Abstract
Loss of functional cells from immunorejection during the early post-transplantation period is an important factor that reduces the efficacy of stem cell-based therapies. Recent studies have shown that transplanted mesenchymal stem cells (MSCs) can exert therapeutic effects by secreting anti-inflammatory and pro-survival trophic factors. We investigated whether co-transplantation of MSCs could improve the survival of other transplanted therapeutic cells. Allogeneic glial-restricted precursors (GRPs) were isolated from the brain of a firefly luciferase transgenic FVB mouse (at E13.5 stage) and intracerebrally transplanted, either alone, or together with syngeneic MSCs in immunocompetent BALB/c mice (n=20) or immunodeficient Rag2−/− mice as survival control (n=8). No immunosuppressive drug was given to any animal. Using bioluminescence imaging (BLI) as a non-invasive readout of cell survival, we found that co-transplantation of MSCs significantly improved (p<0.05) engrafted GRP survival. No significant change in signal intensities were observed in immunodeficient Rag2−/− mice, with transplanted cells surviving in both the GRP only and the GRP+MSC group. In contrast, on day 21 post-transplantation, we observed a 94.2 % decrease in BLI signal intensity in immunocompetent mice transplanted with GRPs alone versus 68.1% in immunocompetent mice co-transplanted with MSCs and GRPs (p<0.05). Immunohistochemical analysis demonstrated a lower number of infiltrating CD45, CD11b+ and CD8+ cells, reduced astrogliosis, and a higher number of FoxP3+ cells at the site of transplantation for the immunocompetent mice receiving MSCs. The present study demonstrates that co-transplantation of MSCs can be used to create a microenvironment that is more conducive to the survival of allogeneic GRPs.
Keywords: Mesenchymal stem cell, co-transplantation, glial-restricted precursor, bioluminescence imaging, cell survival, immunomodulation
Introduction
Stem cell-based therapies have emerged as a promising mode of treatment in neurodegenerative diseases for which there is no effective cure. One of the major obstacles to the success of stem cell-based therapies is the loss of transplanted cells after engraftment (Lawrence et al., 1994; Li and Duncan, 1998; Tambuyzer et al., 2009). Pathogenic immune signaling that results from the disease process itself (Chen and Palmer, 2008), immunorejection (Zhao et al., 2011), or non-immunologic factors, such as the lack of trophic factors in the host microenvironment, greatly reduce transplanted cell survival and functional integration. One way to improve transplanted cell survival and differentiation is to attenuate the host immune response (Chen et al., 2011). Classic immunosuppressive drugs can be used to inhibit activity of the immune system and minimize transplanted cell rejection (Gorelik et al., 2012). However, treatment with immunosuppressive drugs is often associated with low specificity that may lead to toxicity detrimental to both host and grafted cells (Chan et al., 1995; Mohebbi et al., 2009; Oliveira et al., 2004).
Transplanted cell survival can also be improved by supplementing trophic factors (Apostolides et al., 1998) or co-transplantation of “helper cells” engineered to overexpress growth factors such as basic fibroblast growth factor (bFGF) (Liang et al., 2013; Smith and Snyder, 2013).
Mesenchymal stem cells (MSCs) are multipotent stromal cells that play a role in shaping the bone marrow microenvironment where hematopoiesis occurs. Use of MSCs for the improvement of engrafted cell survival is very appealing because in addition to their regenerative abilities, MSCs also exhibit a multitude of positive effects, including the release of pro-survival trophic and anti-inflammatory/immunomodulatory factors (Abboud et al., 1991; Aggarwal and Pittenger, 2005; Jitschin et al., 2013; Wilkins et al., 2009). These cells also have a strong capacity for exosome secretion in response to cellular injuries. (Baglio et al., 2012; Li et al., 2013; Liang et al., 2014). Therapeutic MSCs have been used in several neurological conditions with inflammation, such as multiple sclerosis, amyotrophic lateral sclerosis (Karussis et al., 2010; Vercelli et al., 2008; Zhao et al., 2011), Huntington’s disease (Bantubungi et al., 2008), and spinal cord injury (Torres-Espin et al., 2013), and to enhance engraftment of therapeutic cells such as hematopoietic stem cells (Le Blanc et al., 2007), human embryonic stem cells (Puymirat et al., 2009), oligodendrocyte progenitors (Cristofanilli et al., 2011) and islet cells (Kerby et al., 2013).
Among the therapeutic cell types considered for transplant therapy, glial-restricted precursors (GRPs) have been found to have positive effects in different neurological disorders. GRPs show high A2B5 immunoreactivity and can be differentiated into oligodendrocytes and astrocytes under appropriate differentiation signals (Rao and Mayer-Proschel, 1997; Rao et al., 1998). GRPs, when transplanted into the central nervous system (CNS), can generate extensive remyelination in a rodent models of dysmyelination, preserve electrophysiological function in rats with focal inflammatory spinal cord demyelination (Walczak et al., 2011), and support regeneration in an animal model of spinal cord injury (Haas and Fischer, 2013). However, the long-term survival of these cells post-transplantation remains a challenge. In a previous study it was reported that, in immunocompetent animals, GRP graft survival was compromised despite the use of immunosuppressive drugs (Gorelik et al., 2012). In this study, we aimed to develop a co-transplantation strategy in which the pro-survival and immunomodulatory properties of MSCs can be used to improve the survival of allografted GRPs in mouse brain.
Materials and Methods
Isolation and characterization of MSCs
Syngeneic MSCs were isolated from an aspirate of bone marrow harvested from the tibia and femoral marrow compartments of 4-week-old BALB/c mice (Jacksons Laboratory), the immunocompetent cell recipients in this study. Bone marrow was cultured in a medium with Dulbecco’s modified Eagle’s medium (DMEM) and 20% fetal bovine serum (FBS) for three days at 37 °C in a 5% CO2 incubator. After three days, non-adherent cells were removed and fresh medium was replaced. When primary cultures became 80-90% confluent, cells were treated with TrypLE™ Express (Gibco) for five minutes at 37°C, harvested, washed with 10 mM phosphate buffered saline (PBS), pH=7.4, and replated. An adherent population of MSCs was obtained three weeks after the initiation of culture. For characterization, MSCs (1 × 106 cells) were incubated in 2% FBS in PBS at 4°C for 30 minutes with 1 μl of monoclonal antibody specific for CD34, CD45, CD90, and CD105 (Novus Biological), or left unstained, and were analyzed by FACSCalibur with CellQuest software (Becton Dickinson).
Isolation and characterization of GRPs
Allogeneic GRPs were isolated from the cortical region of the brain of a homozygous firefly luciferase transgenic mouse (FVB background, Jackson Laboratories, strain 008450) at the E13.5 stage, as described elsewhere (Mujtaba et al., 1999). Cells were maintained in serum-free DMEM-F12 medium (Invitrogen) supplemented with N2, B27, bovine serum albumin, and bFGF (Invitogen) as previously described (Lepore et al., 2006). GRPs were characterized using an immunocytochemical assay. In brief, cells were fixed in 4% paraformaldehyde (PFA) for 30 min. Nonspecific binding was blocked by incubating a solution of 10% donkey serum and 0.1% Triton X-100-PBS for two hours at room temperature. Cells were incubated with the appropriate dilutions of primary antibodies in blocking solution overnight at 4°C, then rinsed with PBS and incubated with corresponding secondary antibodies (Alexa Fluor-488 and Alexa Fluro-546, Invitrogen) in blocking buffer for one hour at room temperature. The culture was rinsed three times with PBS and images were obtained with a Zeiss AX10 fluorescence microscope. Primary antibodies used for these experiments were: anti-A2B5 (1:1000, Santa Cruz); anti-Olig1 (1:1000, EMD Milipore); and anti-firefly luciferase (1:3000, GeneTex).
Bioluminescent imaging (BLI) of GRPs in vitro
Transgenic luciferase expressing GRPs were plated into poly L-lysine/laminin-coated 96-well plates at 10,000, 1,000, 100, and 10 cells per well. For bioluminescence measurements, the medium was removed and 0.15 mg/ml D-luciferin in PBS was added. The luminescence signal was measured using an IVIS Spectrum/CT instrument (Perkin Elmer). Images were acquired at one-minute intervals for 10 minutes until peak signal was observed. BLI was quantified by drawing of regions of interest (ROIs) over the wells, with data were expressed as photon flux (p/sec).
Cell transplantation
Twenty immunocompetent male BALB/c (4weeks old, Jacksons Laboratory) and eight male immunodeficient Rag2−/− mice (4-6 weeks old, Taconic) were used in this study. Animals were divided into experimental and control groups. The experimental group was subdivided into: (i) immunocompetent mice transplanted with MSC+GRP (n=10); (ii) immunocompetent mice transplanted with GRP alone (n=10); and a non-immunorejection control group subdivided into (i) immunodeficient mice transplanted with MSC+GRP (n=4); and (ii) immunodeficient mice transplanted with GRP only (n=4).
All animal procedures were conducted according to an approved protocol from our Institutional Animal Care and Use Committee. Animals were anesthetized with 2% isoflurane in oxygen, shaved and stabilized in a Cunningham adaptor mounted on a stereotactic frame (Stoelting). MSCs and GRPs were harvested, washed, and suspended in PBS at a density of 1×105 cells/μl. A 2 μl cell suspension containing 1 μl MSCs and 1μl GRPs or 1 μl GRPs and 1 μl PBS was injected into the brain (AP=0 mm; ML=2.2 mm; DV=2.0 mm) at a rate of 0.5 μl/min using a Hamilton 31G microinjection needle (Hamilton) and a nano-injector (Stoelting). The needle was kept in place for two min after injection to minimize backflow and then withdrawn slowly. Rectal temperature was monitored and maintained between 36.5 and 37.5 °C using a self-regulating heated blanket (Harvard Apparatus) for the duration of surgery. No immunosuppressive drugs were given to any animal before or after surgery. Animals were kept in a normal day-night cycle (12/12 hours) with ad libitum access to food and water.
BLI of transplanted GRPs in vivo
Bioluminescent images were acquired using an IVIS Spectrum/CT instrument (Perkin Elmer). Animals were anesthetized with 2% isoflurane gas in oxygen, and 150 mg/kg D-luciferin (Gold Bioechnology) was injected intraperitoneally (i.p.). Images were acquired 10 min after injection at the peak of the bioluminescence signal. To generate 2D bioluminescent images, no emission filter was used during imaging. Images were quantified by drawing ROIs over the brain region, with data expressed as photon flux (p/sec). To generate 3D bioluminescent images, four spectrally resolved images were acquired using emission filters at 600, 620, 640, and 660 nm, with a bandwidth of 20 nm each. Imaging parameters were an exposure time of 180 s, an aperture of f/1, a FOV=13 cm, and 2048×2048 pixel resolution. Pixel binning was set to an 8×8 bin width for an effective image resolution of 256×256 pixels. Imaging parameters were identical for both 2D and 3D imaging.
Computed tomography (CT)
Micro-CT images were acquired on the same IVIS Spectrum/CT instrument (Perkin Elmer) using 50 kVp x-rays at 1 mA of current, 50 ms exposure time using an aluminum filter. A total of 720 projections spaced 0.5° apart were acquired and the CT volume was reconstructed using Living Image 4.3 software (Perkin Elmer Inc.), at a field of view (FOV) of 12.0×12.0×3.0 cm and an isotropic resolution of 0.15 mm.
Three-dimensional micro-CT/BLI reconstruction
Three-dimensional reconstruction of the bioluminescent signal with superposition on the CT images was performed using the DLIT algorithm in Living Image 4.3 software. The software predefined the bioluminescent source and tissue absorption spectra for the luciferase reporter and mouse tissue. The source distribution was visualized in Living Image using a voxel size of 0.31 mm without smoothing.
(Immuno)histochemistry
At day 21 post-transplantation, mice were anesthetized and perfused intracardially with PBS followed by PBS-buffered 4% PFA. Brains were removed, postfixed overnight at 4°C, and cryopreserved in 30% sucrose. Serial coronal sections, 20 μm thick, were cut using a Thermo Scientific HM 550 Cryostat. For immunohistochemistry, nonspecific binding was blocked by incubating with a solution of 10% donkey serum and 0.1% Triton X-100-PBS for two hours at room temperature. Sections were then incubated overnight at 4°C with the appropriate dilution of primary antibody in blocking solution. The corresponding secondary antibodies (Alexa Fluor-488 and Alexa Fluro-546, Invitrogen) were added at a ratio of 1:200 in blocking solution for two hours at room temperature. Sections were then rinsed with 0.1 M PBS and placed on coverslips with aqueous non-fluorescing mounting medium (Immu-mount, Thermo Scientific). Images were obtained with a Zeiss AX10 fluorescence microscope. Primary antibodies used for these experiments were: anti-firefly luciferase (1:3000, GeneTex); anti-CD45 (1:500,Serotec); anti-CD11b (1:300, Biolegend); anti-GFAP (1:1000, Santa Cruz); anti-CD8 (1:500, Serotec); and anti-FoxP3 (1:1000, Abcam).
Statistical analysis
Data are expressed as mean ± standard error of the mean (SEM). Linear regression analysis was performed to determine the correlation between the variables. A Student’s t-test was used for determining significance, set at p < 0.05 using GraphPad Prism software (version 6.0c).
Results
Isolated MSCs and GRPs express cell-specific markers in vitro
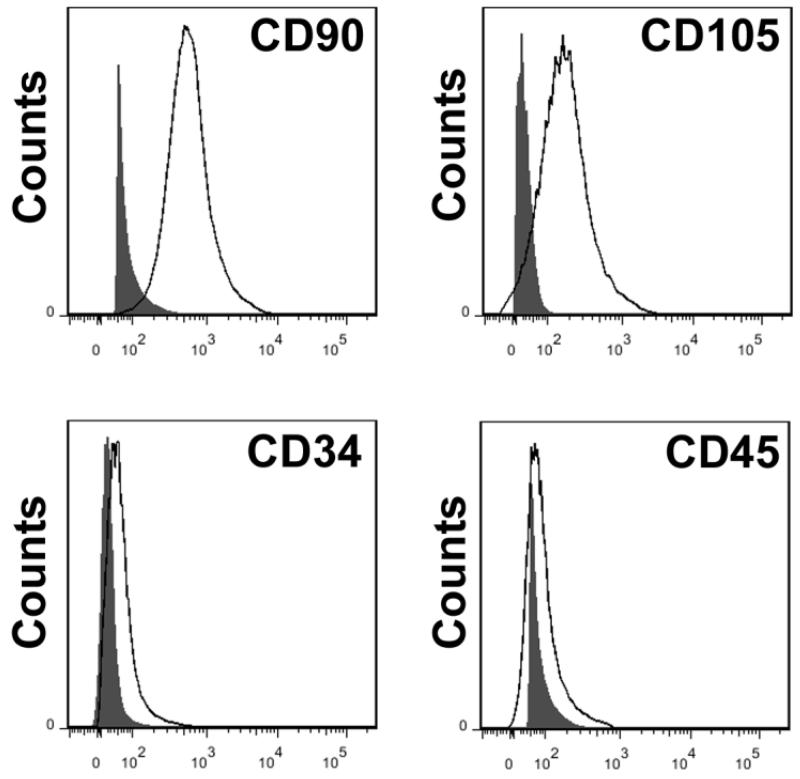
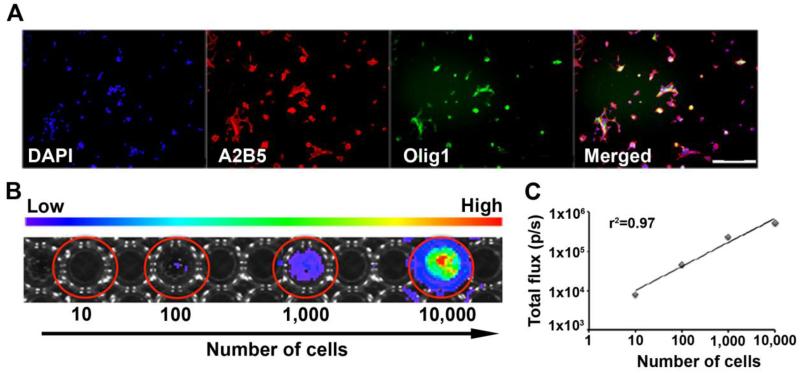
Isolated bone marrow-derived MSCs and brain-derived GRPs were characterized in vitro prior to transplantation. MSCs showed positive expression of the surface antigens CD90 and CD105, and negative expression of the hematopoietic cell-specific antigens CD34 and CD45 at passage four by flow cytometry (Figure 1). Brain-derived GRPs showed positive expression of A2B5 and Olig1 at passage two by immunocytochemistry (Figure 2A).
Figure 1.
Characterization of bone marrow-derived-MSCs by flow cytometry analysis. Shown are histograms of analyzed markers overlaid with unstained controls. MSCs express the specific surface markers CD90 and CD105, while CD34 and CD45 expression was negative, eliminating hematopoietic and endothelial cell contamination in the cell population.
Figure 2.
Characterization of brain-derived GRPs (E13.5) by immunofluorescence and correlation of luciferase reporter gene activity with cell number. (A) Expression of A2B5 protein (red) and Olig1 protein (green). Scale bar= 200μm. (B) BLI of GRPs in vitro at several cell densities. (C) Linear correlation between luciferase expression (number of cells) and BLI signal (r2 =0.97).
Linear correlation between ex vivo BLI signal versus number of GRPs
In vitro BLI analysis of GRPs showed a linear relationship between cell number and BLI signal (R2 = 0.97) (Figure 2B & 2C), validating the use of BLI for quantification of luciferase-expressing GRPs.
Confirmation of accurate injection at the target site
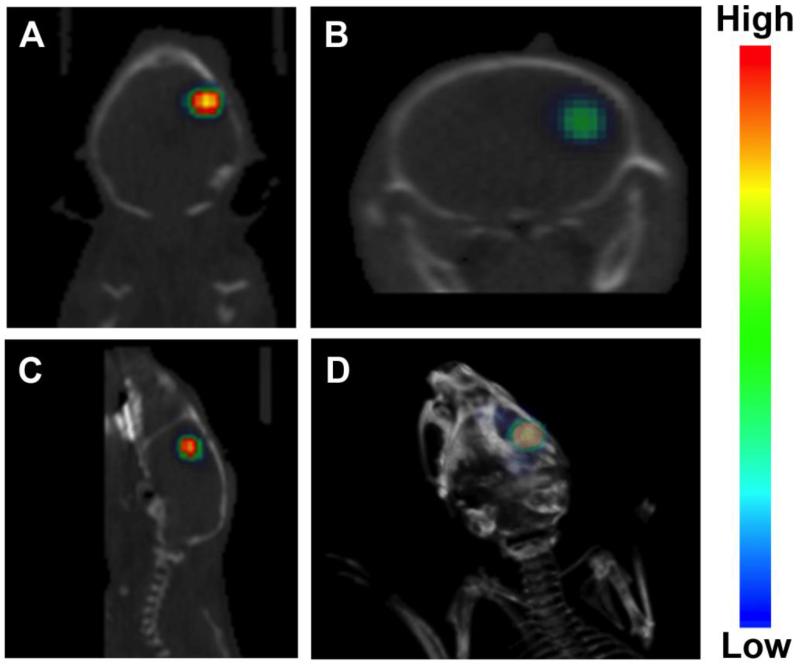
Two- and three-dimensional images generated by co-registering BLI and CT images confirmed the placement of the cells at the site of the targeted injection in the brain. No trace of BLI signal other than the site of transplantation was observed indicating that there was no cell-backflow due to the pressure of the injection, and thus, there was no cell loss during the procedure (Figure 3).
Figure 3.
Co-registration of CT and BL images confirm the correct placement of cells at the site of the targeted injection. Shown are (A) coronal, (B) transaxial, (C) sagittal, and (D) a 3D image of a mouse brain with engrafted cells obtained at day 1 post-transplantation.
GRP survival with and without co-transplantation of syngeneic MSCs
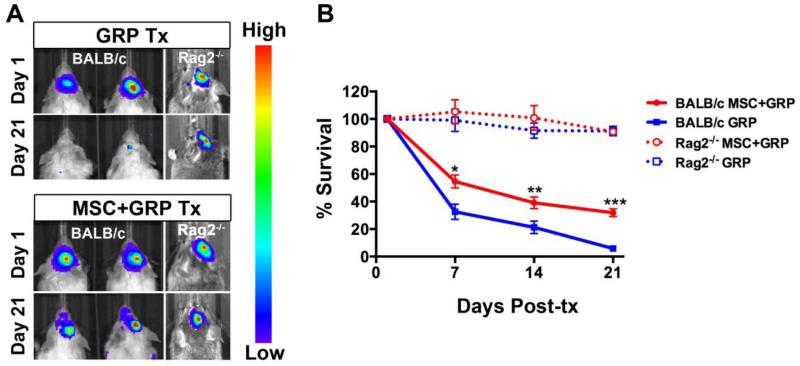
The survival of transplanted luciferase-expressing GRPs was monitored by serial BLI over time. The BLI signal for the survival control group (immunodeficient mice transplanted with MSC+GRP or GRP alone) revealed no significant cell death throughout the study period (Figure 4). For the immunocompetent mice, there was a gradual decline in cell survival for both the MSC+GRP and GRP groups), indicating the occurrence of cell rejection. By day 21, the BLI signal disappeared to background levels for animals transplanted with GRP alone, but was still detectable in animals transplanted with MSC+GRP (Figure 4). For animals transplanted with GRP alone, only 5.8% of the initial signal intensity at three weeks post-transplantation was observed, while the number for animals co-transplanted with MSC+GRP was 39.1% (p<0.05).
Figure 4.
Improved allogeneic GRP graft survival following co-transplantation of syngeneic MSCs. Immunocompetent BALB/c and immunodeficient Rag2−/− mice were used as recipients. (A) BL images of luciferase-expressing GRPs, transplanted alone (upper panel), or together with MSCs (lower panel) at days 1 and 21. (B) Percentage loss of BLI signal compared to day 1 post-transplantation (set as 100%). (*=p<0.5, **= p<0.01, ***= p<0.001, n=10 each).
Syngeneic MSCs effectively suppressed the host immune response
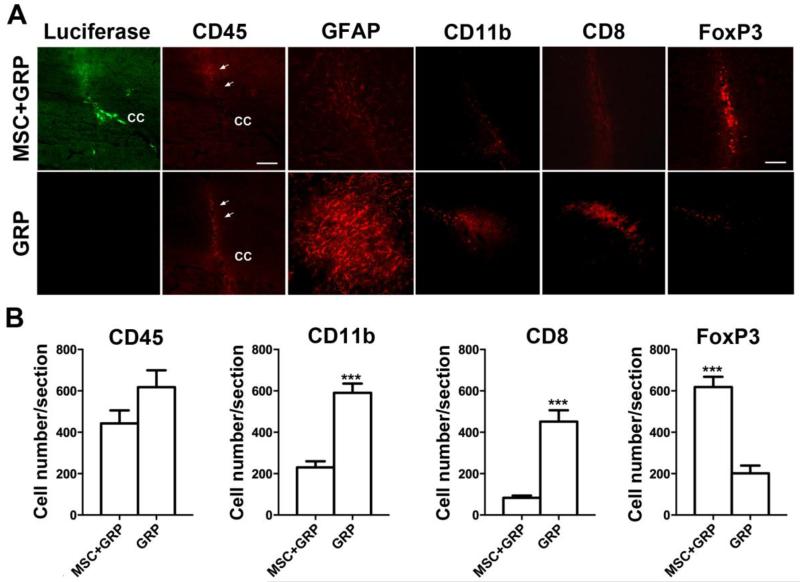
Following the last BLI time point (day 21), animals were sacrificed for histological analysis. Immunohistochemical analysis showed the presence of luciferase-positive cells at the site of injection, three weeks post-transplantation in the animals transplanted with MSC+GRP. No luciferase-positive cells could be observed in animals transplanted with GRP alone (Figure 5A), consistent with the BLI results. Immunostaining for the pan-leukocyte marker CD45 demonstrated a higher infiltration of immune cells along the needle track and at the site of injection in animals transplanted with GRP alone, compared to animals transplanted with MSC+GFP (Figure 5B), although the difference was not significant.
Figure 5.
Co-transplantation of syngeneic MSCs creates a microenvironment that is more conducive to the survival of allogeneic GRPs, as assessed at 21 days post-transplantation. (A) Anti-luciferase staining (green) and anti-CD45 (red) (scale bar = 100μm), anti-GFAP (red), anti-CD11b (red), anti-CD8 (red) and anti-FoxP3 (red) (scale bar = 50μm). Needle track is indicated by arrows, c.c= corpus callosum. (B) Stereological counting of immune cells. Although a higher number of CD45+ cells was observed in mice transplanted with GRPs alone, the difference was not significant (p>0.05). A significant difference in CD11b+ cell numbers was observed between mice transplanted with GRPs alone and those transplanted together with syngeneic MSCs (***p<0.001). Stereological counting of FoxP3+ and CD8+ cells numbers revealed significant differences between animals transplanted with GRPs alone and those transplanted together with syngeneic MSCs (***p<0.001). All images were acquired using the same exposure time, with the data are expressed as mean ± S.E.M.
Immunostaining for other immune cell markers showed a significantly higher cell counts for both CD11b (p<0.001) and CD8 (p<0.001) expression in animals transplanted with GRP alone as compared to those transplanted with MSC+GRP. Animals transplanted with MSC+GRP also displayed a lower degree of astrogliosis (GFAP positivity) around the graft compared to animals transplanted with GRP alone. The higher number of FoxP3-expressing cells for animals co-transplanted with MSCs indicate that these cells have an immunomodulatory (suppressing) role around the graft area (Figure 5A & 5B).
Discussion
Allogeneic cells arguably offer the most practical source for cell therapy of CNS disorders. In contrast to the use of autologous cells, they can be isolated and expanded in bulk in advance of diagnosis, making treatment an immediate option without the need of lengthy cell propagations. In addition, not every donor isolation may yield the proper number of autologous cells. However, one of the major challenges in the use of allogeneic cells is their potential transplant rejection (Li and Duncan, 1998; Tambuyzer et al., 2009). We have chosen to use allogeneic GRPs as they have been widely studied for CNS repair (Goldman, 2005; Haidet-Phillips and Maragakis, 2015; Kim et al., 2012; Rao et al., 1998; Walczak et al., 2011), with lineage-restricted progenitors presenting a better option for controlling the fate of engrafted cells (Rao and Mayer-Proschel, 2000). Furthermore, at our participating institute, allogeneic human GRPs (“Q cells”) recently received FDA approval for a Phase I/II clinical trial in patients with amyotrophic lateral sclerosis. We used syngeneic MSCs as co-transplanted cells because of their inherent immunomodulatory/pro-survival properties. They are relatively easy to isolate in an autologous fashion, equivalent to the syngeneic paradigm in mice, and are now widely used in clinical trials for a variety of immune-mediated diseases.
In this study, we observed that co-transplanting syngeneic bone marrow-derived MSCs creates a microenvironment that is more conducive to the survival of allogeneic GRPs in mouse brain in the absence of immunosuppressive drugs. Here, we did not use immunosuppressants as their effects would mask the action of co-transplanted MSCs. Co-transplanted MSCs only act locally at the transplant site, avoiding the non-specificity and toxicity of immunosupressive drugs (Chan et al., 1995; Mohebbi et al., 2009). It has also been reported that a variety of immunosuppressants failed to fully protect allogeneic GRPs when transplanted in the brain of immunocompetent mice (Gorelik et al., 2012). Nevertheless, further studies may be undertaken to investigate the additional benefit of administering these agents.
We observed no significant change in BLI signal intensity for the immunodeficient Rag2−/− mice (in both the GRP and MSC+GRP groups) but a continuous decline in the immunocompetent mice for both groups. These results indicate that cell death was attributable to immune-mediated cell rejection and not to any other cause such as hypoxia or apoptosis. The rejection of engrafted cells involves a coordinated attack of the innate and the adaptive immune system. It has been previously reported that the initiation of an immune response inside the CNS is comparatively more difficult than for non-CNS sites (Perry, 1998). However, despite the fact that the CNS is an immunoprivileged site, allografts and xenografts are able to activate the innate and adaptive immune systems (Chen and Palmer, 2008). In our study, we also observed infiltration of cells belonging to both the innate (CD11b) and adaptive (CD8) immune systems.
To avoid graft rejection, high doses of immunosuppressive drugs are generally required. Routine systemic immunosuppression is usually not sufficient and often associated with severe toxicity (Craddock, 2000; Cristofanilli et al., 2011). MSCs have shown to possess immunosuppressive properties both in vitro and in vivo (Casiraghi et al., 2013; Uccelli et al., 2006). Hence, their adjunct use in cell transplantation is attractive, as they may obviate the need for immunosuppressive drugs. In previous studies, MSCs have been reported to be immunomodulatory and tolerogenic by mechanisms that involve the release of anti-inflammatory molecules and cell-to-cell contact (Uccelli et al., 2006), and have found uses in several conditions ranging from neurodegenerative disorders to solid organ transplantation (Chao et al., 2009; Hematti, 2008; Karussis et al., 2010; Laroni et al., 2013). In this study, we found that MSCs do, indeed, have the ability to support the survival of allogeneic GRPs by suppressing the host immune response in the absence of immunosuppressive drugs.
CD45 is a common leukocyte antigen expressed by almost all nucleated white cells, including T cells, natural killer (NK) cells, and granulocytes (Penninger et al., 2001). In this study, the reduced number of CD45+ cells in mice transplanted with MSC+GRP, compared to GRP alone at day 21 post-transplantation, indicates that the immune response was attenuated by the presence of MSCs. This finding is an agreement with previously reported studies, which have shown that MSCs influence all components of the immune system, including T-, B-, NK-, monocytic, and dendritic cells (Le Blanc et al., 2007; Uccelli et al., 2006). Bartholomew et al. (2002) also reported that MSCs could suppress lymphocyte reactivity to allogeneic target cells and tissues, and prolong graft survival.
We observed a reduction in both the innate and adaptive host immune response with lower numbers of infiltrating CD11b+ and CD8+ cells at the site of transplantation in the animals transplanted with MSC+GRP. It is well established that MSCs can modify the functional properties of innate and adaptive immune mediators in a manner that it suppresses a range of potentially destructive inflammatory pathways (Griffin et al., 2010). Calkoen et al. also investigated the immunosuppressive effect of MSCs on the proliferation of peripheral blood mononuclear cells, and showed that MSCs isolated from children with systemic juvenile idiopathic arthritis successfully suppressed both the innate and adaptive immune response in vitro (Calkoen et al., 2013). We also noticed a reduced magnitude of astrogliosis in the co-transplanted animals. Cristofanilli and colleagues showed that co-transplantation of syngeneic mouse MSCs with embryonic stem cell derived-allogeneic oligodendrocyte progenitors (ES-OPs) in the corpus callosum of dysmyelinated mice not only improved the survival of engrafted cells but also significantly reduced the astrogliosis and microgliosis at 2 weeks post-transplantation (Cristofanilli et al., 2011). Similarly, Miller et al. found that systemic injection of MSCs into animals with experimental autoimmune encephalomyelitis (EAE) resulted in reduced astrogliosis in the spinal cord (Miller et al., 2010). In this study, a significant increase in FoxP3+ cells in animals co-transplanted with MSC+GRP compared to GRP alone indicates involvement of regulatory T cells (Tregs) in enhanced graft survival. Several studies have shown that recipient-derived FoxP3+ Tregs are involved in transplantation tolerance (Cipriani et al., 2013; Melief et al., 2013; Yan et al., 2014). Other cells have also been used to increase the total number of T regulatory cells, for instance human ES-OPs in experimental autoimmune encephalomyelitis, a mouse model of MS (Kim et al. 2012).
Despite the evidence of immunomodulatory effects exerted by co-transplanted syngeneic MSCs, it is possible that several other factors may have attributed to improved cell survival. For example, MSCs are known to produce several pro-survival trophic factors (Abboud et al., 1991; Wilkins et al., 2009) that may play an important role in enhanced cell survival. Further studies are warranted to reveal the paracrine effects of MSCs on GRP graft survival, and in how far this contributes relatively to immunomodulation. Another limitation of this study is the lack of data beyond day 21 post-transplantation. Animals were sacrificed at this time point in order to investigate the MSC effects at the time point when all cells were lost in the animals transplanted with GRPs alone. It may be possible that MSCs when co-transplanted only delay cell death. Further studies are required to assess the fate of co-transplanted cells at later time points.
Summarized, our study demonstrates that co-transplantation of syngeneic MSCs creates a microenvironment that is favorable for transplanted cell survival, which may be further applied to improve allogeneic cell therapy for treatment of neurodegenerative disorders.
Highlights.
Bioluminescence imaging is well suited to serially monitor transplanted cell survival.
Allogeneic GRPs exhibit improved cell survival when co-transplanted with syngeneic MSCs.
This beneficial effect of MSCs is manifested by reduced immune cell infiltration and reduced astrogliosis.
Acknowledgements
This study was supported by a grant from the National Multiple Sclerosis Society (NMSS RG4994), MSCRFII-0193, MSCRFII0052, and grants 2RO1 NS045062, S10 OD010744, and R01 NS076573 from the National Institutes of Health. The authors thank Mary McAllister for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abboud SL, Bethel CR, Aron DC. Secretion of insulinlike growth factor I and insulinlike growth factor-binding proteins by murine bone marrow stromal cells. J Clin Invest. 1991;88:470–475. doi: 10.1172/JCI115327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- Apostolides C, Sanford E, Hong M, Mendez I. Glial cell line-derived neurotrophic factor improves intrastriatal graft survival of stored dopaminergic cells. Neuroscience. 1998;83:363–372. doi: 10.1016/s0306-4522(97)00369-2. [DOI] [PubMed] [Google Scholar]
- Baglio SR, Pegtel DM, Baldini N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Frontiers in physiology. 2012;3:359. doi: 10.3389/fphys.2012.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantubungi K, Blum D, Cuvelier L, Wislet-Gendebien S, Rogister B, Brouillet E, Schiffmann SN. Stem cell factor and mesenchymal and neural stem cell transplantation in a rat model of Huntington’s disease. Mol Cell Neurosci. 2008;37:454–470. doi: 10.1016/j.mcn.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Calkoen FG, Brinkman DM, Vervat C, van Ostaijen-Ten Dam MM, Ten Cate R, van Tol MJ, Ball LM. Mesenchymal stromal cells isolated from children with systemic juvenile idiopathic arthritis suppress innate and adaptive immune responses. Cytotherapy. 2013;15:280–291. doi: 10.1016/j.jcyt.2012.10.017. [DOI] [PubMed] [Google Scholar]
- Casiraghi F, Perico N, Remuzzi G. Mesenchymal stromal cells to promote solid organ transplantation tolerance. Curr Opin Organ Transplant. 2013;18:51–58. doi: 10.1097/MOT.0b013e32835c5016. [DOI] [PubMed] [Google Scholar]
- Chan CC, Martin DF, Xu D, Roberge FG. Side effects of rapamycin in the rat. J Ocul Pharmacol Ther. 1995;11:177–181. doi: 10.1089/jop.1995.11.177. [DOI] [PubMed] [Google Scholar]
- Chao YX, He BP, Tay SS. Mesenchymal stem cell transplantation attenuates blood brain barrier damage and neuroinflammation and protects dopaminergic neurons against MPTP toxicity in the substantia nigra in a model of Parkinson’s disease. J Neuroimmunol. 2009;216:39–50. doi: 10.1016/j.jneuroim.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Chen Z, Palmer TD. Cellular repair of CNS disorders: an immunological perspective. Hum Mol Genet. 2008;17:R84–92. doi: 10.1093/hmg/ddn104. [DOI] [PubMed] [Google Scholar]
- Chen Z, Phillips LK, Gould E, Campisi J, Lee SW, Ormerod BK, Zwierzchoniewska M, Martinez OM, Palmer TD. MHC mismatch inhibits neurogenesis and neuron maturation in stem cell allografts. PLoS One. 2011;6:e14787. doi: 10.1371/journal.pone.0014787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani P, Di Benedetto P, Liakouli V, Del Papa B, Di Padova M, Di Ianni M, Marrelli A, Alesse E, Giacomelli R. Mesenchymal stem cells (MSCs) from scleroderma patients (SSc) preserve their immunomodulatory properties although senescent and normally induce T regulatory cells (Tregs) with a functional phenotype: implications for cellular-based therapy. Clin Exp Immunol. 2013;173:195–206. doi: 10.1111/cei.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock C. Haemopoietic stem-cell transplantation: recent progress and future promise. Lancet Oncol. 2000;1:227–234. doi: 10.1016/s1470-2045(00)00153-4. [DOI] [PubMed] [Google Scholar]
- Cristofanilli M, Harris VK, Zigelbaum A, Goossens AM, Lu A, Rosenthal H, Sadiq SA. Mesenchymal stem cells enhance the engraftment and myelinating ability of allogeneic oligodendrocyte progenitors in dysmyelinated mice. Stem cells and development. 2011;20:2065–2076. doi: 10.1089/scd.2010.0547. [DOI] [PubMed] [Google Scholar]
- Goldman S. Stem and progenitor cell-based therapy of the human central nervous system. Nat Biotechnol. 2005;23:862–871. doi: 10.1038/nbt1119. [DOI] [PubMed] [Google Scholar]
- Gorelik M, Janowski M, Galpoththawela C, Rifkin R, Levy M, Lukomska B, Kerr DA, Bulte JW, Walczak P. Noninvasive monitoring of immunosuppressive drug efficacy to prevent rejection of intracerebral glial precursor allografts. Cell Transplant. 2012;21:2149–2157. doi: 10.3727/096368912X636911. [DOI] [PubMed] [Google Scholar]
- Griffin MD, Ritter T, Mahon BP. Immunological aspects of allogeneic mesenchymal stem cell therapies. Human gene therapy. 2010;21:1641–1655. doi: 10.1089/hum.2010.156. [DOI] [PubMed] [Google Scholar]
- Haas C, Fischer I. Human astrocytes derived from glial restricted progenitors support regeneration of the injured spinal cord. J Neurotrauma. 2013;30:1035–1052. doi: 10.1089/neu.2013.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidet-Phillips AM, Maragakis NJ. Neural and glial progenitor transplantation as a neuroprotective strategy for Amyotrophic Lateral Sclerosis (ALS) Brain Res. 2015 doi: 10.1016/j.brainres.2015.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hematti P. Role of mesenchymal stromal cells in solid organ transplantation. Transplant Rev (Orlando) 2008;22:262–273. doi: 10.1016/j.trre.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jitschin R, Mougiakakos D, Von Bahr L, Volkl S, Moll G, Ringden O, Kiessling R, Linder S, Le Blanc K. Alterations in the cellular immune compartment of patients treated with third-party mesenchymal stromal cells following allogeneic hematopoietic stem cell transplantation. Stem Cells. 2013;31:1715–1725. doi: 10.1002/stem.1386. [DOI] [PubMed] [Google Scholar]
- Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I, Bulte JW, Petrou P, Ben-Hur T, Abramsky O, Slavin S. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010;67:1187–1194. doi: 10.1001/archneurol.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerby A, Jones ES, Jones PM, King AJ. Co-transplantation of islets with mesenchymal stem cells in microcapsules demonstrates graft outcome can be improved in an isolated-graft model of islet transplantation in mice. Cytotherapy. 2013;15:192–200. doi: 10.1016/j.jcyt.2012.10.018. [DOI] [PubMed] [Google Scholar]
- Kim H, Walczak P, Muja N, Campanelli JT, Bulte JW. ICV-transplanted human glial precursor cells are short-lived yet exert immunomodulatory effects in mice with EAE. Glia. 2012;60:1117–1129. doi: 10.1002/glia.22339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laroni A, Novi G, Kerlero de Rosbo N, Uccelli A. Towards clinical application of mesenchymal stem cells for treatment of neurological diseases of the central nervous system. J Neuroimmune Pharmacol. 2013;8:1062–1076. doi: 10.1007/s11481-013-9456-6. [DOI] [PubMed] [Google Scholar]
- Lawrence JM, Morris RJ, Raisman G. Anatomical evidence that microglia are involved in both the immune presenting and immune attack phases of intracerebral allograft rejection. Neuropathol Appl Neurobiol. 1994;20:203–205. [PubMed] [Google Scholar]
- Le Blanc K, Samuelsson H, Gustafsson B, Remberger M, Sundberg B, Arvidson J, Ljungman P, Lonnies H, Nava S, Ringden O. Transplantation of mesenchymal stem cells to enhance engraftment of hematopoietic stem cells. Leukemia. 2007;21:1733–1738. doi: 10.1038/sj.leu.2404777. [DOI] [PubMed] [Google Scholar]
- Lepore AC, Walczak P, Rao MS, Fischer I, Bulte JW. MR imaging of lineage-restricted neural precursors following transplantation into the adult spinal cord. Exp Neurol. 2006;201:49–59. doi: 10.1016/j.expneurol.2006.03.032. [DOI] [PubMed] [Google Scholar]
- Li DW, Duncan ID. The immune status of the myelin deficient rat and its immune responses to transplanted allogeneic glial cells. J Neuroimmunol. 1998;85:202–211. doi: 10.1016/s0165-5728(98)00006-x. [DOI] [PubMed] [Google Scholar]
- Li T, Yan Y, Wang B, Qian H, Zhang X, Shen L, Wang M, Zhou Y, Zhu W, Li W, Xu W. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem cells and development. 2013;22:845–854. doi: 10.1089/scd.2012.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Ding Y, Zhang Y, Tse HF, Lian Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant. 2014;23:1045–1059. doi: 10.3727/096368913X667709. [DOI] [PubMed] [Google Scholar]
- Liang Y, Agren L, Lyczek A, Walczak P, Bulte JW. Neural progenitor cell survival in mouse brain can be improved by co-transplantation of helper cells expressing bFGF under doxycycline control. Exp Neurol. 2013;247:73–79. doi: 10.1016/j.expneurol.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melief SM, Schrama E, Brugman MH, Tiemessen MM, Hoogduijn MJ, Fibbe WE, Roelofs H. Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages. Stem Cells. 2013;31:1980–1991. doi: 10.1002/stem.1432. [DOI] [PubMed] [Google Scholar]
- Miller RH, Bai L, Lennon DP, Caplan AI. The potential of mesenchymal stem cells for neural repair. Discov Med. 2010;9:236–242. [PubMed] [Google Scholar]
- Mohebbi N, Mihailova M, Wagner CA. The calcineurin inhibitor FK506 (tacrolimus) is associated with transient metabolic acidosis and altered expression of renal acid-base transport proteins. Am J Physiol Renal Physiol. 2009;297:F499–509. doi: 10.1152/ajprenal.90489.2008. [DOI] [PubMed] [Google Scholar]
- Mujtaba T, Piper DR, Kalyani A, Groves AK, Lucero MT, Rao MS. Lineage-restricted neural precursors can be isolated from both the mouse neural tube and cultured ES cells. Developmental biology. 1999;214:113–127. doi: 10.1006/dbio.1999.9418. [DOI] [PubMed] [Google Scholar]
- Oliveira VD, Zankl H, Rath T. Mutagenic and cytotoxic effects of immunosuppressive drugs on human lymphocyte cultures. Exp Clin Transplant. 2004;2:273–279. [PubMed] [Google Scholar]
- Penninger JM, Irie-Sasaki J, Sasaki T, Oliveira-dos-Santos AJ. CD45: new jobs for an old acquaintance. Nat Immunol. 2001;2:389–396. doi: 10.1038/87687. [DOI] [PubMed] [Google Scholar]
- Perry VH. A revised view of the central nervous system microenvironment and major histocompatibility complex class II antigen presentation. J Neuroimmunol. 1998;90:113–121. doi: 10.1016/s0165-5728(98)00145-3. [DOI] [PubMed] [Google Scholar]
- Puymirat E, Geha R, Tomescot A, Bellamy V, Larghero J, Trinquart L, Bruneval P, Desnos M, Hagege A, Puceat M, Menasche P. Can mesenchymal stem cells induce tolerance to cotransplanted human embryonic stem cells? Mol Ther. 2009;17:176–182. doi: 10.1038/mt.2008.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MS, Mayer-Proschel M. Glial-restricted precursors are derived from multipotent neuroepithelial stem cells. Developmental biology. 1997;188:48–63. doi: 10.1006/dbio.1997.8597. [DOI] [PubMed] [Google Scholar]
- Rao MS, Mayer-Proschel M. Precursor cells for transplantation. Prog Brain Res. 2000;128:273–292. doi: 10.1016/S0079-6123(00)28025-4. [DOI] [PubMed] [Google Scholar]
- Rao MS, Noble M, Mayer-Proschel M. A tripotential glial precursor cell is present in the developing spinal cord. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:3996–4001. doi: 10.1073/pnas.95.7.3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GA, Snyder EY. Two cells are better than one: optimizing stem cell survival by co-grafting “helper” cells that offer regulated trophic support. Exp Neurol. 2013;247:751–754. doi: 10.1016/j.expneurol.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Tambuyzer BR, Bergwerf I, De Vocht N, Reekmans K, Daans J, Jorens PG, Goossens H, Ysebaert DK, Chatterjee S, Van Marck E, Berneman ZN, Ponsaerts P. Allogeneic stromal cell implantation in brain tissue leads to robust microglial activation. Immunol Cell Biol. 2009;87:267–273. doi: 10.1038/icb.2009.12. [DOI] [PubMed] [Google Scholar]
- Torres-Espin A, Hernandez J, Navarro X. Gene expression changes in the injured spinal cord following transplantation of mesenchymal stem cells or olfactory ensheathing cells. PLoS One. 2013;8:e76141. doi: 10.1371/journal.pone.0076141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uccelli A, Moretta L, Pistoia V. Immunoregulatory function of mesenchymal stem cells. Eur J Immunol. 2006;36:2566–2573. doi: 10.1002/eji.200636416. [DOI] [PubMed] [Google Scholar]
- Vercelli A, Mereuta OM, Garbossa D, Muraca G, Mareschi K, Rustichelli D, Ferrero I, Mazzini L, Madon E, Fagioli F. Human mesenchymal stem cell transplantation extends survival, improves motor performance and decreases neuroinflammation in mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2008;31:395–405. doi: 10.1016/j.nbd.2008.05.016. [DOI] [PubMed] [Google Scholar]
- Walczak P, All AH, Rumpal N, Gorelik M, Kim H, Maybhate A, Agrawal G, Campanelli JT, Gilad AA, Kerr DA, Bulte JW. Human glial-restricted progenitors survive, proliferate, and preserve electrophysiological function in rats with focal inflammatory spinal cord demyelination. Glia. 2011;59:499–510. doi: 10.1002/glia.21119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins A, Kemp K, Ginty M, Hares K, Mallam E, Scolding N. Human bone marrow-derived mesenchymal stem cells secrete brain-derived neurotrophic factor which promotes neuronal survival in vitro. Stem Cell Res. 2009;3:63–70. doi: 10.1016/j.scr.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Yan Z, Zhuansun Y, Chen R, Li J, Ran P. Immunomodulation of mesenchymal stromal cells on regulatory T cells and its possible mechanism. Exp Cell Res. 2014 doi: 10.1016/j.yexcr.2014.03.013. [DOI] [PubMed] [Google Scholar]
- Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–215. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]