Abstract
Hypoxia ischemia (HI) of the brain in near-term and term infants is a leading cause of infant mortality and lifelong disability but current therapeutic approaches remain limited. Males consistently display greater vulnerability to the deleterious consequences of HI in both humans and animal models. Neurogenesis increases after neonatal HI and offers a potential therapeutic target for recovery. The steroid hormone estradiol has been extensively explored as a neuroprotectant in adult models of stroke but with mixed results. Less consideration has been afforded to this naturally occurring agent in the developing brain, which has unique challenges from the adult. Using a model of term HI in the rat we have explored the impact of this insult on cell genesis in the hippocampus of males and females and the ability of estradiol treatment immediately after insult to restore function. Both short-term (3 days) and long-term (7 days) post-injury were assessed and revealed that only females had markedly increased cell genesis on the short-term but both sexes were increased long-term. A battery of behavioral tests revealed motor impairment in males and compromised episodic memory while both sexes were modestly impaired in spatial memory. Juvenile social play was also depressed in both sexes after HI. Estradiol therapy improved behavioral performance in both sexes but did not reverse a deficit in hippocampal volume ipsilateral to the insult. Thus the effects of estradiol do not appear to be via cell death or proliferation but rather involve other components of neural functioning.
Keywords: hypoxia ischemia, sex differences, rat, estradiol, hippocampus, neurogenesis, neonatal
Introduction
Perinatal hypoxia ischemia (HI) remains a leading global cause of infant mortality and lifelong disability (Fatemi et al., 2009; Graham et al., 2008; Vannucci & Perlman, 1997). Sex differences in severity of functional impairment following HI are well documented in both humans (Lauterbach et al., 2001; Johnston & Hagberg, 2007; Renolleau et al., 2008) and rodents (Arteni et al., 2010; Hill et al., 2011b; 2012; Hill & Fitch, 2012; Smith et al., 2014). Males are more likely to suffer a hypoxic ischemic insult, and recovery is poor relative to females. Males and females diverge in a number of mechanisms that contribute to differences in histological and functional outcomes, including dominant cell death pathways and inflammatory responses induced by HI (Hagberg et al., 2004 Hill et al., 2011a; Nijboer et al., 2007; Renolleau et al., 2007, Chavez-Valdez et al., 2014; Northington et al., 2011; Mirza et al., 2015).
Although the emphasis of much research on perinatal HI focuses on cell death, there is also a well documented increase in cell genesis. The number of neuronal progenitors in the subventricular zone is dramatically and persistently increased following HI (Felling et al., 2006; Yang & Levison, 2006; 2007; Yang et al., 2008). The neurogenic effects can last months, and some of these cells survive and integrate into the ipsilateral striatum in a model of moderate HI brain injury (Felling et al., 2006; Yang et al., 2008). Inhibition of post-HI inflammation can increase or decrease cell genesis, depending on the brain region studied. Indomethacin decreases cell genesis in the medial subventricular zone, but increases cell genesis in the subgranular zone of the dentate gyrus (Covey et al., 2011). Thus, treatment interventions can have opposing effects on this presumed attempt at post-injury repair.
Following on the enormous interest in estradiol as a neuroprotective agent in adult models of stroke (Brown et al. 2009), the potential for a similar effect has been explored in models of perinatal HI. Estradiol reduces the loss of ipsilateral brain weight and volume following perinatal HI, and significantly reduces histopathology scores in the hippocampus and thalamus (Feng et al., 2005; Nunez et al., 2007). Survival of oligodendrocyte progenitors is enhanced by estradiol in vitro, and estradiol treatment reduces loss of white matter volume in vivo (Gerstner et al., 2009). In adult models of ischemic stroke, estrogen action is multifactorial and includes decreased cell death, increased cell genesis and enhanced neurotrophic and anti-inflammatory actions (Brown et al. 2009). However, despite this promise the conflicting and at times contradicting reports on estradiol-mediated neuroprotection in the adult (Wise et al., 2009) cautions against a rush to concluding this naturally occurring compound is a panacea against brain injury.
The experiments presented here assessed hippocampal cell genesis in male and female rat pups subjected to HI at postnatal day (PN) 10. This age is a model of hypoxic ischemic brain injury in term infants based on parallels in cortical development and response to hypoxic ischemia brain injury (Patel et al., 2014). In premature infants, hypoxic ischemic encephalopathy results in white matter damage (Back et al., 2002). Oligodendrocytes are particularly vulnerable to hypoxic ischemic injury in the PN2-PN7 rat (Back et al., 2002; Hagberg et al., 2002). Between postnatal days 9 and 12, patterns of cortical activity begin to parallel that of term infants (Tucker et al., 2009). This is also a developmental phase in which cortical damage induced by HI shifts from columnar to laminar, and the hippocampus becomes increasingly vulnerable (Towfighi et al., 1997). We report that following HI cell genesis is increased in the female hippocampus earlier than detected in males, and is increased in both the contralateral and ipsilateral hemisphere 48 hr after HI. By 7 days post HI, sex differences were largely absent, but a significant increase in cell genesis was evident in both sexes in the ipsilateral hippocampus. We further tested the beneficial effect of estradiol treatment against HI-induced damage by assessing a variety of behaviors and hippocampal volume and found sex-specific as well as sex-neutral neuroprotective effects.
Materials and Methods
Timed pregnant female dams were obtained from Charles River (Baltimore, MD) and checked daily to determine the day of birth which was deemed postnatal day 1 (PN1). Within 24 hr of birth, litters were culled to 10 pups, with equal males and females when possible. On PN10 the dam and pups were placed in a clean cage and transported to the laboratory for either the carotid artery ligation followed by hypoxia or sham surgery. Pups were weighed, sexed and assigned randomly to experimental condition.
Hypoxia Ischemia Procedure
A modified version of the Rice-Vannucci perinatal HI procedure was used (Rice et al., 1981). Pups were gently placed in a supine position with the snout in a small nose cone and anesthetized with 3.0% isoflourane for 1 min, and then 1.5% for the remaining time required to complete the surgery (~4.5 min). The right carotid artery was isolated, ligated twice, and severed between the ligations. The pups were then placed in a large open jar within a circulating water bath (37° C) for 25 min. This was sufficient time for recovery from anesthesia, evidenced by normal breathing and color. Pups were then returned to the dam in a quiet area of the laboratory for 1 hr to permit feeding. To induce hypoxia, pups were placed in glass jars for delivery of 8% O2:92% N2 in the water bath (37° C) for 1 hr. After hypoxia and treatment, pups remained in the large open jar in the water bath for 2 hr to maintain body temperature. Sham operated control pups were anesthetized for the average surgery time, incised and sutured, and treated the same as hypoxic ischemic animals with the exception that they were warmed in room air conditions while litter mates underwent hypoxia.
Experiment 1A and 1B: Sex differences in cell genesis after HI
Male and female rats were subjected to HI or sham surgery as described. Rats were injected with a single dose of bromodeoxyuridine (BrdU; 100 mg/kg; Sigma Aldrich) 48 hr (Figure 2; Experiment 1A) or 7 days (Figure 3; Experiment 1B) after HI. The following day, pups were transcardially perfused with 0.9% saline and 4% paraformaldehyde. Brains were post-fixed for 48 hr and allowed to sink in 30% sucrose prior to cryosectioning. Rats with severe injury, indicated by formation of a cyst in the right hemisphere were excluded from analysis. For Experiment 1A, the number of pups in each group was: Male Control n=8, Female Control n=8, Male HI n=7, Female HI n=7. For Experiment 1B, the number of pups per group was: Male Control n=5, Male HI n=7, Female Control n=7, Female HI n=5.
Figure 2.
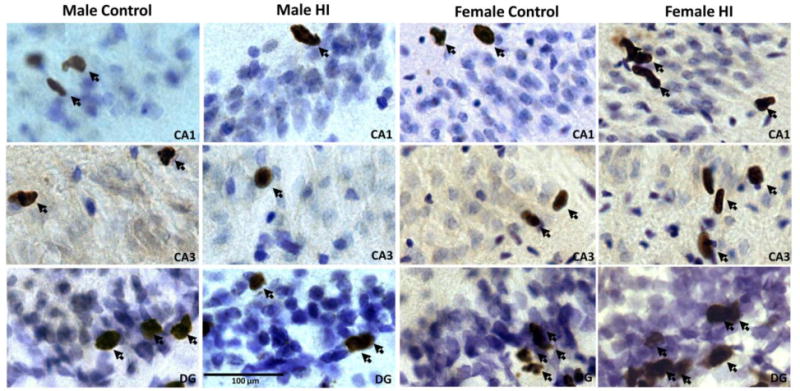
BrdU photographs of BrdU immunohistochemistry in male and female rat pups subjected to HI at PN10 and perfused 72 hr later. HI induced increases in cell genesis in the female brain earlier than males. All images were taken of the ipsilateral (i.e., right) hemisphere. Examples of cells and cell clusters considered immunopostive for BrdU are highlighted with arrows. DG=dentate gyrus.
Figure 3.

Cell genesis in the hippocampus increases in females bilaterally compared to males following HI 48 hr after HI. Cell genesis was estimated in CA1, CA3 and the dentate gyrus (DG) of pups subjected to HI on PN10. Mean (±SEM) estimated BrdU+ cells in the hippocampus of rat pups treated with BrdU (100 mg/kg) 2 days after HI and sacrificed 24 hr later were quantified. Contra=Hemisphere contralateral to carotid artery ligation; lpsi=hemisphere ipsilateral to carotid artery ligation. * indicate a significant difference in HI females compared to same sex controls and males.
Immunohistochemistry and Stereological Quantification: Twelve series of 45 μM sections were collected throughout the rostrocaudal extent of the hippocampus. For BrdU staining, sections were mounted to slides and heated in 0.1 M citric acid (pH 6.0). Slides were then rinsed in PBS, incubated in trypsin for 10 min, denatured in 2 M HCl:PBS for 30 min, rinsed, incubated with mouse antibodies to bromodeoxyuridine (BrdU) cells (BD Biosciences diluted 1:500) with 0.5% Tween-20. The next day, slides were rinsed, incubated with biotinylated anti-mouse (1:200, Vector) for 60 min, rinsed, incubated with avidin-biotin complex (1:500; Vector), rinsed and reacted in 0.01% DAB. Slides were counterstained with cresyl violet, dehydrated, cleared and coverslipped. Stereological Quantification of BrdU+ cells: Unbiased sterology was used to estimate the number of BrdU+ cells in the hippocampus using the optical dissector method (West et al., 1991). StereoInvestigator software (MBFbioscience, Williston, VT) was used to delineate CA1, CA3 and the dentate gyrus (DG) in each hemisphere. CA1 included the layers oriens, pyramidal cell layer, striatum radiatum and lacunosum molecular (as described in Long et al., 1998). Boundaries of CA3 were defined by the dense pyramidal cell layer and included the areas of dense BrdU+ cells just outside of the cresyl-stained pyramidal cell layer but not extending into less cell dense areas of the neuropil. A similar approach was used to define CA3 was used to quantify cell genesis in the DG. Analysis of BrdU+ cells in pups treated 48 hr after HI was conducted on the left and right hemisphere of 4 sections of the dorsal hippocampus. BrdU+ cells were quantified in both hemispheres in 6 sections of the dorsal hippocampus in pups treated with BrdU 7 days after HI. The counting frame area was 448 μm with a minimum of 35 counting sites per subregion. The average mounted tissue thickness was 20.5 μm. Optical dissector quantification schemes (i.e., dissector dimensions and sampling frequency) were determined based on pilot work in sham operated, which established parameters yielding a coefficient of error less than 0.1. An estimation of total cells per subregion was generated for each animal.
Experiment 2: The effect of estradiol on functional outcomes in male and female rats
Pups were subjected to HI or sham surgery as described. Immediately after HI, pups were randomly assigned to treatment condition, balancing sex and surgery condition within litter. Each pup received a subcutaneous injection of sesame oil or estradiol in sesame oil (10 μg) subcutaneously immediately after hypoxia. This treatment was repeated 24 hr later. The dose of estradiol is equivalent to that given to adults to induce sexual receptivity but is 10X’s lower than that given to newborns to induce masculinization. The timing was designed to increase circulating levels of estradiol within hrs of HI and to maintain levels for up to 48 hrs. Pups were weaned at PN21, and housed with same-sex littermates in groups of 2–3 for the duration of the experiment. Pups were tested on a series of behavioral assays. The number of rats in each group was: Male Control+Oil n=8, Male Control+E2 n=8, Male HI+Oil n=9, Male HI+E2 n=9, Female Control+Oil n=7, Female Control+E2 n=7, Female HI+Oil n=8, Female HI+E2 n=8. The experimental timeline is presented in Figure 1.
Figure 1.
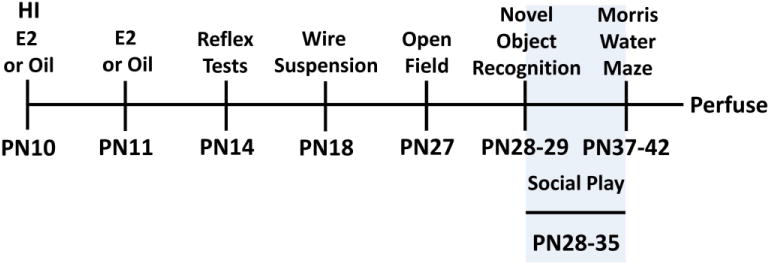
Experimental timeline for Experiment 2. Animals were subjected to HI or sham surgery on PN10 and treated with estradiol (E2) or oil immediately after hypoxia and 24 hr later. Rats were then tested on a series of behavioral tasks. Social play was tested in the dark phase on PN28-35.
Reflex and Motor testing: Righting Reflex
On PN14, pups were tested for simple reflexes. To test righting reflexes, or the ability to turn from their back upright to their feet, pups were placed on their backs on a heating pad (36° C). This simple test has been used to detect motor deficits after HI by others (i.e., Fan et al., 2005) and can detect deficits in motor coordination in young rodents presumably due to subcortical dysfunction (i.e., Altman & Sudarshan, 1975). Each pup was released, and the amount of time taken to turn over in seconds was recorded. This was repeated 3 times, with a 3 min interval between each trial and the average determined.
Negative geotaxis
Also on PN14, each rat was placed on a ramp with a 25 degree incline, head pointing downwards. This assessment determines whether the rat can restore postural stability by coordinated movements to point their head upwards. Though interpretation of this behavioral tendency has been called into question (see Motz & Alberts, 2005), we consistently find that uninjured pups quickly reorient their position to point their heads up at this age. Versidry was placed on the incline to enable traction. The time to turn to face up the slope was measured 3 times, with a 3 min interval between trials and the average determined.
Suspension on a wire
On PN18, the rats’ front paws were placed on a metal dowel suspended above a padded bin. The trial period was a maximum of 90 seconds. Rats were considered successfulif they were able to bring their rear paws to the dowel and hold on (Dean et al., 2012). If the rat fell, it was considered to have failed. The percentage of rats in each group to successfully grasp the dowel with their hindlimbs was calculated. This test has also been used to detect motor deficits after neonatal HI (Fan et al., 2005). We find that this behavior is transiently disrupted by HI in the developing rat as reported by others (i.e., Hermans et al., 1993).
Open Field
On PN27, rats were placed in an open plexiglass arena (49 cm length, 37 cm wide, 24 cm high) under bright lights for 5 min. The floor of the arena was delineated into a perimeter and a center region, with lines dissecting the perimeter. The session was taped and scored offline. The amount of time each rat spent in the center of the arena, number of lines crossed and latency to enter the center were measured.
Novel Object Recognition
On PN28-29, rats were placed in the same behavior arena as was used for the open field test, but without a gridded floor and under dim lighting to permit acclimation to the testing chamber. On PN30, two identical objects were placed in the arena (i.e., ink bottles, flask stoppers). The rat was allowed to explore freely for 5 min. Following a 1 hr retention interval, one object was replaced with a novel one in the same location as the object it replaced. The rat was again permitted to explore freely for 5 min. The now familiar object was tested with a novel object in the same location 24 hr later. Rats are capable of retention of the familiar object for at least 24 hr at this age (Reger et al., 2009). All test sessions were video recorded and scored offline. The amount of time the rat spent investigating each object was recorded. To be considered object exploration, the rat had to be touching and/or sniffing the object and not looking elsewhere. Data are presented as (Time spent exploring novel object-Time spent exploring familiar object)/Total time spent exploring both objects. Therefore, a ratio of 0 means the rats spent the same amount of time with both objects. A ratio of 0.5 means the rat spent twice as much time with the novel object.
Social Play
On PN28-35, rats were separated from their cagemates 1–1.5 hr before onset of the dark cycle. During this time, each rat received a distinct marking on the back. At the end of this period of social isolation, same sex groups, consisting of 4–5 rats with at least 1 rat from each experimental condition, were placed in the behavior arena with cobb bedding under red lights. After a 30 sec delay, video recording began. Rats remained in the play arena for 10 min per session. Play behavior was scored offline. The number of playful approaches and successful pinnings of another rat were counted for the first 5 min of sessions 1–4, 6 and 8, omitting the first 30 sec of video to permit the rats to acclimate to the arena. Play behavior was robust at this time, and persisted for at least 5–8 min.
Morris Water Maze
On PN37-38, rats began acclimation to the Morris Water Maze task. Each day, rats were placed in the opaque pool with the submerged, invisible escape platform. For acclimation, the pool was enclosed in a plain curtain, with no spatial cues. Rats were placed in the pool from various starting points and allowed to search for the platform for a maximum of 90 s. If the rat did not locate the platform, it was gently guided to the platform. Each acclimation day consisted of 4 trials. The platform was moved to a different location for each trial. This non-spatial training reduces expression of sex differences in performance strategy that can obscure assessment of spatial learning ability (Perrot-Sinal et al, 1996; Morris, 1984). The following day, spatial cues were placed on the curtain surrounding the pool. Rats were given 4 daily training trials each day for 3 days (PN39-41), with the starting point varying on each trial. The maximum trial duration was 90 s. The latency to reach the platform was measured. On PN42, rats returned to the pool for a 90 sec probe trial in which the escape platform was removed. The probe trial was video recorded and the amount of time spent in each quadrant was recorded and measured offline.
Hippocampal Volume
The day after the probe trial (PN43), rats were perfused and brains collected. One male sham operated rat from the oil treatment group was excluded from histological analysis due to poor perfusion. No other rats were excluded, irrespective of the magnitude of injury. A 1:12 series was collected through the rostrocaudal extent of the hippocampus. Sections were 45 μm thick and mounted to slides. Sections were hydrated in descending concentrations of ethanol and then placed in 0.1% cresyl violet with 1% acetic acid. Sections were then placed in 1% acetic acid in water until white matter tracts were visible and the principle cell layers of the hippocampus were clear. Sections were dehydrated in ethanol baths, placed in xylene and coverslipped. For each rat, 8–9 sections were analyzed for total hippocampal volume in each hemisphere using Cavalieri method. Sections were stained with cresyl violet and hippocampal volume was measured using Neurolucida software (MBFbioscience, Williston, VT).
Statistical Analyses
SPSS version 18 was used for all statistical analyses (Armonk, NY). Repeated measures ANOVA with BrdU positive cells in the Contralateral and Ipsilateral hippocampus as the within subjects factors and Sex and Surgery as the between subjects factors was conducted for CA1, CA3 and the dentate gyrus (DG) for Experiment 1A and 1B. Behavioral tests were also analyzed by ANOVA with hormone treatment included as a factor. Males and females were analyzed separately in order to retain sufficient power to detect within sex effects.
Results
Experiment 1A: HI induces early and bilateral cell genesis in the hippocampus of females but not males
Representative photographs of BrdU immunohistochemistry are presented in Figure 2 for animals examined 3 days post HI. BrdU+ cells can be seen in CA1, CA3 and the DG.
CA1
Hypoxia-ischemia increased cell genesis in the ipsilateral CA1 regions in females, but not males, within 48 hours of injury compared to non-injured controls [F(1,25)=5.188, p<.032, main effect of HI, F(1,25)=9.551, p<.005, sex effect, F(1,25)=7.387, p<.012, interaction]. Moreover, cell genesis was higher in females subjected to HI compared to control females and males, and this was evident in both the ipsilateral and contralateral CA1. There was no significant difference between hemispheres in any of the experimental groups (Figure 3, left panel).
CA3
Cell genesis was similarly higher in CA3 of the female, but not male, hippocampus after HI [F(1,25)=7.41, p<.012, injury effect; F(1,25)=8.90, p<.006, sex effect] (Figure 3, middle panel). Cell genesis was bilaterally increased in females, with a greater increase ipsilateral to the carotid artery ligation, while cell genesis in the contralateral hemisphere was slightly but still significantly elevated in males [F(1,25)=4.737, p<.039, hemisphere by sex interaction, F(1,25)=7.36, p<.012, HI by sex interaction].
DG
The effect of HI on cell genesis in the DG was influenced by a complex interplay of sex and cerebral hemisphere (Figure 3, right panel). There was more cell genesis in females following HI, and this was greatest in the ipsilateral hemisphere, yet both hemispheres in HI females contained more BrdU+ cells compared to HI males. [F(1,25)=6.36, p<.019, effect of sex; [F(1,25)=7.71, p<.01, sex × HI interaction;, [F(1,25)=4.47, p<.04, effect of hemisphere, [F(1,25)=6.13, p<.021; hemisphere × sex interaction; [F(1,25)=6.51, p.017, Hemisphere × Sex × HI interaction].
Taken together these data indicate cell genesis was higher in all three subregions of the hippocampus in females within 2 days after HI, and importantly, the increased cell genesis was present in both the ipsi- and contralateral hemispheres of females.
Experiment 1B: Both males and females exhibit increased cell genesis in the ipsilateral hippocampus seven days after HI
By a week after HI, sex differences in cell genesis were no longer detected, but the number of BrdU immunoreactive cells was increased in the ipsilateral hemisphere in both sexes in CA1 and CA3 (Figure 4).
Figure 4.

Cell genesis in the hippocampus is similarly increased in the ipsilateral hemisphere of males and females one week after HI. Cell genesis was estimated in CA1, CA3 and the dentate gyrus (DG) of pups subjected to HI on PN10. Mean (±SEM) estimated BrdU+ cells in the hippocampus of rat pups treated with BrdU (100 mg/kg) 7 days after HI and sacrificed 24 hr later were quantified. Contra=Hemisphere contralateral to carotid artery ligation; lpsi=hemisphere ipsilateral to carotid artery ligation. * indicates a significant increase in cell genesis in the ipsilateral hemisphere compared to the contralateral hemisphere and control rats.
CA1
Both sexes exhibited similar increases in cell genesis in CA1 ipsilateral to the carotid artery ligation [F(1,20)=9.628, p<.006, effect of HI, [F(1,20)=22.164, p<.0001, effect of hemisphere; F(1,20)=13.78, p<.001, hemisphere × HI interaction] (Figure 4, left panel).
CA3
Cell genesis was also increased in the ipsilateral CA3 in rats experiencing HI irrespective of sex, [F(1,20)=5.49. p<.03, HI × hemisphere interaction]. (Figure 4, middle panel). Thus, by one week after HI, males and females exhibit similar increases in cell genesis in ammons horn, and this increase is restricted to the ipsilateral hemisphere.
DG
There was no effect of HI or Sex on the number of BrdU+ cells in the dentate gyrus but there was an unexpected significant effect of Hemisphere such that cell genesis was slightly but consistently higher in the contralateral hemisphere in all groups regardless of HI [F(1,20)=6.21, p<.022, effect of hemisphere] (Figure 4, right panel).
Taken together these data indicate that by 7 days after HI the sex difference in cell genesis previously observed has dissipated, as has the increase previously observed in the DG. Moreover, the bilateral increases in cell genesis observed in females on the short-term were now limited to the contralateral hemisphere but found in both sexes. Our results also demonstrate the developmental decline in postnatal hippocampal cell genesis between PN12 and PN17. This has been previously reported by others (Muramatsu et al., 2007).
Experiment 2: Estradiol treatment has mixed effects on functional outcomes and hippocampal volume Righting reflex
Males were more susceptible to HI effects on the righting reflex test than females, being significantly impaired, while females were only marginally impaired [F(1,30)=14.003, p<.001, effect of HI in males; F(1,26)=3.66, p<.067, effect of HI in females]. Males experiencing HI benefited from estradiol treatment while in females, estradiol treatment decreased righting latency regardless of surgical condition [F(1,30)=4.815, p<.036, sex × treatment interaction in males; F(1,26)=6.76, p<.015, effect of treatment in females]. Thus although the effect of HI on this measure is small, males are impaired while females are not. (Figure 5A).
Figure 5.

Males are more impaired on simple motor tasks after HI than females. A) Mean time (±SEM) to right from a supine position 96 hr after HI. B) Mean time (±SEM) to right from facing downward on an incline 96 hr after HI C) Percent of rats capable of bringing their hindpaws to a metal dowel and hang on. * indicate a significant effect of HI compared to same sex controls, # indicate a significant effect of estradiol.
Negative Geotaxis
Males subjected to HI exhibited a deficit in the negative geotaxis task (Figure 5) and this was improved by treatment with estradiol [F(1,30)=7.12, p<.012, effect of HI; F(1,30)=3.96, p<.05, effect of treatment]. There were no effects of HI or hormone treatment in females (Figure 5B).
Wire Suspension
Only males were significantly impaired in the ability to bring the rear paws up to a metal dowel and hold on (Pearson Chi Square, χ2(1,34) = 6.349, p<.019; Figure 5C). Estradiol treatment increased the number of males capable of holding onto the dowel in this simple motor task [χ2(1,34) = 3.78, p<.05]. There were no effects of HI or hormone treatment on female performance.
Open Field
The open field test detected a significant effect of HI in males evidenced by decreased time spent in the center of the field [F(1,30)=14.17, p<.001, effect of HI] (Figure 6A), HI did not significantly affect open field behavior in females (Figure 6B). Sham operated females spent very little time in the center, making effects of surgery or treatment more difficult to detect. There were no group differences in the number of lines crossed or the latency to enter the center of the arena in either sex. However, though too variable to meet significance, estradiol tended to increase the latency to enter the center of the arena in females irrespective of surgery condition [F(1,26)=3.46, p<.07, effect of treatment]. HI also did not significantly decrease time spent in center in females [F(1,26)=2.61, p<0.11].
Figure 6.
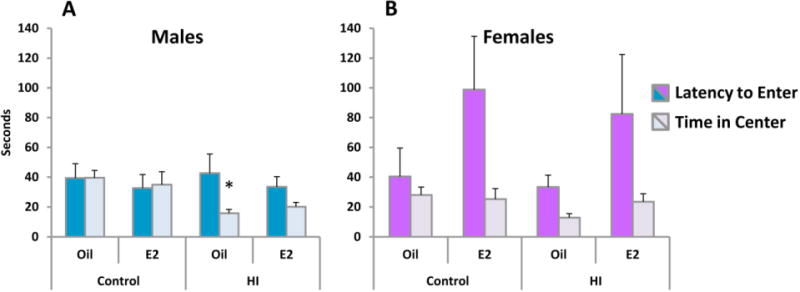
HI reduces time spent in the center of an open field in males. On PN27 rats were tested in an open field under bright illumination. The mean (±SEM) time taken to enter the center of the arena and time spent in the center were determined for each group. HI reduced the time spent in the center in males. * indicated a significant difference from same sex controls, p<.05. Note that while HI appears to reduce time in center in females, this did not reach statistical significance.
Novel Object Recognition
Males experiencing HI showed no evidence of object memory and this was reversed by estradiol treatment [F(1,30)=10.17, p<.003, effect of HI; F(1,30)=8.76, p<.006, effect of treatment; F(1,30)=4.81, p<.036, HI × treatment interaction]. HI did not disrupt object memory in females (Figure 7). Poor memory expression persisted in males experiencing HI in the 24 hr retention test [F(1,30)=9.99, p<.004; effect of HI], [F(1,30)=7.77, p<.009, effect of treatment]. Again there were no effects on memory in females. Although it appears that retention in the 24 hr test was poorer in HI females treated with oil, and improved by estradiol, this did not reach statistical significance.
Figure 7.

Novel object recognition (NOR) is disrupted by HI in males only and this was reversed by estradiol. On PN30-31, rats were tested on the novel object recognition task. Time spent invesitgating a new object versus a familiar one was measured. A discrimination ratio of 0 indicates no difference in time spent exploring each object. * indicated a significant difference from same sex controls, # indicates a significant effect of estradiol.
Social Play
Social play increased in all groups over days, as rats became both anticipatory of play and more familiar with play partners and conditions. In males, prior HI dampened the increase in play across days, but treatment with estradiol improved play behavior [F(5,150)=40.96, p<.0001, effect of day; F(1,30)=62.24, p<.0001, effect of HI; F(5,150)=2.34, p<.044, HI × day interaction; F(1,30)=9.19, p<.005, effect of treatment; F(1,30)=10.26, p<.003, HI × treatment interaction] (Figure 8A). In females, prior HI also reduced play and this was improved by treatment with estradiol [F(1,26)=31.75, p<.0001; effect of HI, F(1,26)=6.42, p<.018, effect of treatment; F(1,26)=4.36, p<.047, HI × treatment interaction] (Figure 8C).
Figure 8.
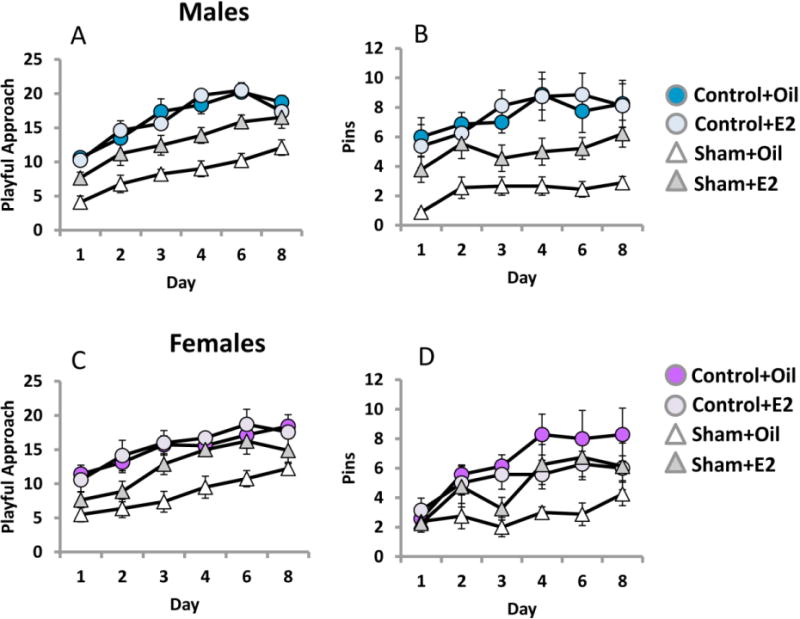
Social play is reduced by HI in males and females. Rat play behavior was recorded on PN28-35. Estradiol enhanced play in males and females subjected to HI. The number of playful approaches and successful pinnings of another rat were tallied in a 5 min video and averaged for each group.
The number of successful pins also increased over days in males for all groups, but similar to playful approaches this behavior was reduced by prior HI and rescued by estradiol treatment [F(5,150)=5.02, p<.0001; effect of day; F(1,30)=29.54, effect of HI; [F(1,30)=4.05, p<.05, effect of treatment] (Figure 8B). Estradiol treated females subjected to HI also made more successful pins than HI females treated with oil, and reached levels of sham operated estradiol treated females [3-way interaction; F(5,130)=2.39, p<.04] (Figure 8D). Overall, this pattern of results reveals that animals subject to HI exhibit deficits in social play behavior but that estradiol treatment prevents those deficits from appearing in both sexes. This measure detects deficits in females that are robust enough to permit detection of a beneficial effect of estradiol.
Morris Water Maze
The mean response latency for each group was calculated for each trial to assess learning (Figure 9). Reduced response latencies across trials in males confirmed their ability to learn the task (Figure 9A), but this was impaired in those previously experiencing HI [F(11,330)=11.24, p<.0001, effect of trial; F(1,30)=4.19, p<.05 effect of HI]. Unlike social play, there was no effect of treatment with estradiol on response latencies. A similar pattern was evident in females in that they improved across trials but those subject to HI improved less [F(11,286)=5.74, p<.0001, effect of trial; F(1,26)=8.31, p<.008 effect of HI] and there was no effect of estradiol treatment (Figure 9C). These analyses confirm that rats in all conditions decreased their escape latencies across trials, and were capable of spatially guided navigation.
Figure 9.
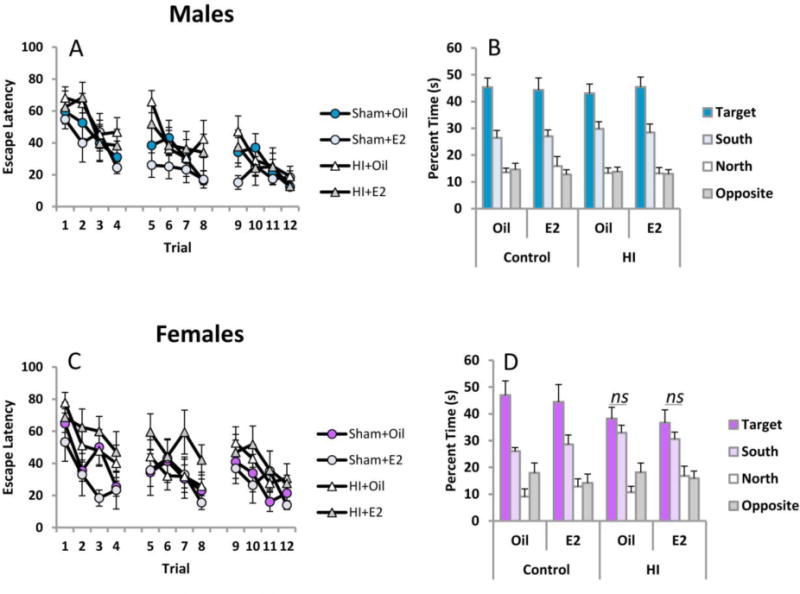
Both males and females exhibit a modest impairment on the Morris Water Maze task. Rats were trained to locate an invisible platform using extramaze cues on PN39-41. Retention was assessed By measuring the time spent in each quadrant of the pool in the absence of the escape platform.
During the probe trial the quadrant previously housing the escape platform during training is referred to as the target quadrant. Other quadrants are referred to relative to the target quadrant (i.e., opposite, north and south). Time spent in the target quadrant is a further index of learning and memory retention. Males spent more time searching for the escape platform in the target quadrant regardless of HI or treatment, confirming that males in each condition recalled the platform location [F(3,90)=81.27, p<.0001, effect of quadrant] (Figure 9B). Females also recalled the platform location regardless of HI or treatment [F(3,78)=38.57, p<.0001, effect of quadrant]. However, inspection of Figure 9D suggests that HI females did not restrict their search to the target quadrant relative to the south quadrant and this was confirmed by paired sample t-tests [HI+Oil t(7)=1.03, p<.33, and HI+E2 t(7)=.93, p <.38]. All other groups did significantly discriminate between these two quadrants, suggesting retention in females was more susceptible to HI-induced deficits than males.
Hippocampal Volume
As expected, in males there was a significant difference in volume of the hippocampus between the two hemispheres induced by HI (Figure 10, top left panel), but there was no effect of estradiol treatment [F(1,29)=12.34, p<.001, effect of hemisphere; F(1,29)=11.71, p<.001, effect of HI; F(1,29)=6.88, p<.014, HI × hemisphere interaction]. The contralateral hemisphere volume was analyzed separately and found to be significantly reduced by HI relative to sham operated controls, but again not impacted by estradiol treatment [F(1,29)=6.05, p<.02, effect of HI; F(1,29)=2.25, p>.05, effect of treatment]. Parallel analysis of the ipsilateral hemisphere detected a significant effect of HI [F(1,29)=10.43, p<.003] but no effect of treatment.
Figure 10.
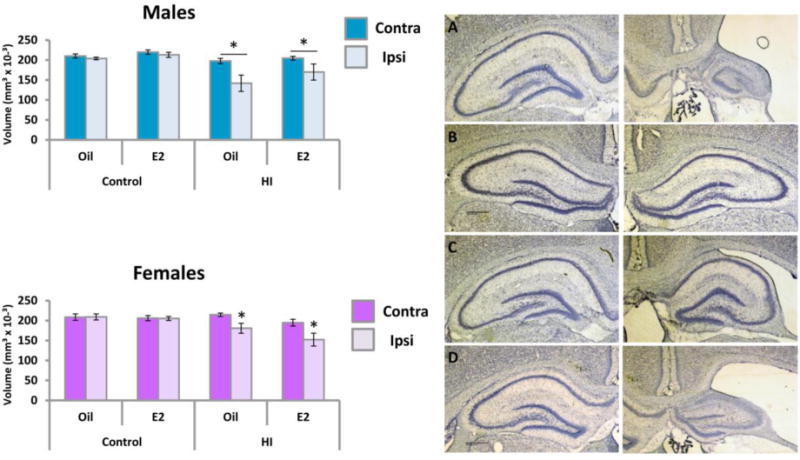
Hippocampal volume is reduced in both the contralateral and ipsilateral hemisphere of male. Left Panel: Mean volume (±SEM) of the contralateral and ipsilateral hippocampus. Right Panel: Representative photographs of the dorsal hippocampus on PN43. A) Male Hl+Oil B) Male Hl+Estradiol C) Female Hl+Oil D) Female Hl+Estradiol. * indicate a significant difference from same sex controls.
In females there was also a significant difference in the size of the hemispheres and this was again impacted by HI with no effect of treatment (Figure 10, bottom panel) but only in the ipsilateral hemisphere [F(1,26)=22.13, p<.001, effect of hemisphere; F(1,26)=21.81, p<.001, hemisphere × HI interaction, F(1,26)=12.04, p<.002, effect of HI on the ipsilateral hemisphere]. Analysis of the contralateral hemisphere found no effects of HI or treatment, Overall, these results suggest females are less vulnerable to the effects of HI on hippocampal volume in both hemispheres as compared to males.
Discussion
Perinatal HI occurs in as many as 0.2% to 0.4% of live births and has life long devastating consequences from extensive neuronal and oligodendrocyte cell loss (Geddes et al., 2001; Gonzalez & Ferriero, 2008; Wu et al., 2004). There are limited therapeutic options for prevention of the enduring cognitive and locomotor impairments incurred (Scafidi and Gallo, 2008). Only restorative therapeutics offer hope of recovery from irreversible cell loss. Following HI there is extensive cell proliferation in some regions for extended durations after the insult, but survival rates are generally low (Yang et al., 2007). However it is essential to carefully characterize both the proliferation and survival profile after HI in both males and females as their basal rate of cell genesis and response to injury can vary. Moreover, sex differences in the timing of cell genesis in response to HI might contribute to sex differences in responses to therapeutic interventions (e.g., Fan et al., 2011; Hagberg et al., 2004; Hill et al., 2011a; Nijboer et al., 2007; Renolleau et al., 2007). Attempts to maximize survival of neural precursors would then require different timing between the sexes.
The steroid hormone estradiol has been explored as a therapeutic post-stroke in adult women, with mixed results. The fetal and neonatal brain also experiences high levels of estradiol exposure and we therefore explored whether this naturally occurring signaling molecule could be of therapeutic benefit in a model of neonatal ischemic stroke. The developing brain provides unique challenges that are directly relevant to estrogen action, including sex differences in neonatal hippocampal neurogenesis and maturation of GABAergic inhibition. In early postnatal development, males show twice the rate of proliferation in the hippocampus as females (Bowers et al., 2010). Both estradiol and its precursor, testosterone, potently modulate proliferation in the neonatal hippocampus in a sex-dependent manner (Zhang et al., 2008, Waddell et al., 2013). Of particular interest to the current report is the observation that up to 80% of the surviving newborn cells in the male brain will differentiate into neurons, whereas only 40% will do so in females (Bowers et al,, 2010). Thus estradiol appears to exert a dual effect of promoting proliferation and differentiation. The mechanisms by which estrogens and androgens induce cell genesis and/or differentiation are currently unknown and this sex difference is transient, being essentially gone by the first week of life and so does not persist until the time points examined here. However, injury frequently induces a recapitulation of earlier development events and that lead us to examine sex differences in the effects of HI on hippocampal cell genesis. The switch in GABA action from predominantly depolarizing to hyperpolarizing as maturation proceeds also differs between the sexes (Cherubini et al., 2011; Galanopoulou, 2008). We have found the progression of GABA action to differ in males and females such that females switch to hyperpolarizing at an earlier age, and estradiol treatment delays that switch (Nunez & McCarthy, 2007). Thus estradiol treatment given to injured males and females of the same age could have profoundly different effects. Together these observations highlight the need to understand basic mechanisms of brain development and recovery in males and females, as well as the multiplicity of ways in which steroid hormones can modulate those mechanisms.
We explored the rate of cell genesis at relatively short (2-days) versus long (7-days) time periods following HI induced in 10-day old male and female rat pups. The rates of cell genesis were significantly higher in females in CA1, CA3 and dentate gyrus on the short-term, but not the long term since by 7-days after injury both males and females exhibited elevated cell genesis in Ammon’s horn but not in the dentate gyrus. In the adult neural progenitor cells are limited to the proliferative zone of the dentate gyrus but we have previously observed neurogenesis in Ammon’s horn of neonates less than one week old. Whether this capability persists until the later ages examined here is unknown. Moreover, we did not determine the cellular fate of those cells being born in CA1 and CA3 seven days after HI and it is possible they are of non-neuronal origin (i.e. microglia).
We also conducted extensive behavioral testing to assess if functional end points were differentially sensitive in males and females. Males exhibited subtle but significant changes in motor ability on basic tasks such as the righting reflex and negative geotaxis. Males were also reliably less capable of bringing the rear paws to a metal dowel to hang on in a wire hang task. Estradiol treatment following HI improved performance on these tasks in males, but no significant deficits were detected in females. HI similarly increased anxiety-like behavior in males only, reducing the total time spent in the center of the arena. These results do not rule out a potentially protective effect of estradiol in females, however, since we did not detect impairments.
Assessment of short-term memory of a familiar object indicated males subjected to HI were severely impaired whereas females were unaffected. Estradiol treatment following HI improved object recognition in males. This form of incidental learning tests episodic memory through the natural tendency of rodents to investigate novel stimuli more than familiar ones (Ennaceur & Delacour, 1988). Acquisition and retention of the familiar object relies on the perirhinal cortex (Barker & Warburton, 2011, 2015). Though the role of the hippocampus is less clear, hippocampal lesions can reduce expression of this behavior at long retention intervals (see Clark et al., 2000). Perinatal HI reduced hippocampal c-fos expression in adulthood, demonstrating a long term depressive effect on activity in surviving hippocampal neurons (Souza et al., 2014). Whether this suppression extends to females or neocortical areas is unknown but such effects on surviving neurons might underlie functional deficits not readily explained by volume of tissue loss. In contrast to male vulnerability in episodic memory, both sexes were slightly impaired by HI in the canonical test for spatial learning, the Morris Water Maze (Morris, 1984). These results highlight the potential disconnect between seemingly beneficial changes in histological measures and some behavioral outcomes.
Social play behavior was assessed in same sex but mixed treatment groups and both males and females subject to HI played less than their unaffected cage mates. Thus there are both sex differences and sex similarities in the functional impact of HI. Estradiol treatment increased social play in both males and females subjected to HI. Social interaction is critical for development of communication skills, cognitive ability, and is a potent behavioral reinforcer (reviewed in Trezza et al., 2011). Play behavior dramatically increases between 18 and 28 days of age and peaks around 32 days (Pellis, 1981). Restoration of motivation to communicate and play with others during this critical adolescent window likely improves many behaviors in the long term. Inactivation of the frontal cortices reduces social play dramatically, suggesting that perinatal HI suppresses activity in the prefrontal cortices (van Kerkhof et al., 2013). Normal social play further requires a balance between activity in the prefrontal cortices and the striatum (van Kerkhof et al., 2013). These neural circuits appear to be equally vulnerable in males and females subjected to HI.
In addition to measures of cell genesis and functional outcome, we also quantified the volume of both hippocampal hemispheres following HI. Hippocampal volume was reduced in both the contralateral and ipsilateral hemisphere of males and there was a lesser reduction in size of the ipsilateral hemisphere in females following neonatal HI. In children treated for respiratory failure as neonates, reduced hippocampal volume correlates with poor memory performance, without overt neurological symptoms (Cooper et al., 2015), consistent with what we observed here where males had greater reduction in hippocampal volume and performed worse on memory tasks than females following neonatal HI. Zhu et al. (2006) found no sex difference in gross morphology of injury in mice injured at PND5 or PND9, but in adulthood, females exhibited less loss of brain volume in response to early life HI than males (Zhu et al., 2006). Tissue loss is more consistent and global in the male brain; injury magnitude was reported to be dramatically more variable in females (Chavez-Valdez et al., 2014; Northington et al., 2011). Also consistent with our findings, a model of term HI (i.e., PN10) found females lost less total hemisphere volume compared to males, and this sex difference persisted into adulthood (Mirza et al., 2015). HI-induced seizures were also lower in females and there was lower infiltration by peripheral leukocytes on the ipsilateral side. Serum cytokine levels were elevated in males longer than in females and thus neuroinflammation may be an important contributor to male vulnerability (Mirza et al., 2015).
Estradiol is a steroid hormone derived from the aromatization of precursor testosterone, and therefore often referred to as a gonadal steroid, yet the brain contains high levels of the aromatase enzyme. The amount of enzyme and its activity are heterogeneously distributed and the many variables controlling both of these aspects remain poorly understood (Charlier et al., 2013; Jacobson et al., 1997; Tabatadze et al., 2014). Nonetheless there is great appeal in using estrogen mimetics as therapeutics given its potency at promoting both cell proliferation and survival. Overall, estradiol treatment proved to be protective from a functional perspective in both males and females. This is consistent with other studies but expands on those by modeling injury at birth via carotid artery ligation. Pretreatment with estradiol was neuroprotective against an ischemic insult in CA1 in a slice preparation (Raval et al., 2006) and protected against volume loss in the hippocampus in vivo (Nunez et al., 2007). Male rats subjected to HI show poor auditory processing compared to females and administration of testosterone to females in the early postnatal period masculinized this sensitivity to HI-induced auditory processing deficits (Hill et al., 2011). Testosterone can be neuroprotective by acting as a precursor to estradiol, or it can act directly via androgen receptors but in this case can enhance damage in some models of brain injury (Nunez et al., 2008; Zup et al, 2014).
We did not explore the effect of estradiol on neurogenesis per se. Future experiments will examine sex differences in long-term survival of cells generated in the hippocampus post-HI. Direct comparison of the sexes is complicated by the divergence of timing of increased cell genesis and may require multiple treatments with BrdU to gain resolution on this issue. Our results are limited in that we cannot determine the fate of the cells at such short BrdU-injection to sacrifice intervals. However, these experiments reveal a novel sex difference that might inform the observed sex differences in functional outcomes and guide future experimental design to most definitively compare the sexes. The early and bilateral increase in females might promote recovery, and reduce functional deficits in the long-term. This might also contribute to the fact that females did not exhibit a loss in hippocampal volume in the contralateral hemisphere, while males did. Surprisingly there was no impact of estradiol treatment on hippocampal volume. Thus the neuroprotective effects of estradiol are more subtle than a simple prevention of cell death or enhancement of restorative cell genesis. Given the multitude of effects endogenous estradiol has on the developing brain (McCarthy, 2008), this is not necessarily surprising but it does highlight that there are many potential means by which estradiol treatment can improve outcomes following neonatal HI.
Acknowledgments
This work was supported by NIH NICHD P01 HD016596 to MCM & MMM
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interests: The authors have no competing interests.
References
- Altman J, Sudarshan K. Postnatal development of locomotion of the laboratory rat. Anim Behav. 1975;23:896–920. doi: 10.1016/0003-3472(75)90114-1. [DOI] [PubMed] [Google Scholar]
- Arteni NS, Pereira LO, Rodrigues AL, Lavinsky D, Achaval ME, Netto CA. Lateralized and sex-dependent behavioral and morphological effects of unilateral neonatal cerebral hypoxia–ischemia in the rat. Behav Brain Res. 2010;210:92–98. doi: 10.1016/j.bbr.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Back SA, Han BH, Luo NL, Chricton CA, Xanthoudakis S, Tam J, Arvin KL, Holtzman DM. Selective vulnerability of late oligodendrocyte progenitors to hypoxia ischemia. J Neurosci. 2002;22:455–463. doi: 10.1523/JNEUROSCI.22-02-00455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GR, Warburton EC. When is the hippocampus involved in recognition memory? J Neurosci. 2011;31:10721–10731. doi: 10.1523/JNEUROSCI.6413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers M, Waddell J, McCarthy MM. A developmental sex difference is mediated by endogenous oestradiol. Biol Sex Diff. 2010;1:1–13. doi: 10.1186/2042-6410-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CM, Suzuki S, Jelks KA, Wise PM. Estradiol is a potent protective, restorative and trophic factor after brain injury. Semin Reprod Med. 2009;27:240–249. doi: 10.1055/s-0029-1216277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier TD, Cornil CA, Balthazart J. Rapid modulation of aromatase activity in the vertebrate brain. J Exp Neurosci. 2013;7:31–37. doi: 10.4137/JEN.S11268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez-Valdez R, Martin LG, Razdan S, Gauda EB, Northington FJ. Sexual dimorphism in BDNF signaling after neonatal hypoxia-ischemia and treatment with necrostatin-1. Neuroscience. 2014;260:106–119. doi: 10.1016/j.neuroscience.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerubini E, Griguoli M, Safiulina V, Logastena L. The depolarizing action of GABA controls early network activity in the developing hippocampus. Mol Neurobiol. 2011;43:97–106. doi: 10.1007/s12035-010-8147-z. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JM, Gadian DG, Jentschke S, Goldman A, Munoz M, Pitts G, Banks T, Chong WK, Hoskote A, Deanfield J, Baldeweg T, de Haan M, Mishkin M, Vargha-Khadem F. Neonatal hypoxia, hippocampal atrophy, and memory impairment: Evidence of a causal sequence. Cerebral Cortex. 2015;25:1469–1476. doi: 10.1093/cercor/bht332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey MV, Loporchia D, Buono KD, Levison SW. Opposite effect of inflammation on subventricular zone versus hippocampal precursors in brain injury. Ann Neurol. 2011;70:616–626. doi: 10.1002/ana.22473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean SL, Knutson JF, Krebs-Kraft DL, McCarthy MM. Prostaglandin E2 is an endogenous modulator of cerebellar development and complex behavior during a sensitive postnatal period. Eur J Neurosci. 2012;35:1218–1229. doi: 10.1111/j.1460-9568.2012.08032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1. Behavioral Data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Fan X, Heijnen CJ, Van der Kouij MA, Groenendall F, van Bel F. Beneficial effect of erythropoietin on sensorimotor function and white matter after hypoxia-ischemia in neonatal mice. Pediatric Research. 2011;69:56–61. doi: 10.1203/PDR.0b013e3181fcbef3. [DOI] [PubMed] [Google Scholar]
- Fan LW, Lin S, Pang Y, Lei M, Zhang F, Rhodes PG, Cai Z. Hypoxia-ischemia induced neurological dysfunction and brain injury in the neonatal rat. Behavioural Brain Research. 2005;165:80–90. doi: 10.1016/j.bbr.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Fatemi A, Wilson MA, Johnston MV. Hypoxic-ischemic encephalopathy in the term infant. Clin Perinatol. 2009;36:835–858. doi: 10.1016/j.clp.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felling RJ, Snyder MJ, Romanko MJ, Rothstein RP, Ziegler AN, Yang Z, Givogri MI, Bongarzone ER, Levison SW. Neural stem/progenitor cells participate in the regenerative response to perinatal hypoxia/ischemia. J Neurosci. 2006;26:4359–4369. doi: 10.1523/JNEUROSCI.1898-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Fratkins JD, LeBlanc MH. Estrogen attenuates hypoxic-ischemic brain injury in neonatal rats. Eur J Pharmacol. 2005;507:77–86. doi: 10.1016/j.ejphar.2004.11.039. [DOI] [PubMed] [Google Scholar]
- Galanopoulou AS. Sexually dimorphic expression of KCC2 and GABA function. Epilespy Research. 2008;80:99–113. doi: 10.1016/j.eplepsyres.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes R, Vannucci RC, Vannucci SJ. Delayed cerebral atrophy following moderate hypoxia–ischemia in the immature rat. Dev Neurosci. 2001;23:180–185. doi: 10.1159/000046140. [DOI] [PubMed] [Google Scholar]
- Gerstner B, Lee J, DeSilva TM, Jensen FE, Volpe JJ, Rosenberg PA. 17b-estradiol protect against hypoxic/ischemic white matter damage in the neonatal rat brain. Journal of Neuroscience Research. 2009;87:2078–2086. doi: 10.1002/jnr.22023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez FF, Ferriero DM. Therapeutics for neonatal brain injury. Pharmacol Ther. 2008;120:43–53. doi: 10.1016/j.pharmthera.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Graham EM, Ruis KA, Hartman AL, Northington FJ, Fox HE. A systematic review of the role of intrapartum hypoxia-ischemia in the causation of neonatal encephalopathy. Am J Obstet Gynecol. 2008;199:587–595. doi: 10.1016/j.ajog.2008.06.094. [DOI] [PubMed] [Google Scholar]
- Hagberg HH, Peebles D, Mallard C. Models of white matter injury: comparison of infectious, hypoxic-ischemic and excitotoxic insults. Ment Retard Dev Disabil Res Rev. 2002;8:30–38. doi: 10.1002/mrdd.10007. [DOI] [PubMed] [Google Scholar]
- Hagberg H, Wilson MA, Matsushita H, Zhu C, Lange M, Gustavsson M, Poitras MF, Dawson TM, Dawson LV, Northington F, Johnston MV. PARP-1 gene disruption in mice preferentially protects males from perinatal brain injury. J Neurochem. 2004;90:1068–1075. doi: 10.1111/j.1471-4159.2004.02547.x. [DOI] [PubMed] [Google Scholar]
- Hermans RH, McGivern RF, Chen W, Longo LD. Altered adult sexual behavior in the male rat following chronic prenatal hypoxia. Neurotoxicol Teratol. 1993;15:353–363. doi: 10.1016/0892-0362(93)90051-o. [DOI] [PubMed] [Google Scholar]
- Hill CA, Alexander ML, McCollough LD, Fitch RH. Inhibition of X-linked inhibitor of apoptosis with embelin differentially affects male versus female behavioral outcome following neonatal hypoxia-ischemia in rats. Developmental Neuroscience. 2011a;33:494–504. doi: 10.1159/000331651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CA, Threlkeld SW, Fitch RH. Early testosterone modulated sex differences in behavioral outcome following neonatal hypoxia ischemia in rats. International Journal of Developmental Neuroscience. 2011b;29:381–388. doi: 10.1016/j.ijdevneu.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CA, Fitch RH. Sex differences in mechanism and outcome of neonatal hypoxia-ischemia in rodent models: Implications for sex-specific neuroprotection in clinical neonatal practice. Neurology Research International. 2012 doi: 10.1155/2012/867531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson NA, Ladle DR, Lephart ED. Aromatase cytochrome P450 and 5 alpha-reductase in the amygdala and cortex of perinatal rats. Neuroreport. 1997;8:2529–2533. doi: 10.1097/00001756-199707280-00022. [DOI] [PubMed] [Google Scholar]
- Johnston MV, Haberg H. Sex and the pathogenesis of cerebral palsy. Dev Med Child Neurol. 2007;49:74–78. doi: 10.1017/s0012162207000199.x. [DOI] [PubMed] [Google Scholar]
- Kinnavane L, Albasser MM, Aggleton JP. Advances in the behavioural testing and network imaging of rodent recognition memory. Behav Brain Res. 2015;285:67–78. doi: 10.1016/j.bbr.2014.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterbach MD, Raz S, Sander CJ. Neonatal hypoxic risk in preterm birth infants: the influence of sex and severity of respiratory distress on cognitive recovery. Neuropsy. 2001;15:411–420. [PubMed] [Google Scholar]
- Long JM, Kalehua AN, Muth NJ, Hengemihle JM, Jucker M, Calhoun ME, Ingram DK, Mouton PR. Stereological estimation of total microglia number in mouse hippocampus. J Neurosci Methods. 1998;84:101–108. doi: 10.1016/s0165-0270(98)00100-9. [DOI] [PubMed] [Google Scholar]
- McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88:91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza M, Ritzel R, Xu Y, McCullough LD, Liu F. Sexually dimorphic outcomes and inflammatory responses in hypoxic-ischemic encephalopathy. J Neuroinflammation. 2015;12:32. doi: 10.1186/s12974-015-0251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Motz BA, Alberts JR. The validity and utility of geotaxis in young rodents. Neurotoxicology and Teratology. 2005;27:529–533. doi: 10.1016/j.ntt.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Müller MM, Middelanis J, Meier C, Surbek D, Berger R. 17β-estradiol protects 7-day old rats from acute brain injury and reduces the number of apoptotic cells. Reprod Sci. 2013;20:253–261. doi: 10.1177/1933719112452471. [DOI] [PubMed] [Google Scholar]
- Muramatsu R, Ikegaya Y, Matsuki N, Koyama R. Neonatally born granule cells numberically dominate adult mice dentate gyrus. Neurosci. 2007;148:593–598. doi: 10.1016/j.neuroscience.2007.06.040. [DOI] [PubMed] [Google Scholar]
- Nijboer CHA, Groenendaal F, Kavelaars A, Hagberg HH, van Bel F, Heijnen CJ. Gender-specific neuroprotection by 2-iminobiotin after hypoxia-ischemia in the neonatal rat via a nitric oxide independent pathway. J Cereb Blood Flow Metab. 2007;27:282–292. doi: 10.1038/sj.jcbfm.9600342. [DOI] [PubMed] [Google Scholar]
- Northington FJ, Chavez-Valdez R, Graham EM, Razdan S, Gauda EB, Martin LJ. Necrostatin decreases oxidative damage, inflammation and injury after neonatal HI. J Cereb Blood Flow Metab. 2011;31:178–189. doi: 10.1038/jcbfm.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez JL, McCarthy MM. Evidence for an extended duration of GABA-mediated excitation in the developing male versus female hippocampus. Dev Neurobiol. 2007;67:1879–1890. doi: 10.1002/dneu.20567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez JL, McCarthy MM. Androgens predispose males to GABAA-mediated excitotoxicity in the developing hippocampus. Exp Neuro. 2008;210:699–708. doi: 10.1016/j.expneurol.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez J, Yang Z, Grandys T, Mark I, Levison SW. 17beta-estradiol protects the neonatal brain from hypoxia-ischemia. Exp Neurol. 2007;208:269–276. doi: 10.1016/j.expneurol.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panskepp J. The ontogeny of play in rats. Dev Psychobio. 1981;14:327–332. doi: 10.1002/dev.420140405. [DOI] [PubMed] [Google Scholar]
- Patel SD, Pierce L, Ciardiello AJ, Vannucci SJ. Neonatal encephalopathy: pre-clinical studies in neuroprotection. Biochem Soc Trans. 2014;42:564–568. doi: 10.1042/BST20130247. [DOI] [PubMed] [Google Scholar]
- Perrot-Sinal TS, Kostenuik MA, Ossenkopp KP, Kavaliers M. Sex differences in performance in the Morris water maze and the effects of initial nonstationary hidden platform training. Behav Neurosci. 1996;110:1309–1320. doi: 10.1037//0735-7044.110.6.1309. [DOI] [PubMed] [Google Scholar]
- Raval AP, Bramlett H, Perez-Pinzon MA. Estrogen preconditioning protects the hippocampal CA1 against ischemia. Neurosci. 2006;141:1721–1730. doi: 10.1016/j.neuroscience.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Reger ML, Hovda DA, Giza CC. Ontogeny of rat recognition memory measured by the novel object recognition task. Dev Psychobiol. 2009;51:672–678. doi: 10.1002/dev.20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renolleau S, Fau S, Charriaut-Marlangue C. Gender-related differences in apoptotic pathways after neonatal cerebral ischemia. Neuroscientist. 2008;14:46–52. doi: 10.1177/1073858407308889. [DOI] [PubMed] [Google Scholar]
- Renolleau S, Fau S, Goyenvalle C, Joly LM, Chauvier D, Jacotot E, Mariani J, Charriaut-Marlangue C. Specific caspase inhibitor QVD-OPh prevents neonatal stroke in P7 rat: a role for gender. J Neurochem. 2007;100:1062–1071. doi: 10.1111/j.1471-4159.2006.04269.x. [DOI] [PubMed] [Google Scholar]
- Rice JE, 3rd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9:131–41. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- Scafidi J, Gallo V. New concepts in perinatal hypoxia ischemia encephalopathy. Curr Neurol Neurosci Rep. 2008;8:130–138. doi: 10.1007/s11910-008-0021-2. [DOI] [PubMed] [Google Scholar]
- Smith AL, Alexander M, Rosenkrantz TS, Sadek ML, Fitch RH. Sex differences in behavioral outcome following neonatal hypoxia ischemia: Insights from a clinical meta-analysis and a rodent model of induced hypoxic ischemic brain injury. Exp Neurol. 2014;254:54–67. doi: 10.1016/j.expneurol.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Souza A, Dussan-Sarria JA, Medeiros LF, Souza AC, Oliveira C, Scarabelot VL, Adachi LN, Winkelmann-Duarte EC, Phillippi-Martins BB, Netto CA, Caumo W, Torres IL. Neonatal hypoxic-ischemic encephalopathy reduces c-Fos activation in the rat hippocampus: evidence of a long lasting effect. Int J Neurosci. 2014;38:213–222. doi: 10.1016/j.ijdevneu.2014.09.003. [DOI] [PubMed] [Google Scholar]
- Tabatadze N, Sato SM, Woolley CS. Quantitative analysis of long-form aromatase mRNA in the male and female rat brain. PLoS One. 2014;9(7):e100628. doi: 10.1371/journal.pone.0100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towfighi J, Mauger D, Vannucci RC, Vannucci SJ. Influence of age on the cerebral lesions in a immature rat model of cerebral hypoxia-ischemia: a light microscopic study. Dev Brain Res. 1997;100:149–160. doi: 10.1016/s0165-3806(97)00036-9. [DOI] [PubMed] [Google Scholar]
- Trezza V, Campolongo P, Vanderschuren LJMJ. Evaluating the rewarding nature of social interactions in laboratory animals. Dev Cog Neurosci. 2011;1:444–458. doi: 10.1016/j.dcn.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker AM, Aquilina K, Chakkarapani E, Hobbs CE, Thoresen M. Pediatric Res. 2009;65:62–66. doi: 10.1203/PDR.0b013e3181891316. [DOI] [PubMed] [Google Scholar]
- van Kerkhof LVM, Damsteegt R, Trezza V, Voorn P, Vanderschuren LJMJ. Social Play Behavior in Adolescent Rats is Mediated by Functional Activity in Medial Prefrontal Cortex and Striatum. Neuropsychopharm. 2013;38:1899–1909. doi: 10.1038/npp.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucci RC, Perlman JM. Interventions for perinatal hypoxic-ischemic encephalopathy. Pediatrics. 1997;100:1004–1014. doi: 10.1542/peds.100.6.1004. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Brain injury in the premature infant: overview of clinical aspects, neuropathology, and pathogenesis. Semin Pediatr Neurol. 1998;5:135–151. doi: 10.1016/s1071-9091(98)80030-2. [DOI] [PubMed] [Google Scholar]
- Wu YW, Backstrand KH, Zhao S, Fullerton HJ, Johnston SC. Declining diagnosis of birth asphyxia in California: 1991–2000. Pediatrics. 2004;114:1584–1590. doi: 10.1542/peds.2004-0708. [DOI] [PubMed] [Google Scholar]
- Waddell J, Bowers JM, Edwards NS, Jordan CL, McCarthy MM. Dysregulation of neonatal hippocampal cell genesis in the androgen insensitive Tfm rat. Horm Behav. 2013;64:144–52. doi: 10.1016/j.yhbeh.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Wise PM, Suzuki S, Brown CM. Estradiol: a hormone with diverse and contradictory neuroprotective actions. Dialogues Clin Neurosci. 2009;11:297–303. doi: 10.31887/DCNS.2009.11.3/pmwise. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Covey MV, Bitel CL, Ni L, Jonakait GM, Levison SW. Sustained neocortical neurogenesis after neonatal hypoxic/ischemic injury. Ann Neurol. 2007;61:199–208. doi: 10.1002/ana.21068. [DOI] [PubMed] [Google Scholar]
- Yang Y, Levison SW. Hypoxia/ischemia expands the regenerative capacity of progenitors in the perinatal subventricular zone. Neuroscience. 2006;139:555–564. doi: 10.1016/j.neuroscience.2005.12.059. [DOI] [PubMed] [Google Scholar]
- Yang Z, Levison SW. Perinatal hypoxic/ischemic brain injury induces persistent production of striatal neurons from subventricular zone progenitors. Dev Neurosci. 2007;29:331–340. doi: 10.1159/000105474. [DOI] [PubMed] [Google Scholar]
- Yang Z, You Y, Levison SW. Neonatal hypoxic/ischemic brain injury induces production of calretinin-expressing interneurons in the striatum. J Comp Neurol. 2008;511:19–33. doi: 10.1002/cne.21819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JM, Konkle ATM, Zup SL, McCarthy MM. Impact of sex and hormones on new cells in the developing rat hippocampus: a novel source of sex dimorphism? European Journal of Neuroscience. 2008;27:791–800. doi: 10.1111/j.1460-9568.2008.06073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Xu F, Wang X, Shibata M, Uchiyama Y, Blomgren K, Hagberg H. Different apoptotic mechanisms are activated in male and female brains after neonatal hypoxia-ischemia. Journal of Neurochemistry. 2006;96:1016–1027. doi: 10.1111/j.1471-4159.2005.03639.x. [DOI] [PubMed] [Google Scholar]
- Zup SL, Edwards NS, McCarthy MM. Sex- and age-dependent effects of androgens on glutamate-induced cell death and intracellular calcium regulation in the developing hippocampus. Neurosci. 2014;281:77–87. doi: 10.1016/j.neuroscience.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]


