Abstract
Purpose
We compared overall survival and influencing factors between Asian American women as a whole and by subgroup with white women with cervical cancer.
Methods
Cervical cancer data were from the Surveillance, Epidemiology, and End Results registry; socioeconomic information was from the Area Health Resource File. We used standard tests to compare characteristics between groups; the Kaplan-Meier method with log-rank test to assess overall survival and compare it between groups; and Cox proportional hazards models to determine the effect of race and other covariates on overall survival (with/without age-stratification).
Results
Being 3.3 years older than white women at diagnosis (p<0.001), Asian American women were more likely to be in a spousal relationship, had more progressive disease, and were better off socioeconomically. Women of Filipino, Japanese, and Korean origin had similar clinical characteristics compared with white women. Asian American women had higher 36- and 60-month survival rates (p=0.004 and p=0.013, respectively), higher overall survival rates (p=0.049), and longer overall survival durations after adjusting for age and other covariates (hazard ratio=0.77, 95% confidence interval: 0.68–0.86). Overall survival differed across age strata between the two racial groups. With the exception of women of Japanese or Korean origin, Asian American women grouped by geographic origin had better overall survival than white women.
Conclusions
Although Asian American women, except those of Japanese or Korean origin, had better overall survival than white women, their older age at cervical cancer diagnosis suggests that they have less access to screening programs.
Keywords: cervical cancer, Asian Americans, healthcare disparities, race, ethnicity, survival
INTRODUCTION
Cervical cancer is the second leading cause of cancer-related death among women aged 20 to 39 years and has remained a public health problem in the United States. In 2013 alone, 12,340 new cases of cervical cancer were diagnosed, and approximately 4,030 women died of the disease (1, 2). Survival durations differ among patients with cervical cancer owing to many factors (3–5), but race or ethnicity is one of the most common predictors of survival. For example, African American women have been shown to have an increased risk of death from cervical cancer compared with white women, whereas Hispanic women have a decreased risk (3, 4). Although numerous studies have reported survival disparities for African American and Hispanic women compared with white women (5, 6), nationwide cervical cancer survival rates for other racial and ethnic groups, including Asian American women, have not been reported.
The Asian American community has rapidly grown over the past decade; a four-fold increase in the population size was observed between 2000 and 2010. Fewer Asian Americans than non-Hispanic whites had health insurance coverage (82% versus 88% in 2011) (7). As a result, Asian Americans were less likely to access healthcare services for prevention and treatment, including cervical cancer screening programs. This disparity represents a significant public health problem, as cancer is the leading cause of death among Asian American women (8). This group has a disproportionately high rate of cancers of infectious origin, including cervical cancer (9); between 1990 and 2008, invasive cervical cancer was one of the five most common cancers in some subgroups of Asian American women, including those of Cambodian and Korean origin (10). Furthermore, Southeast Asian women, who have the highest rates of cervical cancer in the United States, have the lowest rates of Papanicolaou smear testing (11). In general, cervical cancer is a serious problem in the fast-growing Asian American population, requiring study on a national scale. Numerous studies have addressed the cervical cancer screening among Asian American women (12–15). However, there is a lack of knowledge on the survival outcomes of Asian American women diagnosed with cervical cancer. Additionally, for the purposes of cancer control, it is imperative to report disaggregated data of the Asian American population since this population is heterogeneous and dynamic (16, 17). Consequently, analyses of cervical cancer survival outcomes among subgroups of the Asian American women at the national level would be of paramount importance.
In this study, we investigated cervical cancer overall survival durations and factors influencing survival among Asian American women and among women of Asian subgroups in comparisons with non-Hispanic white women. We sought to explore the potential effects of demographics, socioeconomic patterns, and clinical characteristics, which have been suggested as predictors of survival in previous studies (18–22). We expect that findings from this study would help to better design health interventions and guide future research for cervical cancer prevention and control.
MATERIALS AND METHODS
Study population and data source
We used records from the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) registry for the years 1996–2012 (23). The registry represented approximately 28% of the U.S. population and had wide geographic coverage. The validity and completeness of the SEER database make it a well-known, high-quality source of cancer statistics (24). The year 1996 was chosen as our starting point because from this year onward, specific ethnic information about patients with Asian origins was recorded. Because patients were not identifiable, our study received exempt status from the Institutional Review Board of The University of Texas MD Anderson Cancer Center.
We extracted the records of Asian American women (including those of Indian/Pakistani, Chinese, Filipino, Japanese, Kampuchean, Korean, Laotian, or Vietnamese origin) and non-Hispanic white women (white women) who had a confirmed diagnosis of cervical cancer during the study period. We collected information about each patient’s disease stage at diagnosis (“SEER historic stage A”), tumor histologic classification, demographic characteristics, and initial treatment. Socioeconomic status was determined using data from the Area Health Resource File database, based on state county code. Information about poverty, education, income, and unemployment at the county level were used to estimate a composite socioeconomic index, as described elsewhere (22). This index was categorized into four quartiles, the lowest of which was the most socioeconomically disadvantaged. We used the rural/urban continuum code to describe the population density of the counties in which the patients resided. Additionally, as a surrogate variable to estimate the proportion of our cohort that was foreign-born, we determined the percentage of the population of each woman’s county-of-residence born outside of the United States and categorized the percentages into 1 of 4 relative quartiles, from lowest to highest. For both databases, we excluded records with missing values for key covariates, including marital status, age at diagnosis, histologic classification of the tumor, type of treatment, and socioeconomic status. Cancer survival outcome variables included vital status and time-to-event (i.e., the time from the date of diagnosis until death, censoring, or last follow-up).
Statistical analysis
We used the chi-square test to examine differences by race in the distribution of categorical variables such as demographic characteristics, tumor histologic classification, and type of treatment. The Wilcoxon rank-sum test was used to assess differences in the median values of continuous variables (e.g., age at diagnosis).
We used the Kaplan-Meier method to investigate overall survival within each racial group, and the log-rank test to examine differences between the groups. Overall survival was calculated in months from the time of diagnosis to death or last follow-up. Patients who were still alive at last follow-up were censored. We compared overall survival rates between racial groups at key time points (36 months and 60 months).
We also conducted univariable and multivariable analyses using Cox proportional hazards regression modeling with overall survival as the evaluable endpoint. The effect of race on overall survival was examined with the presence of all covariates. This effect was further examined in different age groups and in subgroups of Asian American women, which were categorized by geographic area of origin and sample size. To evaluate the proportional hazards assumptions for this Cox model, we used Schoenfeld residual analysis.
All analyses were performed in SAS 9.4 (SAS, Cary, NC); p < 0.05 was considered statistically significant.
RESULTS
Study cohort
We identified the records of 11,902 women with a cervical cancer diagnosis between 1996 and 2011 (with last follow-ups through 2012) in the SEER database. We excluded 1,252 SEER records (11%) that had missing values for at least one variable and another 176 records with missing information in the Area Health Resource File database that prevented us from determining socioeconomic status. Marital status and disease stage at diagnosis were the variables most often missing (718 [6%] for marital status, stage at diagnosis 637 [5%], and treatment type (145 [1%]). Some records were missing values for more than one variable.
We included the records of 10,474 women in the analysis. Among these women, 9,408 (90%) were white, and 1,066 (10%) were Asian American (Table 1). Of the Asian American women, 344 (32%) were of Filipino origin, 236 (22%) were of Chinese origin, 249 (23%) were of North Asian origin (146 of Japanese and 103 of Korean origin), and 206 (19%) were of Southeast Asian origin (137 Vietnamese, 21 Laotian, 26 Kampuchean, and 22 Thai). Only 31 women (3%) were of Asian Indian/Pakistani origin. Three hundred forty-nine (33%) of the Asian American women and 3,452 (37%) of the white women had died by the last follow-up, and 60% of all deaths were related to cervical cancer. The proportions of Asian American women and white women who died of cervical cancer did not differ (p = 0.969).
Table 1.
Demographic, socioeconomic and clinical characteristics of women with cervical cancer of Asian American group and of Asian subgroups in a comparison to white women (1996–2012).
| No. (%) | |||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Total cohort n = 10,474 |
White n = 9,408 |
Asian American n = 1,066 |
Filipino n = 344 |
Chinese n = 236 |
North Asian n = 249 |
Southeast Asian n = 206 |
| Mean age at diagnosis, years (standard deviation) | 50.4 (15.7) |
50.1 (15.7) |
53.4 (14.8) p<0.001 |
51.4 (14.1) p=0.028 |
54.3 (16.5) p<0.001 |
54.8 (15.9) p<0.001 |
53.6 (12.7) p<0.001 |
| Median age at diagnosis, years | 48 | 47 | 51 | 49 | 50 | 51 | 52 |
| Age, years | p<0.001 | p=0.009 | p=0.008 | p<0.001 | p<0.001 | ||
| <35 | 1,618 (15) | 1,532 (16) | 86 (8) | 33 (10) | 26 (11) | 15 (6) | 10 (5) |
| 35–44 | 2,697 (26) | 2,444 (26) | 253 (24) | 90 (26) | 56 (24) | 62 (25) | 41 (20) |
| 45–54 | 2,472 (24) | 2,184 (23) | 288 (27) | 98 (28) | 51 (22) | 66 (27) | 64 (31) |
| 55–64 | 1,599 (15) | 1,406 (15) | 193 (18) | 51 (15) | 36 (15) | 39 (16) | 55 (27) |
| ≥65 | 2,088 (20) | 1,842 (20) | 246 (23) | 72 (21) | 67 (28) | 67 (27) | 36 (17) |
| Marital status | p<0.001 | p<0.001 | p<0.001 | p=0.174 | p=0.022 | ||
| Single | 2,109 (20) | 1,963 (21) | 146 (14) | 45 (13) | 26 (11) | 41 (16) | 31 (15) |
| Married/domestic partner | 5,428 (52) | 4,780 (51) | 648 (61) | 210 (61) | 156 (66) | 139 (56) | 124 (60) |
| Divorced/separated/widowed | 2,937 (28) | 2,665 (28) | 272 (26) | 89 (26) | 54 (23) | 69 (28) | 51 (25) |
| Stage at diagnosis | p<0.001 | p=0.419 | p=0.046 | p=0.473 | p<0.001 | ||
| Localized | 5,587 (53) | 5,060 (54) | 527 (49) | 175 (51) | 129 (55) | 125 (50) | 90 (44) |
| Regional | 3,722 (36) | 3,285 (35) | 437 (41) | 132 (38) | 92 (39) | 96 (39) | 99 (48) |
| Distant | 1,165 (11) | 1,063 (11) | 102 (10) | 37 (11) | 15 (6) | 28 (11) | 17 (8) |
| Histologic classification | p=0.024 | p=0.582 | p=0.267 | p=0.608 | p=0.009 | ||
| Squamous cell carcinoma | 6,696 (64) | 5,994 (64) | 702 (66) | 223 (65) | 145 (61) | 166 (67) | 146 (71) |
| Adenoma or adenocarcinoma | 2,448 (23) | 2,233 (24) | 215 (20) | 74 (22) | 53 (22) | 53 (21) | 30 (15) |
| Other | 1,330 (13) | 1,181 (13) | 149 (14) | 47 (14) | 38 (16) | 30 (12) | 30 (15) |
| Vital status at last follow-up | p=0.011 | p=0.179 | p=0.157 | p=0.662 | p=0.130 | ||
| Alive | 6,673 (64) | 5,956 (63) | 717 (67) | 230 (67) | 160 (68) | 161 (65) | 141 (68) |
| Dead | 3,801 (36) | 3,452 (37) | 349 (33) | 114 (33) | 76 (32) | 88 (35) | 65 (32) |
| Treatment | p<0.001 | p=0.069 | p=0.671 | p=0.099 | p=0.003 | ||
| No treatment | 491 (5) | 456 (5) | 35 (3) | 10 (3) | 12 (5) | 5 (2) | 6 (3) |
| Surgery | 4,599 (44) | 4,180 (44) | 419 (39) | 139 (40) | 97 (41) | 103 (41) | 70 (34) |
| Radiation | 2,813 (27) | 2,481 (26) | 332 (31) | 108 (31) | 70 (30) | 73 (29) | 73 (35) |
| Surgery and radiation | 2,571 (25) | 2,291 (24) | 280 (26) | 87 (25) | 57 (24) | 68 (27) | 57 (28) |
| Socioeconomic status1 | p<0.001 | p<0.001 | p<0.001 | p<0.001 | p<0.001 | ||
| Quartile 1 | 2,670 (25) | 2,586 (27) | 84 (8) | 30 (9) | 9 (4) | 19 (8) | 22 (11) |
| Quartile 2 | 3,173 (30) | 2,902 (31) | 271 (25) | 77 (22) | 56 (24) | 63 (25) | 61 (30) |
| Quartile 3 | 1,699 (16) | 1,578 (17) | 121 (11) | 46 (13) | 14 (6) | 28 (11) | 31 (15) |
| Quartile 4 | 2,932 (28) | 2,342 (25) | 590 (55) | 191 (55) | 157 (67) | 139 (56) | 92 (45) |
| Foreign-born2 | p<0.001 | p<0.001 | p<0.001 | p<0.001 | p<0.001 | ||
| Quartile 1 | 3,084 (29) | 3,022 (32) | 62 (6) | 37 (11) | 1 (0) | 16 (6) | 8 (4) |
| Quartile 2 | 2,242 (21) | 2,139 (23) | 103 (10) | 34 (10) | 9 (4) | 33 (13) | 22 (11) |
| Quartile 3 | 2,117 (20) | 1,991 (21) | 126 (12) | 37 (11) | 19 (8) | 28 (11) | 34 (17) |
| Quartile 4 | 3,031 (29) | 2,256 (24) | 775 (73) | 236 (67) | 207 (88) | 172 (69) | 142 (69) |
| Residence population density | p<0.001 | p=0.941 | p<0.001 | p=0.403 | p<0.001 | ||
| County population ≥1,000,000 | 5,709 (55) | 5,013 (53) | 696 (65) | 184 (53) | 198 (84) | 126 (51) | 164 (80) |
| County population <1,000,000 | 4,765 (45) | 4,395 (47) | 370 (35) | 160 (47) | 38 (16) | 123 (49) | 42 (20) |
Socioeconomic status (based on county-level poverty, education, income, and unemployment levels) was categorized into 1 of 4 relative quartiles within the study sample. Quartile 1 represents the lowest socioeconomic status, or most socioeconomically disadvantaged, and quartile 4 represents the highest socioeconomic status, or most socioeconomically advantaged.
Percentage of the population of the woman’s county of residence born outside the United States, categorized into 1 of 4 relative quartiles, from lowest to highest percentage.
The distribution of age at the time of diagnosis was fairly even among those aged 35–44 years, 45–54 years, and 65 years and older; however, there were much fewer women in both the youngest group (younger than 35 years, 1,618 [15%]) and those aged 55–64 years (1,599 [15%]). More than half of the women (5,428 [52%]) were married or living with a partner, and a similar fraction had stage 1 disease at diagnosis (5,587 [53%]). Regardless of race, most women were treated with surgery alone (4,599 [44%]) or in combination with radiation (2,571 [25%]). The proportions of women who were most socioeconomically disadvantaged (quartile 1) and those who were most socioeconomically advantaged (quartile 4) were similar (quartile 1: 2,670 [25%]; quartile 4: 2,932 [28%]), and the third quartile was the smallest (1,699 [16%]).
We found that several demographic, socioeconomic and clinical characteristics differed between the Asian American and white groups, but the results were not necessarily similar when subgroups of Asian American women were compared with white women (Table 1). Asian American women were about 3.3 years older than white women at the time of diagnosis (Asian American women mean age: 53.4 years; white women mean age: 50.1 years; p < 0.001). Similarly, compared with white women, women of Asian subgroups demonstrated significantly higher age at diagnosis. Asian American women were more likely to live with a spouse or domestic partner than were white women (Asian American: 61%; white: 51%; p < 0.001). Among Asian American women, those of North Asian origin had the lowest marriage rate (56%), which did not differ significantly from that of white women. In addition, the proportion of regional or distant disease of Asian American women (51%) was significantly higher than that of white women (46%; p = 0.002). However, the regional or distant disease proportions of women of Filipino or North Asian origin did not differ from those of white women. Asian American women and white women had tumors with different histologic classification (p = 0.024). However, several subgroups of Asian American women, including those of Filipino, Chinese, and North Asian origin, had histologic classification distributions that did not differ significantly from those of white women. The proportions of Asian American women and white women who had squamous cell carcinoma (66% and 64%, respectively) did not differ significantly (p = 0.089). More than half of the Asian American women (55%) but only a quarter of the white women (25%) were in the highest relative socioeconomic status quartile. Approximately half of women of North Asian and Filipino origin and white women lived in metro areas (of one million population or more); however, the proportion living in metro areas were much larger in other Asian subgroups (range, 65% – 84%). In general, women of North Asian and Filipino origin, but not those in other subgroups of Asian American women, had clinical characteristics similar to those of white women.
Univariable analysis
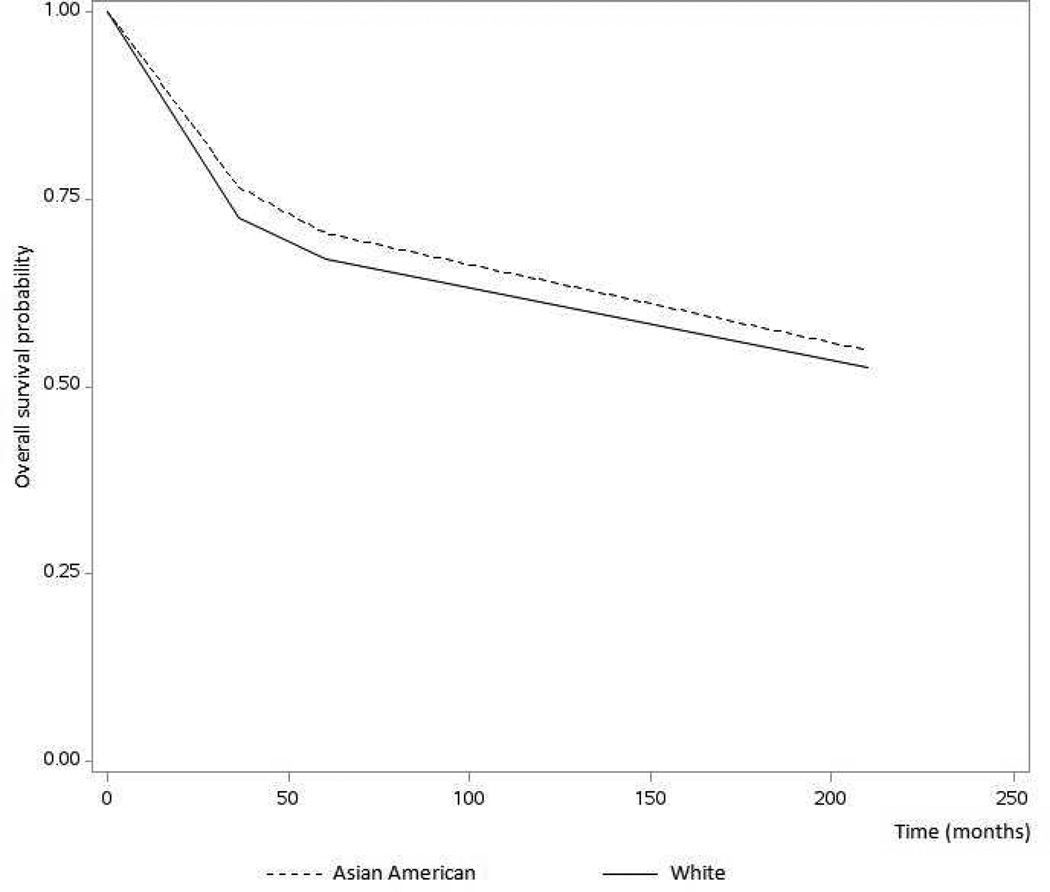
The median overall survival duration for all women was 62 months (range, 0–203 months). The Kaplan-Meier overall survival curves for Asian American women and white women are shown in Figure 1. Asian American women had a higher overall survival probability than white women did (p = 0.049), and the overall survival rates of Asian American women were better than those of white women at both 36 months (0.77 vs 0.73; p = 0.004) and 60 months (0.70 vs 0.67; p = 0.033).
Figure 1.
Kaplan-Meier curves comparing overall survival probabilities between Asian American women and white women diagnosed with cervical cancer. Overall survival significantly differed between the two groups (p = 0.049).
Multivariable analysis
The multivariable analysis showed that race was predictive of overall survival after controlling for marital status, age, stage at diagnosis, type of treatment, histologic classification, socioeconomic status, county-of-residence population density, and place of birth (Table 2). Consistent with the Kaplan-Meier analysis, the Cox model demonstrated that Asian American women had better overall survival than white women (hazard ratio [HR] = 0.77, 95% confidence interval [CI] =0.68–0.86). In addition, women of Filipino, Chinese, or Southeast Asian origin, but not those of North Asian origin, had better survival compared with white women, with HRs ranging from 0.62 to 0.82 (Table 3). Subgroup analysis of the 31 Asian Indian/Pakistani women was not performed because of the limited sample size.
Table 2.
Results of the multivariable Cox regression model assessing the effects of race and other covariates on the overall survival of 10,474 Asian American women and white women with cervical cancer
| Covariate | Parameter estimate |
Standard error |
Hazard ratio |
95% confidence interval |
|---|---|---|---|---|
| Marital status | ||||
| Single | Ref. | |||
| Married/domestic partner | −0.144 | 0.047 | 0.87 | 0.79–0.95 |
| Divorced/separated/widowed | 0.074 | 0.050 | 1.08 | 0.98–1.19 |
| Age at diagnosis, years | ||||
| <35 | Ref. | |||
| 35–44 | 0.396 | 0.083 | 1.49 | 1.26–1.75 |
| 45–54 | 0.696 | 0.080 | 2.01 | 1.71–2.35 |
| 55–64 | 0.811 | 0.082 | 2.25 | 1.92–2.65 |
| ≥65 | 1.391 | 0.080 | 4.02 | 3.44–4.70 |
| Stage at diagnosis | ||||
| Localized | Ref. | |||
| Regional | 0.690 | 0.049 | 1.99 | 1.81–2.20 |
| Distant | 1.752 | 0.055 | 5.77 | 5.17–6.43 |
| Histologic classification | ||||
| Squamous cell carcinoma | Ref. | |||
| Adenoma or adenocarcinoma | 0.078 | 0.044 | 1.08 | 0.99–1.18 |
| Other | 0.408 | 0.046 | 1.50 | 1.38–1.65 |
| Treatment | ||||
| No surgery | Ref. | |||
| Surgery | −2.146 | 0.074 | 0.12 | 0.10–0.14 |
| Radiation | −0.853 | 0.058 | 0.43 | 0.38–0.48 |
| Surgery and radiation | −1.487 | 0.062 | 0.23 | 0.20–0.26 |
| Socioeconomic status1 | ||||
| Quartile 1 | Ref. | |||
| Quartile 2 | −0.043 | 0.046 | 0.96 | 0.88–1.05 |
| Quartile 3 | −0.063 | 0.055 | 0.94 | 0.84–1.05 |
| Quartile 4 | −0.160 | 0.055 | 0.85 | 0.76–0.95 |
| Foreign-born2 | ||||
| Quartile 1 | Ref. | |||
| Quartile 2 | 0.018 | 0.049 | 1.02 | 0.93–1.12 |
| Quartile 3 | 0.057 | 0.051 | 1.06 | 0.96–1.17 |
| Quartile 4 | 0.009 | 0.059 | 1.01 | 0.90–1.13 |
| Residence population density | ||||
| County population ≥1,000,000 | Ref. | |||
| County population <1,000,000 | 0.024 | 0.037 | 1.02 | 0.95–1.10 |
| Race | ||||
| White | Ref. | |||
| Asian American | −0.267 | 0.060 | 0.77 | 0.68–0.86 |
Socioeconomic status (based on county-level poverty, education, income, and unemployment levels) was categorized into 1 of 4 relative quartiles within the study sample. Quartile 1 represents the lowest socioeconomic status, or most socioeconomically disadvantaged, and quartile 4 represents the highest socioeconomic status, or most socioeconomically advantaged.
Percentage of the population of the woman’s county of residence born outside the United States, categorized into 1 of 4 relative quartiles, from lowest to highest percentage.
Table 3.
Results of the multivariable Cox regression models comparing overall survival outcomes between women in Asian American subgroups and white women with cervical cancer.
| Hazard ratio (95% confidence interval) | ||||
|---|---|---|---|---|
| Characteristics | Filipino women, n = 344 | Chinese women, n = 236 | North Asian women, n = 249 | Southeast Asian women, n = 206 |
| Marital status | ||||
| Single | Ref. | Ref. | Ref. | Ref. |
| Married/domestic partner | 0.86 (0.79–0.95) | 0.87 (0.79–0.96) | 0.87 (0.79–0.96) | 0.87 (0.79–0.96) |
| Divorced/separated/widowed | 1.09 (0.98–1.20) | 1.09 (0.99–1.21) | 1.08 (0.98–1.19) | 1.08 (0.98–1.19) |
| Age at diagnosis, years | ||||
| Younger than 35 | Ref. | Ref. | Ref. | Ref. |
| 35–44 | 1.53 (1.29–1.80) | 1.50 (1.27–1.78) | 1.55 (1.31–1.83) | 1.56 (1.32–1.85) |
| 45–54 | 2.06 (1.75–2.42) | 2.05 (1.74–2.42) | 2.10 (1.78–2.48) | 2.12 (1.79–2.50) |
| 55–64 | 2.37 (2.01–2.80) | 2.37 (2.01–2.80) | 2.41 (2.03–2.85) | 2.44 (2.06–2.89) |
| 65 or older | 4.22 (3.60–4.96) | 4.26 (3.63–5.01) | 4.37 (3.71–5.14) | 4.43 (3.76–5.22) |
| Stage at diagnosis | ||||
| Localized | Ref. | Ref. | Ref. | Ref. |
| Regional | 1.97 (1.78–2.17) | 2.00 (1.81–2.21) | 1.98 (1.79–2.19) | 1.99 (1.80–2.20) |
| Distant | 5.70 (5.10–6.38) | 5.72 (5.11–6.40) | 5.67 (5.07–6.34) | 5.68 (5.07–6.35) |
| Histologic classification | ||||
| Squamous cell carcinoma | Ref. | Ref. | Ref. | Ref. |
| Adenoma or adenocarcinoma | 1.05 (0.96–1.15) | 1.04 (0.95–1.13) | 1.03 (0.95–1.13) | 1.02 (0.94–1.12) |
| Other | 1.42 (1.29–1.56) | 1.42 (1.29–1.56) | 1.41 (1.29–1.55) | 1.42 (1.29–1.56) |
| Treatment | ||||
| No surgery | Ref. | Ref. | Ref. | Ref. |
| Surgery | 0.12 (0.10–0.14) | 0.12 (0.10–0.14) | 0.12 (0.10–0.13) | 0.12 (0.10–0.14) |
| Radiation | 0.43 (0.38–0.49) | 0.42 (0.38–0.48) | 0.42 (0.37–0.47) | 0.42 (0.38–0.48) |
| Surgery and radiation | 0.23 (0.20–0.26) | 0.22 (0.20–0.25) | 0.22 (0.20–0.25) | 0.22 (0.20–0.25) |
| Socioeconomic status1 | ||||
| Quartile 1 | Ref. | Ref. | Ref. | Ref. |
| Quartile 2 | 0.97 (0.88–1.06) | 0.96 (0.88–1.06) | 0.97 (0.89–1.06) | 0.97 (0.89–1.07) |
| Quartile 3 | 0.94 (0.84–1.05) | 0.94 (0.84–1.05) | 0.93 (0.83–1.04) | 0.94 (0.84–1.05) |
| Quartile 4 | 0.86 (0.77–0.96) | 0.86 (0.77–0.97) | 0.86 (0.77–0.97) | 0.85 (0.76–0.95) |
| Foreign-born2 | ||||
| Quartile 1 | Ref. | Ref. | Ref. | Ref. |
| Quartile 2 | 1.01 (0.94–1.11) | 1.01 (0.91–1.11) | 1.01 (0.92–1.11) | 1.02 (0.92–1.12) |
| Quartile 3 | 1.05 (0.95–1.17) | 1.08 (0.97–1.19) | 1.08 (0.97–1.19) | 1.07 (0.97–1.19) |
| Quartile 4 | 1.01 (0.90–1.13) | 1.01 (0.90–1.14) | 1.00 (0.89–1.13) | 1.02 (0.90–1.15) |
| Residence population density | ||||
| County population ≥1,000,000 | Ref. | Ref. | Ref. | Ref. |
| County population <1,000,000 | 1.01 (0.94–1.09) | 1.02 (0.94–1.10) | 1.02 (0.94–1.09) | 1.01 (0.94–1.09) |
| Race | ||||
| White | Ref. | Ref. | Ref. | Ref. |
| Asian American | 0.82 (0.68–1.00) | 0.77 (0.61–0.97) | 0.82 (0.66–1.02) | 0.62 (0.49–0.80) |
| Univariable analysis3 (Asian American subgroup women compared with White) | Indifferent p = 0.434 |
Indifferent p = 0.267 |
Indifferent p = 0.957 |
Indifferent p = 0.072 |
Socioeconomic status (based on county-level poverty, education, income, and unemployment levels) was categorized into 1 of 4 relative quartiles within the study sample. Quartile 1 represents the lowest socioeconomic status, or most socioeconomically disadvantaged, and quartile 4 represents the highest socioeconomic status, or most socioeconomically advantaged.
Percentage of the population of the woman’s county of residence born outside the United States, categorized into 1 of 4 relative quartiles, from lowest to highest percentage.
Univariable analyses compared survival outcomes of women from each of the Asian American subgroups with white women without controlling for any other covariates.
High socioeconomic status positively affected overall survival outcomes; although overall survival outcomes did not differ among women in socioeconomic status quartiles 1, 2, or 3, women in quartile 4, who had the highest socioeconomic status, had improved overall survival outcomes (HR = 0.85, 95% CI = 0.76–0.95; Table 2). High socioeconomic status also had a positive impact on the overall survival durations of the subgroups of Asian American women (Table 3). The proportions of the county-of-residence population born outside the United States differed significantly between Asian American women (73% in the highest quartile) and white women (24% in the highest quartile; p < 0.001); however, this factor did not affect survival outcomes. The survival outcomes of women with squamous cell carcinoma and those of women with adenoma or adenocarcinoma did not differ, but compared with women who had squamous cell carcinoma, those with other types of tumors had poorer survival outcomes (HR = 1.50, 95% CI = 1.38–1.65; Table 2). The analyses comparing the survival outcomes on the basis of the histologic classification of subgroups of Asian American women with those of white women yielded similar findings.
Age at diagnosis affected the overall survival outcomes of Asian American women and white women differently. In the age-stratified analysis, heterogeneity was evident in the overall survival comparisons between the two racial groups (Table 4). Among women younger than 35 years, Asian American women were approximately twice as likely as white women to die (HR =2.42, 95% CI = 1.47–4.00). However, among women 55 years or older, Asian American women consistently had better overall survival than white women, with HRs ranging from 0.59 to 0.61. For Cox model specification, the Schoenfeld residual analysis provided evidence that the proportional hazards assumption held.
Table 4.
Results of the multivariable Cox regression models comparing overall survival outcomes between Asian American women and white women with cervical cancer across age strata.
| Hazard ratio (95% confidence interval) | |||||
|---|---|---|---|---|---|
| Characteristics | Younger than 35 years, n = 1,618 |
35–44 years, n = 2,697 |
45–54 years, n = 2,472 |
55–64 years, n = 1,599 |
65 years or older, n = 2,088 |
| Marital status | |||||
| Single | Ref. | Ref. | Ref. | Ref. | Ref. |
| Married/domestic partner | 0.94 (0.69–1.28) | 0.73 (0.60–0.89) | 0.80 (0.67–0.96) | 1.06 (0.85–1.31) | 0.98 (0.79–1.21) |
| Divorced/separated/widowed | 1.29 (0.82–2.03) | 0.74 (0.57–0.96) | 1.08 (0.89–1.31) | 1.07 (0.85–1.33) | 1.36 (1.11–1.66) |
| Stage at diagnosis | |||||
| Localized | Ref. | Ref. | Ref. | Ref. | Ref. |
| Regional | 2.46 (1.57–3.86) | 2.40 (1.84–3.15) | 2.08 (1.68–2.58) | 1.88 (1.49–2.38) | 1.65 (1.43–1.90) |
| Distant | 8.68 (5.33–14.12) | 8.52 (6.35–11.41) | 7.95 (6.28–10.06) | 5.45 (4.20–7.08) | 3.60 (3.04–4.26) |
| Histologic classification | |||||
| Squamous cell carcinoma | Ref. | Ref. | Ref. | Ref. | Ref. |
| Adenoma or adenocarcinoma | 0.95 (0.65–1.39) | 0.90 (0.70–1.16) | 1.01 (0.83–1.21) | 0.98 (0.80–1.21) | 1.28 (1.12–1.46) |
| Other | 1.41 (0.99–2.01) | 1.53 (1.22–1.92) | 1.77 (1.46–2.14) | 1.51 (1.23–1.85) | 1.44 (1.23–1.68) |
| Treatment | |||||
| No surgery | Ref. | Ref. | Ref. | Ref. | Ref. |
| Surgery | 0.15 (0.08–0.29) | 0.13 (0.08–0.20) | 0.09 (0.07–0.13) | 0.14 (0.10–0.19) | 0.15 (0.12–0.18) |
| Radiation | 1.19 (0.63–2.56) | 0.75 (0.49–1.13) | 0.33 (0.25–0.44) | 0.41 (0.32–0.52) | 0.36 (0.31–0.43) |
| Surgery and radiation | 0.60 (0.32–1.12) | 0.34 (0.23–0.52) | 0.16 (0.12–0.21) | 0.22 (0.17–0.28) | 0.18 (0.15–0.22) |
| Socioeconomic status1 | |||||
| Quartile 1 | Ref. | Ref. | Ref. | Ref. | Ref. |
| Quartile 2 | 0.99 (0.67–1.47) | 1.00 (0.78–1.26) | 0.88 (0.73–1.06) | 0.91 (0.74–1.12) | 0.99 (0.86–1.14) |
| Quartile 3 | 0.84 (0.53–1.32) | 1.06 (0.80–1.40) | 0.92 (0.73–1.16) | 0.92 (0.73–1.17) | 0.92 (0.76–1.10) |
| Quartile 4 | 1.00 (0.63–1.60) | 0.88 (0.66–1.17) | 0.78 (0.62–0.98) | 0.85 (0.65–1.10) | 0.87 (0.73–1.04) |
| Foreign-born2 | |||||
| Quartile 1 | Ref. | Ref. | Ref. | Ref. | Ref. |
| Quartile 2 | 0.62 (0.39–0.97) | 0.97 (0.75–1.25) | 1.10 (0.90–1.35) | 0.89 (0.71–1.11) | 1.19 (1.02–1.39) |
| Quartile 3 | 0.93 (0.59–1.46) | 1.05 (0.80–1.36) | 0.99 (0.80–1.23) | 1.00 (0.80–1.26) | 1.18 (1.01–1.39) |
| Quartile 4 | 0.82 (0.49–1.38) | 0.81 (0.59–1.10) | 1.12 (0.88–1.43) | 1.02 (0.78–1.33) | 1.08 (0.90–1.31) |
| Residence population density | |||||
| County population ≥1,000,000 | Ref. | Ref. | Ref. | Ref. | Ref. |
| County population <1,000,000 | 0.93 (0.66–1.31) | 0.99 (0.81–1.21) | 0.98 (0.84–1.15) | 0.96 (0.81–1.13) | 1.08 (0.96–1.22) |
| Race | |||||
| White | Ref. | Ref. | Ref. | Ref. | Ref. |
| Asian American | 2.42 (1.47–4.00) | 1.21 (0.89–1.65) | 0.92 (0.74–1.16) | 0.61 (0.46–0.81) | 0.59 (0.49–0.72) |
| Univariable analysis3 (Asian American compared with White) | Worse p < 0.001 |
Indifferent p = 0.934 |
Indifferent p = 0.281 |
Better p < 0.001 |
Better p < 0.001 |
Socioeconomic status (based on county-level poverty, education, income, and unemployment levels) was categorized into 1 of 4 relative quartiles within the study sample. Quartile 1 represents the lowest socioeconomic status, or most socioeconomically disadvantaged, and quartile 4 represents the highest socioeconomic status, or most socioeconomically advantaged.
Percentage of the population of the woman’s county of residence born outside the United States, categorized into 1 of 4 relative quartiles, from lowest to highest percentage.
Univariable analyses compared survival outcomes between the two racial groups without controlling for any other covariates.
DISCUSSION
To the best of our knowledge, our study is among the first to evaluate overall survival in Asian American cervical cancer patients at a national level. Our results showed that, after adjustment for covariates including marital status, age at diagnosis, stage at diagnosis, histologic classification, treatment type, and socioeconomic status, Asian American women as a whole had better overall survival rates than white women did. However, women of North Asian origin and white women had similar survival durations. Additionally, both Asian American women as a whole and those grouped by geographical place of origin were older than white women at the time of diagnosis.
In a study of Asian American women with cervical cancer (including those of Chinese, Filipino, Vietnamese, Korean, South Asian (Asian Indian and Pakistani) and Japanese origin) in California between 1988 and 2004, Bates and colleagues found that Asian American women had better survival outcomes than non-Hispanic white women (HRs varied between 0.57 and 0.79 compared with white women) (25). This is consistent with our findings. By contrast, another study of cervical cancer patients, also in California, showed no difference in overall survival between white women and women of Asian origin (26). Because very few studies have examined survival in Asian American women with cervical cancer, findings among studies may vary with the covariates selected.
In our study, Asian American women tended to be older than white women at cervical cancer diagnosis, which may indicate disparity in these groups’ access to healthcare services, particularly screening. Lim and colleagues also observed that ethnic minorities often live in neighborhoods with limited access to healthcare services [2]. A 2007 study showed that 81% of non-Hispanic white women, but only 70% of Asian American women, had a Papanicolaou test (15). Asian American women in our study had better socioeconomic status than white women, but socioeconomic status may not always correlate with access to preventive services. Additionally, because previous successful reductions in cervical cancer incidence and mortality have been credited to early detection of neoplasia, health promotion programs may consider tailoring their communication messages, particularly those concerning screening, to specific minority groups such as Asian Americans.
Low rates of cervical cancer screening and inadequate follow-up of abnormal testing results were found to be associated with low socioeconomic status, structural factors, and cultural or personal barriers (27). Our results indicated that Asian American women were socioeconomically better off than white women, suggesting that interventions to improve screening rates among Asian American women should focus on resolving cultural or personal barriers and structural factors. In addition to a language barrier, Asian American women often hold beliefs or values that may not support a healthy lifestyle (15). For example, they may believe that getting sick or getting cancer is a matter of luck, and consequently may procrastinate or not believe that preventive services are important (15, 27, 28). Structural barriers may include a shortage of mobile services in hard-to-reach areas. Evidence from a study in Santa Clara, California, showed that media-assisted education in combination with lay health worker communication may improve screening rates among Vietnamese American women (29).
Our findings would contribute to the research aimed at reducing health disparities in the United States. The Asian American population is one of the fastest growing populations in the nation (30). Thus, continuing health disparities in this group will greatly affect societal infrastructure and lead to substantial burdens on the system. The expansion of state and federal cancer screening programs is consistent with the Healthy People 2020 agenda, in which achieving health equity, eliminating disparities, and improving the health of all groups is one of the four overarching goals. Evidence has shown an increase of 81.2% in patient use of screening between 1991 and 2005 as a result of the national partnerships and outreach interventions under the National Breast and Cervical Cancer Early Detection Program (aimed at underserved areas and individuals) (27). This increased screening may partially explain the similarity in the overall survival rates of cervical cancer patients in the first, second, and third socioeconomic status quartiles in the present study.
Our analysis indicated that place of birth did not affect overall survival in Asian American women with cervical cancer. In 2010, most foreign-born people in the United States were from Asia (28%) or Mexico (29%) (31). Previous studies have revealed that nativity affects the survival of Hispanic women with cervical cancer, and this effect is known as the “Hispanic paradox.” Montealegre and colleagues found that, among women with late-stage cervical cancer, foreign-born Hispanic women had better survival than U.S.-born Hispanic women (32). Similarly, Gomez et al. found that, among Californian women of high socioeconomic status, those who were foreign-born had lower cervical cancer mortality rates than those who were born in the United States (33). Asian American women with cervical cancer may be subject to a similar effect, and additional research is needed to investigate this possibility. Future analysis should examine the relationship between nativity and cervical cancer-specific death. Determining patients’ place of birth at the individual level, rather than the community level, would ensure more accurate results.
We also observed that Asian American women as a whole were more likely than white women to live with their spouses or domestic partners at the time of diagnosis. Because family support plays a crucial role in improving treatment outcomes for cancer patients (9, 34), spousal relationships may contribute to improved survival among patients with cervical cancer. Further study is needed to examine this relationship.
Heterogeneous characteristics among Asian subgroups were demonstrated in both patient characteristics (demographic, socioeconomic, and clinical) and the final survival outcomes. Women of both North Asian origin and Filipino origin had similar clinical characteristics with those of white women, but women of Filipino origin had better survival outcome than white women while the survival outcomes of women of North Asian origin did not differ with those of white women. Our study demonstrated the similarity in tumor characteristics, disease stage, and marital status could explain the indifference of the survival outcomes between women of North Asian origin and white women. Other reasons could include the degree of “eastern view” of women of North Asian origin that was not examined in the present study. In a population-based study, Wang et al. defined “eastern view” as a measure of thought in “I think staying healthy is a matter of luck more than anything else”, and thought in “It is generally better to take care of your own health than to go to the doctor.” This study found that women of Japanese or Korean origin had the least “eastern view” compared with other Asian subgroups (including women of Vietnamese, Chinese, and Filipino origin) (15). Future research may examine the association between the degree of “eastern view” among North Asian women and cervical cancer survival outcomes. Additionally, women of Japanese origin in this population-based study had similar rate of having Papanicolaou tests in the past two years with white women. Consequently, increasing of cervical screening would potentially be of paramount importance for other Asian subgroups but not women of Japanese origin.
Our study had some limitations. The final sample excluded patients whose marital status was unknown. We decided to keep this variable in our model because previous studies have shown that spousal relationships influence cancer survivorship (35, 36). Also, because socioeconomic information was not available at the individual level, we utilized community-level socioeconomic data. Our model also did not account for comorbid diseases and conditions (e.g., hypertension, smoking, obesity) that have been reported to have a strong relationship with cancer survivorship (37–40). Although our study sample (extracted from SEER registry) is among the largest used to date to study cervical cancer nationwide, it did not represent the whole Asian American population in the United States. Of the 10 places with the highest populations of Asian Americans in the United States, (7) half, including New York, Chicago, and Houston, were not included in our sample. Therefore, future research should focus on these geographical areas. In addition, the results of our subgroup analyses may be at risk for being unstable because of the limited sample sizes (41). In the future, including longer follow-up times and additional patients from the SEER registry and/or using advanced statistical methods for sample matching could address this limitation. Another helpful approach might be to investigate the simultaneous effects of covariates on overall survival, as previous studies have suggested that certain covariates for overall survival (e.g., disease stage and type of treatment; race and socioeconomic status) are interdependent (9, 42).
CONCLUSIONS
We found a pronounced difference in age at diagnosis among Asian American and white women. Compared with white women, women of Filipino, Japanese or Korean had similar clinical characteristics, but women of other Asian subgroups did not. Although overall survival durations were better among Asian American (except for those of Japanese and Korean origin) than white women after controlling for demographic, clinical, and socioeconomic factors, Asian American women may have reduced access to screening programs and thus are being diagnosed at older ages. We suggest that additional study to identify ways to improve Asian American women’s access to care and screening is needed. Future studies would need to report the data by Asian subgroups to fully address the heterogeneity of this Asian American women population. The findings of our study also suggest that potential effective health promotion and behavioral interventions to improve cervical screening should focus on addressing cultural or personal barriers and structural factors.
ACKNOWLEDGMENTS
The authors thank Erica Goodoff and Joseph Munch for editorial contributions; Jinhai Huo, PhD, MD, MPH, and Jun Yu, MS, for statistical advice; and Rana Banton for administrative assistance.
Funding sources: Our study was partially funded by grants P01 CA082710 (SBC, KRD, ZDM, VTN) and R25 CA57712 (VTN) from the National Cancer Institute/National Institutes of Health and by research funding (VTN) from the Center for Health Promotion and Prevention Research at the University of Texas School of Public Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of conflicts: None.
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
CONTRIBUTORS
VTN and SBC conceptualized the research question. VTN analyzed the data and wrote the first draft of the manuscript. VTN, SBC, KRD, WC, and ZDM contributed to interpreting the results and drafting the manuscript. All authors have approved the final version of the manuscript.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Ronit E, Landrine H. Cancer Disparities: Causes and Evidence-Based Solutions. Vol. 2010. New York, USA: Springer; 2010. [Google Scholar]
- 3.Rauh-Hain JA, Clemmer JT, Bradford LS, Clark RM, Growdon WB, Goodman A, et al. Racial disparities in cervical cancer survival over time. Cancer. 2013;119(20):3644–3652. doi: 10.1002/cncr.28261. [DOI] [PubMed] [Google Scholar]
- 4.Eggleston KS, Coker AL, Williams M, Tortolero-Luna G, Martin JB, Tortolero SR. Cervical cancer survival by socioeconomic status, race/ethnicity, and place of residence in Texas, 1995–2001. J Womens Health (Larchmt) 2006;15(8):941–951. doi: 10.1089/jwh.2006.15.941. [DOI] [PubMed] [Google Scholar]
- 5.Coker AL, Eggleston KS, Du XL, Ramondetta L. Ethnic disparities in cervical cancer survival among Medicare eligible women in a multiethnic population. Int J Gynecol Cancer. 2009;19(1):13–20. doi: 10.1111/IGC.0b013e318197f343. [DOI] [PubMed] [Google Scholar]
- 6.Siegel R, Naishadham D, Jemal A. Cancer statistics for Hispanics/Latinos, 2012. CA Cancer J Clin. 2012;62(5):283–298. doi: 10.3322/caac.21153. [DOI] [PubMed] [Google Scholar]
- 7.U.S. Department of Commerce. The Asian population: 2010. [Accessed on November 11, 2014];2012 Available from: http://www.census.gov/prod/cen2010/briefs/c2010br-11.pdf.
- 8.Centers for Disease Control and Prevention. Leading causes of death by race/ethnicity, All females - United States, 2013. [Accessed on September 17, 2015];2015 Available from: http://www.cdc.gov/women/lcod/2013/WomenRace_2013.pdf.
- 9.Chen MS., Jr Cancer health disparities among Asian Americans: what we do and what we need to do. Cancer. 2005;104(12 Suppl):2895–2902. doi: 10.1002/cncr.21501. [DOI] [PubMed] [Google Scholar]
- 10.Gomez SL, Noone AM, Lichtensztajn DY, Scoppa S, Gibson JT, Liu L, et al. Cancer incidence trends among Asian American populations in the United States, 1990–2008. J Natl Cancer Inst. 2013;105(15):1096–1110. doi: 10.1093/jnci/djt157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor VM, Schwartz SM, Jackson JC, Kuniyuki A, Fischer M, Yasui Y, et al. Cervical cancer screening among Cambodian-American women. Cancer Epidemiol Biomarkers Prev. 1999;8(6):541–546. [PubMed] [Google Scholar]
- 12.Benard VB, Royalty J, Saraiya M, Rockwell T, Helsel W. The effectiveness of targeting never or rarely screened women in a national cervical cancer screening program for underserved women. Cancer Causes Control. 2015;26(5):713–719. doi: 10.1007/s10552-015-0542-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee J, Carvallo M. Socioecological perspectives on cervical cancer and cervical cancer screening among Asian American women. J Community Health. 2014;39(5):863–871. doi: 10.1007/s10900-014-9887-x. [DOI] [PubMed] [Google Scholar]
- 14.Fang CY, Ma GX, Tan Y. Overcoming Barriers to Cervical Cancer Screening Among Asian American Women. N Am J Med Sci (Boston) 2011;4(2):77–83. doi: 10.7156/v4i2p077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang JH, Sheppard VB, Schwartz MD, Liang W, Mandelblatt JS. Disparities in cervical cancer screening between Asian American and Non-Hispanic white women. Cancer Epidemiol Biomarkers Prev. 2008;17(8):1968–1973. doi: 10.1158/1055-9965.EPI-08-0078. [DOI] [PubMed] [Google Scholar]
- 16.Gomez SL, Glaser SL, Horn-Ross PL, Cheng I, Quach T, Clarke CA, et al. Cancer research in Asian American, Native Hawaiian, and Pacific Islander populations: accelerating cancer knowledge by acknowledging and leveraging heterogeneity. Cancer Epidemiol Biomarkers Prev. 2014;23(11):2202–2205. doi: 10.1158/1055-9965.EPI-14-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen AB, Chawla N, Noone AM, Srinivasan S. Disaggregated data and beyond: future queries in cancer control research. Cancer Epidemiol Biomarkers Prev. 2014;23(11):2266–2272. doi: 10.1158/1055-9965.EPI-14-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woods LM, Rachet B, Coleman MP. Origins of socio-economic inequalities in cancer survival: a review. Ann Oncol. 2006;17(1):5–19. doi: 10.1093/annonc/mdj007. [DOI] [PubMed] [Google Scholar]
- 19.Newmann SJ, Garner EO. Social inequities along the cervical cancer continuum: a structured review. Cancer Causes Control. 2005;16(1):63–70. doi: 10.1007/s10552-004-1290-y. [DOI] [PubMed] [Google Scholar]
- 20.Coker AL, Du XL, Fang S, Eggleston KS. Socioeconomic status and cervical cancer survival among older women: findings from the SEER-Medicare linked data cohorts. Gynecol Oncol. 2006;102(2):278–284. doi: 10.1016/j.ygyno.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 21.Murphy M, Goldblatt P, Thornton-Jones H, Silcocks P. Survival among women with cancer of the uterine cervix: influence of marital status and social class. J Epidemiol Community Health. 1990;44(4):293–296. doi: 10.1136/jech.44.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robert SA, Strombom I, Trentham-Dietz A, Hampton JM, McElroy JA, Newcomb PA, et al. Socioeconomic risk factors for breast cancer: distinguishing individual- and community-level effects. Epidemiology. 2004;15(4):442–450. doi: 10.1097/01.ede.0000129512.61698.03. [DOI] [PubMed] [Google Scholar]
- 23.National Cancer Institute. Surveillance, Epidemiology and End Results. [Accessed on March 04, 2014];2013 Available from: www.seer.cancer.gov.
- 24.Lairson DR, Parikh RC, Cormier JN, Du XL. Cost-utility analysis of platinum-based chemotherapy versus taxane and other regimens for ovarian cancer. Value Health. 2014;17(1):34–42. doi: 10.1016/j.jval.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Bates JH, Hofer BM, Parikh-Patel A. Cervical cancer incidence, mortality, and survival among Asian subgroups in California, 1990–2004. Cancer. 2008;113(10 Suppl):2955–2963. doi: 10.1002/cncr.23752. [DOI] [PubMed] [Google Scholar]
- 26.Lim JW, Ashing-Giwa KT. Examining the effect of minority status and neighborhood characteristics on cervical cancer survival outcomes. Gynecol Oncol. 2011;121(1):87–93. doi: 10.1016/j.ygyno.2010.11.041. [DOI] [PubMed] [Google Scholar]
- 27.Koh HK. Toward the elimination of cancer disparities. New York, USA: Springer; 2009. [Google Scholar]
- 28.Grewal K, Stewart DE, Grace SL. Differences in social support and illness perceptions among South Asian and Caucasian patients with coronary artery disease. Heart Lung. 2010;39(3):180–187. doi: 10.1016/j.hrtlng.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 29.Mock J, McPhee SJ, Nguyen T, Wong C, Doan H, Lai KQ, et al. Effective lay health worker outreach and media-based education for promoting cervical cancer screening among Vietnamese American women. Am J Public Health. 2007;97(9):1693–1700. doi: 10.2105/AJPH.2006.086470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ortman JM, Guarneri CE. United States population projections: 2000 to 2050. [Accessed on 10/17/2014];2009 Available from: http://www.census.gov/population/projections/files/analytical-document09.pdf.
- 31.U.S. Census Bureau. The Foreign-Born Population in the United States: 2010. 2012 [Google Scholar]
- 32.Montealegre JR, Zhou R, Amirian ES, Follen M, Scheurer ME. Nativity disparities in late-stage diagnosis and cause-specific survival among Hispanic women with invasive cervical cancer: an analysis of Surveillance, Epidemiology, and End Results data. Cancer Causes Control. 2013;24(11):1985–1994. doi: 10.1007/s10552-013-0274-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomez N, Guendelman S, Harley KG, Gomez SL. Nativity and neighborhood characteristics and cervical cancer stage at diagnosis and survival outcomes among Hispanic women in california. Am J Public Health. 2015;105(3):538–545. doi: 10.2105/AJPH.2014.302261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmadi A, Hashemi Nazari SS, Mobasheri M. Does ethnicity affect survival following colorectal cancer? A prospective, cohort study using Iranian cancer registry. Med J Islam Repub Iran. 2014;28:83. [PMC free article] [PubMed] [Google Scholar]
- 35.Morgan MA, Small BJ, Donovan KA, Overcash J, McMillan S. Cancer patients with pain: the spouse/partner relationship and quality of life. Cancer Nurs. 2011;34(1):13–23. doi: 10.1097/NCC.0b013e3181efed43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldzweig G, Andritsch E, Hubert A, Walach N, Perry S, Brenner B, et al. How relevant is marital status and gender variables in coping with colorectal cancer? A sample of middle-aged and older cancer survivors. Psychooncology. 2009;18(8):866–874. doi: 10.1002/pon.1499. [DOI] [PubMed] [Google Scholar]
- 37.Seamon LG, Tarrant RL, Fleming ST, Vanderpool RC, Pachtman S, Podzielinski I, et al. Cervical cancer survival for patients referred to a tertiary care center in Kentucky. Gynecol Oncol. 2011;123(3):565–570. doi: 10.1016/j.ygyno.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Waggoner SE, Darcy KM, Fuhrman B, Parham G, Lucci J, 3rd, Monk BJ, et al. Association between cigarette smoking and prognosis in locally advanced cervical carcinoma treated with chemoradiation: a Gynecologic Oncology Group study. Gynecol Oncol. 2006;103(3):853–858. doi: 10.1016/j.ygyno.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 39.Koo KC, Yoon YE, Rha KH, Chung BH, Yang SC, Hong SJ. Low body mass index is associated with adverse oncological outcomes following radical prostatectomy in Korean prostate cancer patients. Int Urol Nephrol. 2014;46(10):1935–1940. doi: 10.1007/s11255-014-0729-7. [DOI] [PubMed] [Google Scholar]
- 40.Frumovitz M, Jhingran A, Soliman PT, Klopp AH, Schmeler KM, Eifel PJ. Morbid obesity as an independent risk factor for disease-specific mortality in women with cervical cancer. Obstet Gynecol. 2014;124(6):1098–1104. doi: 10.1097/AOG.0000000000000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holland AT, Palaniappan LP. Problems with the collection and interpretation of Asian-American health data: omission, aggregation, and extrapolation. Ann Epidemiol. 2012;22(6):397–405. doi: 10.1016/j.annepidem.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Franzini L, Williams AF, Franklin J, Singletary SE, Theriault RL. Effects of race and socioeconomic status on survival of 1,332 black, Hispanic, and white women with breast cancer. Ann Surg Oncol. 1997;4(2):111–118. doi: 10.1007/BF02303792. [DOI] [PubMed] [Google Scholar]