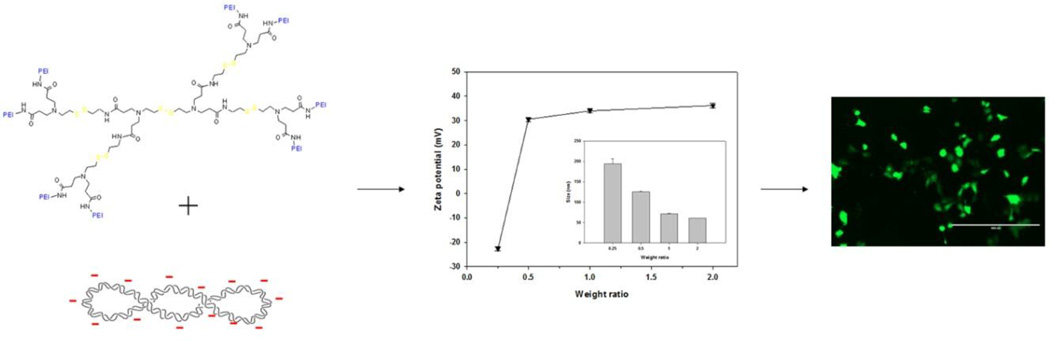
Abstract
Branched poly(ethylenimine) (PEI) 25 kDa is an efficient gene delivery vector with outstanding gene condensation ability and great endosome escape activity. However, it also induces higher cytotoxicity. Transfection efficiency and toxicity of PEI are highly dependent upon their molecular weight and structure. We developed a bioreducible poly(ethylenimine) (PEI (-s-s-)) derived from low molecular weight PEI (1.8 kDa) for efficient gene delivery. Bioreducible core molecule is expected to increase molecular weight and reduce the cytotoxicity of the copolymer. PEI (-s-s-) polyplexes showed higher transfection efficiency and lower cytotoxicity compared to branched PEI 25 kDa, Lipofectamine® 2000 and, FuGENE® 6. In addition, PEI (-s-s-) derivative (16 kDa) formed stable polyplexes with a zeta-potential value of +34 mV and polyplex size of 61 nm. PEI (-s-s-) derivative (16 kDa) showed excellent transfection efficiency: 3.6 times higher than branched PEI 25 kDa in HeLa cells and 7.4 times higher than Lipofectamine® 2000 in H9C2 cell. The derivatives also showed lower cytotoxicity compared with Lipofectamine® 2000 and PEI 25 kDa in various cell types. In addition, newly synthesized PEI (-s-s-) derivatives have high reproducibility.
Keywords: Biodegradation, Cytotoxicity, Gene expression, Poly(ethylenimine), Dendrimer
Graphical Abstract
1. Introduction
Gene therapy has potential in treating many diseases, such as cancers, infectious diseases, and immune system disorders. Techniques for directly killing diseased cells, producing or inhibiting disease-related protein, and regulating the immune system have been applied to these diseases using gene therapy [1–4]. Efficient delivery of therapeutic genes to target cells is the most important steps of gene therapy [2, 5]. Both viral and non-viral delivery systems have been used for gene delivery [6]. Compared with viral vectors, non-viral vectors have advantages such as large-scale production, low immune responses, flexible loading capacity of therapeutic genes, and stability of the vector [7, 8]. The development of efficient and safe non-viral delivery vectors is an important issue in non-viral gene therapy [9, 10].
The typical non-viral vectors are cationic lipids and polymers. Among the cationic polymers, branched poly(ethylenimine) (PEI) 25 kDa is one of the most popular and inexpensive gene delivery vectors. Because of its high amine density, PEI shows outstanding gene encapsulation efficiency and great endosome escape activity. However, it could destabilize the cell membrane, which will cause cytotoxicity to the cell [11–14]. Transfection efficiency and toxicity of cationic polymer are highly dependent upon molecular weight and structure. For example, high molecular weight (HMW) PEI shows a high transfection efficiency but, it also induces higher cytotoxicity. Therefore, high toxicity of HMW PEI limits transfection to in vitro and in vivo conditions. Contrast, low molecular weight (LMW) PEI has lower cytotoxicity, but its transfection efficiency is very poor. Therefore, LMW PEI cannot be used as a non-viral gene delivery vector [15, 16].
There have been several approaches to decrease the toxicity, while maintaining the merits of high transfection efficiency by combining PEI with biodegradable polymer to make an ideal non-viral vector. One way to overcome the cytotoxicity of cationic polymer is the introduction of biodegradable bonds such as ester and disulfide-bonds [17]. Nam et al. reported that the cytotoxicity of dendrimers could be decreased through the introduction of ester bond [18]. However, ester bonds are readily hydrolyzed in an aqueous environment. Contrast, disulfide bonds are not reduced until they are exposed to reducing agents such as β-mercaptoethanol (BME), dithiothritol (DTT), and glutathione (GSH) [19]. Therefore, disulfide-linked polymers are more stable than ester-linked polymers in the extracellular environment. In addition, disulfide bonds can be cleaved by glutathione in the intracellular cytoplasm [20]. Ou et al. synthesized and evaluated a family of bioreducible poly(disulfide amine)s as polymeric gene vectors. In that study, they showed that transfection efficiency and toxicity of cationic polymer are highly dependent upon molecular weight and structure [21]. Low molecular weight polymer required high weight ratio to indicate a high transfection efficiency. This was also associated with cytotoxicity. The disulfide-containing PEI derivatives were also reported by several groups. For example, Peng et al. reported that higher transfection efficiency could be obtained via thiolation of low molecular weight PEI (800 Da). In 2010, Koo et al. have synthesized biodegradable branched PEI by crosslinking linear PEIS which showed high transfection efficiency. Moreover, disulfide-containing PEIs were synthesized via click chemistry by Liu et al. [22–27].
Branched PEI consists 25, 50 and 25 % of primary, secondary, and tertiary amine groups [28]. Because PEI has many primary amine groups, it can be easily modified to optimize its gene delivery activity and cytotoxicity [29]. To overcome the limitations of current PEI gene delivery systems, LMW PEI was connected with biodegradable core molecule. Bioreducible PEI (PEI (-s-s-)) was developed to impart biodegradable characteristics while maintaining the amine density.
Generally, the controlled synthesis of dendrimers results low polydispersity while linear synthetic polymer has a high polydispersity. Moreover, dendrimer has well-defined numbers of terminal groups for the conjugation. In this study, we hypothesized that the conjugation of PEI 1.8 kDa with dendritic core molecule would increase the molecular weight of PEI, and that increasing molecular weight would also increase the transfection efficiency. In addition, we expected that polymers would have high stability because using a disulfide bond rather than ester bond. We also expected that polymers would have a low polydispersity (P.D.) value because of the well-defined structure and surface functionality of dendritic core molecules. In this study, we described: 1) the synthesis and characterization of this novel bioreducible PEI derivatives; 2) its polyplex formation; 3) transfection efficiency; 4) cytotoxicity.
2. Materials and methods
2.1. Materials
Branched polyethylenimine (bPEI 1.8 kDa, Mw 1,800 Da) was purchased from Polysciences (Warrington, PA). Branched polyethylenimine (bPEI 25 kDa, Mw 25,000 Da), methanol (MeOH), cystamine dihydrochloride, triethylamine (TEA), methyl acrylate (MA), magnesium sulfate, diethyl ether, and thiazolyl blue tetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, MO). BCA protein assay kit was purchased from Pierce (Rocford, IL). The Luciferase assay system, reporter lysis buffer, and FuGENE® 6 were purchased from Promega (Madison, WI). Dulbecco’s modified Eagle’s medium (DMEM), Opti-MEM®, Dulbecco′s phosphate buffered saline (DPBS), TrypLE™ Express, SYBR safe DNA gel stain, and Lipofectamine® 2000 were purchased from Invitrogen (Carlsbad, CA). Fetal bovine serum (FBS) was purchased from Seradigm (Radnor, PA). Dialysis membranes were purchased from Spectrum Laboratories (Rancho Dominguez, CA).
2.2. Preparation of bioreducible poly(ethylenimine)
2.2.1. Synthesis of 1st core molecule
Methyl acrylate (0.213 mol) was mixed with 10 mL of MeOH. Cystamine dihydrochloride (8.881 mmol) and TEA (0.018 mol) were dissolved in 20 mL of MeOH and cystamine solution was added dropwise to methyl acrylate solution over 30 min. After two days of reaction at room temperature under a nitrogen atmosphere, MeOH and TEA were removed by evaporation. A viscous light yellow liquid interspersed with white precipitate was dissolved in diethyl ether and extracted with water. Diethyl ether layers were dried over magnesium sulfate, filtered, and evaporated (yield: 82.97%, theoretical mass: 496.6 Da).
2.2.2. Synthesis of PEI (-s-s-) 7.6 kDa
bPEI 1.8 kDa (8.055 mmol) was mixed with 50 mL of MeOH. The 1st core molecule (0.201 mmol) was dissolved in 20 mL of MeOH and the 1st core solution was added to bPEI solution dropwise over 30 min. After three days of reaction at room temperature under a nitrogen atmosphere, MeOH was removed by evaporation. A viscous liquid was dissolved in water and dialyzed (MWCO = 3,500 Da). Then, the product was filtered, and lyophilized (yield: 68.40%).
2.2.3. Synthesis of 2nd core molecule
Cystamine dihydrochloride (0.040 mol) and TEA (0.081 mol) were dissolved in 100 mL of MeOH. The 1st core molecule (1.007 mmol) was dissolved in 50 mL of MeOH and the 1st core solution was added to cystamine solution dropwise over 30 min. After four days of reaction at 4 °C under a nitrogen atmosphere, MeOH and TEA were removed by evaporation. The mixture was dissolved in water and dialyzed (MWCO= 500 Da). Then, the sample was lyophilized (yield: 50.72%, theoretical mass: 977.6 Da).
Methyl acrylate (0.068 mol) was mixed with 12 mL of MeOH. The lyophilized sample (0.853 mmol) and TEA (6.824 mmol) were dissolved in 8 mL of MeOH and sample solution was added dropwise over 30 min. After two days of reaction at room temperature under a nitrogen atmosphere, MeOH and TEA were removed by evaporation. Then, the product was extracted with diethyl ether and evaporated (yield: 74.66%, theoretical mass: 1666.3 Da).
2.2.4. Synthesis of PEI (-s-s-) 16 kDa
bPEI 1.8 kDa (9.602 mmol) was mixed with 50 mL of MeOH. The 2nd core molecule (0.120 mmol) was dissolved in 20 mL of MeOH and the 2nd core solution was added to bPEI solution dropwise over 30 min. After five days of reaction at room temperature under a nitrogen atmosphere, MeOH was removed by evaporation. A product was dissolved in water and dialyzed (MWCO = 10,000 Da). Then, the product was filtered and lyophilized (yield: 58.74%).
2.2.5. Synthesis of 3rd core molecule
Cystamine dihydrochloride (0.048 mol) and TEA (0.096 mol) were dissolved in 100 mL of MeOH. The 2nd core molecule (0.600 mmol) was dissolved in 50 mL of MeOH and the 2nd core solution was added to cystamine solution dropwise over 30 min. After four days of reaction at 4 °C under a nitrogen atmosphere, MeOH and TEA were removed by evaporation. The mixture was dissolved in water and dialyzed (MWCO= 1,000 Da). Then, the sample was lyophilized (yield: 47.55%, theoretical mass: 2628.2 Da).
Methyl acrylate (0.053 mol) was mixed with 12 mL of MeOH. The lyophilized sample (0.329 mmol) and TEA (5.264 mmol) were dissolved in 8 mL of MeOH and sample solution was added dropwise over 30 min. After three days of reaction at room temperature under a nitrogen atmosphere, MeOH and TEA were removed by evaporation. Then, the product was extracted with diethyl ether and evaporated (yield: 72.45%, theoretical mass: 4005.7 Da).
2.2.6. Synthesis of PEI (-s-s-) 32 kDa
bPEI 1.8 kDa (7.987 mmol) was mixed with 50 mL of MeOH. The 3rd core molecule (0.050 mmol) was dissolved in 20 mL of MeOH and the 3rd core solution was added to bPEI solution dropwise over 30 min. After five days reaction at room temperature under a nitrogen atmosphere, MeOH was removed by evaporation. . A product was dissolved in water and dialyzed (MWCO = 10,000 Da). Then, the product was filtered and lyophilized (yield: 52.69%).
2.3. Physical properties
The molecular weight of PEI derivatives was estimated by size exclusion chromatography (SEC) using AKTA FPLC system (Superdex 75 column). Poly[N-(2-hydroxypropyl)methacrylamide] (pHPMA) was used as molecular-weight standards. Acetate buffer (0.1 M ammonium acetate, 30% acetonitrile, pH 5.5) was used as eluent. Concentration of polymer was 3 mg/mL and flow rate was 0.4 mL/min. In order to validate SEC results, static light scattering method was used for the measurement of molecular weight of PEI derivatives.
PEI (-s-s-) derivatives have internal disulfide bonds, which are degraded in the reductive environment. To determine the impact of the reducing agent, PEI (-s-s-) 7.6 kDa was incubated in 10 mM DTT which is a well-known reducing agent. After one hour of incubation at room temperature, the molecular weight of PEI (-s-s-) 7.6 kDa was measured by using the Nano ZS (Malvern Instruments Ltd., Worcestershire, UK).
2.4. Gel retardation assay
0.5 µg of plasmid DNA (pDNA, gWiz-Luc) was mixed with PEI derivatives in 10 µL of HEPES buffered saline (10 mM HEPES, 1 mM NaCl, pH 7.4) at various weight ratios, and incubated 30 min. After 30 min of incubation at room temperature, 2 µL of loading dye was added to each sample and then the samples were analyzed by electrophoresis on a 0.7% agarose gel plate containing SYBR safe gel staining solution.
2.5. Particle size and surface charge measurement
The average size and zeta-potential of each sample of bioreducible PEI derivatives with 4 µg pDNA (gWiz-Luc) at 25°C were examined using the Nano ZS with a He-Ne laser (633 nm). 100 µL of polyplex solutions were prepared in HEPES buffered saline (10 mM HEPES, 1 mM NaCl, pH 7.4) at various weight ratios ranging from 0.25 to 2, respectively. After 30 min of incubation, sample solutions were diluted to a final volume of 600 µL before the measurement.
2.6. Cell lines and culture
Human lung adenocarcinoma epithelial cell line (A549), human cervical cancer cell line (HeLa), and rat cardiomyoblast cell line (H9C2) were grown in DMEM containing 10% FBS. The cells were maintained on plastic tissue culture dishes at 37 °C in a humidified atmosphere containing 5% CO2.
2.7. Luciferase expression and cytotoxicity
3.0×104 cells were seeded in 24-well plates in 500 µL of medium (DMEM) containing 10% FBS for a day before transfection. 0.5 µg of pDNA (gWiz-Luc) was complexed with PEI (-s-s-) derivatives in 50 µL of serum free medium (Opti-MEM®) and incubated for 30 min at room temperature. Lipofectamine® 2000 and FuGENE® 6 were used as controls (incubation time: 15 min). To create an in vitro environment that more accurately mimics in vivo conditions, the medium was replaced by 450 µL of medium (DMEM) containing 50% FBS and the polyplexes were added. For FBS free condition, the medium was replaced by 450 µL of serum free medium (DMEM) and the polyplexes were added. Following four hours treatment of polyplexes, the medium was replaced by 500 µL of fresh medium (DMEM) containing 10% FBS, and plates were incubated for 2 days at 37 °C.
For the luciferase assay, the cells were washed with DPBS and lysed with 200 µL of reporter lysis buffer for 30 min at room temperature. The luciferase activity of 20 µL cell lysate was evaluated by using Tecan Infinite M200 Pro spectrophotometer (Tecan Group Ltd., Männedorf, Switzerland) and the total protein concentration was measured by using a Micro BCA assay reagent kit (Pierce, Rockford, IL).
For cytotoxicity assay of polyplexes, 20 µL of filtered MTT solution (2 mg/mL in DPBS) was added to each well and incubated further for 2 h. After incubation, the medium was removed from the well and 500 µL DMSO was added to dissolve insoluble formazan crystals. The absorbance was measured at 565 nm using a Tecan Infinite M200 Pro spectrophotometer and the cell viability was calculated as a percentage relative to untreated control cells. All experiments were performed in triplicate.
2.8. GFP expression
A549 (1.0×105 cells/well) cells were seeded in 12-well plates with 1 mL of medium (DMEM) containing 10% FBS for a day before transfection. 1.0 µg of pDNA (gWiz-GFP) was complexed with PEI (-s-s-) derivatives in 100 µL of serum free medium (Opti-MEM®), and incubated for 30 min at room temperature. Lipofectamine® 2000 and FuGENE® 6 were used as controls (incubation time: 15 min). The medium was replaced by 900 µL of serum free medium (DMEM), and polyplex was added. After four hours of incubation, the cells were washed with DPBS and the medium was replaced by 1 mL of fresh medium (DMEM) containing 10% FBS, and then incubated further for 2 days at 37 °C. The GFP expression was evaluated by using an EVOS microscope (AMG, Bothell, WA).
2.9. Cytotoxicity of polymers
Cells were seeded at 5000 cells/well in 96-well plates in 90 µL of DMEM containing 10% FBS and incubated at 37°C for one day. Then 10 µL of polymer solution at various concentrations was added and cells were incubated for 2 days before assay.
To measure the cytotoxicity of PEI (-s-s-), MTT assays were performed. 10 µL of filtered MTT solution (2 mg/mL in DPBS) was added to each well and incubated further for 2 h. After incubation, the medium was removed from the well and 100 µL DMSO was added to dissolve insoluble formazan crystals. The absorbance was measured at 565 nm using a Tecan Infinite M200 Pro spectrophotometer and the cell viability was calculated as a percentage relative to untreated control cells. All experiments were performed in quintuplicate.
3. Results and discussion
3.1. Synthesis of bioreducible poly(ethylenimine) derivatives
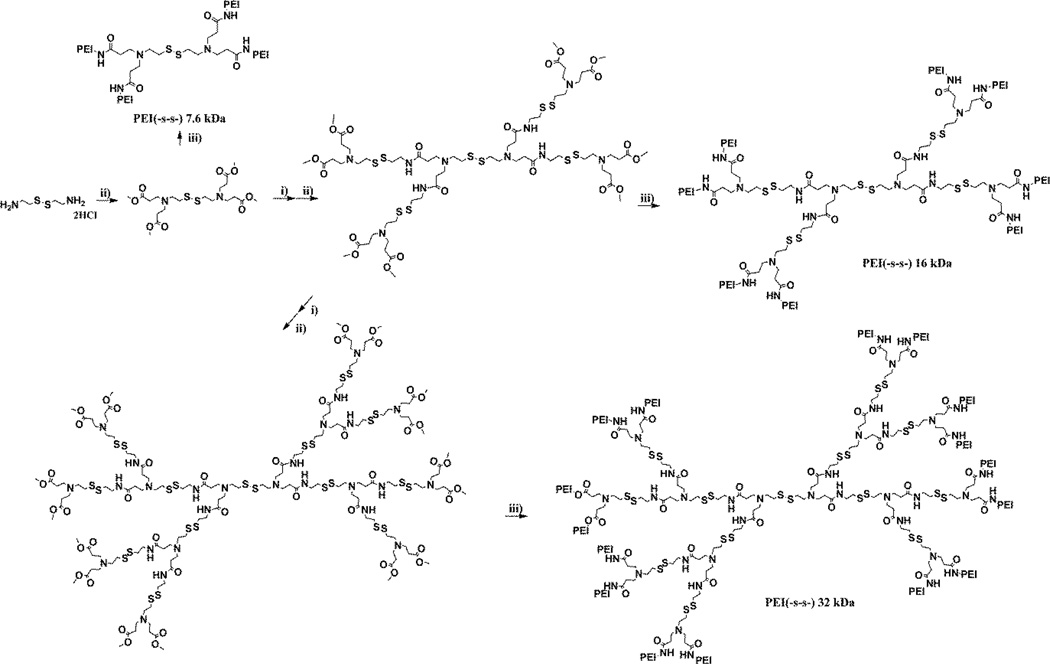
Figure 1 shows the molecular structure of the bioreducible poly(ethylenimine) derivatives. Michael addition-amidation reactions were performed repeatedly for the synthesis of core molecules containing disulfide bonds [30]. After synthesizing the dendritic core molecule, bPEI 1.8 kDa was conjugated with core molecule. Each reaction step was monitored by Thin-Layer Chromatography (TLC) and 1H NMR using the methoxy proton signal. For 1H NMR analysis, the samples were dissolved either in MeOD or D2O. Because of the unique 1H NMR peak for methoxy group of methyl acrylate (δ 3.66), reactions were easy to identify. The 1H NMR results of higher molecular weight polymers showed similar patterns with a 7.6 kDa polymer. The 1H NMR spectrum of the each polymer is shown in figure S1.
Cystamine dihydrochloride (400 MHz, D2O)
δ 3.05 (-SCH2CH2NH3+), δ 3.42 (-SCH2CH2NH3+)
1st core molecule (400 MHz, MeOD)
δ 2.48 (-NCH2CH2CO-), δ 2.80 (-SCH2CH2N-, -NCH2CH2CO-), δ 3.66 (-O-CH3)
Bioreducible PEI (-s-s-) 7.6 kDa (400 MHz, D2O)
δ 2.28 (-NCH2CH2CO-; core), δ 2.45~2.75 (-NCH2CH2N-; PEI), δ 2.98~3.38 (-SCH2CH2N-, -NCH2CH2CO-; core)
Figure 1.
The synthesis scheme and structure of bioreducible poly(ethylenimine) derivatives. i) Cystamine dihydrochloride, ii) methyl acrylate, iii) bPEI 1.8 kDa
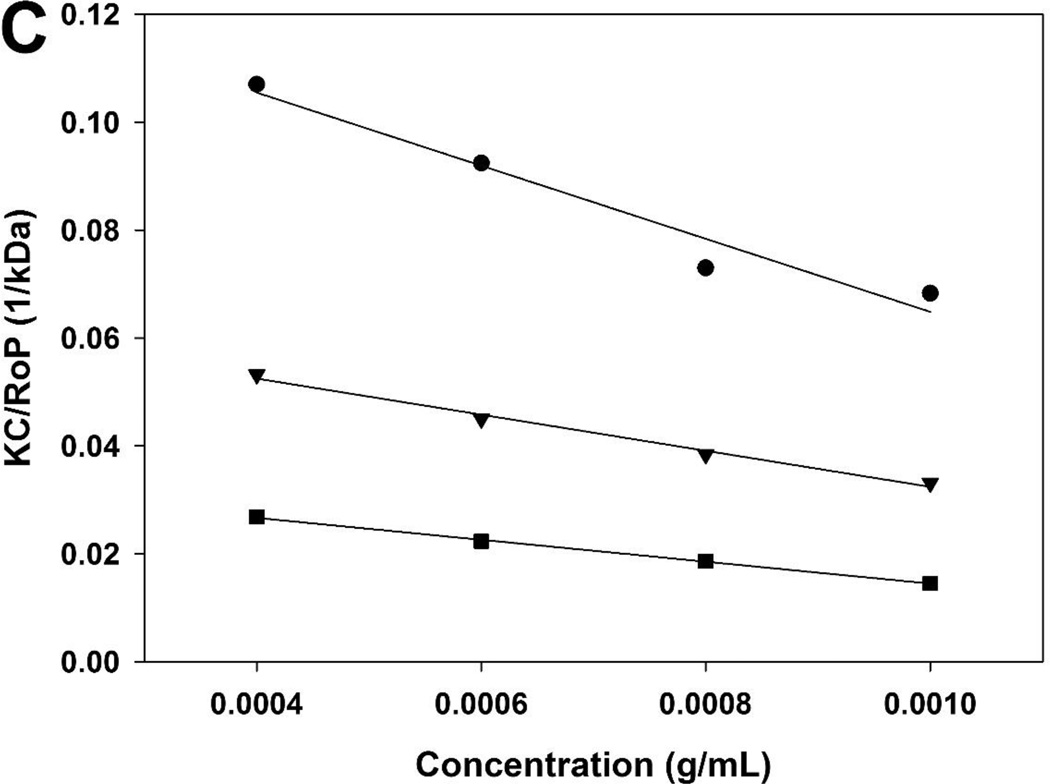
The molecular weight was calculated size exclusion chromatography (SEC) data and static light scattering data (Table 1, figure 2). Based on the results, one bPEI 1.8 kDa had been conjugated to each primary amine branches of the dendritic core molecule. The molecular weight of bioreducible poly(ethylenimine) derivatives was estimated to be 7.6 kDa (P.D. = 1.20), 16.3 kDa (P.D. = 1.26), and 32.6 kDa (P.D. = 1.24), respectively. P.D. values of step polymerization reactions were typically around 2.0. However, P.D. values of PEI (-s-s-) derivatives were about 1.26. This represents that PEI (-s-s-) derivatives have high reproducibility.
Table 1.
Molecular weights and second virial coefficients (A2) of PEIs
| Size exclusion chromatography | Static light scattering | |||
|---|---|---|---|---|
| kDa | polydispersity | kDa | A2 (mL mol/g2) | |
| PEI(-s-s-) 7.6 kDa | 7.6 | 1.20 | 7.57 ± 0.41 | −0.0336 |
| PEI(-s-s-) 16 kDa | 16.3 | 1.26 | 15.20 ± 0.82 | −0.0168 |
| PEI(-s-s-) 32 kDa | 32.6 | 1.24 | 31.30 ± 1.70 | −0.0081 |
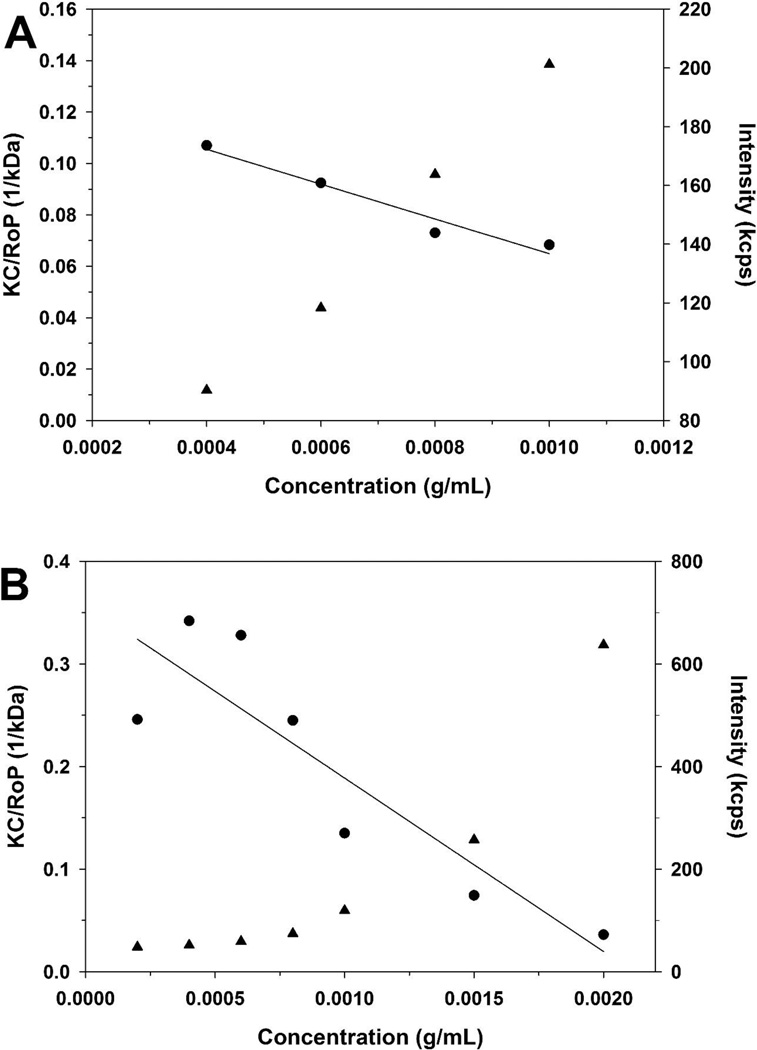
Figure 2.
Debye plots for PEI (-s-s-) 7.6 kDa before (A) and after (B) DTT treatment. KC/RθP (●) and scattering intensity (▲). (C) Debye plots for PEI (-s-s-) 7.6 kDa (●), 16 kDa (▼) and 32 kDa (■)
3.2. Degradation study
Because of the low extracellular concentrations of GSH, disulfide bonds are generally stable during the circulation, however undergo rapid cleavage in the intracellular cytoplasm. Therefore, disulfide-based cationic gene delivery polymers have been developed by several groups and, disulfide-based polymers usually showed low toxicity and rapid gene release profiles in the intracellular cytoplasm [22, 24]. PEI (-s-s-) derivatives have internal disulfide bonds, which are degraded in the reductive environment. To determine the impact of the reducing agent, PEI (-s-s-) 7.6 kDa was incubated in DTT solution. After one hour of incubation at room temperature, the molecular weight of the polymer was measured by using the Nano ZS (figure 2). Static light scattering experiments can be used to determine the molecular weight.
As a result, the molecular weight of PEI 7.6 kDa was calculated as 7.57 ± 0.41 kDa (R2 = 0.96, A2 = −0.0336 mL mol/g2) before DTT treatment. After DTT treatment, the average molecular weight of polymer was decreased (2.79 ± 0.30 kDa, R2 = 0.79, A2 = −0.0846 mL mol/g2). Because it is a mixture of different polymers after DTT treatment, a correlation coefficient (R2) was only 0.79 after DTT treatment.
3.3. Particle formation and stability
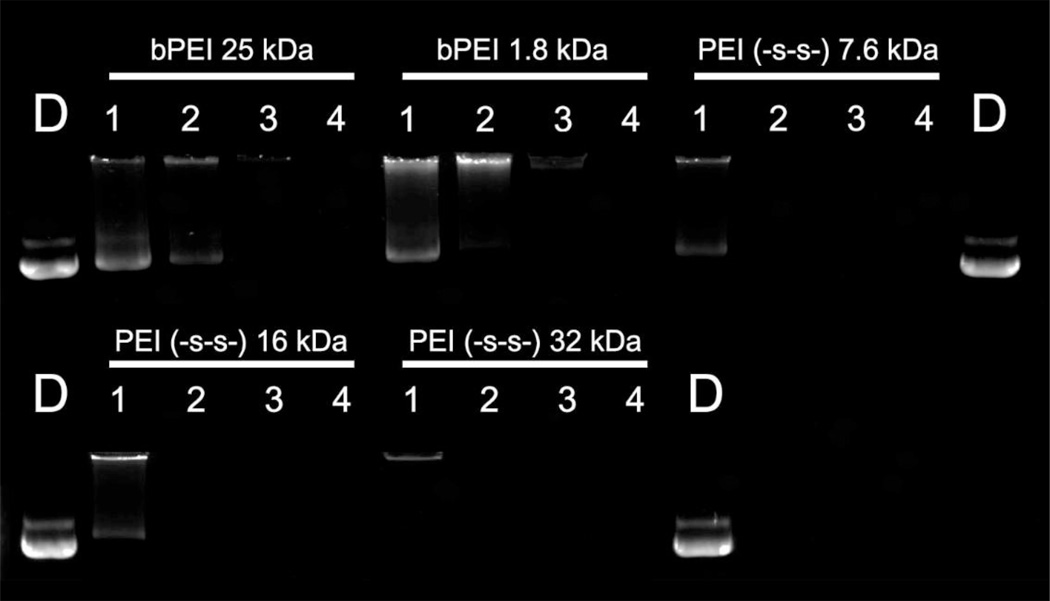
The formation of nano-sized polyplexes with a positive charge is the most important factor in polymeric gene delivery [31, 32]. To confirm the polyplex formation, polyplexes were prepared at various weight ratios ranging from 0.25 to 2. Non-degradable branched PEI 25 kDa and 1.8 kDa were used as controls. First, the self-assembly of the synthesized polymers with pDNA was investigated by gel retardation assay at 37 °C. As shown in figure 3, all synthesized polymers showed a complete retardation of pDNA at a weight ratio of 0.25.
Figure 3.
Agarose gel electrophoresis retardation assay of PEI derivatives with 0.5 µg pDNA. pDNA only (lane D), weight ratios of polymer/pDNA = 0.25, 0.5, 1 and 2 (lanes 1, 2, 3 and 4, respectively). The negative particles migrated toward the positive electrode under the electric field.
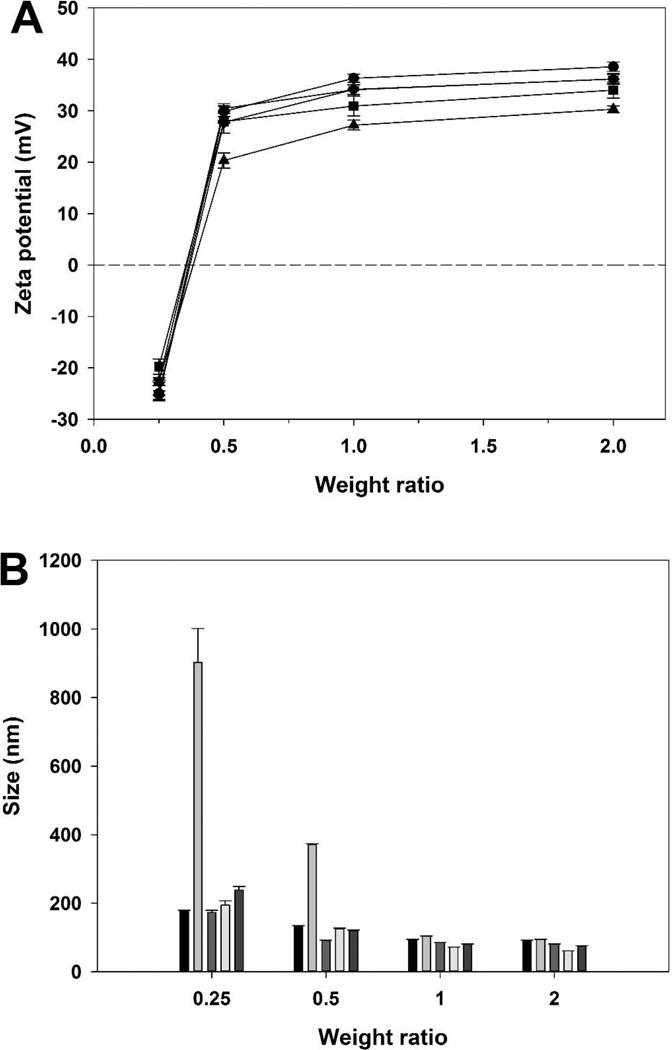
Then, the particle size and the surface charge of polyplexes were measured to determine the condensing ability of the synthesized polymers. The surface charge increased with increasing weight ratios for all polymers (figure 4A). Surface charge of the PEI (-s-s-) 7.6 kDa polyplex (36.20 ± 0.93 mV), the PEI (-s-s-) 16 kDa polyplex (34.03 ± 1.56 mV), and the PEI (-s-s-) 32 kDa polyplex (36.17 ± 1.06 mV) were stronger than the bPEI 1.8 kDa (30.32 ± 0.66 mV) and weaker than the bPEI 25 kDa (38.57 ± 0.90 mV) at weight ratio of 2. In addition, the particle size decreased with increasing weight ratios (figure 4B). The particle sizes of PEI (-s-s-) polyplexes (7.6 kDa: 80.95 ± 0.30 nm, 16 kDa: 61.04 ± 0.14 nm, and 32 kDa: 74.35 ± 1.99 nm) were smaller than bPEI 25 kDa (90.82 ± 1.30 nm) and 1.8 kDa (94.22 ± 0.86 nm). These results represent that condensing ability of PEI (-s-s-) was better than non-degradable branched bPEI 25 kDa and 1.8 kDa.
Figure 4.
The zeta potential (A) and size (B) analysis of PEI derivatives/pDNA polyplexes at various weight ratios. PEI 25 kDa (●,  ), PEI 1.8 kDa (▲,
), PEI 1.8 kDa (▲,  ), PEI (-s-s-) 7.6 kDa (▼,
), PEI (-s-s-) 7.6 kDa (▼,  ), PEI (-s-s-) 16 kDa (■,
), PEI (-s-s-) 16 kDa (■,  ), and PEI (-s-s-) 32 kDa (♦,
), and PEI (-s-s-) 32 kDa (♦,  ). Results are expressed as mean ± standard deviation (n=3).
). Results are expressed as mean ± standard deviation (n=3).
3.4. Transfection efficiency and cytotoxicity of polyplexes
The transfection efficiency of PEI (-s-s-) derivatives was evaluated in the A549, HeLa, and H9C2 cells using luciferase (gWiz-Luc) and GFP (gWiz-GFP) genes. bPEI 25 kDa, bPEI 1.8 kDa, Lipofectamine® 2000, and FuGENE® 6 were used as controls. In order to find the optimal gene transfer condition for each polymer, the transfection experiments were performed at various weight ratios. The selected conditions were as follows:
Table 2.
Selected weight ratios for transfection experiments
| Cell line | A549 | HeLa | H9C2 | ||||
|---|---|---|---|---|---|---|---|
| Polymer | serum | 50% | 0% | 50% | 0% | 50% | 0% |
| PEI 25 kDa | 1 | 1 | 1 | 1 | 1 | 1 | |
| Lipofectamine® 2000 | 2 | 2 | 2 | 2 | 2 | 2 | |
| FuGENE® 6 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | |
| PEI 1.8k Da | 8 | 4 | 6 | 4 | 8 | 6 | |
| PEI(-s-s-) 7.6 kDa | 8 | 4 | 6 | 4 | 8 | 6 | |
| PEI(-s-s-) 16 kDa | 8 | 4 | 6 | 4 | 8 | 6 | |
| PEI(-s-s-) 32 kDa | 8 | 4 | 6 | 4 | 8 | 6 | |
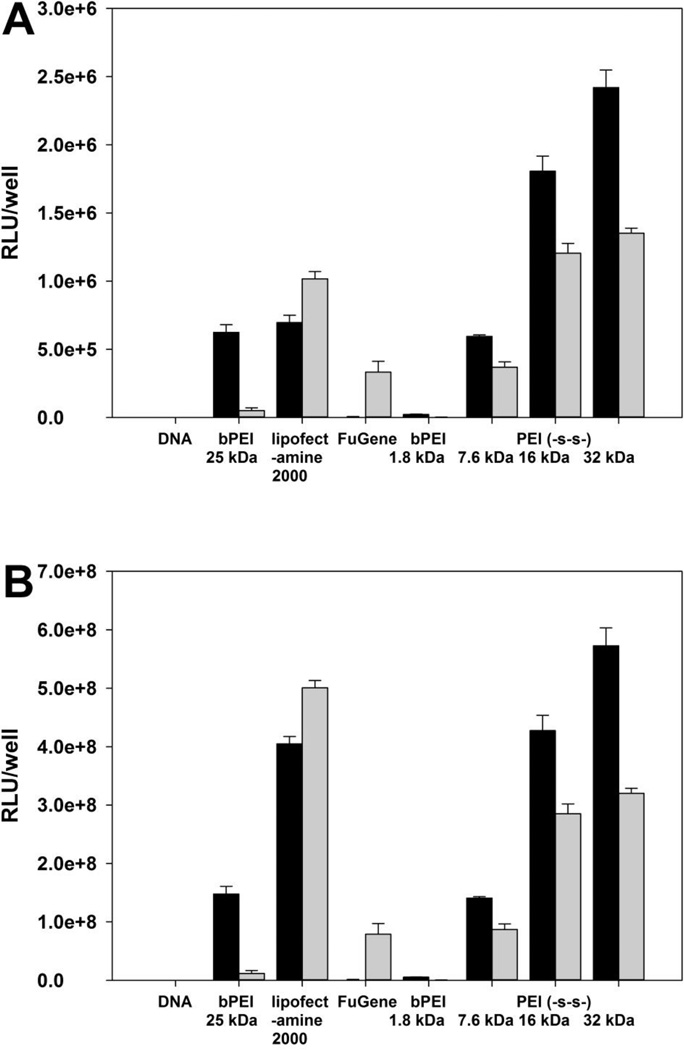
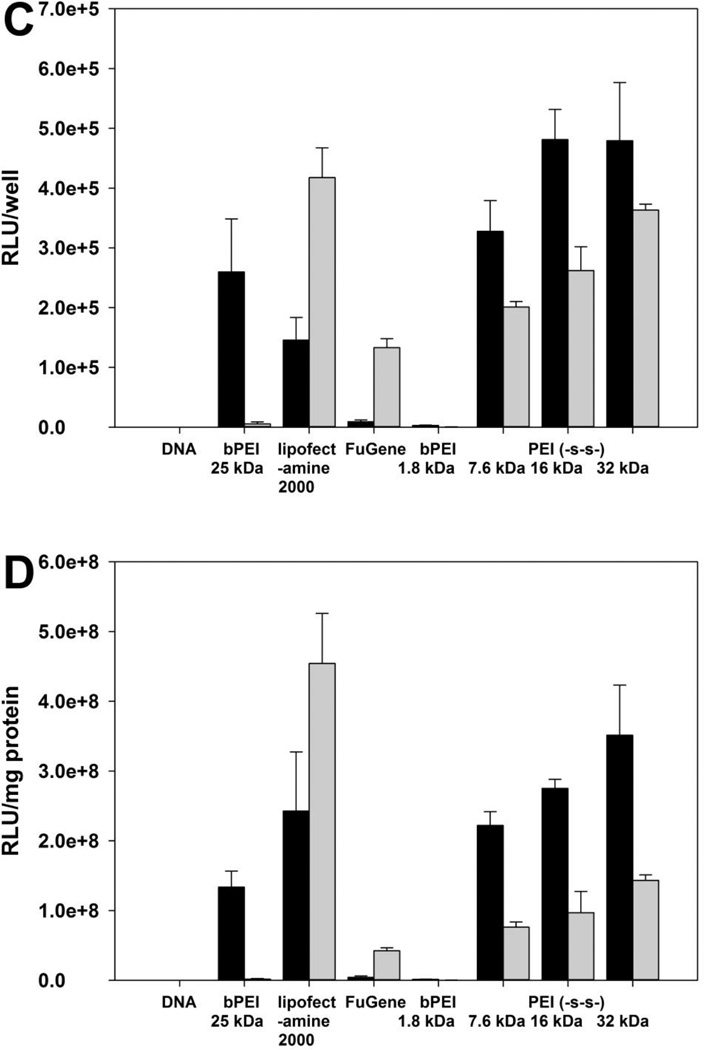
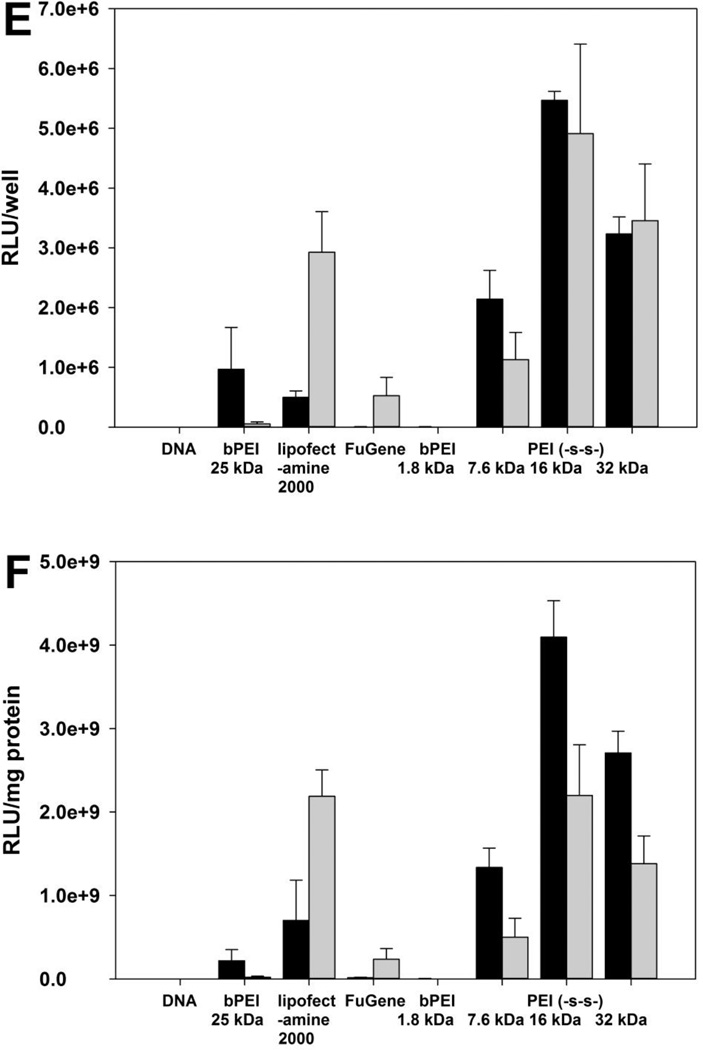
Whereas bPEI 1.8 kDa did not show the transfection efficiency, PEI (-s-s-) derivatives showed higher transfection efficiency at weight ratio of 4 for A549 and HeLa cell line, and 6 for H9C2 cell line (figure 5). Due to the cytotoxicity of polyplexes, the total amount of cellular protein from Lipofectamine® 2000 treated groups were much lower than other polymer treated groups. In order to prevent misinterpretation of results, the transfection efficiency was reported both RLU/well (A, C, E) and RLU/mg protein (B, D, F). In the HeLa cell line, PEI (-s-s-) 16 kDa and 32 kDa showed similar transfection efficiency at weight of 4 (figure 5C). The order of transfection efficiency was PEI (-s-s-) 32 kDa ≥ PEI (-s-s-) 16 kDa > PEI (-s-s-) 7.6 kDa > bPEI 25 kDa > Lipofectamine® 2000 > FuGENE® 6 ≈ bPEI 1.8 kDa (RLU/well). The transfection efficiency of PEI (-s-s-) polyplexes followed a similar order in the A549 cell line (figure 5 A, B). However, the transfection efficiency of PEI (-s-s-) 32 kDa was lower than PEI (-s-s-) 16 kDa in the H9C2 cell line (figure 5E, F) due to their cytotoxicity (figure 7C). As a result, PEI (-s-s-) derivatives exhibited an improved transfection efficiency in all three cell lines. Especially, PEI (-s-s-) 16 kDa gave 3.3 times higher gene expression than Lipofectamine® 2000 in HeLa cell and 11.0 times higher than Lipofectamine® 2000 in H9C2 cell.
Figure 5.
Transfection experiment results at various weight ratios in A549 cells (A, B), HeLa cells (C, D), and H9C2 cells (E, F). Results are expressed as mean ± standard deviation (n=3). Black bars for (−) FBS, gray bars for (+) FBS.
Figure 7.
Cytotoxicity assay of polyplexes in A549 cells (A), HeLa cells (B), and H9C2 cells (C) by MTT assay. Results are expressed as mean ± standard deviation (n=3).
To mimic in vivo conditions, the transfection efficiency was also evaluated in the presence of serum. As demonstrated in figure 5, the transfection efficiency of polymers slightly decreased at 50% FBS conditions. However, the transfection efficiency of PEI (-s-s-) derivatives showed much higher levels compared with PEI 25kDa, PEI 1.8kDa and FuGENE® 6. Interestingly, Lipofectamine® 2000 showed increased transfection efficiency in the 50% serum condition. However, the cytotoxicity induced by Lipofectamine® 2000 is still remained a big problem.
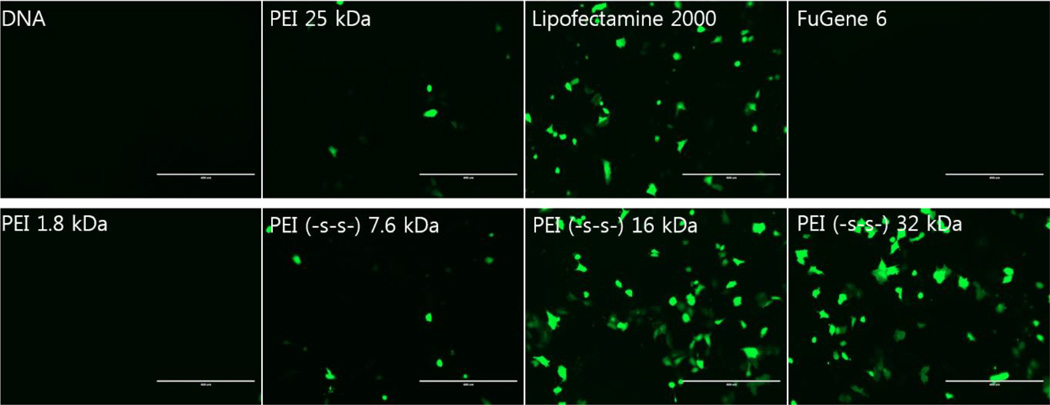
Transfection results with GFP gene showed similar results in A549 cells (figure 6). PEI (-s-s-) 16 kDa and 32 kDa showed higher transfection efficiency than other polymers at a weight ratio of 4. The same result was observed in other cells (data not shown).
Figure 6.
Green fluorescent protein expression levels in A549 cells
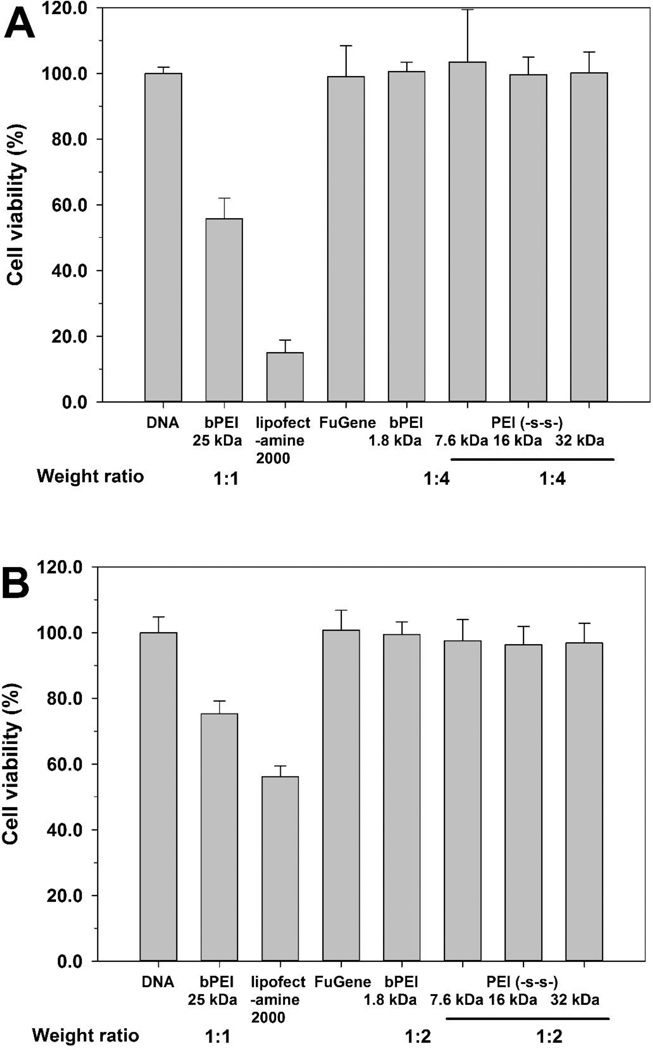
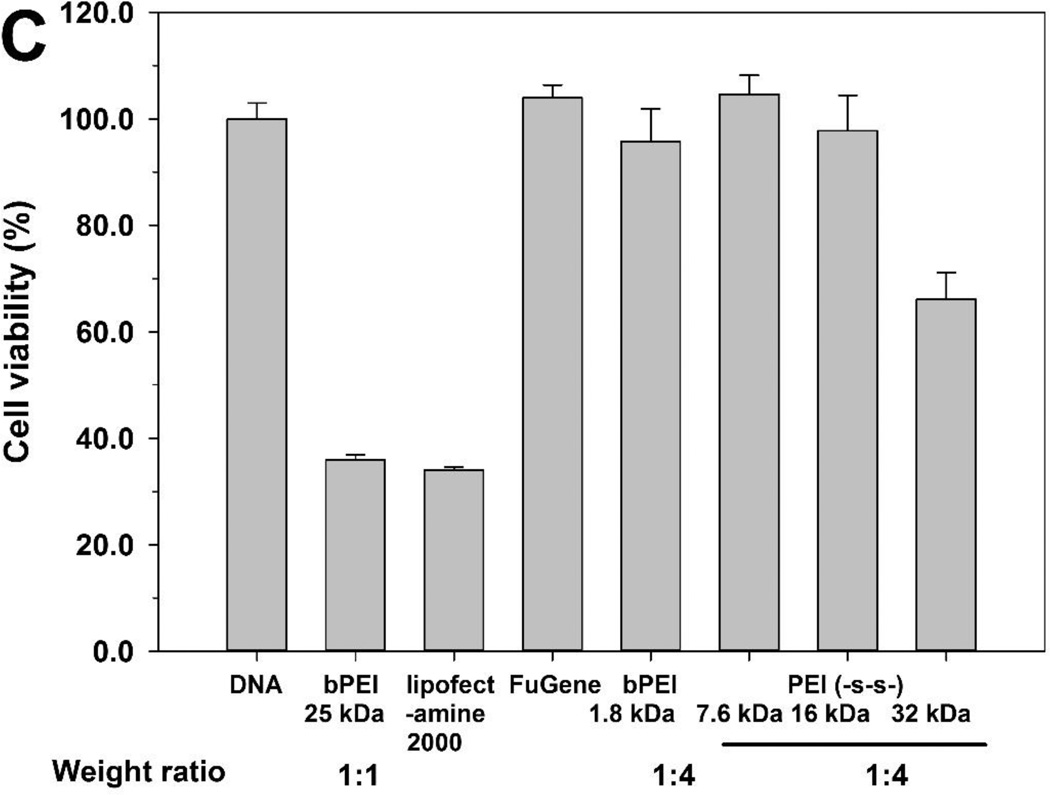
The cytotoxicity of polyplexes is a very important factor in polymeric gene delivery. The cytotoxicity of polyplexes is highly correlated with their surface charge and molecular weight. Generally, polyplex toxicity increases with increasing surface charge and molecular weight. However, PEI (-s-s-) derivatives exhibited a relatively low cytotoxicity due to their biodegradability (figure 7).
3.5. Cytotoxicity of polymer
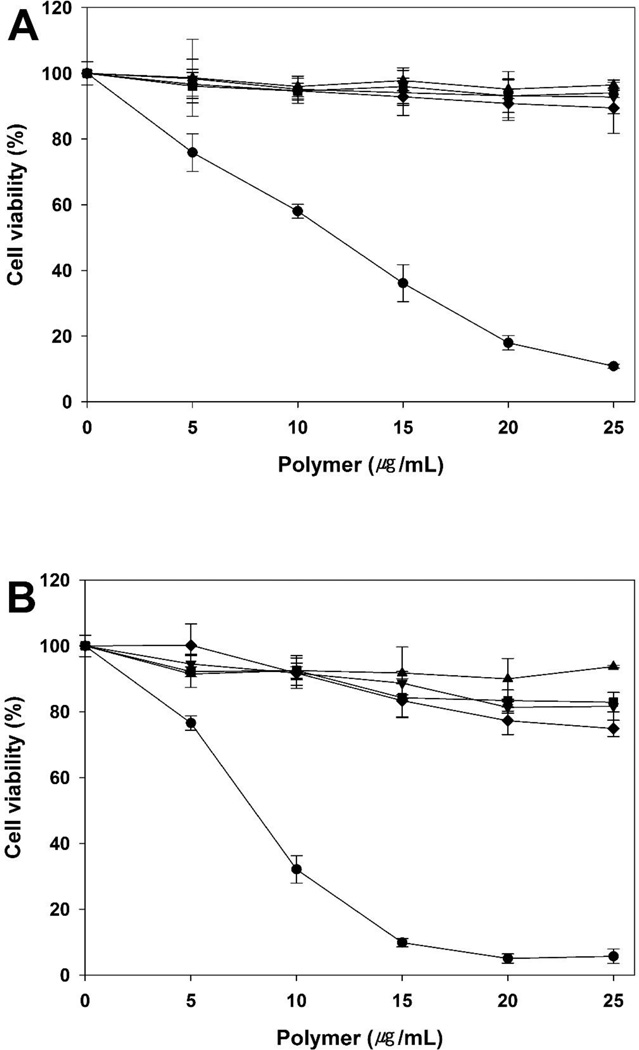
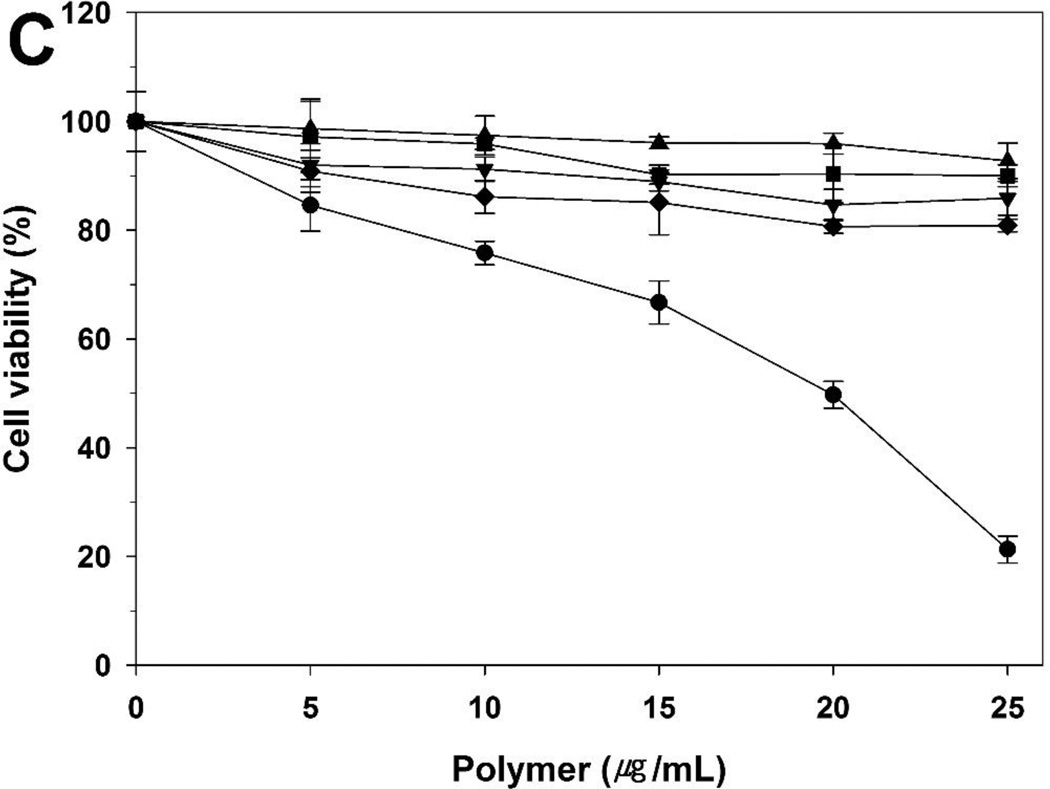
The cytotoxicity of polymers was evaluated in the A549, HeLa, and H9C2 cells by MTT assay (figure 8). bPEI 25 kDa and 1.8 kDa were used as controls. Normally, cell viability tends to decrease with increasing molecular weight. Therefore, PEI (-s-s-) derivatives showed slightly increased cytotoxicity compared to PEI 1.8 kDa. The order of cell viability was bPEI 1.8 kDa > PEI (-s-s-) 7.6 kDa >PEI (-s-s-) 16 kDa > PEI (-s-s-) 32 kDa » bPEI 25 kDa in all three cells. However, PEI (-s-s-) derivatives were almost non-toxic, while the relative cell viability with bPEI 25 kDa was below 20%. Moreover, despite the higher molecular weight, PEI (-s-s-) 32 kDa showed lower cytotoxicity than bPEI 25 kDa due to its biodegradability. From the results, we observed that bPEI 25 kDa was highly toxic and PEI (-s-s-) derivatives were much less toxic in all three cell lines.
Figure 8.
Cytotoxicity assay in A549 cells (A), HeLa cells (B), and H9C2 cells (C) at various concentrations of PEI derivatives by MTT assay. PEI 25 kDa (●), PEI 1.8 kDa (▲), PEI (-s-s-) 7.6 kDa (▼), PEI (-s-s-) 16 kDa (■), and PEI (-s-s-) 32 kDa (♦). Results are expressed as mean ± standard deviation (n=5).
4. Conclusion
Bioreducible cationic copolymers containing 1.8 kDa PEI and dendritic core molecule were synthesized and investigated for efficient gene delivery. Generally, the molecular weight and charge density of polymer affect the cytotoxicity and gene transfer activity. Enhanced transfection efficiency is expected from an increased molecular weight and decreased cytotoxicity is expected from the degradation of the core molecule in the intracellular cytoplasm. Three types of newly synthesized core molecule containing disulfide bonds have increased the molecular weight of PEI. The obtained PEI (-s-s-) derivatives showed higher transfection efficiency, as compared to non-degradable PEI 25 kDa, 1.8 kDa, Lipofectamine®, and FuGENE® 6. Moreover, the PEI (-s-s-) derivatives had relatively lower cytotoxicity due to the degradability of the polymers. In addition, these polymers showed great plasmid condensing ability. The above results lead us to conclude that the bioreducible poly(ethylenimine) has great potential as a gene carrier, especially PEI (-s-s-) 16 kDa.
Supplementary Material
Acknowledgments
This work was financially supported by NIH CA177932. The authors would like to thank Joshua P. Jones for help with preparing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Touchefeu Y, Harrington KJ, Galmiche JP, Vassaux G. Review article: gene therapy, recent developments and future prospects in gastrointestinal oncology. Aliment Pharmacol Ther. 2010;32:953–968. doi: 10.1111/j.1365-2036.2010.04424.x. [DOI] [PubMed] [Google Scholar]
- 2.Verma IM, Somia N. Gene therapy - promises, problems and prospects. Nature. 1997;389:239–242. doi: 10.1038/38410. [DOI] [PubMed] [Google Scholar]
- 3.Strayer DS, Akkina R, Bunnell BA, Dropulic B, Planelles V, Pomerantz RJ, Rossi JJ, Zaia JA. Current status of gene therapy strategies to treat HIV/AIDS. Mol Ther. 2005;11:823–842. doi: 10.1016/j.ymthe.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 4.Lewin AS, Glazer PM, Milstone LM. Gene therapy for autosomal dominant disorders of keratin. J Investig Dermatol Symp Proc. 2005;10:47–61. doi: 10.1111/j.1087-0024.2005.10207.x. [DOI] [PubMed] [Google Scholar]
- 5.Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. Non-viral vectors for gene-based therapy. Nat Rev Genet. 2014;15:541–555. doi: 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- 6.Lundstrom K, Boulikas T. Viral and non-viral vectors in gene therapy: technology development and clinical trials. Technol Cancer Res Treat. 2003;2:471–486. doi: 10.1177/153303460300200513. [DOI] [PubMed] [Google Scholar]
- 7.Kay MA, Liu D, Hoogerbrugge PM. Gene therapy. Proc Natl Acad Sci U S A. 1997;94:12744–12746. doi: 10.1073/pnas.94.24.12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mastrobattista E, Bravo SA, van der Aa M, Crommelin DJ. Nonviral gene delivery systems: From simple transfection agents to artificial viruses. Drug Discov Today Technol. 2005;2:103–109. doi: 10.1016/j.ddtec.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Niidome T, Huang L. Gene therapy progress and prospects: nonviral vectors. Gene Ther. 2002;9:1647–1652. doi: 10.1038/sj.gt.3301923. [DOI] [PubMed] [Google Scholar]
- 10.Al-Dosari MS, Gao X. Nonviral gene delivery: principle, limitations, and recent progress. AAPS J. 2009;11:671–681. doi: 10.1208/s12248-009-9143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo X, Huang L. Recent advances in nonviral vectors for gene delivery. Acc Chem Res. 2012;45:971–979. doi: 10.1021/ar200151m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nayerossadat N, Maedeh T, Ali PA. Viral and nonviral delivery systems for gene delivery. Adv Biomed Res. 2012;1:1–11. doi: 10.4103/2277-9175.98152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamazaki Y, Nango M, Matsuura M, Hasegawa Y, Hasegawa M, Oku N. Polycation liposomes, a novel nonviral gene transfer system, constructed from cetylated polyethylenimine. Gene Ther. 2000;7:1148–1155. doi: 10.1038/sj.gt.3301217. [DOI] [PubMed] [Google Scholar]
- 14.Fischer D, Bieber T, Li Y, Elsasser HP, Kissel T. A novel non-viral vector for DNA delivery based on low molecular weight, branched polyethylenimine: effect of molecular weight on transfection efficiency and cytotoxicity. Pharm Res. 1999;16:1273–1279. doi: 10.1023/a:1014861900478. [DOI] [PubMed] [Google Scholar]
- 15.Jia L, Li Z, Zhang D, Zhang Q, Shen J, Guo H, Tian X, Liu G, Zheng D, Qi L. Redox-responsive catiomer based on PEG-ss-chitosan oligosaccharide-ss-polyethylenimine copolymer for effective gene delivery. Polym Chem. 2013;4:156–165. [Google Scholar]
- 16.Huang H, Yu H, Tang G, Wang Q, Li J. Low molecular weight polyethylenimine cross-linked by 2-hydroxypropyl-gamma-cyclodextrin coupled to peptide targeting HER2 as a gene delivery vector. Biomaterials. 2010;31:1830–1838. doi: 10.1016/j.biomaterials.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Gosselin MA, Guo W, Lee RJ. Efficient gene transfer using reversibly cross-linked low molecular weight polyethylenimine. Bioconjug Chem. 2001;12:989–994. doi: 10.1021/bc0100455. [DOI] [PubMed] [Google Scholar]
- 18.Nam HY, Nam K, Hahn HJ, Kim BH, Lim HJ, Kim HJ, Choi JS, Park JS. Biodegradable PAMAM ester for enhanced transfection efficiency with low cytotoxicity. Biomaterials. 2009;30:665–673. doi: 10.1016/j.biomaterials.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Ahn CH, Chae SY, Bae YH, Kim SW. Biodegradable poly(ethylenimine) for plasmid DNA delivery. J Control Release. 2002;80:273–282. doi: 10.1016/s0168-3659(01)00547-8. [DOI] [PubMed] [Google Scholar]
- 20.Saito G, Swanson JA, Lee KD. Drug delivery strategy utilizing conjugation via reversible disulfide linkages: role and site of cellular reducing activities. Adv Drug Deliv Rev. 2003;55:199–215. doi: 10.1016/s0169-409x(02)00179-5. [DOI] [PubMed] [Google Scholar]
- 21.Ou M, Xu R, Kim SH, Bull DA, Kim SW. A family of bioreducible poly(disulfide amine)s for gene delivery. Biomaterials. 2009;30:5804–5814. doi: 10.1016/j.biomaterials.2009.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koo H, Jin GW, Kang H, Lee Y, Nam K, Zhe Bai C, Park JS. Biodegradable branched poly(ethylenimine sulfide) for gene delivery. Biomaterials. 2010;31:988–997. doi: 10.1016/j.biomaterials.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Peng Q, Zhong Z, Zhuo R. Disulfide cross-linked polyethylenimines (PEI) prepared via thiolation of low molecular weight PEI as highly efficient gene vectors. Bioconjug Chem. 2008;19:499–506. doi: 10.1021/bc7003236. [DOI] [PubMed] [Google Scholar]
- 24.Kim WJ, Chang CW, Lee M, Kim SW. Efficient siRNA delivery using water soluble lipopolymer for anti-angiogenic gene therapy. J Control Release. 2007;118:357–363. doi: 10.1016/j.jconrel.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Jiang X, Xu L, Wang X, Hennink WE, Zhuo R. Novel reduction-responsive cross-linked polyethylenimine derivatives by click chemistry for nonviral gene delivery. Bioconjug Chem. 2010;21:1827–1835. doi: 10.1021/bc100191r. [DOI] [PubMed] [Google Scholar]
- 26.Choi S, Lee KD. Enhanced gene delivery using disulfide-crosslinked low molecular weight polyethylenimine with listeriolysin o-polyethylenimine disulfide conjugate. J Control Release. 2008;131:70–76. doi: 10.1016/j.jconrel.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Chen P, Shen J. The development and characterization of a glutathione-sensitive cross-linked polyethylenimine gene vector. Biomaterials. 2006;27:5292–5298. doi: 10.1016/j.biomaterials.2006.05.049. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi S, Hiroishi K, Tokunoh M, Saegusa T. Chelating properties of linear and branched poly(ethylenimines) Macromolecules. 1987;20:1496–1500. [Google Scholar]
- 29.Kircheis R, Wightman L, Wagner E. Design and gene delivery activity of modified polyethylenimines. Adv Drug Deliv Rev. 2001;53:341–358. doi: 10.1016/s0169-409x(01)00202-2. [DOI] [PubMed] [Google Scholar]
- 30.Tomalia DA, Huang B, Swanson DR, Brothers Ii HM, Klimash JW. Structure control within poly(amidoamine) dendrimers: size, shape and regio-chemical mimicry of globular proteins. Tetrahedron. 2003;59:3799–3813. [Google Scholar]
- 31.Kim TI, Rothmund T, Kissel T, Kim SW. Bioreducible polymers with cell penetrating and endosome buffering functionality for gene delivery systems. J Control Release. 2011;152:110–119. doi: 10.1016/j.jconrel.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pouton CW, Seymour LW. Key issues in non-viral gene delivery. Adv Drug Deliv Rev. 2001;46:187–203. doi: 10.1016/s0169-409x(00)00133-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.