Abstract
Background
The Scn5a-encoded voltage-gated sodium channel NaV1.5 is expressed in human jejunum and colon. Mutations in NaV1.5 are associated with gastrointestinal motility disorders. The rat gastrointestinal tract expresses voltage-gated sodium channels, but their molecular identity and role in rat gastrointestinal electrophysiology are unknown.
Methods
The presence and distribution of Scn5a-encoded NaV1.5 was examined by PCR, Western blotting and immunohistochemistry in rat jejunum. Freshly dissociated smooth muscle cells were examined by whole cell electrophysiology. Zinc finger nuclease was used to target Scn5a in rats. Lentiviral-mediated transduction with shRNA was used to target Scn5a in rat jejunum smooth muscle organotypic cultures. Organotypic cultures were examined by sharp electrode electrophysiology and RT-PCR.
Key Results
We found NaV1.5 in rat jejunum and colon smooth muscle by Western blot. Immunohistochemistry using two other antibodies to different portions of NaV1.5 revealed the presence of the ion channel in rat jejunum. Whole cell voltage-clamp in dissociated smooth muscle cells from rat jejunum showed fast activating and inactivating voltage-dependent inward current that was eliminated by Na+ replacement by NMDG+. Constitutive rat Scn5a knock out resulted in death in utero. NaV1.5 shRNA delivered by lentivirus into rat jejunum smooth muscle organotypic culture resulted in 57% loss of Scn5a mRNA and several significant changes in slow waves, namely 40% decrease in peak amplitude, 30% decrease in half-width and 7 mV hyperpolarization of the membrane potential at peak amplitude.
Conclusions & Inferences
Scn5a-encoded NaV1.5 is expressed in rat gastrointestinal smooth muscle and it contributes to smooth muscle electrophysiology.
Keywords: jejunum, smooth muscle, sodium channel, rat
Graphical Abstract

INTRODUCTION
Ion channels are critical for the normal operation of the gastrointestinal (GI) tract. Abnormalities in ion channel function lead to GI pathologies,(1) and drugs targeting ion channels are viable therapies for these pathologies.(2) Voltage-gated sodium (NaV) channels are a sine qua non requirement for the function of neurons, cardiac, and skeletal myocytes. NaV channels are also found in some GI smooth muscles, but their roles in smooth muscle tissues remain unclear.(3–7) Job first noted the importance of a Na+ influx current in the cat jejunum for the depolarization phase of cyclic electrical events that drive mechanical activity, known as the electrical slow wave.(8) Several subsequent studies in the small bowel of cats, dogs, rabbits and humans used ion substitution experiments in which replacement of the majority of extracellular Na+ led to complete loss of slow waves.(7, 9–11) However, since these effects took minutes to hours to develop, some authors suggested that the impact of Na+ replacement on slow waves was indirect.(9) In studies on cat and dog colon Na+-free solution did not eliminate the slow waves.(12, 13) In situations where slow waves remained, Na+ substitution led to significant changes in the electrical function in both small bowel and colon. These changes included hyperpolarization of the resting potential(7, 9, 13) and changes to slow wave parameters, including significantly decreased amplitude,(9, 12, 13) rate of rise,(12, 13) duration,(12) and frequency.(13) Conversely, increasing extracellular Na+ resulted in depolarization of the resting potential(9) and increased slow wave amplitude.(13) While Na+ replacement is simple and can point towards the role of Na+ in regulation of GI smooth muscle function, it is limited by its significant impact on several ionic gradients by coupled transport with Ca2+, K+, or Cl− ions, alterations in ion mobility due to ion size and charge mismatch, and osmotic effects.(12)
Pharmacologic block of NaV channels is an alternative approach to determine the role of NaV channels in smooth muscle function. At less than 1 μM, TTX blocks TTX-sensitive NaV channels, such as neuronal NaV channels, but not TTX-resistant NaV channels, such as the SCN5A-encoded NaV1.5. Therefore, TTX is used extensively to block neuronal activity. At less than 1 μM, TTX did not influence the resting membrane potential or upstroke amplitude and rate of rise of the electrical slow wave.(12) While TTX increased slow wave duration and decreased frequency, these effects were attributed to the blockade of the tonic inhibitory nerve activity.(12) Unlike TTX, local anesthetics block most NaV channel isoforms and compound such as QX-314 and lidocaine which block NaV channels in smooth muscle cells. In the human jejunum, lidocaine and QX-314 had differing effects from TTX, in that they hyperpolarized the resting membrane potential and decreased slow wave rate of rise and frequency.(7) Therefore, smooth muscle NaV channels influence several important parameters of smooth muscle electrophysiology. However, studies that use ion substitution or even pharmacologic block are limited by their lack of specificity for mechanisms and channel isoforms.
Studies show that both TTX-sensitive and TTX-resistant NaV channels are present in GI smooth muscle.(2) A particular NaV channel isoform, SCN5A-encoded NaV1.5, is found in smooth muscle cells (SMC) and interstitial cells of Cajal (ICC) of human jejunum(5, 7, 14) and colon.(15) Mutations in SCN5A or associating proteins like telethonin lead to GI diseases.(16, 17) Importantly, a subset of irritable bowel syndrome (IBS) patients have SCN5A mutations that lead to abnormal NaV1.5 function (1, 18, 19), and restoration of NaV1.5 function can normalize bowel habits.(1) NaV1.5 is not present in GI smooth muscle of all species, and notably it is absent in mouse intestinal smooth muscle.(14) Therefore, the mouse is not an appropriate experimental animal for studying the role of Nav1.5 in gastrointestinal motility. Previous studies in rats showed the presence of NaV currents in the colon,(20) but the identity of these channels or presence of NaV currents in the rat small bowel has not been reported. The aim of this study was to determine whether NaV1.5 channels are present in rat jejunum and whether specific inhibition of these channels impacts the electrophysiological properties of the tissue in order to gain insight on the functional role of NaV1.5 in gastrointestinal smooth muscle.
METHODS
All animal procedures were done according to protocols approved by the Mayo Clinic Institutional Animal Care and Use Committee and by the Medical College of Wisconsin Institutional Animal Care and Use Committee.
Preparation of samples and immunoblotting
Flash frozen rat heart, jejunum, and colon were homogenized in 500 μl homogenization buffer (0.025 M Tris, 0.15 M NaCl, 0.001 M EDTA, 1% NP-40, 5% glycerol, protease inhibitors, PMSF, pH 7.4) using a handheld homogenizer. The homogenates were centrifuged at high speed and the supernatant protein concentration quantitated by bicinchoninic acid assay. 100 μg of jejunum and colon and 10 μg of heart were separated on a 4–15% tris-glycine gel (BioRad) and the proteins transferred to nitrocellulose (0.45 μm). Nitrocellulose blots were blocked for 1 hour at 4°C in 5% non-fat dry milk/Tris Buffered Saline + Tween (5%NFDM/TTBS), followed by overnight incubation at 4°C with anti-Nav1.5 Ig (Covance) (Supplementary Table 1). Blots were washed three times in TTBS (3 × 10 minutes) and incubated for 2 hours at 4°C in secondary antibody (donkey anti-rabbit; Jackson Laboratories) (Supplementary Table 1). Then, blots were washed three more times in TTBS (3 × 10 minutes), developed using the BioRad Clarity ECL kit, and imaged with the BioRad Gel Documentation System and accompanying software.
Immunolabeling for NaV1.5
Rat jejunum tissue fixed with 4% paraformaldehyde was immunolabeled as 12 μm cryosections using standard techniques and including all necessary controls as described previously.(21) Briefly, sections from flash frozen tissues were washed in 0.1 M phosphate buffered saline (PBS), fixed, then incubated in 0.1M glycine/PBS to quench the aldehydes. Frozen sections were blocked and permeabilized using 0.3% Triton X100, 1% bovine serum albumin in PBS. Antibodies were diluted in blocking solution. To confirm specificity of tissue labeling, each anti-SCN5A primary antibody (Supplementary Table 1) was incubated with the respective immunogens overnight prior to use for immunohistochemistry. In each experiment, samples were exposed to secondary antibody (Supplementary Table 1) in the absence of primary antibody to test for non-specific staining. Experiments were repeated on tissues from n = 3 individual rats.
Cell dissociation
To obtain cells for whole cell electrophysiology, rat small intestine was cut open along its length and laid flat to remove mucosa from the muscle layer. Next, minced pieces of muscle were stirred at 400 rpm with a papain cocktail (1.2 mg/mL papain, 0.3 mg/mL DTT, and 0.6 mg/mL fatty acid-free BSA in 10 mL Ca2+- and Mg2+-free Hanks) in a test tube warmed in a 32°C water bath. After 3 minutes, cells were spun at 1000 rpm for 90 s, the supernatant was replaced with a collagenase cocktail (0.8–1.0 mg/mL Collagenase Type II (Worthington 4176), 4.0–5.0 mg/mL DTT, and 1.0 mg/mL fatty acid-free BSA in 10 mL Ca2+- and Mg2+-free Hanks), and the cells were again stirred in the 32 °C water bath as before. After 5–10 min, cells were rinsed twice with Hanks as above and resuspended in modified minimal essential media (10.4 g/L MEM (Sigma, St. Louis, MO) containing (in mM): 4.17 Na+, 0.5 Ca2+, 0.5 Mg2+, 2 Cl−, 4.17 HCO3−, 10 HEPES, pH 7.2, 280 mOSM).
Whole cell electrophysiology
Inward currents from freshly dissociated myocytes from the rat jejunum tunica muscularis were recorded by whole cell electrophysiology. KG12 glass (Kimble Garner) was pulled on a P-97 puller (Sutter Instruments), and electrodes were coated with R6101 compound (Dow Corning). Inward currents were recorded at a sampling rate of 20 kHz with Axopatch 200B, Digidata 1440, and Cyberamp 300 hardware and Clampex 10 software (Molecular Devices Corp., Sunnyvale, CA). Steady-state activation of inward currents was recorded by 50-ms sequential depolarizations from a −100 mV holding potential to −80 through +35 mV in 5-mV intervals. Steady-state inactivation was recorded by holding cells at −130 mV, pulsing for 3 s to −130 through −25 mV in 5-mV intervals, then to −130 mV for 0.1 ms to reset capacitance artifact, and finally to −30 mV. The extracellular solution contained (in mM): 150 Na+ or NMDG+, 2.5 Ca2+ or Mn2+, 5 K+, and 10 HEPES at pH 7.35 and 300 mmol/kg osmolality. The intracellular solution contained (in mM): 145 Cs+, 5 Na+, 5 Mg2+, 125 CH3SO3−, 35 Cl−, 2 EGTA, and 10 HEPES at pH 7.0 and 290 mmol/kg osmolality. The predicted liquid junction potential was subtracted during analysis. Chemicals were purchased from Sigma-Aldrich Co. (St. Louis, MO). Data were analyzed in Clampfit 10 (Molecular Devices Corp., Sunnyvale, CA), Excel 2010 (Microsoft), and SigmaPlot 12.3. Steady-state activation and inactivation were fit with a 3-parameter sigmoid (Boltzmann) function: y = a/(1+e((x0−x)/b)), where a = 100%, b = slope δV, and x0 = V1/2, the voltage of half-maximal activation or inactivation.
Organotypic cultures
The tunica muscularis from the jejunum of 6–8 weeks old Dark Agouti rats was quickly dissected out and loosely pinned onto sterile 60 mm dishes covered by a thin layer of Sylgard (Dow Corning Corp., Midland, MI). After 5 rapid washes in medium composed of M199 (Thermo Fisher, Waltham MA) without phenol red, supplemented with 4.5 g/l of glucose (Sigma–Aldrich, St. Louis, MO), 10% FBS, 2 mM L-glutamine and 2% antibiotic–antimycotic (Invitrogen Grand Island, NY), the tissue was incubated for 3 hours at 37°C for equilibration. For lentiviral-mediated transduction with short hairpin RNA (shRNA), the tissues were treated with non-targeting (NT) or SCN5A targeting, shRNA lentiviral transduction particles (Mission Sigma) (T-KD) with a multiplicity of infection (MOI) of 2. An untreated (UT) control was cultured in the same conditions. A total of n = 5 knockdown experiments were done on tissues from individual rats. For the next 3 days, tissues were washed and fresh culture medium was added daily. After 3 days, one tissue each from the non-targeting shRNA (NT), SCN5A targeting shRNA (T-KD) and untreated (UT) tissues was used for electrical recording and one tissue was frozen immediately in liquid nitrogen for qRT-PCR to assess the level of knockdown of SCN5A. Recordings were made from 5–8 cells for each of the 3 different conditions (UT, NT and KD) per animal, corresponding to ~19–30 cells per animal.
Quantitative RT-PCR
After 72 hours, the tissue was collected and total RNA was extracted with RNA-Bee solution (Tel-Test Inc., Friendswood, TX) according to the manufacturer’s instructions. RT was performed using the SuperScript VILO enzyme (Invitrogen, Grand Island, NY). The reaction protocol consisted of annealing at 25 °C for 10 min, followed by one cycle at 42 °C for 60 min and terminated by incubation at 85 °C for 5 min. The cDNA was then used for real time PCR in the LightCycler 480 instrument using the SYBR Green I Master Mix (Roche, Indianapolis, IN) and primers specific for the rat SCN5A gene (Supplementary Table 2). The standard protocol used was the following: 5 min at 95 °C, followed by 45 cycles of 10 s at 95 °C, 10 s at 60 °C and 10 s at 72 °C. Melting curves generated with a dissociation protocol were used to ensure the specificity of primers for a single product for each reaction. The expression of SCN5A was normalized to β-actin and the level of knockdown was calculated using the ΔΔCt method. Levels of RNA were measured in triplicate from each knockdown experiment on tissues from n = 5 rats.
Sharp electrode electrophysiology
Electrophysiological experiments were recorded from lentiviral-treated and control muscle strips. Sharp microelectrode recordings were made through the circular muscle side in the presence of 2 μM nifedipine (Sigma-Aldrich, St. Louis, MO) to minimize muscle contraction. The muscle strips were pinned with the circular side facing upward using sterile, fine wire pins (California Fine Wire Company, Grover Beach, CA) on a Sylgard elastomer (Dow Corning Corp.) coated, custom made recording chamber (60 mm × 15 mm) for an inverted microscope (Olympus IX70). The electrophysiological recording chamber was constantly perfused with an oxygenated Krebs solution aerated with 97% O2/3% CO2 at 37°C (±0.5°C). Glass capillary microelectrodes (borosilicate glass tube, 1.2 mm OD, 0.6 mm ID, 75 mm length, FHC Inc., Bowdoin, ME) were pulled using a P-97 micropipette puller (Sutter Instruments, Novato, CA). Microelectrodes filled with 3 M KCl had tip resistances ranging between 70 and 90 MΩ. Transmembrane potentials were measured using an Axoclamp 2B amplifier and a Digidata 1440A acquisition system, and stored on a computer running Axoscope 10.0 software (Molecular Devices Corp., Sunnyvale, CA). Signals were recorded at a sampling rate of 2 kHz (interval of 500 μs).
Solutions and drugs
Muscle strips were constantly perfused with an oxygenated Krebs solution composed of the following (in mM): NaCl 120.3; KCl 5.9; MgCl2 1.2; NaHCO3 15.5; NaH2PO4 1.2; glucose 11.5; CaCl2 2.5 (pH was 7.35–7.45 when bubbled with 97% O2 + 3% CO2 at 37±0.5°C). Nifedipine (Sigma-Aldrich, St Louis, MO) was dissolved in ethanol at a stock concentration of 10 mM.
Data analysis and statistics for electrophysiology
A stable impalement for a ~30 second time period consisting of at least 10 slow wave events was considered for data analysis. All the raw slow wave recordings were low-pass filtered using a Butterworth (8-pole) digital filter in Clampfit 10.3.1.5 software (Molecular Devices Corp., Sunnyvale, CA) with a cut-off of 60 Hz. Data were analyzed off-line using Clampfit 10.3.1.5 software (Molecular Devices Corp., Sunnyvale, CA) for the following slow wave parameters: (i) resting membrane potential (Em, mV), (ii) peak slow wave amplitude (mV), (iii) membrane potential (MP) at peak amplitude (Em-Pk, mV); (iv) half-width (ms), (v) rise slope 10%–90% (mV/ms), (vi) rise time 10%–90% (ms) and (vii) instantaneous frequency (Hz). In 8 out of the 81 cells studied, the RMPs were adjusted for the electrode pullout potential upon withdrawal of the electrode from the cell. Data are expressed as means ± standard errors of the mean (SEM). Statistical significance was determined by Graphpad Prism using the appropriate statistical test with corrections for multiple comparisons where necessary, as indicated in the results section. P values of less than 0.05 were considered statistically significant. The values from different cells (n) were analyzed and reported as means ± SEM. The ‘n’ value refers to the count of separate cells recorded from tissue strips; and the ‘N’ value refers to the animal count.
Generation of SCN5A Knockout (KO) Rat
Nav1.5 was knocked out in Dark Agouti rats using zinc finger nuclease (ZFN) technology to target exon 4 in the rat Scn5a gene using previously reported techniques.(22, 23) Briefly, ZFN constructs specific for the rat SCN5A gene, which is found on chromosome 8 at location 8q32, were designed, assembled, and validated by Sigma-Aldrich. The construct targeted the sequence TGCACCATCCTGACCAActGCGTGTTCATGGCCCAG to generate a break in the genome. mRNA encoding the ZFNs was injected into 1-cell Dark Agouti rat embryos. Injected embryos were transferred to pseudo-pregnant females, and genotyped using the following primers that flank the target sequence, ZFN Primer F, AGGGGCTTCTGTGGCTTTAT and ZFN Primer R CTCTCAGGCAACACTAGGGC, in order to identify animals with frameshift deletions in the targeted sequence. Two founders were identified with deletions affecting the SCN5A gene; one with a 28bp frameshift deletion and the other with a 110 bp deletion of exon 4 and intron 4 in the SCN5A gene. The founder with the 28 bp deletion was selected for further study since this was confirmed to create a non-functional protein and was backcrossed to Dark Agouti male rats. Rats containing the mutated alleles were identified by PCR amplification from genomic DNA extracted from ear punches using the primers shown above.
RESULTS
NaV1.5 protein is found in rat jejunum and colon
We first examined Nav1.5 expression in detergent-soluble lysates from rat heart, jejunum, and colon by immunoblotting (Supplementary Table 1). Nav1.5 expression was evident in both jejunum and colon homogenates with heart used as a positive control (Figure 1A). The intensities of the NaV1.5 bands for rat jejunum and colon were 51% and 48%, respectively, when compared to the heart controls.
Figure 1.
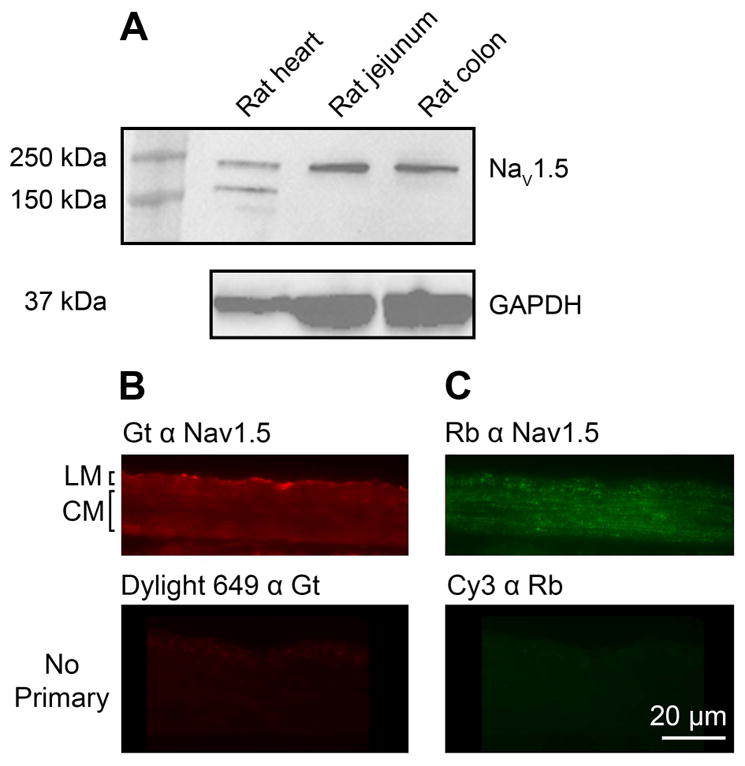
NaV1.5 is present in rat GI tract. A) Protein immunoblots from rat heart, jejunum and colon tunica muscularis demonstrate expression of Nav1.5. B) and C) Immunoreactivity for Nav1.5 in the muscularis propria of rat jejunum. Immunolabeling was detected in the longitudinal (LM) and circular (CM) muscle layers using both goat (Gt) α Nav1.5 antibody (B) and rabbit (Rb) α Nav1.5 (C) antibodies directed against different epitopes on the Scn5a protein (upper panels). No signal is detected when the tissues were incubated with only the secondary antibodies (lower panels). Tissues were labeled at the same time and images were collected with the same exposure times.
NaV1.5 immunoreactivity is present in the circular smooth muscle layer of the rat jejunum
We then used two antibodies to different portions of the NaV1.5 molecule for immunohistochemistry (Supplementary Table 1). Immunolabeling using both antibodies - one raised to an epitope in the C-terminal region of human SCN5A (goat anti-Nav1.5, Figure 1B) and another raised to the intracellular loop between the first two domains of the rat protein (rabbit anti-Nav1.5, Figure 1C) showed immunoreactivity in the muscularis propria of rat jejunum. Although there was also some signal from other regions of the tissue, such as in the serosa when using the goat anti-Nav1.5 antibody (Figure 1B), the only overlapping signal was in the muscularis propria. In addition, the labeling within the muscularis propria appeared more slightly more punctate when using the rabbit anti-Nav1.5 antibody, which we attribute to the different locations of the two epitopes. No signal was detected when the primary antibodies were omitted (Figure 1B & C) and the labeling in the muscularis propria was significantly reduced when the primary antibodies were pre-incubated with the antigenic peptide (Supplementary Figure 1).
Voltage-dependent inward sodium current found in rat jejunum myocytes
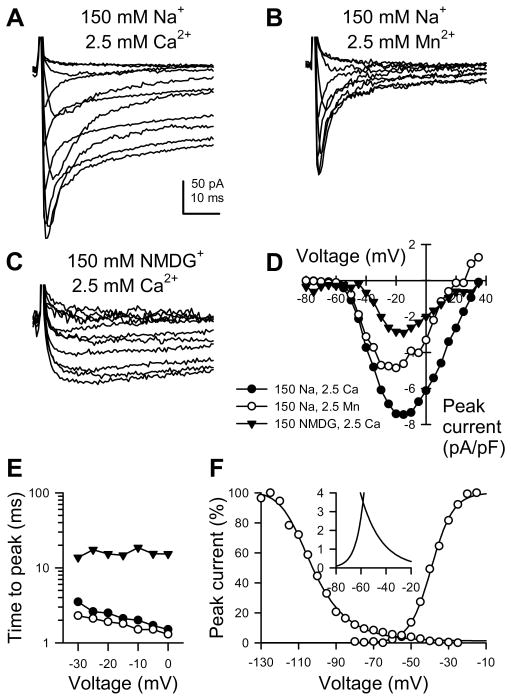
To determine inward conductances present in rat jejunum circular smooth muscle cells, inward currents were recorded by whole cell electrophysiology with high intracellular Cs+ to block outward K+ currents. Serial depolarizations revealed an inward current peaking at −20 mV and reversing at +35 mV with faster kinetics at more negative potentials (Figure 2A). To decipher whether the total inward current was contributed by multiple ionic conductances, extracellular Ca2+ was replaced with Mn2+ revealing a current with fast activation and inactivation kinetics (Figure 2B). Also, extracellular Na+ was replaced with NMDG+, leaving a current of slower kinetics (Figure 2C). Although the half points of voltage-dependence for steady-state activation for these components were separated by just 15 mV (Figure 2D, fast, −30 mV; slow, −15 mV), time-to-peak current (Figure 2E) confirmed the visual differences seen in A–C. The voltage dependence for steady-state activation and inactivation of the fast component showed a window current (−70 to −30 mV) at voltages in the range of Em for jejunum smooth muscle cells in these recordings that resembled that from the Na+ channel NaV1.5 present in human and dog jejunum myocytes.(5, 14)
Figure 2.
Inward currents in rat jejunum circular smooth muscle myocytes. A–C, Representative inward currents elicited by serial depolarizations from −100 mV, recorded from a rat jejunum myocyte bathed in extracellular solution containing 150 mM Na+ (A–B) or NMDG+ (C) and 2.5 mM Ca2+ (A,C) or Mn2+ (B), thereby showing inward currents contributed by Na+ and Ca2+ together (A), only Na+ (B), or only Ca2+ (C). D–F, ●, Na+ and Ca2+ components together; ○, Na+ component only; ▼, Ca2+ component only. D, Current-voltage plot of peak currents at voltage steps from −80 to +35 mV, as shown in A–C. E, Time to peak current versus voltage of step potential. F, Steady-state activation and inactivation of Na+ component of inward current. Inset, window current.
SCN5A Knockout is Lethal in Utero
To determine the role of NaV1.5 in rat GI smooth muscle we created a rat NaV1.5 knock out (KO). No rats homozygous for the SCN5A knockout allele were obtained by breeding heterozygous rats derived from the founding line. We genotyped 113 progeny from 19 litters and found 39 (34%) that had two wild type alleles and 74 (66%) that were heterozygous for the mutant allele. Examination of embryos from timed pregnancies identified runted or dead fetuses in the uterus of female rats that were found to be homozygous for the knockout allele. We genotyped 20 fetuses, of which 2 (10%) were wild type animals, 11 (55%) were heterozygous for the mutant allele and 7 (35%) were homozygous knockout animals. The homozygous knockout fetuses died prior to embryonic day 16 and were of a size and developmental appearance of embryonic day 8–9 rats whereas wild type and heterozygous fetuses appeared normal. At embryonic day 16, the mean crown length for the knockout fetuses was 4.8 ± 0.42 mm compared to 11.0 ± 2.8 mm for wild type and heterozygotes (mean ± SD, n = 5, P < 0.05, t test). Morphologically, the knockout fetuses exhibited very little tissue differentiation, the neural tube was not closed, and there was no evidence of even a primitive gastrointestinal tract. Since we could not obtain SCN5A knockout animals, we proceeded to test the effect of knocking down SCN5A expression in explant tissues (organotypic cultures) from adult rats.
Knockdown of SCN5A in rat jejunum organotypic cultures
To determine the role of NaV1.5 in tissue we used a shRNA lentiviral approach to knock down NaV1.5 in organotypic cultures prepared from rat jejunum smooth muscle strips. We optimized the protocol for the knockdown of SCN5A using Mission transduction shRNA-SCN5A and control shRNA-NT (non-targeting control shRNA) and determined that, at a multiplicity of infection (MOI) of 2 with a 3 day treatment, we obtained about 57.2% ± 0.07 knockdown of SCN5A RNA expression as assessed by quantitative RT-PCR (n=5; P<0.05 unpaired t test) (Figure 3A).
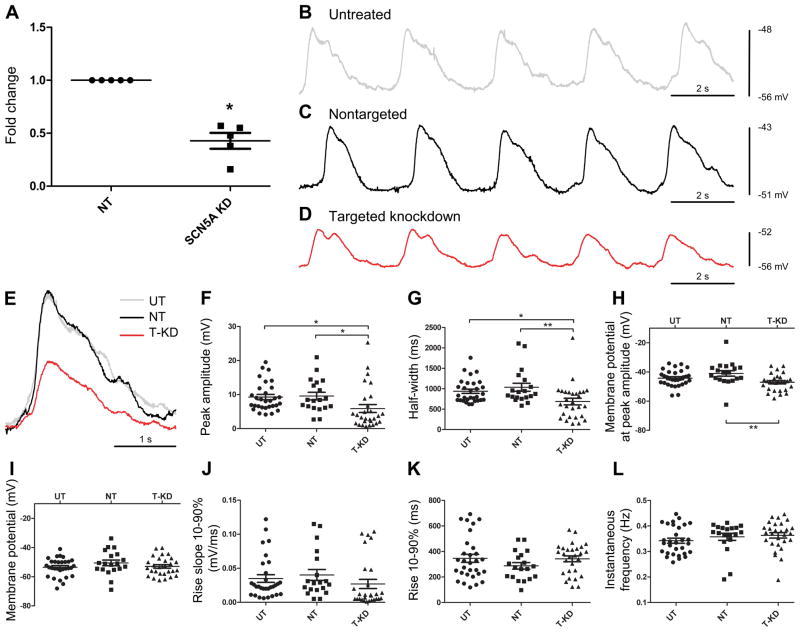
Figure 3.
Knockdown of Scn5a in organotypic cultures alters electrical slow waves. A) Organotypic cultures were transduced with either non-targeting (NT) shRNA lentiviral particles or with shRNA lentiviral particles targeted to knock down Scn5a for 3 days. The mRNA for SCN5A was significantly reduced after transduction with shRNA- Scn5a (Scn5a KD), compared to non-targeting shRNA (NT) (42.8 ± 0.07, n=5, *P<0.05, unpaired t-test). B–D) Representative traces for small intestinal organotypic culture tissue strips untreated (UT) (B), treated with shRNA-NT (C) particles and treated with shRNA-SCN5A (D) particles for 3 days. Panel (E) Superimposition of the single slow wave event on the rising phase of the respective tissue recordings. The scatter plot panels in F – L describe the mean peak amplitude (F), half-width (G), membrane potential at peak amplitude (H), membrane potential (I), rise slope (10–90%) (J), rise (10–90%) (K) and instantaneous frequency (L), respectively in untreated, UT (n=30, N=4), non-targeted, NT (n=19, N=4) and targeted knock down, T-KD (n=28, N=4) tissues. Values are expressed as means±SEM. Statistical differences were noted for all three parameters (E–G) (*P<0.05, One-way ANOVA with Bonferroni post-test).
Effect on electrical activity of knocking down SCN5A (Nav1.5) expression using shRNA lentiviral particles
We performed intracellular sharp microelectrode recordings to assess the functional consequence of knocking down SCN5A mRNA expression. We recorded spontaneous electrical activity from organotypic cultures of control tissues, untreated (UT, Figure 3B); tissues treated with non-targeted shRNA (NT, Figure 3C); and tissues treated with shRNA-SCN5A (targeted knock down, T-KD, Figure 3D) lentiviral particles. Superimposition of the single slow wave event on the rising phase of the respective tissue recordings visually demonstrates differences with NaV1.5 knock down (Figure 3E). We quantified several parameters, focusing on the electrophysiological parameters that have been previously reported to be dependent on Na+ flux. These included resting membrane potential (Em, mV), peak slow wave amplitude (mV), slow wave half-width (ms), rise slope 10%–90% (mV/ms), rise time 10%–90% (ms), and instantaneous frequency (Hz). Additionally, given the existence of a gradient of membrane potential and the corresponding variability in slow wave amplitudes across the muscle layer of the intestine, we also examined the membrane potential at peak amplitudes (Em-Pk, mV).
We observed significant differences in several slow wave parameters in organotypic cultures treated with shRNA-SCN5A (T-KD) compared to the tissues treated with non-targeted shRNA (NT) and untreated (UT) tissues. Specifically, there was an approximately 40% decrease in slow wave peak amplitude (Figure 3F; UT: 9.26±0.77 mV, n=30, N=4 and NT: 9.56±1.12 mV, n=19, N=4 vs. T-KD: 5.90±1.17 mV, n=28, N=4, P<0.05, One-way ANOVA with Bonferroni post-test) and an approximately 30% decrease in half-width time (Figure 3G; UT: 936.9±51.88 ms, n=30, N=4, NT: 1034±98.36 ms, n=19, N=4 vs. T-KD: 684.7±81.69 ms, n=28, N=4, P<0.05, One-way ANOVA with Bonferroni post-test). The membrane potential (Em) at peak amplitude (Em-Pk, mV) of the slow wave was significantly more negative in SCN5A - T-KD compared to NT (Figure 3H; UT: −44.26±1.00, n=30, N=4 vs. T-KD, P>0.05; NT: −40.99±1.88, n=19, N=4 vs. T-KD: −47.08±1.10, n=28, N=4, P<0.05, One-way ANOVA with Bonferroni post-test).
The other electrical slow wave parameters did not show significant differences between the T-KD and NT or UT tissues. These included membrane potential (Em) (Figure 3I; UT: −53.53±1.12 mV, n=30, N=4, NT: −50.55±1.95 mV, n=19, N=4 vs. T-KD: −52.98±1.20 mV, n=28, N=4, P>0.05, One-way ANOVA with Bonferroni post-test), rise slope 10%–90% (Figure 3J; UT: 0.035±0.006 mV/ms, n=30, N=4, NT: 0.040±0.008 mV/ms, n=19, N=4 vs. T-KD: 0.027±0.007 mV/ms, n=28, N=4, P>0.05, One-way ANOVA with Bonferroni post-test), rise time 10%–90% (Figure 3K; UT: 346±31.38 ms, n=30, N=4, NT: 287±25.55 ms, n=19, N=4 vs. T-KD: 343±23.16 ms, n=28, N=4, P>0.05, One-way ANOVA with Bonferroni post-test) and instantaneous frequency (Figure 3L; UT: 0.34±0.010 Hz, n=30, N=4, NT: 0.36±0.014 Hz, n=19, N=4 vs. T-KD: 0.36±0.011 Hz, n=28, N=4, P>0.05, One-way ANOVA with Bonferroni post-test).
DISCUSSION
This study demonstrates that the Scn5a -encoded voltage-gated Na+ channel Nav1.5 is present in rat jejunum smooth muscle cells and that NaV1.5 contributes to the electrophysiological properties of the small intestine. These conclusions are based on the demonstration of a voltage-gated Na+ current in dissociated smooth muscle cells, the amplification of message for Scn5a from the muscularis propria and the identification of Nav1.5-like immunoreactivity in the muscularis propria by immunohistochemistry and immunoblotting as well as the observation that knocking down Nav1.5 expression alters the properties of the electrical slow wave in the organotypically cultured tunica muscularis.
The currents recorded in isolated smooth muscle cells had similar properties to those previously found in human and dog smooth muscle cells,(5, 14, 24) human ICC (7) and rat colon smooth muscle cells.(20) The currents exhibited fast activation and inactivation, were resistant to block by Ca2+ channel blockers, and impermeant to NMDG+.
In addition to NaV1.5 presence with immunoblotting, two different antibodies detected Nav1.5 immunoreactivity by immunohistochemistry, suggesting that the signal was not due to non-specific binding of the antibodies and is consistent with our observations in human tissue.(24) The immunolabeling for Nav1.5 was found in both the longitudinal and circular muscle layers of rat jejunum, whereas in the human tissue we have previously found that both the mRNA and the protein for Nav1.5 were restricted to the circular smooth muscle.(24) These differences in Nav1.5 expression between rats and humans add to our previous reports on the species differences in expression of the currents and mRNA for this channel, which include the absence of Scn5a expression in mouse jejunum smooth muscle.(14)
The effect of knocking down expression of Scn5a using lentivirus expressing targeted shRNA suggests a physiological role for this channel in normal gastrointestinal function. We achieved an average of 60% knockdown in Scn5a mRNA expression compared to treatment with shRNA and this knockdown was sufficient to have significant physiologic effects. The electrophysiological effects of the knockdown included reduction in electrical slow wave amplitude and half width time. Also, the activation kinetics and voltage range for the window currents of Nav1.5 reported in this study indicate that the channels are available at the smooth muscle resting potential.
Complete knockdown of Scn5a expression might have revealed additional effects on the electrical properties of small intestinal smooth muscle, but since constitutive knockout of the gene was lethal and could not be rescued using cardiac specific promoter to drive a knocked in Scn5a gene, we could not test this hypothesis. Our observations do support a role for Scn5a in fetal development beyond contributing to electrical activity in the heart and gastrointestinal tract. We also confirmed a previous report showing a gene dosage effect on Scn5a RNA levels in the hearts of animals with a single knockout allele in mice,(25) an effect that was not observed for SCN5A RNA levels in the rat jejunum (data not shown). The data presented here using shRNA-mediated knockdown do not support a major contribution of Nav1.5 to membrane resting potential in rat smooth muscle, since knockdown of the channel in organotypic cultures with shRNA did not significantly alter the membrane potential of the smooth muscle. In human jejunum we previously found that pharmacologic sodium channel blockade caused a hyperpolarization of the resting potential(7) and others had found a similar hyperpolarization in cat jejunum using ion replacement.(9, 13) The absence of an effect of NaV1.5 knock down on resting membrane potential in this study may reflect either a species difference or the incomplete knockdown achieved in this study. Our data do provide evidence that activation of Nav1.5 is involved in the upstroke of the electrical slow wave. The peak amplitudes of the slow waves were smaller after targeted knock down of Nav1.5 in organotypic cultures compared to the slow waves recorded after treatment with non-targeted shRNA of tissues or in un-treated tissues cultured at the same time from the same rats. Since the membrane potential did not change, the reduced amplitude of the slow wave was due to the cells depolarizing to a significantly more negative peak voltage in the knock down tissues. The half width of the events was also significantly smaller after targeted knockdown of Nav1.5. Smaller peaks may contribute to this effect by potentially resulting in decreased rise in intracellular Ca2+ and reduced activation of the Ca2+ dependent conductances that contribute to the plateau component of the electrical slow wave in ICC, most likely the Ca2+-activated Cl− channel, Ano1.(26) The consequences of these effects lead us to conclude that Nav1.5 may alter contractility in the rat small intestine in two ways. Since Nav1.5 contributes to the initial, fast-rising depolarization of the slow wave in smooth muscle, it will dictate the rate of voltage-dependent Ca2+ channel activation, increase the likelihood of smooth muscle action potentials super-imposed on the slow wave, and therefore greater Ca2+ entry and initiation of intracellular Ca2+ release in smooth muscle cells. Further, a shorter slow wave duration limits the duration of Ca2+ influx and therefore the ability of the smooth muscle to achieve intracellular Ca2+ levels sufficient to initiate muscle contractions.
In summary, the voltage-gated Na+ channel Nav1.5, encoded by the Scn5a gene, is expressed in rat GI smooth muscle and regulates the electrical slow wave. The role of Nav1.5 in the rat small intestine is consistent with reduced smooth muscle excitability and decreased motility and also the constipation phenotype in patients with loss of function mutations in Scn5a.(1) The identification of rats as an animal model for these studies significantly improves the ability to study the precise role of this channel in the gastrointestinal tract.
Supplementary Material
KEY MESSAGES.
This study establishes the expression and function of SCN5A-encoded NaV1.5 in rat gastrointestinal smooth muscle.
The SCN5A-encoded voltage-gated sodium channel NaV1.5 is found in the human GI tract, where it regulates smooth muscle electrical properties. Mutations in SCN5A are found in patients with irritable bowel syndrome (IBS). The goal of this work was to determine the presence and functional significance of NaV1.5 in rat jejunum.
We used immunohistochemistry, Western blotting, PCR, SCN5A knock down by shRNA in rat jejunum organotypic cultures and whole cell and tissue electrophysiology.
We found that NaV1.5 is expressed in rat jejunum smooth muscle and that knock down of NaV1.5 resulted in a decrease in slow wave amplitude and half-width. This study establishes rat gastrointestinal tract as an important model for study of the SCN5A-encoded NaV1.5 in GI physiology and pathophysiology.
Acknowledgments
The authors would like to thank Gary Stoltz for tissue dissection and cell dissociation and Kristy Zodrow for secretarial assistance. This work was supported by NIH DK 57266, DK 57061 DK84567 and HL 083422.
Abbreviations
- shRNA
short hairpin RNA
- DAPI
4′,6-diamidino-2-phenylindole
- NMDG+
N-methyl D-glucamine
- RT-PCR
Reverse transcription PCR
Footnotes
Competing Interests: the authors have no competing interests.
CONTRIBUTIONS: AB - study design, analysis and interpretation of data, drafting of the manuscript, critical revision of important intellectual content; SJG - study design, analysis and interpretation of data, drafting of the manuscript, critical revision of important intellectual content; AM - acquisition, analysis and interpretation of data, drafting of the manuscript; PRS - study design, acquisition, analysis and interpretation of data, drafting of the manuscript, critical revision of important intellectual content; SAS - acquisition and analysis of data, drafting of the manuscript; LS, SH, STE, CEB - acquisition and interpretation of data; PJM, FK - acquisition and interpretation of data, drafting of the manuscript; GF - study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis, obtained funding, administrative, technical, and material support, study supervision.
References
- 1.Beyder A, Mazzone A, Strege PR, Tester DJ, Saito YA, Bernard CE, Enders FT, Ek WE, et al. Loss-of-function of the voltage-gated sodium channel NaV1. 5 (channelopathies) in patients with irritable bowel syndrome. Gastroenterology. 2014;146:1659–1668. doi: 10.1053/j.gastro.2014.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beyder A, Farrugia G. Targeting ion channels for the treatment of gastrointestinal motility disorders. Therap Adv Gastroenterol. 2012;5:5–21. doi: 10.1177/1756283X11415892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiong Z, Sperelakis N, Noffsinger A, Fenoglio-Preiser C. Changes in calcium channel current densities in rat colonic smooth muscle cells during development and aging. Am J Physiol. 1993;265:C617–625. doi: 10.1152/ajpcell.1993.265.3.C617. [DOI] [PubMed] [Google Scholar]
- 4.Xiong Z, Sperelakis N, Noffsinger A, Fenoglio-Preiser C. Ca2+ currents in human colonic smooth muscle cells. Am J Physiol. 1995;269:G378–385. doi: 10.1152/ajpgi.1995.269.3.G378. [DOI] [PubMed] [Google Scholar]
- 5.Holm AN, Rich A, Miller SM, Strege P, Ou Y, Gibbons S, Sarr MG, Szurszewski JH, et al. Sodium current in human jejunal circular smooth muscle cells. Gastroenterology. 2002;122:178–187. doi: 10.1053/gast.2002.30346. [DOI] [PubMed] [Google Scholar]
- 6.Strege PR, Holm AN, Rich A, Miller SM, Ou Y, Sarr MG, Farrugia G. Cytoskeletal modulation of sodium current in human jejunal circular smooth muscle cells. Am J Physiol Cell Physiol. 2003;284:C60–66. doi: 10.1152/ajpcell.00532.2001. [DOI] [PubMed] [Google Scholar]
- 7.Strege PR, Ou Y, Sha L, Rich A, Gibbons SJ, Szurszewski JH, Sarr MG, Farrugia G. Sodium current in human intestinal interstitial cells of Cajal. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1111–1121. doi: 10.1152/ajpgi.00152.2003. [DOI] [PubMed] [Google Scholar]
- 8.Job DD. Ionic basis of intestinal electrical activity. Am J Physiol. 1969;217:1534–1541. doi: 10.1152/ajplegacy.1969.217.5.1534. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Prosser CL, Job DD. Ionic dependence of slow waves and spikes in intestinal muscle. Am J Physiol. 1969;217:1542–1547. doi: 10.1152/ajplegacy.1969.217.5.1542. [DOI] [PubMed] [Google Scholar]
- 10.El-Sharkawy TY, Daniel EE. Ionic mechanisms of intestinal electrical control activity. Am J Physiol. 1975;229:1287–1298. doi: 10.1152/ajplegacy.1975.229.5.1287. [DOI] [PubMed] [Google Scholar]
- 11.Connor JA, Prosser CL, Weems WA. A study of pace-maker activity in intestinal smooth muscle. J Physiol. 1974;240:671–701. doi: 10.1113/jphysiol.1974.sp010629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barajas-Lopez C, Den Hertog A, Huizinga JD. Ionic basis of pacemaker generation in dog colonic smooth muscle. J Physiol. 1989;416:385–402. doi: 10.1113/jphysiol.1989.sp017767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wienbeck M, Christensen J. Cationic requirements of colon slow waves in the cat. Am J Physiol. 1971;220:513–519. doi: 10.1152/ajplegacy.1971.220.2.513. [DOI] [PubMed] [Google Scholar]
- 14.Strege PR, Mazzone A, Kraichely RE, Sha L, Holm AN, Ou Y, Lim I, Gibbons SJ, et al. Species dependent expression of intestinal smooth muscle mechanosensitive sodium channels. Neurogastroenterol Motil. 2007;19:135–143. doi: 10.1111/j.1365-2982.2006.00844.x. [DOI] [PubMed] [Google Scholar]
- 15.Neshatian L, Strege PR, Rhee P-L, Kraichely RE, Mazzone AM, Bernard CE, Cima RR, Larson DW, et al. Ranolazine inhibits voltage-gated mechanosensitive sodium channels in human colon circular smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2015 doi: 10.1152/ajpgi.00051.2015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poh YC, Beyder A, Strege PR, Farrugia G, Buist ML. Quantification of gastrointestinal sodium channelopathy. J Theor Biol. 2012;293C:41–48. doi: 10.1016/j.jtbi.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazzone A, Strege PR, Tester DJ, Bernard CE, Faulkner G, De Giorgio R, Makielski JC, Stanghellini V, et al. A mutation in telethonin alters Nav1. 5 function. J Biol Chem. 2008;283:16537–16544. doi: 10.1074/jbc.M801744200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Locke GR, 3rd, Ackerman MJ, Zinsmeister AR, Thapa P, Farrugia G. Gastrointestinal symptoms in families of patients with an SCN5A-encoded cardiac channelopathy: evidence of an intestinal channelopathy. Am J Gastroenterol. 2006;101:1299–1304. doi: 10.1111/j.1572-0241.2006.00507.x. [DOI] [PubMed] [Google Scholar]
- 19.Saito YA, Strege PR, Tester DJ, Locke GR, 3rd, Talley NJ, Bernard CE, Rae JL, Makielski JC, et al. Sodium channel mutation in irritable bowel syndrome: evidence for an ion channelopathy. Am J Physiol Gastrointest Liver Physiol. 2009;296:G211–218. doi: 10.1152/ajpgi.90571.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiong Z, Sperelakis N, Noffsinger A, Fenoglio-Preiser C. Fast Na+ current in circular smooth muscle cells of the large intestine. Pflugers Arch. 1993;423:485–491. doi: 10.1007/BF00374945. [DOI] [PubMed] [Google Scholar]
- 21.Bernard CE, Gibbons SJ, Gomez-Pinilla PJ, Lurken MS, Schmalz PF, Roeder JL, Linden D, Cima RR, et al. Effect of age on the enteric nervous system of the human colon. Neurogastroenterol Motil. 2009;21:746–e746. doi: 10.1111/j.1365-2982.2008.01245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geurts AM, Cost GJ, Remy S, Cui X, Tesson L, Usal C, Menoret S, Jacob HJ, et al. Generation of gene-specific mutated rats using zinc-finger nucleases. Methods Mol Biol. 2010;597:211–225. doi: 10.1007/978-1-60327-389-3_15. [DOI] [PubMed] [Google Scholar]
- 23.Moreno C, Hoffman M, Stodola TJ, Didier DN, Lazar J, Geurts AM, North PE, Jacob HJ, et al. Creation and characterization of a renin knockout rat. Hypertension. 2011;57:614–619. doi: 10.1161/HYPERTENSIONAHA.110.163840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ou Y, Gibbons SJ, Miller SM, Strege PR, Rich A, Distad MA, Ackerman MJ, Rae JL, et al. SCN5A is expressed in human jejunal circular smooth muscle cells. Neurogastroenterol Motil. 2002;14:477–486. doi: 10.1046/j.1365-2982.2002.00348.x. [DOI] [PubMed] [Google Scholar]
- 25.Papadatos GA, Wallerstein PM, Head CE, Ratcliff R, Brady PA, Benndorf K, Saumarez RC, Trezise AE, et al. Slowed conduction and ventricular tachycardia after targeted disruption of the cardiac sodium channel gene Scn5a. Proc Natl Acad Sci U S A. 2002;99:6210–6215. doi: 10.1073/pnas.082121299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kito Y, Suzuki H. Properties of pacemaker potentials recorded from myenteric interstitial cells of Cajal distributed in the mouse small intestine. J Physiol. 2003;553:803–818. doi: 10.1113/jphysiol.2003.051334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.