Abstract
In the attempt to elucidate if the “peripheral sink hypothesis” could be a potential mechanism of action for tau removal in passive immunotherapy experiments, we have examined tau levels in serum of chronically injected JNLP3 and tg4510 transgenic animals. Measurement of tau in serum of mice treated with tau antibodies is challenging due to the antibody interference in sandwich ELISAs. To address this issue we have developed a heat treatment protocol at acidic pH to remove interfering molecules from serum, with excellent recovery of tau. The present data show that pan-tau and conformational antibodies do increase tau in mouse sera. However, these concentrations in serum do not consistently correlate with reductions of tau pathology in brain, suggesting that large elevations of tau species measured in serum are not predictive of efficacy. Here we describe a reliable method to detect tau in serum of transgenic animals that have undergone tau immunotherapy. Levels of tau in human serum are below the sensitivity of current assays, although artifactual signals are common. The method may be useful in similarly treated humans, a situation in which false positive signals are likely.
Keywords: Tau in serum, tau immunotherapy, extracellular tau, tau ELISA, HAMA
Introduction
Tau is a natively unfolded, soluble protein that binds to and promotes assembly and stabilization of microtubules required for axonal transport and growth. In human tauopathies such as Alzheimer’s disease (AD) the accumulation of hyperphosphorylated tau protein within the somatodendritic compartment of neurons leads to the formation of well-defined fibers called paired helical filaments (PHFs), the core of the neurofibrillary tangles (NFTs) (Jeganathan, et al., 2008,Mandelkow and Mandelkow, 2012,Witman, et al., 1976). In recent years, there have been promising studies describing how “extracellular tau” might be the culprit in propagation of tau pathology as disease progresses (Clavaguera, et al., 2009,de Calignon, et al., 2012,Frost, et al., 2009,Holmes, et al., 2014,Kfoury, et al., 2012,Wu, et al., 2013). Even though it is not clear yet which pathological species of tau to target, recent studies have suggested that an immunotherapy approach can effectively prevent or possibly block the progression of tau pathology in transgenic mouse models (Asuni, et al., 2007,Boutajangout, et al., 2010,Chai, et al., 2011,d'Abramo, et al., 2013,Theunis, et al., 2013,Yanamandra, et al., 2015,Yanamandra, et al., 2013).
In the present study, we have examined tau levels in the serum of transgenic mice following chronic injections of antibody in order to explore the “peripheral sink hypothesis” as a mechanism of action for reduction of brain tau pathology. Being able to track changes in serum tau levels occurring as a result of antibody therapy might clarify the way tau immunotherapy works, together with acquiring a biomarker of pathology to monitor in blood.
Measurement of tau in serum of mice treated with tau antibodies is challenging due to the antibody interference in sandwich ELISAs. Hence, a heat treatment at acidic pH was set up and validated using mouse and human specimens. JNPL3 and Tg4510 mice, chronically injected either with pan-tau phospho-tau or conformational antibodies, showed a dramatic reduction of the apparent “tau” ELISA signal in serum after the heat protocol was applied. Surprisingly, pan-tau Abs were the only ones able to consistently increase tau concentrations in serum, even though no strong correlation with tau brain load has been found (d’Abramo et al, submitted). Furthermore, dose-response experiments showed that higher concentrations of pan-tau (DA9) or conformational (MC1) antibodies were able to further raise tau in serum following the concentration profile, again with no correlations of serum tau levels to changes in brain tau pathology. In acute experiments using pan-tau Abs, serum tau increases rapidly, peaking at 2 days post-injection.
Being able to accurately measure tau in human specimens represents a major goal in the AD field. The presence of HAMA (human antibodies against mouse immunoglobulin) in human plasma has been described in 30% of the population, and consequent interference by human anti-globulin antibodies in immunoassay has been reported in several studies (Dillman, et al., 1986,Maiolini, et al., 1980,Pimm, et al., 1985,Primus, et al., 1988,Schroff, et al., 1985,Thompson, et al., 1986). In the attempt to translate our research into clinical applications, we have extended our method to human CSF and serum. While a nearly perfect correlation between heated/not-treated samples was seen in CSF, serum tau levels dropped to zero after pre-treating. These results support the idea that even though our assay is not sensitive enough to detect tau in human serum, we are able to completely eliminate the assay interference present in serum, a first step toward enabling reliable detection of serum tau in the future.
2. Materials and Methods
2.1 Transgenic animals
JNPL3 transgenic animals were purchased from Taconic (Lewis, et al., 2000). Cohorts of female JNPL3 were used as a model of hyperphosphorylation and aggregation of tau protein. This transgenic line expresses human MAPT (4R0N) with the P301L mutation driven by the mouse prion promoter and develops neurofibrillary tangles in an age and gene–dose dependent manner, as early as 4.5 months.
The tg4510 transgenic animals were purchased and housed at MERCK laboratories (Santacruz, et al., 2005). This transgenic model carries the MAPT (4R0N) with the P301L mutation driven by the CamKII promoter element.
Starting at 3 months of age, JNPL3 mice were treated for 4 months with weekly intraperitoneal injections of purified tau monoclonal antibodies or saline. The antibodies were used at a dose of 10mg/Kg or 40mg/Kg, and 1X phosphate buffered saline was used as a negative control. The Tg4510 mice were treated for 10 weeks with10mg/kg antibody starting at 12 weeks of age. For JNPL3 the treated groups included 16 animals, the control group 29 mice, while the baseline cohort consisted of 22 mice sacrificed at 3 months of age. In the tg4510 experiments all the cohorts included 20 animals.
Euthanasia of mice was performed by decapitation under deep isoflurane anesthesia. Approval for the work reported here was obtained from the Feinstein Institute IACUC under protocol numbers 2007-029 and 2015-018.
2.2 Tau monoclonal antibodies
All monoclonal antibodies used were produced and purified in our laboratory, and have been divided into different groups, based on specificity for pathological tau: the pan-tau antibodies DA9 (AAs 102–140), and DA31 (AAs 150–190); two phospho-tau antibodies targeting phospho-epitopes present on both normal adult brain and PHF-tau, CP13 (pSer202), and RZ3 (pThr231); a phospho-Ab directed to a phosphorylation site found just on PHF-tau, PG5 (pSer409); and one conformation-specific antibody MC1 that recognizes the epitope created by the folding of the N-terminus into the vicinity of the third repeat domain of tau. The antibodies used in the study are all IgG1 isotype, except for PG5, which is an IgG3. Information about the relative affinities of these antibodies has been provided in a previous publication (d'Abramo, et al., 2013).
Assays of antibody levels in serum were performed using plates coated with peptides specific either for DA31 (tau amino acids 150–190) or DA9 (tau amino acids 102–149). Sera samples were diluted 1:100 and added to the plate for 1h, followed by a goat antimouse IgG (heavy and light) HRP-secondary antibody diluted 1:500 in 5% milk. Biorad HRP substrate kit was used and added for 30’ and plates read at 415nm.
2.3 Low –Tau sandwich ELISA
Low-Tau sandwich ELISA was performed as already published (Acker, et al., 2013). 96-well plates (Nunc) were respectively coated with DA31 at a final concentration of 6µg/ml in coating buffer, for at least 48h at 4°C. After washing 3X in wash buffer, the plates were blocked for 1h at room temperature using StartingBlock Blocking buffer (Thermo Scientific) to avoid non-specific binding. Each plate was then washed 5X and 50µl of the appropriate sample was added to the wells, with 50µl of DA9-HRP detection antibody. Plates were incubated O/N shaking at 4°C and then washed 9X in wash buffer. 1-Step ULTRA TMB-ELISA (Thermo Scientific) was added for 30’ at room temperature before stopping the reaction with 100 µl 2M H2SO4. Plates were read with an Infinite m200 plate reader (Tecan) at 450nm.
2.4 DNP ELISA
The monoclonal antibody Anti-Dinitrophenol (DNP) (IgG1) was purchased from Abnova Corporation. This “irrelevant” antibody was used to set up an assay able to further demonstrate the non-specific signal detected when loading the straight sera of immunized mice on tau ELISA. The method completely overlaps with the DA31 assay described in section 2.3 except for the coating (capture) antibody, which is anti-DNP.
2.5 Human specimens
De-identified samples of plasma and CSF from healthy controls and patients with Alzheimer’s disease were obtained from the clinical research program of the Litwin-Zucker Center.
2.6 Sodium Acetate (NaOAc) and Heat Treatment of serum, plasma or CSF samples
Utilizing the heat stability of tau, samples were prepared following a protocol previously described (Primus, et al., 1988). Briefly, CSF, plasma, or mouse serum samples were diluted 1:3 in 0.2M NaOAc, pH 5.0 and heated at 90°C for 15 min. After heat treatment, samples were allowed to cool at 4°C for 15 min, and then spun at 15,000g for 10 min. Supernatant was collected and 7µl of 1M Tris buffer per 100µl of supernatant were added to samples to neutralize pH; samples were further diluted 1:2 in 20% Superblock, giving a total final dilution of 1:6. Sera not treated with NaOAc were removed from the initial aliquot and diluted 1:6 with 20% Superblock. All samples were stored at −80°C until use. DA31 Elisa to detect total tau was used as described above.
2.7 Statistical Analysis
Statistical analyses were performed with dedicated software (GraphPad Prism vs6). Data were analyzed by Unpaired t-test with Welch’s correction, with significance level set at p<0.05. Linear regression analysis was performed for all correlations.
3. Results
3.1 The NaOAc/heat-treatment does not affect tau concentration in serum
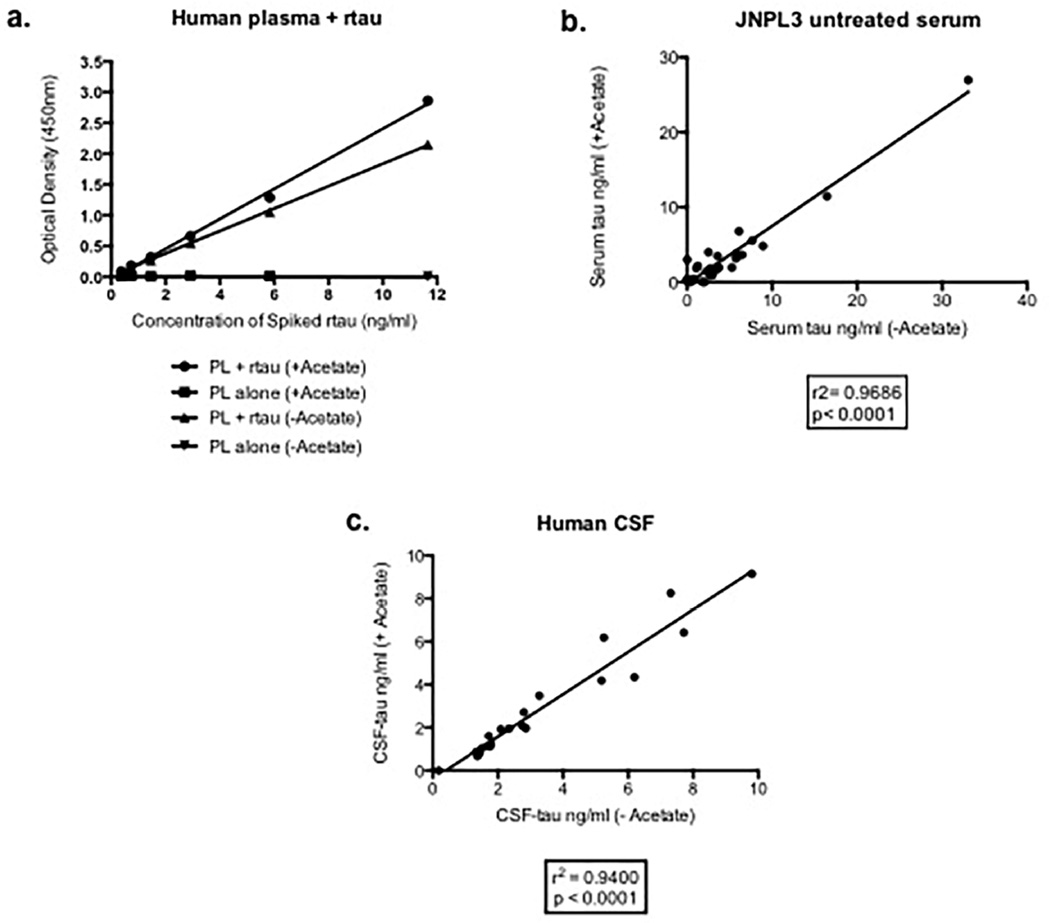
Measurement of tau in serum of mice treated with tau antibodies is challenging due to the antibody interference in sandwich ELISAs. The NaOAc heat-treatment protocol described in the “Materials and Methods” section has been validated using different control specimens. Preliminary experiments were conducted in order to determine the recovery of tau in serum. Human serum was spiked with recombinant tau (0–12ng/ml) and assayed with the total tau ELISA showing that heat-treatment did not affect tau detection (Figure 1a). In addition, when analyzing the amount of tau in control (untreated) JNPL3 mouse sera, we have been able to show a strong correlation (r2=0.9686) between “with or without” heat-preparation samples (Figure 1b). Additionally, human CSF subjected to the same heat preparation protocol showed no differences (r2=0.9400) in terms of tau immunoreactivity in the assay (Figure 1c).
Figure 1. Validation of the NaOAc/heat treatment method using mouse and human samples.
a, Human plasma (PL) is spiked with recombinant tau (rtau) followed by Na Acetate treatment: no signal is detected in plasma alone with or without Na Acetate (+ or – Acetate) treatment; plasma spiked with rtau produces the same signal with or without Na Acetate treatment. Values are expressed as optical densities (OD). b, tau concentrations in sera from control P301L mice show near perfect correlation (r2=0.9686) with or without Na Acetate. Serum tau is expressed as ng/ml. c, tau concentration measured in human cerebrospinal fluid (CSF) does not change on treatment with Na Acetate (r2=0.9400). CSF-tau is expressed as ng/ml.
3.2 “Tau” ELISA signal detected in untreated sera from antibody-treated JNPL3 mice is dramatically elevated, with NaOAc/heat-treatment suppressing the ELISA artifacts
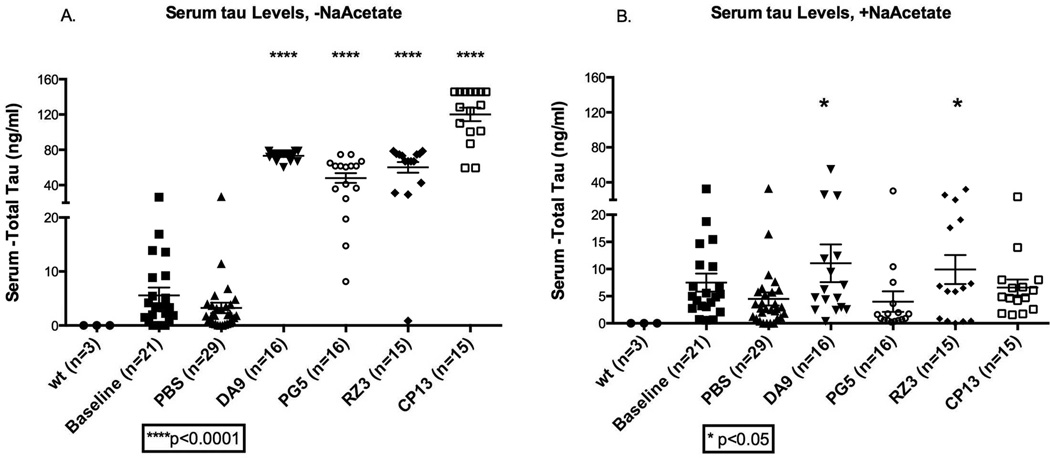
After confirming the efficacy of our method in assaying tau on controls, we tested the sera belonging to JNPL3 mice injected weekly with the tau-Abs (DA9, RZ3, PG5 and CP13) over a 4 months period. Apparent tau levels in the untreated sera of the antibody-injected animals were considerably elevated (>60ng/ml), together with some reactivity found in the baseline and PBS mice groups (~2–5ng/ml) (Figure 2a). Further analysis of the tau signal detected in the DA9 injected-mice group suggested that the elevated signal was an artifact. If the “tau-DA9 complex” present in serum had bound the capturing antibody on the ELISA plate, no signal would have been expected, since this complex would prevent binding of the HRP-coupled DA9 that is used as the detection antibody in our assay. Given that the heat-treatment previously described allows removal of interfering molecules, including immunoglobulins, without affecting tau stability in serum, all sera from the P301L mice immunotherapy experiment were tested according to the heat-protocol. Following heat treatment, ELISA signals were dramatically reduced, being comparable to the untreated samples, but with DA9 and RZ3 groups still being slightly elevated compared to PBS-injected animals (*p=0.039 and *p=0.036 respectively) (Figure 2b).
Figure 2. Serum tau in JNPL3 mice immunized with tau antibodies.
a, If no treatment with NaOAc/heat is applied, the amount of tau in the serum of animals immunized with DA9, PG5, RZ3 or CP13 is much higher than in the baseline and PBS cohorts, (****p<0.0001). b, after treatment, sera tau levels are reduced but mice treated with DA9 and RZ3 have a slight increase of tau in serum (*p<0.05).serum tau is expressed as ng/ml. The bars on the figure show mean ± standard deviation.
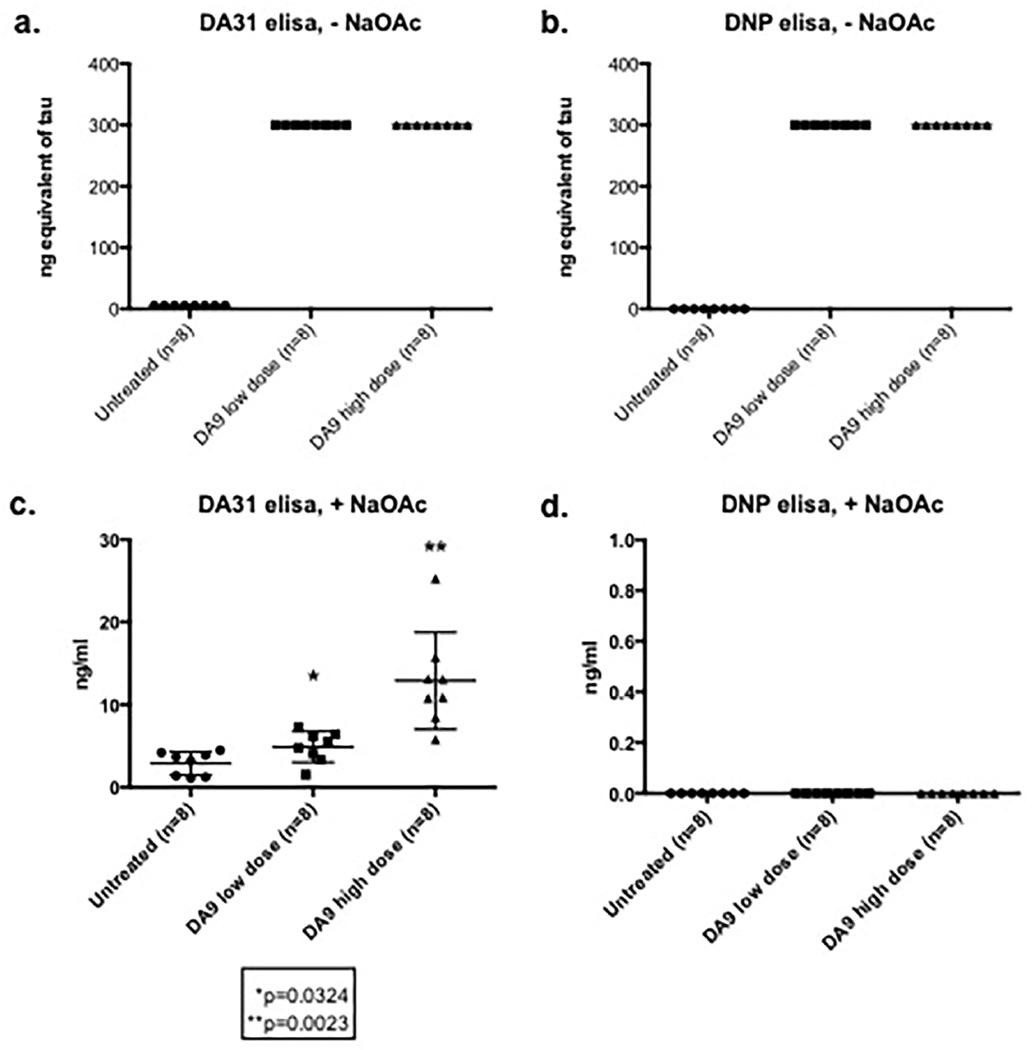
The very high artifactual signal in the serum of immunized mice was thought to be due to the presence of mouse antibodies to mouse immunoglobulins (“MAMI”). To confirm this, an “irrelevant antibody” was selected to build an immunosorbent assay. Anti-DNP (Anti-Dinitrophenol) was used instead of DA31 as coating antibody, while DA9-HRP was kept as detection antibody in both assays. Untreated sera obtained from controls and DA9 injected animals were loaded on both ELISAs showing a complete overlap of the assays (Figure 3 a,b). On the contrary, the NaOAc/heat treated sera exhibit a dose response signal in the tau assay, with complete suppression of the positivity in the “DNP ELISA” (Figure 3 c,d).
Figure 3. Comparison between DA31 and DNP immunosorbent assays on straight and NaOAc/heat treated sera.
a,b Untreated sera from control and DA9 immunized mice were tested on both total tau (DA31) and DNP assays exhibiting saturating signals : ng equilvalent refers to what the assay signal would be if it were tau. c,d Sera processed with the NaOAc/heat treatment show a dose response effect on DA31 ELISA (*p=0.0324 DA9 low dose, **p=0.0023 DA9 high dose), while the DNP signal is reset to zero. The bars on the figure show mean ± standard deviation.
3.3 In tg4510 mice, DA9 and DA31 increase tau in NaOAc/heat extracted serum
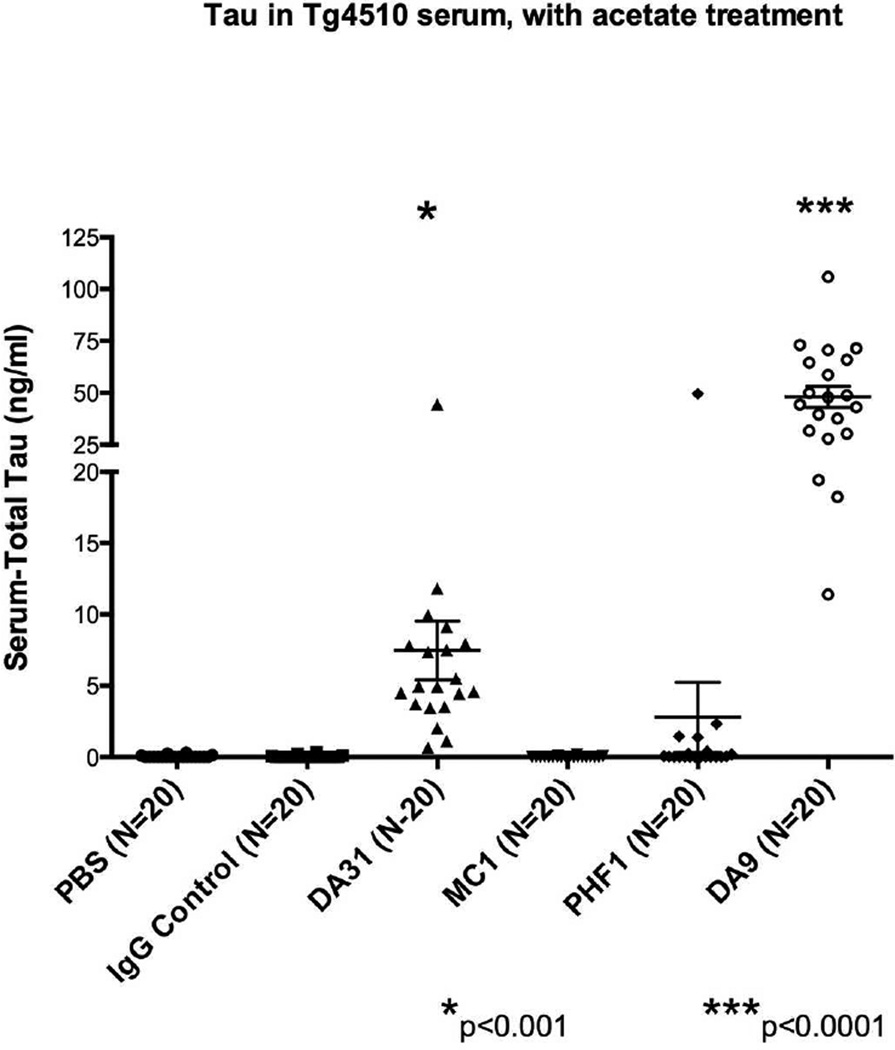
Using the NaOAc/heat protocol on serum from tg4510 mice demonstrated that serum tau reached significantly higher levels than control groups after treating with DA31 (***p<0.001) or DA9 (****p<0.0001) (Figure 4). Unlike the JNPL3 mice, the tau transgene in this model is under the control of the CamKII promoter, a neuronal specific promoter element, and consequently the two control groups, PBS and IgG, do not show any sign of tau leaking from peripheral tissues.
Figure 4. Serum tau in tg4510 mice immunized with tau antibodies.
Tau in serum reaches higher levels than control groups when immunizing with DA31 (***p<0.001) or DA9 (****p<0.0001). Serum tau is expressed as ng/ml. The bars on the figure show mean ± standard deviation.
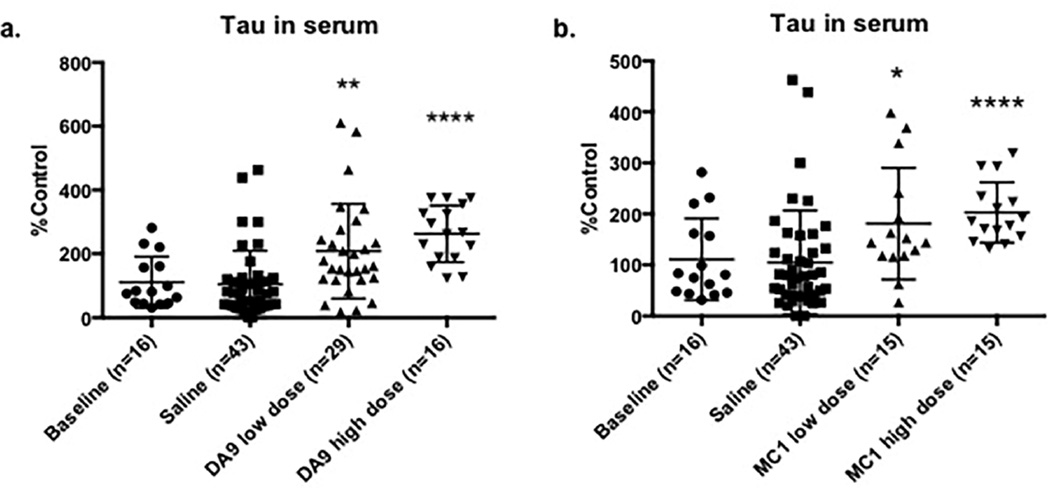
3.4 In JNPL3 mice, dose-response experiments testing DA9 and MC1 result in increased levels of tau in serum
Dose-response experiments were performed in order to verify if increased concentration of antibody would produce any difference in clearing tau pathology from brain. DA9 and MC1, respectively tau linear sequence and conformational Abs, were each injected at low and high doses: 10mg/Kg and 40mg/Kg respectively. Evaluation of NaOAc/heat extracted serum following DA9 (preliminary data on smaller cohorts described in section 3.2) or MC1 at higher doses resulted in extensive increases in serum tau levels compared to the saline injected groups (DA9 high dose ****p<0.0001, MC1 high dose ****p<0.0001). The lower doses of injected antibodies also showed significant elevation of serum tau, but to a lower extent than the higher doses (DA9 low dose **p=0.0021, MC1 low dose *p=0.0269) (Figure 5).
Figure 5. Serum tau in dose-response experiments using DA9 or MC1 antibody.
a, DA9 significantly raises tau levels in serum compared to the control cohorts, following a concentration profile (DA9 low dose **p=0.0021, DA9 high dose ****p<0.0001); b, same behavior is registered for MC1 (MC1 low dose *p=0.0269, MC1 high dose ****p<0.0001). serum tau. Serum tau is expressed as % Control since the data belong to separate experiments. The bars on the figure show mean ± standard deviation.
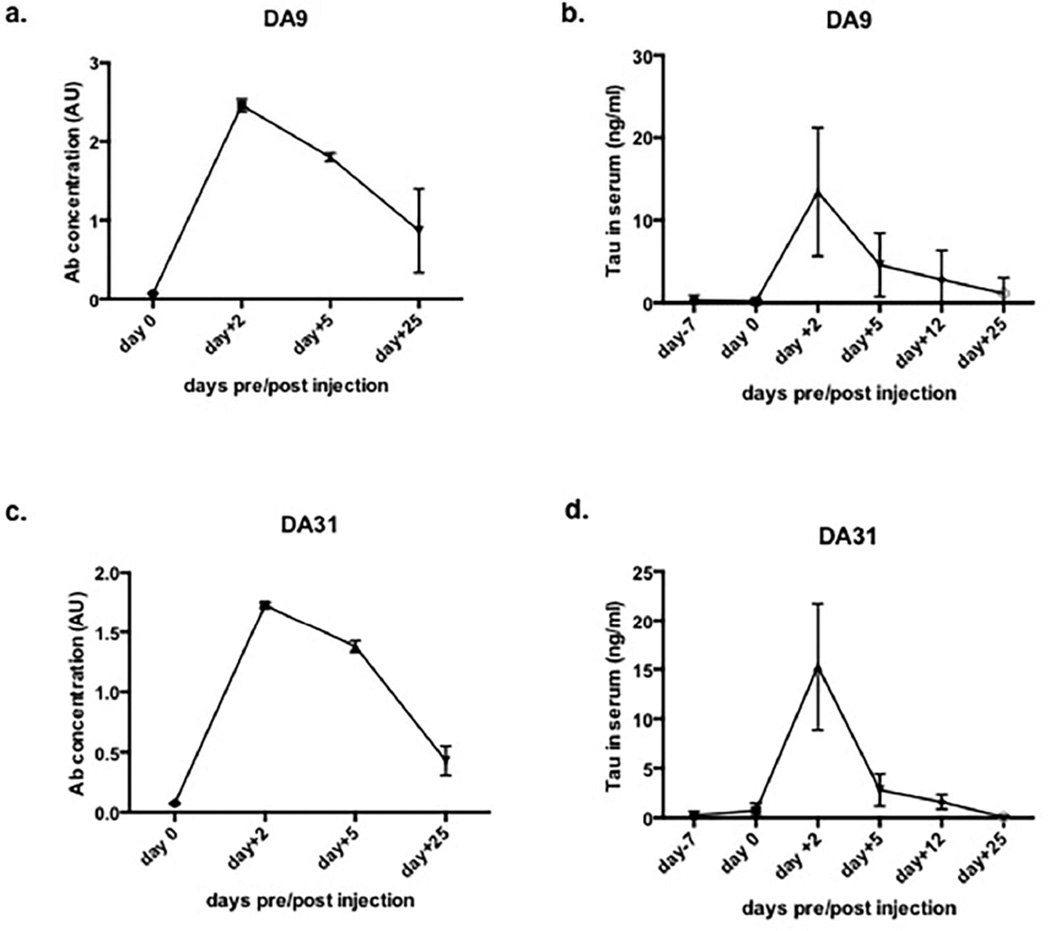
3.5 In acute experiments using pan-tau antibodies DA9 and DA31, serum tau reaches a peak at 2 days post-injection and rapidly decreases
An acute experiment was performed in order to clarify the effect of the pan-tau antibodies in modulating tau levels in serum. A small cohort of female JNPL3 mice were injected with 10mg/Kg of DA9 or DA31 and monitored during a 25 days time frame. Mice were bled at different time points: one week before the injection (day -7), and on days 0, 2, 5, 12, and 25 post-injection. The antibodies levels in serum were analyzed following the same time points, with DA9 being slightly more stable than DA31 (Figure 6 a,c). As displayed in Figures 6b, and 6d tau fluctuations follow a specific pattern with a peak detected at 2 days post-injection and a rapid drop of concentration at 5 and 12 days post injection, reaching the zero at 25 days after the administration.
Figure 6. In acute experiment, JNPL3 mice are dosed with a single injection of DA9 or MC1 and monitored for tau and Abs concentration in serum.
a, DA9 concentration in serum, pre and post injection. b, tau in serum of DA9 injected mice. c, DA31 concentration in serum pre and post administration. d, tau in serum of DA31 injected mice.
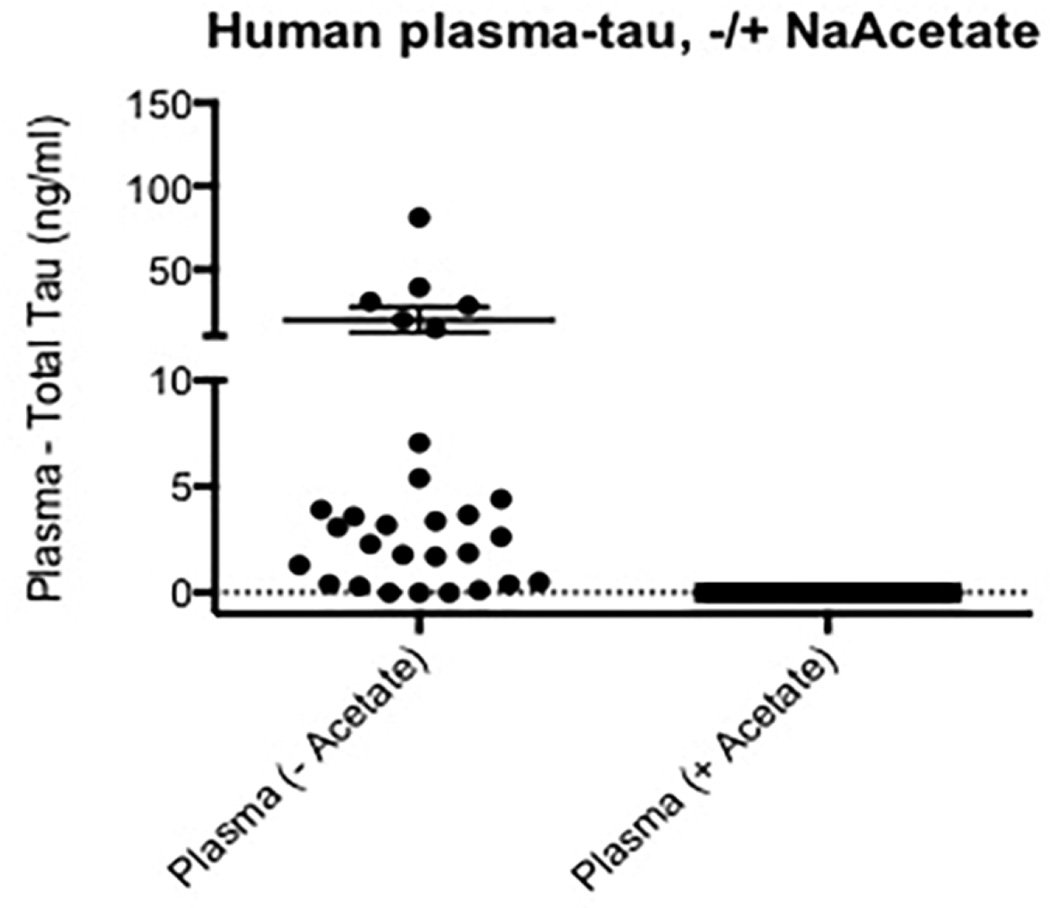
3.6 Human plasma shows provocative differences in tau reactivity after NaOAc/heat-treatment
In our study, the human specimens included plasma from AD and healthy controls. While heat-treatment did not affect tau reactivity on CSF samples, plasma samples displayed an impressive reduction of the apparent tau signals after undergoing the heat extraction protocol (Figure 7).
Figure 7. Human plasma reactivity in tau ELISA.
NaOAc/heat treatment removes all the interfering molecules that produce false positives in human serum, resulting in lack of signal in the total tau ELISA. Plasma tau is expressed as ng/ml. The solid circles represent samples from healthy controls, and the open circles samples from patients with Alzheimer’s disease. This distinction cannot be seen on the right side of the figure (plasma +acetate) as all 31 values are zero.
4. Discussion
Experiments using acute brain slices from tau transgenic mice (Congdon, et al., 2013) have suggested that IgG could enter neurons by clathrin-mediated endocytosis following binding to low-affinity FcγII/III receptors. In the same direction a recent study (Collin, et al., 2014) has recently described the neuronal uptake of tau/pSer422 Ab in a passive immunotherapy study. On the contrary, a previous study in our lab has shown that monoclonal antibodies targeting tau pathology are not observed in the cell bodies of neurons (d'Abramo, et al., 2013) at the doses we employ (10mg/kg). In the present study, we have investigated the hypothesis that tau could be released into plasma, as a result of a brain clearance mechanism, following immunotherapy treatments. Hence, we have examined total tau levels in serum of JNPL3 and tg4510 mice after 4-months of antibody exposure. We observed that measurements of tau in serum of mice treated with tau antibodies are challenging due to the antibody interference in sandwich ELISAs. In the first set of experiments, when testing the sera belonging to JNPL3 mice treated with tau- Abs (DA9, RZ3, PG5 and CP13), we immediately noticed that tau ELISA signals in the straight sera of the immunized animals appeared considerably elevated, together with some reactivity found in controls. The presence of serum tau in the JNPL3 baseline and PBS cohorts did not come as a surprise and depends essentially on the expression of tau under the mouse prion promoter, probably resulting in tau leaking from tissues other than brain. Trying to decipher these data, we realized that the apparent tau signal detected in the DA9 immunized-mice group was an artifact. In fact, even if the “tau-DA9 complex” in serum had bound the capturing antibody on the ELISA plate, no signal should have been registered, since HRP-coupled DA9 is used as the detecting antibody in our assay and the presence of the injected DA9 bound to tau should preclude binding of the detection antibody. The most common explanation is that the increased tau signal is the result of a false positive bridge created by an antibody response against the antibodies injected, similar to the presence of HAMA (human antibodies against mouse immunoglobulin) in human plasma. Although it is not usual for mice to make antibodies to murine immunoglobulins, the presence of small amounts of denatured antibody and the injection schedule may explain this reactivity. Although the finding that antibodies are produced against antibodies is not novel, this is the first experiment that explores the issue in Alzheimer’s disease immunotherapy studies. Therefore, in this study, we have tested a NaOAc/heat treatment protocol aimed to remove interfering molecules, especially immunoglobulins from serum and reaching effectively 100% recovery of tau in animal and human control samples. Anti-DNP was used as “irrelevant” antibody to coat plates instead of DA31: as expected both assays gave strong positive signals when testing the untreated sera of immunized mice, indicating a non-specific signal. On the contrary, the NaOAc/heat treated sera resulted in a detectable signal just in the total tau ELISA, but not in the anti-DNP assay. All sera from JNPL3 animals and from a parallel experiment conducted on tg4510 mice were further tested according to the NaOAc/heatprotocol: tau dramatically dropped to the control levels, except for the cohorts injected with DA9, DA31 and RZ3 still exhibiting some elevation of tau levels. Furthermore, dose-response experiments showed that higher concentrations of pan-tau (DA9) or conformational (MC1) antibodies were able to raise tau in serum to a greater extent, following a concentration profile. However, no significant correlation between tau brain load and tau concentration in serum was found (d’Abramo et al, submitted), questioning the “peripheral sink hypothesis” as a mechanism of clearance for tau in immunotherapy treatments. At present, it is unclear why pan-tau and conformational antibodies consistently raise the protein levels in serum, and we can just give voice to some speculations. Is tau in serum quickly degraded resulting in difficulties in detecting it? Do pan-tau and conformational antibodies recognize and “stabilize” a specific form of tau in serum? To better explore this issue, acute experiments using tau sequence Abs was performed. Serum tau fluctuations reached a peak at 2 days post-injection and dramatically decreased at day 5. The average post-injection levels at 2 days has been calculated to be around 15 ng/ml, the same range of concentration usually obtained after 16 weeks of chronic injection in JNPL3 mice. This implies that in acute conditions we reach very quickly the same kind of tau levels detected after 4 months of chronic injection, followed by a prompt decline. At this short time point, it is highly improbable that the tau species detected in serum derives from NFTs. Furthermore, considering the lack of a strong correlation between sera and brain data in the chronic experiments, it is plausible that tau detected in serum includes species with high affinities for both sequence and conformational antibodies, potentially extracellular forms of tau easily reached by the Abs and quickly released from brain and discharged into CSF and blood flow. The fact that these tau species have high affinity for both sequence and conformational antibodies, resulting in serum “stabilization”, provides additional information about the nature of the proteins released.
Considering the urgent need for sensitive technologies able to reliably capture AD biomarkers in blood, a small part of the study focused on trying to detect tau in human serum. The CSF data demonstrated the complete efficacy of this method in detecting tau with nearly perfect correlation between heat-treated and untreated samples. However, in serum the NaOAc/heat treatment dramatically cancelled any “tau signal”, suggesting that tau concentration in human blood is below the detection limit of our assay. On the other hand, we are now able to eliminate any background signal avoiding false positive data. Besides all the open questions, in this study we have set up a detailed and accurate method to measure tau in serum of transgenic animals that have undergone passive immunotherapy treatment. The future combination between the heat protocol and a more sensitive tau immunosorbent technology will represent the turning point towards a reliable and accurate detection of tau in human serum.
Highlights.
There are obvious and not-so-obvious artifacts in the measurement of tau in the serum of transgenic mice given tau immunotherapy.
Taking advantage of the heat-stability of tau, a simple sodium acetate/heat protocol has been used, with excellent recovery of tau and complete removal of immunoglobulins..
Tau sequence and conformational antibodies raise tau levels in serum
In human serum tau levels are below detection, but the immunoglobulin artifacts can be suppressed.
Acknowledgments
Supported by NIA grant R37 AG022102.
Abbreviations
- AD
Alzheimer’s disease
- Abs
Antibodies
- NFTs
neurofibrillary tangles
- PHF
paired helical filaments
- NaOAc/heat
Sodium Acetate heat treatment
- HAMA
human antibodies against mouse immunoglobulin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acker CM, Forest SK, Zinkowski R, Davies P, d'Abramo C. Sensitive quantitative assays for tau and phospho-tau in transgenic mouse models. Neurobiol Aging. 2013;34(1):338–350. doi: 10.1016/j.neurobiolaging.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asuni AA, Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J Neurosci. 2007;27(34):9115–9129. doi: 10.1523/JNEUROSCI.2361-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau prevents cognitive decline in a new tangle mouse model. J Neurosci. 2010;30(49):16559–16566. doi: 10.1523/JNEUROSCI.4363-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai X, Wu S, Murray TK, Kinley R, Cella CV, Sims H, Buckner N, Hanmer J, Davies P, O'Neill MJ, Hutton ML, Citron M. Passive immunization with anti-Tau antibodies in two transgenic models: reduction of Tau pathology and delay of disease progression. J Biol Chem. 2011;286(39):34457–34467. doi: 10.1074/jbc.M111.229633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, Fraser G, Stalder AK, Beibel M, Staufenbiel M, Jucker M, Goedert M, Tolnay M. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11(7):909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin L, Bohrmann B, Göpfert U, Oroszlan-Szovik K, Ozmen L, Grüninger F. Neuronal uptake of tau/pS422 antibody and reduced progression of tau pathology in a mouse model of Alzheimer's disease. Brain. 2014;137(Pt 10):2834–2846. doi: 10.1093/brain/awu213. [DOI] [PubMed] [Google Scholar]
- Congdon EE, Gu J, Sait HB, Sigurdsson EM. Antibody uptake into neurons occurs primarily via clathrin-dependent Fcγ receptor endocytosis and is a prerequisite for acute tau protein clearance. J Biol Chem. 2013;288(49):35452–35465. doi: 10.1074/jbc.M113.491001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Abramo C, Acker CM, Jimenez HT, Davies P. Tau passive immunotherapy in mutant P301L mice: antibody affinity versus specificity. PLoS One. 2013;8(4):e62402. doi: 10.1371/journal.pone.0062402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Calignon A, Polydoro M, Suárez-Calvet M, William C, Adamowicz DH, Kopeikina KJ, Pitstick R, Sahara N, Ashe KH, Carlson GA, Spires-Jones TL, Hyman BT. Propagation of tau pathology in a model of early Alzheimer's disease. Neuron. 2012;73(4):685–697. doi: 10.1016/j.neuron.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillman RO, Beauregard JC, Halpern SE, Clutter M. Toxicities and side effects associated with intravenous infusions of murine monoclonal antibodies. J Biol Response Mod. 1986;5(1):73–84. [PubMed] [Google Scholar]
- Frost B, Jacks RL, Diamond MI. Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem. 2009;284(19):12845–12852. doi: 10.1074/jbc.M808759200. doi:M808759200 [pii] 10.1074/jbc.M808759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes BB, Furman JL, Mahan TE, Yamasaki TR, Mirbaha H, Eades WC, Belaygorod L, Cairns NJ, Holtzman DM, Diamond MI. Proteopathic tau seeding predicts tauopathy in vivo. Proc Natl Acad Sci U S A. 2014;111(41):E4376–E4385. doi: 10.1073/pnas.1411649111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeganathan S, von Bergen M, Mandelkow EM, Mandelkow E. The natively unfolded character of tau and its aggregation to Alzheimer-like paired helical filaments. Biochemistry. 2008;47(40):10526–10539. doi: 10.1021/bi800783d. [DOI] [PubMed] [Google Scholar]
- Kfoury N, Holmes BB, Jiang H, Holtzman DM, Diamond MI. Transcellular Propagation of Tau Aggregation by Fibrillar Species. J Biol Chem. 2012;287(23):19440–19451. doi: 10.1074/jbc.M112.346072. doi:M112.346072 [pii] 10.1074/jbc.M112.346072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, Van Slegtenhorst M, Gwinn-Hardy K, Paul Murphy M, Baker M, Yu X, Duff K, Hardy J, Corral A, Lin WL, Yen SH, Dickson DW, Davies P, Hutton M. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet. 2000;25(4):402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- Maiolini R, Bagrel A, Chavance C, Krebs B, Herbeth B, Masseyeff R. Study of an enzyme immunoassay kit for carcinoembryonic antigen. Clin Chem. 1980;26(12):1718–1722. [PubMed] [Google Scholar]
- Mandelkow EM, Mandelkow E. Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harb Perspect Med. 2012;2(7):a006247. doi: 10.1101/cshperspect.a006247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimm MV, Perkins AC, Armitage NC, Baldwin RW. The characteristics of blood-borne radiolabels and the effect of anti-mouse IgG antibodies on localization of radiolabeled monoclonal antibody in cancer patients. J Nucl Med. 1985;26(9):1011–1023. [PubMed] [Google Scholar]
- Primus FJ, Kelley EA, Hansen HJ, Goldenberg DM. "Sandwich"-type immunoassay of carcinoembryonic antigen in patients receiving murine monoclonal antibodies for diagnosis and therapy. Clin Chem. 1988;34(2):261–264. [PubMed] [Google Scholar]
- Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E, Forster C, Yue M, Orne J, Janus C, Mariash A, Kuskowski M, Hyman B, Hutton M, Ashe KH. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309(5733):476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroff RW, Foon KA, Beatty SM, Oldham RK, Morgan AC. Human anti-murine immunoglobulin responses in patients receiving monoclonal antibody therapy. Cancer Res. 1985;45(2):879–885. [PubMed] [Google Scholar]
- Theunis C, Crespo-Biel N, Gafner V, Pihlgren M, López-Deber MP, Reis P, Hickman DT, Adolfsson O, Chuard N, Ndao DM, Borghgraef P, Devijver H, Van Leuven F, Pfeifer A, Muhs A. Efficacy and safety of a liposome-based vaccine against protein Tau, assessed in tau.P301L mice that model tauopathy. PLoS One. 2013;8(8):e72301. doi: 10.1371/journal.pone.0072301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RJ, Jackson AP, Langlois N. Circulating antibodies to mouse monoclonal immunoglobulins in normal subjects--incidence, species specificity, and effects on a two-site assay for creatine kinase-MB isoenzyme. Clin Chem. 1986;32(3):476–481. [PubMed] [Google Scholar]
- Witman GB, Cleveland DW, Weingarten MD, Kirschner MW. Tubulin requires tau for growth onto microtubule initiating sites. Proc Natl Acad Sci U S A. 1976;73(11):4070–4074. doi: 10.1073/pnas.73.11.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JW, Herman M, Liu L, Simoes S, Acker CM, Figueroa H, Steinberg JI, Margittai M, Kayed R, Zurzolo C, Di Paolo G, Duff KE. Small misfolded Tau species are internalized via bulk endocytosis and anterogradely and retrogradely transported in neurons. J Biol Chem. 2013;288(3):1856–1870. doi: 10.1074/jbc.M112.394528. doi:M112.394528 [pii] 10.1074/jbc.M112.394528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanamandra K, Jiang H, Mahan TE, Maloney SE, Wozniak DF, Diamond MI, Holtzman DM. Anti-tau antibody reduces insoluble tau and decreases brain atrophy. Ann Clin Transl Neurol. 2015;2(3):278–288. doi: 10.1002/acn3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanamandra K, Kfoury N, Jiang H, Mahan TE, Ma S, Maloney SE, Wozniak DF, Diamond MI, Holtzman DM. Anti-tau antibodies that block tau aggregate seeding in vitro markedly decrease pathology and improve cognition in vivo. Neuron. 2013;80(2):402–414. doi: 10.1016/j.neuron.2013.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]