrophile from the undesired α-face (presumably due to developing syn-pentane interactions of the electrophile with the angular vinyl group). Ultimately, it was found that an aldol-Cannizzaro sequence on the intermediate aldehyde, effected using KOH and formaldehyde, furnishes a geminal bis-methylene diol which was functionalized as the bis-mesylate (see 27), where the C4 stereocenter is ablated. At this stage, in accord with the precedent of Wiesner,34 alkylation of the carbamate nitrogen was accomplished with KOtBu to forge the piperidine ring and reconstitute the C4 stereocenter (see 27). Displacement of the remaining mesylate group with methoxide, reinstallation of the nitrogen protecting MOC group (which is partially cleaved during the methoxide displacement) and removal of the MOM group provides 28. Phenol 28 was advanced to an intermediate analogous to 21 (8 steps) and then to liljestrandinine using a sequence analogous to that described for 23 → 2 (3 steps; see the SI for details). Overall, the synthesis of liljestrandinine proceeds in 29 steps from diene 8 and dienophile 9.
Figure 3.
Figure 3A: Reaction sequence for the synthesis of liljestrandinine. Reagents and conditions: 1. Oxalyl chloride (2. 9 equiv.), DMSO (6.2 equiv.), Et3N (12 equiv.), CH2Cl2, −78 °C to r.t., 1 h, 95%. 2. Formaldehyde (21 equiv.), 2N KOH, MeOH, r.t., 15 h, 96%. 3. MsCl (3.5 equiv.), pyridine, 0 °C to r.t., 2 h, 78%. 4. KOtBu (5 equiv.), THF, 50 °C, 4 h. 5. 0.5M NaOMe in MeOH, 120 °C, 24 h. 6. Methyl chloroformate (20 equiv.), K2CO3 (40 equiv.), acetone, reflux, 20 h; 2N HCl, isopropanol, r.t., 4.5 h, 26% yield over 3 steps.
Figure 3B: Enantioselective Diels–Alder cycloaddition approach employing dienophile 29.
An enantioselective Diels-Alder cycloaddition
The chemical syntheses of weisaconitine D and liljestrandinine described here rely on subsequent diastereoselective installation of all stereocenters from the four contiguous stereocenters that are introduced in the Diels–Alder reaction between diene 8 and dienophile 9. As such, a catalytic, enantioselective, Diels–Alder cycloaddition would enable enantioselective access to the natural products. In this regard, initial attempts to render the cycloaddition between 8 and 9 enantioselective with the aid of chiral, non-racemic, Lewis acid catalysts (e.g., using the method of Mezzetti)35,36 resulted in low enantioselectivity and non-specific decomposition (primarily of diene 8 under the acidic conditions). Ultimately, success was attained using 2937 (for which we have developed a new, scalable, synthesis; see the SI) as a dienophile. This dienophile has enhanced reactivity because of an added intramolecular H-bond,38 as well as a more highly organized transition state (see 30 for a model) that places the enantio-discriminating substituents (e.g., the t-butyl group of the bis-oxazoline ligand) proximal to the reacting dienophile double bond. In the event, a 68% yield of cycloadduct 31 (92% ee; >20:1 d.r.; see CYLview) was obtained using the conditions described in Figure 3B. Furthermore, 31 is easily converted to 32, which intercepts the racemic syntheses described in Figures 2 and 3A.
A web-based network analysis program
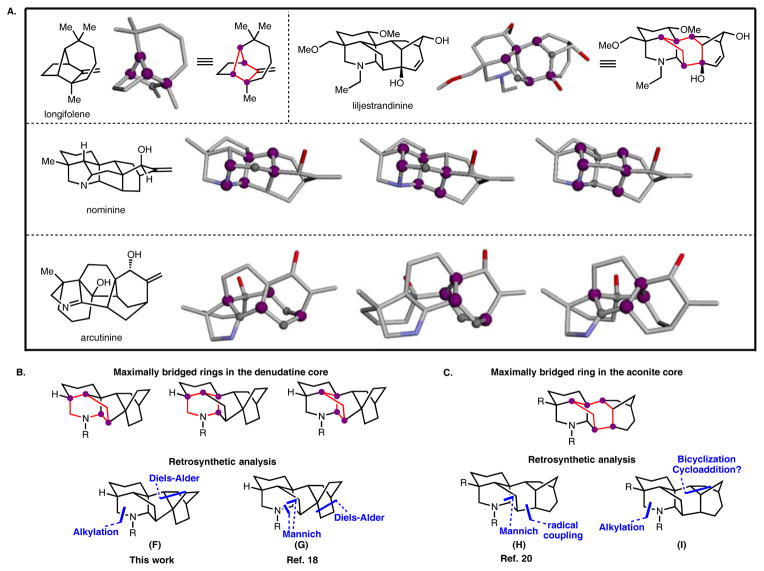
Finally, our iterative application of Corey’s network analysis approach to initiating a strategy for the syntheses of weisaconitine D and liljestrandinine has inspired us to develop general ways to conduct such analyses. Past implementations of network analysis in retrosynthesis, especially in the identification of the maximally bridged ring, have been carried out in a probabilistic manner, which invariably heightens the risk of errors.39,40 To overcome this shortcoming, we have developed a web-based deterministic graphing program that permits the identification of the maximally bridged ring (or rings) for any molecule using the Chemistry Development Kit (CDK) software library41,42 (see Figure 4A for the output of a test set; see the SI for more details). The algorithm we have developed for this purpose is guaranteed to identify the maximally bridged ring each time it is run. The program allows control of several criteria (e.g., the number of atoms that comprise the maximally bridged ring or that span bridging atoms in the maximally bridged ring). The program outputs the maximally bridged ring or in the case of ties (e.g., for nominine and arcutinidine in Figure 4A), all maximally bridged rings.
Figure 4.
Figure 4A: Selected molecules of a test set analyzed using the newly developed graphing program to detect the maximally bridging ring. The program output is a pdb image in gray. The maximally bridging ring is indicated by a combination of gray and purple spheres. The purple spheres represent bridgehead atoms in the maximally bridging ring and the gray spheres represent other atoms in the maximally bridging ring. ChemDraw renditions of the graphing program output are provided for longifolene and liljestrandinine. For an extensive test set, see the SI. 3D views of the output of the test set are located at http://www.cadrerl.com/ring/. To use the program go to http://www.cadrerl.com/maxbridge. Figure 4B: ChemDraw renditions of the program output. Conducted for the denudatine core and other key retrosynthetic disconnections applied in this work and in Ref. 18. Figure 4C: ChemDraw renderings of the program output for the aconite core and key retrosynthetic disconnections applied in Ref. 20 and in this work.
While many considerations are taken into account in retrosynthetic analyses of topologically complex molecules, the role of network analysis can often unlock novel strategic disconnections. For example, consider the denudatine core (Figure 4B), which contains three rings that each possesses four bridgehead atoms. By focusing on these rings for disconnection, maximum retrosynthetic simplification (i.e., removal of bridging chains and fused rings) is achieved in the least amount of steps with our approach (see F). A retrosynthetic analysis of the aconite framework, informed by network analysis (Figure 4C) suggests that disconnections represented by I would provide maximum simplification. These latter strategic disconnections, which guided our approach to the syntheses of weisaconitine D and liljestrandinine, also indicate that a direct bicyclization to construct the bicyclo[3.2.1] moiety would provide the maximum benefit. Efforts to achieve this type of bicyclization are the subject of our ongoing studies. The creation of this web-based program should further facilitate the use of network analysis in developing retrosyntheses of other architecturally complex molecules and enable the identification of an efficient path to their syntheses.
Conclusion
In sum, our preparation of the denudatine core and total syntheses of weisaconitine D and liljestrandinine presented herein reaffirm the utility of complex molecule synthesis as a driver for the implementation of chemical synthesis strategies that advance the field. Our approach offers a plan for the synthesis of a subset of C-18 and C-19 diterpenoid alkaloids and sets the stage to access related secondary metabolites including those in the C-20 family. The web-based deterministic graphing program developed to analyze these topologically complex molecules, which builds on the work of Corey, should find utility beyond this initial intent and may prove valuable in the analysis and synthesis of other architecturally challenging molecules (http://www.cadrerl.com/maxbridge).
Supplementary Material
Acknowledgments
This project was supported by award no. RO1 GM084906 from the National Institute of General Medical Sciences. C.J.M. acknowledges a National Science Foundation graduate fellowship. G.M.G. is grateful to the NIH (5F31GM095238) for a graduate fellowship. T.P.L. and K.G.M.K. acknowledges postdoctoral fellowships from the NSERC (Canada). We are grateful to Prof. Xiao-Yu Liu (Sichuan University, China) for copies of 1H and 13C NMR spectra for liljestrandinine (3). X-Ray crystallography was performed by A. DiPasquale (University of California Berkeley; NIH Shared Instrumentation Grant S10-RR027172). The Cambridge Crystallographic Data Centre (CCDC) contains the supplementary crystallographic data under 1402704, 1402818, 1402820, 1403763 (displayed in the manuscript with CYLview developed by Prof. Claude Y. Legault, Dept. of Chemistry, Université Sherbrooke). These data can be obtained free of charge from www.ccdc.cam.ac.uk/data_request/df. The AV-600, AV-500, DRX-500, and AVB-400 NMR instruments were partially supported by NIH grant SRR023679A, NIH grant 1S10RR016634-01, NSF grant CHE 9633007, and NSF grant CHE-0130862 respectively. Dr. Ethan Fisher (Pfizer) is acknowledged for contributions toward a practical synthesis of diene 8. We thank Kyle Owens (UCB) for help with generating sdf files and Anthony Chen (UCB), Naomi Kelly (UCB) and Kaarin Evens (Amgen Scholar at UCB in 2014) for the preparation of starting materials. Dr. Manuel Weber (UCB) is acknowledged for computational calculations pertaining to allylic alcohol 21 and its isomer.
Footnotes
Author Contributions
Authorship was assigned on the basis of the contributions of each author to the work described in the manuscript (detailed below) following a discussion involving all authors. R.S. wrote the manuscript and all authors contributed to the reading and editing of the manuscript. C.J.M, G.M.G, J.C.L, T.P.L, S.K, and K. M. G. K. conducted the chemical reactions described in the manuscript. S. K., K. M. G. K., and C. J. M. compiled the Supporting Information. Revisions and contributions to the Supporting Information were made by T.P. L. and G. M. G. J. Q., R. L. and R.S. conceptualized the graphing program, which was executed by J. Q. and R. L. J. Q., R. L. and R.S. also prepared the portion of the Supporting Information describing the graphing program. C. J. M. completed the synthesis and characterization of weisaconitine D. Significant contributions en-route to this end included conversion of the [2.2.2] to the [3.2.1] bicycle (i.e., 19→21), the formal hydromethoxylation sequence (21→23) and developing robust conditions for the aryl conjugate addition (7→12, with K. M. G. K.) and Hofmann rearrangement (13→14). G. M. G. developed steps 3–17 in the synthesis of [2.2.2] bicycle 19. Significant contributions en route to this end included establishing a large-scale synthesis of 10, synthesis of piperidine 16, Diels–Alder cycloaddition of 17, and stereoselective reduction of ketone 18. J. C. L. completed the synthesis of liljestrandinine. Significant contributions en-route to this end included conceptualization of the nitrile as a nitrogen atom surrogate, developing conditions for the conjugate addition of the functionalized arene (7→12) and establishing the sequence described for the conversion of 23→2 and 15→28. T. P. L. contributed to the conceptualization of the synthetic route with significant synthetic contributions made to the early portion of the synthesis including the establishment of a large-scale synthesis of 10. S. K. developed the enantioselective Diels Alder reaction (i.e., synthesis of 32) and also completed the optimization, scale up, and characterization of liljestrandinine. K. G. M. K. completed the optimization, scale up, and characterization of weisaconitine D and also optimized the conjugate addition (7→12, with C. J. M.) and the construction of piperidine 16.
References
- 1.Nusim S, editor. Active Pharmaceutical Ingredients: Development, Manufacturing, and Regulation, Second Edition (Drugs and the Pharmaceutical Sciences) CRC Press; 2009. [Google Scholar]
- 2.Schaefer B. Natural Products in the Chemical Industry. Springer-Verlag; Berlin Heidelberg: 2014. pp. 209–518. [Google Scholar]
- 3.Farina V, Reeves JT, Senanayake CH, Song JJ. Asymmetric synthesis of active pharmaceutical ingredients. Chem Rev. 2006;106:2734–2793. doi: 10.1021/cr040700c. [DOI] [PubMed] [Google Scholar]
- 4.dos Santos Pinheiro AE, Antunes OAC, Fortunak JMD. A survey of the syntheses of active pharmaceutical ingredients for antiretroviral drug combinations critical to access in emerging nations. Antiviral Res. 2008;79:143–165. doi: 10.1016/j.antiviral.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Shenvi RA, O’Malley DP, Baran PS. Chemoselectivity: The mother of invention in total synthesis. Acc Chem Res. 2009;42:530–541. doi: 10.1021/ar800182r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hudlický T, Reed J. The Way of Synthesis. Wiley-VCH; Weinheim: 2007. [Google Scholar]
- 7.Service RF. Race for molecular summits. Science. 1999;285:184–187. doi: 10.1126/science.285.5425.184. [DOI] [PubMed] [Google Scholar]
- 8.Negishi E. Magical power of transition metals: Past, present and future (Nobel lecture) Angew Chem Int Ed. 2011;50:6738–6764. doi: 10.1002/anie.201101380. [DOI] [PubMed] [Google Scholar]
- 9.White MC. C–H bond functionalization and synthesis in the 21st century: A brief history and prospectus. Synlett. 2012;23:2746–2748. [Google Scholar]
- 10.Kwok R. Five hard truths for synthetic biology. Nature. 2010;463:288–290. doi: 10.1038/463288a. [DOI] [PubMed] [Google Scholar]
- 11.MacMillan J, Beale MH. Diterpene biosynthesis. Comp Nat Prod Chem. 1999;2:217–243. [Google Scholar]
- 12.Koehn FE, Carter GT. The evolving role of natural products in drug discovery. Nat Rev Drug Discov. 2005;4:206–220. doi: 10.1038/nrd1657. [DOI] [PubMed] [Google Scholar]
- 13.Corey EJ, Howe WJ, Orf HW, Pensak DA, Petersson G. General methods of synthesis analysis – Strategic bond disconnections for bridged polycyclic structures. J Am Chem Soc. 1975;97:6116–6124. [Google Scholar]
- 14.Chan TYK. Aconite poisoning. Clin Toxicol. 2009;47:279–285. doi: 10.1080/15563650902904407. [DOI] [PubMed] [Google Scholar]
- 15.Caterall WA, Castéle S, Yarov-Yarovoy V, Yu FH, Konoki K, Scheuer T. Voltage-gated ion channels and gating modifier toxins. Toxicon. 2007;49:124–141. doi: 10.1016/j.toxicon.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 16.Anger T, Madge DJ, Mulla M, Riddall D. Medicinal chemistry of neuronal voltage-gated sodium channel blockers. J Med Chem. 2001;44:115–137. doi: 10.1021/jm000155h. [DOI] [PubMed] [Google Scholar]
- 17.Vakhitova YV, Farafontova EI, Khisamutdinova RY, Yunusov VM, Tsypysheva IP, Yunusov MS. A study of the mechanism of the antiarrhythmic action of Alapinin. Bioorg Khim. 2013;39:92–101. doi: 10.1134/s1068162013010111. [DOI] [PubMed] [Google Scholar]
- 18.Nishiyama Y, Han-ya Y, Yokoshima S, Fukuyama T. Total synthesis of (−)-lepenine. J Am Chem Soc. 2014;136:6598–6601. doi: 10.1021/ja503023h. [DOI] [PubMed] [Google Scholar]
- 19.Cherney EC, Lopchuk JM, Green JC, Baran PS. A unified approach to ent-atisane diterpenes and related alkaloids: Synthesis of (−)-methyl atisenoate, (−)-isoatisine, and the hetidine skeleton. J Am Chem Soc. 2014;136:12592–12595. doi: 10.1021/ja507321j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi Y, Wilmot JT, Nordstrøm LU, Tan DS, Gin DY. Total synthesis, relay synthesis, and structural confirmation of the C18-norditerpenoid alkaloid neofinaconitine. J Am Chem Soc. 2013;135:14313–14320. doi: 10.1021/ja4064958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiesner K, Tsai TYR, Huber K, Bolton SE, Vlahov R. Total synthesis of talatisamine, a delphinine type alkaloid. J Am Chem Soc. 1974;96:4990–4992. [Google Scholar]
- 22.Prabhakaran J, Lhermitte H, Das J, Sasi-Kumar TK, Grierson DS. The synthesis of a sulfone containing analogue of the esperamicin-A(1) aglycone: A hetero Diels-Alder approach. Synlett. 2000;5:658–662. [Google Scholar]
- 23.Marx JN, Cox JH, Norman LR. 2-Carbomethoxycyclopent-2-enone. J Org Chem. 1972;37:4489–4491. doi: 10.1021/jo00799a602. [DOI] [PubMed] [Google Scholar]
- 24.Bandyopadhyaya AK, Manion BD, Benz A, Taylor A, Rath NP, Evers AS, Zorumski CF, Mennerick S, Covey DF. Neurosteroid analogues. 15. A comparative study of the anesthetic and GABAergic actions of alphaxalone. Δ16-alphaxalone and their corresponding 17-carbonitrile analogues. Bioorg Med Chem Lett. 2010;20:6680–6684. doi: 10.1016/j.bmcl.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yokota S, Miyamoto S. Insect pest control agents containing hexahydrocyclopentapyranones. Japanese Patent 2008-024670, A. Jpn Kokai Tokkyo Koho. 2008 Feb 7;
- 26.Lee J, Kim M, Chang S, Lee HY. Anhydrous hydration of nitriles to amides using aldoximes as the water source. Org Lett. 2009;11:5598–5601. doi: 10.1021/ol902309z. [DOI] [PubMed] [Google Scholar]
- 27.Quideau S, Pouysegu L, Deffieux D, Ozanne A, Gagnepain J, Fabre I, Oxoby M. Iodine-mediated and electrochemical oxidative transformations of 2-methoxy- and 2-methylphenols. ARKIVOC. 2003;vi:106–119. [Google Scholar]
- 28.Cheng H, Xu L, Chen DL, Chen QH, Wang FP. Constructuion of the functionalized B/C/D ring system of C-19-diterpenoid alkaloids via intramolecular Diels-Alder reaction followed by Wagner-Meerwein rearrangement. Tetrahedron. 2012;68:1171–1176. [Google Scholar]
- 29.Fürstner A, Davies PW. Catalytic carbophilic activation: Catalysis by platinum and gold π acids. Angew Chem Int Ed. 2007;46:3410–3449. doi: 10.1002/anie.200604335. [DOI] [PubMed] [Google Scholar]
- 30.Evans DA, Fu GC, Hoveyda AH. Rhodium-catalyzed hydroboration of olefins – The documentation of regiochemical and stereochemical control in cyclic and acyclic systems. J Am Chem Soc. 1988;110:6917–6918. [Google Scholar]
- 31.Isayama S, Mukaiyama T. Novel method for the preparation of triethylsilyl peroxides from olefins by the reaction with molecular oxygen and triethylsilane catalyzed by bis(1,3-diketonato)cobalt(II) Chem Lett. 1989;18:573–576. [Google Scholar]
- 32.Isayama S. An efficient method for the direct peroxygenation of various olefinic compounds with molecular oxygen and triethylsilane catalyzed by a cobalt(II) complex. Bull Chem Soc Jpn. 1990;63:1305–1310. [Google Scholar]
- 33.Cuerva JM, Campana AG, Justicia J, Rosales A, Oller-Lopez JL, Robles R, Cardenas DJ, Bunuel E, Oltra JE. Water: The ideal hydrogen-atom source in free-radical chemistry mediated by Ti-III and other single-electron transfer metals. Angew Chem Int Ed. 2006;45:5522–5526. doi: 10.1002/anie.200600831. [DOI] [PubMed] [Google Scholar]
- 34.Wiesner K. Total synthesis of racemic talatisamine. Pure Appl Chem. 1975;41:93–112. [Google Scholar]
- 35.Schotes C, Mezzetti A. Asymmetic Diels-Alder reactions of unsaturated β-ketoesters catalyzed by chiral ruthenium PNNP complexes. J Am Chem Soc. 2010;132:3652–3653. doi: 10.1021/ja910039e. [DOI] [PubMed] [Google Scholar]
- 36.Schotes C, Althaus M, Aardoom R, Mezzetti A. Asymmetric Diels-Alder and Ficini reactions with alkylidene β-ketoesters catalyzed by chiral ruthenium PNNP complexes: Mechanistic insight. J Am Chem Soc. 2012;134:1331–1343. doi: 10.1021/ja210372u. [DOI] [PubMed] [Google Scholar]
- 37.Oyama H, Orimoto K, Niwa T, Nakada M. Highly enantioselective catalytic asymmetric Mukaiyama-Michael reactions of cyclic α-alkylidene-β-oxo imides. Tetrahedron: Asymmetry. 2015;26:262–270. [Google Scholar]
- 38.Orimoto K, Oyama H, Namera Y, Niwa T, Nakada M. Catalytic asymmetric [4+2] cycloadditions and Hosomi-Sakurai reactions of α-alkylidene-β-keto imides. Org Lett. 2013;15:768–771. doi: 10.1021/ol303381c. [DOI] [PubMed] [Google Scholar]
- 39.Corey EJ, Cheng X-M. The Logic of Chemical Synthesis. Wiley; New York: 1989. [Google Scholar]
- 40.Hoffmann RW. Elements of Synthesis Planning. Springer-Verlag; Berlin Heidelberg: 2009. [Google Scholar]
- 41.Steinbeck C, Han Y, Kuhn S, Horlacher O, Luttmann E, Willighagen E. The chemistry development kit (CDK): An open-source Java library for chemo- and bioinformatics. J Chem Inf Comput Sci. 2003;43:493–500. doi: 10.1021/ci025584y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steinbeck C, Hoppe C, Kuhn S, Floris M, Guha R, Willighagen EL. Recent developments of the chemistry development kit (CDK) – An open-source Java library for chemo- and bioinformatics. Curr Pharm Des. 2006;12:2111–2120. doi: 10.2174/138161206777585274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.