Abstract
Background
This study sought to systematically investigate the derivation of late outgrowth endothelial progenitor cells (late EPC) and mesenchymal stem cells (MSC) from umbilical cord blood (UCB) and to examine their therapeutic effects on myocardial infarction (MI).
Methods
The expression of angiogenic genes were determined by qRT-PCR. Myocardial infarction (MI) was induced in rats, and cells were directly transplanted into the border regions of ischemic heart tissue.
Results
Culture of UCB mononuclear cells yielded two distinct types of cells by morphology after 2 weeks in the same culture conditions. These cells were identified as late EPC and MSC, and each was intramyocardially injected into rat hearts after induction of MI. Echocardiography and histologic analyses demonstrated that both EPC and MSC improved cardiac function and enhanced vascularization, although fibrosis was reduced only in the EPC transplanted hearts. Different paracrine factors were enriched in EPC and MSC. However, once injected into the hearts, they induced similar types of paracrine factors in the heart. Transplanted EPC or MSC were mostly localized at the perivascular areas. This study demonstrated that EPC and MSC can be simultaneously derived from UCB under the same initial culture conditions, and that common paracrine factors are involved in the repair of MI.
Conclusion
Late EPC and MSC are effective for infarct repair, apparently mediated through common humoral mechanisms.
Keywords: umbilical cord blood, late outgrowing endothelial progenitor cells, mesenchymal stem cells, acute myocardial infarction, angiogenesis
Introduction
During the past decade, adult bone marrow (BM) was the major source of stem or progenitor cells such as hematopoietic stem cells (HSC), endothelial progenitor cells (EPC), and mesenchymal stem cells (MSC) for cell-based therapy. However, the aspiration of BM is an invasive procedure associated with risks such as infection, bleeding, or pain. Compared to BM, UCB has various benefits for clinical application due to the lack of viral contamination and easy access without invasive procedures or long-term storage [1]. Therefore, UCB has emerged as an attractive and alternative source for stem or progenitor cells for clinical application [2–4].
To date, at least two distinctive types of cultured EPC have been reported: early and late outgrowth EPC. Early EPC are typically derived from culture of mononuclear cells (MNC) for less than a week and are characterized by a low proliferation rate [5–8]. Late outgrowth EPC, also called outgrowth endothelial cells, late EPC, or endothelial colony forming cells, emerge as colonies after culture of MNC for 2 weeks or more and are characterized by cobblestone morphology and high proliferative potential [9–11]. These two types of EPC express different sets of cell surface markers. Early EPC express the pan-leukocyte marker CD45 and monocytic/macrophage markers CD11b/CD14. Late EPC express most of the endothelial cell markers but not hematopoietic cell markers. MSC are another representative adult stem cell which are frequently derived from BM and possess multipotency and paracrine effects [12, 13]. Over the past decade, BM-derived early EPC or MSC were shown to improve cardiovascular ischemia or infarct repair [14–19]. However, most studies with EPC or MSC derived from UCB remain at the level of in vitro cell characterization [20–26]. While several studies reported therapeutic effects of UCB-derived early EPC on myocardial infarction (MI), no studies have investigated the therapeutic potential of UCB-derived late EPC or MSC. Only a few studies are available which explored in vitro cardiomyogenic differentiation potential of UCB-derived MSC [27].
Accordingly, the present study sought to characterize UCB-derived late EPCs and -MSCs and determine their therapeutic effects in myocardial infarction and the underlying mechanisms.
Materials and Methods
Derivation and characterization of EPC and MSC
UCB was collected after receiving informed consent from the birthing mother using the guidelines approved by institutional review boards of Yonsei University. MNC were collected according to the previously published method [28]. Briefly, MNC were obtained by Ficoll-Hypaque (Amersham Biosciences, Sunnyvale, CA) density gradient centrifugation. The MNC were harvested from the interface, washed two to three times, plated on cell culture dishes coated with 0.01% human fibronectin (Sigma, St. Louis, MO) at a density of 0.5 to 1×106 cells/cm2, and cultured in endothelial growth medium-2 (EGM-2, Cambrex, Walkersville, MD). Non-adherent cells were removed during medium changes after 72 hours and the adherent cells were fed twice a week with fresh culture medium. Cell expansion culture was performed with EGM-2 for another three months.
Flow cytometry
Flow cytometry analyses of EPC and MSC were performed at passage 5 of culture as we previously described [29]. For flow cytometry analyses, we used FITC- or PE-conjugated Abs against CD29, CD31, CD34, CD44, CD45, CD73, CD90, CD105, CD144, CD146, vWF, HLA-DR (all from BD), KDR and Tie-2 (from R&D systems). The fluorescence intensities of the cells were evaluated by an FC 500 flow cytometer (BD) and the data were analyzed with FlowJo 7.
Tube formation assay
To investigate the tube formation potential, EPC and MSC were seeded onto Matrigel-coated dishes (BD) and cultured in complete EBM-2 medium at a concentration of 1 × 104 cells/well. After 24 hours of incubation, endothelial network formation was examined and representative fields were photographed under fluorescence microscopy.
Induction of myocardial infarction and cell transplantation
Male Sprague-Dawley rats (LabAnimal. Korea) and nude rats (Charles River Laboratories, Wilmington, MA) aged 8 weeks were used. All animal protocols were approved by the Institutional Animal Care and Use Committees at the Yonsei University and Emory University. For physiologic and functional studies we used Sprague-Dawley rats and for mechanistic studies we switched to nude rats to better elucidate mechanisms. Rats were anesthetized with Zoletil 50 (100mg/kg, Virbac), then intubated and ventilated using a small animal ventilation apparatus (Harvard apparatus, MA). MI was induced by ligation of the left anterior descending coronary artery as described previously [28]. One hour after MI, the rats received 5 × 106 EPC or MSC suspended in 50 µl of PBS intramyocardially. The same volume of PBS without cells was injected as a control. The cells or PBS were injected with a 31-gage needle attached to an insulin syringe.
Echocardiography
Echocardiography was performed 4 weeks after cell transplantation. The rats were anesthetized with inhalation of isoflurane. The echocardiographic images were obtained using Vivid 7 echocardiographic machine with 12 MHz phased array transducer (GE Medical Systems, Milwaukee, WI)[30]. Left ventricular (LV) M-mode and two dimensional imaging at the papillary muscle level were obtained by parasternal short axis view. The end-systolic inter-ventricular septum (IVSs), end-diastolic inter ventricular septum (IVSd), LV end-systolic dimension (LVESD), LV end-systolic posterior wall (LVPWs), and LV end-diastolic posterior wall (LVPWd) were measured on M-mode echocardiograms. These parameters allowed calculation of the LV fractional shortening (FS %) using the following equation: FS % = [(LVEDD−LVESD)/LVEDD] × 100%. The LV ejection fraction (EF %) was measured by a modified Quinone’s method. All measurements were averaged for three consecutive cardiac cycles and all the parameters were analyzed by an observer blinded to the experiment.
Histological analysis
Rats were euthanized 2 or 4 weeks after cell transplantation. Hearts were harvested and fixed in 4% paraformaldehyde and incubated overnight in 15% sucrose solution. The tissues were embedded in OCT compound (Sakura Finetek), snap-frozen in liquid nitrogen, and sectioned at 10 – 20 µm thickness as described previously by our laboratory [28]. For capillary density measurement, four frozen sections of ischemic tissues were stained with primary biotinylated isolectin B4 (ILB4) (1:250, Vector Laboratories, Inc., Burlingame, CA) and secondary streptavidin Alexafluor 488 (1:400, Invitrogen). Five fields from four tissue sections were randomly selected, and the number of capillaries was counted in each field. Photographs were taken using fluorescent inverted microscopy or confocal microscopy. To evaluate apoptosis, TdT-mediated dUTP nick-end labeling (TUNEL) reaction was performed using a fluorescein in situ cell death detection kit (Roche-Molecular). To investigate proliferative cells, immunostaining with anti-Ki-67 antibody (Novocastra Laboratories) was performed. Details of the above procedures were described in our prior publications [28, 31].
Real-time RT-PCR (qRT-PCR)
qRT-PCR assay was performed as described previously [32]. Peri-infarct myocardial tissues were harvested and pulverized to extract RNA or protein. Total RNA was extracted using RNA-Stat (Iso-Tex Diagnostics) according to the manufacturer’s instructions. cDNA was synthesized from the extracted RNA (500 ng) with Taqman Reverse Transcription Reagents (Applied Biosystems) and subjected to real-time polymerase chain reaction using human and rat-specific primers (Supplemental Table 1). qPCR was performed on a 7500 Fast Real-Time PCR system (Applied Biosystems) using gene expression master mix and the TaqMan method (Applied Biosystems). Relative mRNA expression of target gene normalized to GAPDH was calculated as previously described [29]. Relative RNA expression was determined using the formula Rel Exp = 2−ΔCT (fold difference), where ΔCt = (Ct of target genes) − (Ct of endogenous control gene, GAPDH) in experimental samples [29, 32].
Statistical analysis
All data were presented as mean ± SD. Statistical analyses were performed with Student’s t-test for comparisons between two groups, and ANOVA followed by Bonferroni’s correction for more than two groups using SPSS version 11.0. P < 0.05 is considered statistical significance.
Results
Culture derivations of late EPCs and MSCs
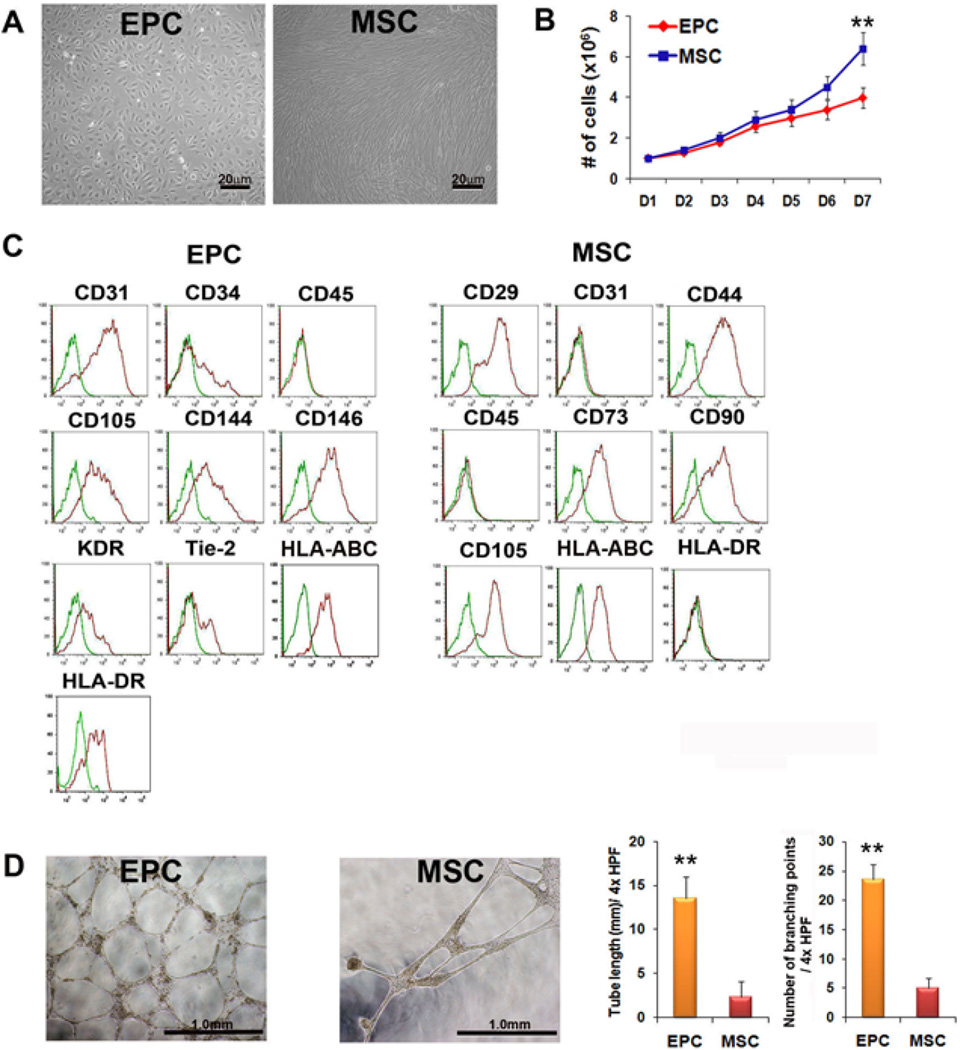
After culturing UCB-derived MNC for 14–21 days under conventional EPC culture conditions, two types of dominant cell populations emerged in different dishes: cobblestone-like cells (identified as EPC) and spindle-shaped cells (identified as MSC) (Figure 1A). Due to unknown reasons, one type of cell usually dominates in one dish within several weeks. Cells of each type were further cultured and the distinction became clearer. Spindle-shaped cells or MSC were continuously cultured without significant signs of senescence or morphologic changes for more than 21 passages (over 3 months) and were expanded more than 1000-fold. EPC continued to proliferate for 4 weeks, albeit showing less proliferative potential. The mean doubling time of EPC and MSC were approximately 34 and 26 hours, respectively (Figure 1B). Twenty six units out of 48 units of cord blood (66%) generated EPC and 13 units out of 37 units (35%) generated MSC during cultivation.
Figure 1. Cell Biological characteristics and tube formation potential of EPC and MSC.
(A) Morphology of UCB-derived late outgrowth EPC and MSC at passage 5. (B) Growth curves of EPC and MSC at passage 5. Cells were plated at 1 × 104/cm2 in T-25 tissue flask. (C) Immunophenotypic characterization of EPC and MSC by flow cytometry analysis. (D) Tube length and the number of branching points after Matrigel tube formation assay. **P < 0.01.
Characterization of late EPC and MSC
Flow cytometry was used to characterize the two cell types. Cobblestone cells expressed CD31 (PECAM-1), CD34, CD105 (Endoglin), CD144 (VE-Cadherin), CD146 (MCAM), KDR (VEGFR-2), TIE-2, HLA-ABC, and HLA-DR, and were negative for CD45 (leukocyte common antigen), suggesting a late EPC phenotype (Figure 1C). The spindle-shaped cells expressed CD29 (Integrin-β1), CD44 (hyaluronate receptor), CD73 (NT5E), CD90 (Thy-1), and HLA-ABC, but not CD31, CD45 or HLA-DR, showing a conventional MSC phenotype. To investigate the in vitro vessel-forming capabilities, we conducted tube formation assays on Matrigel. The results showed that both EPC and MSC formed tubular structures and EPC showed more branching points (5.8 fold) and larger tube lengths (4.7 fold) compared to MSC (Figure 1D).
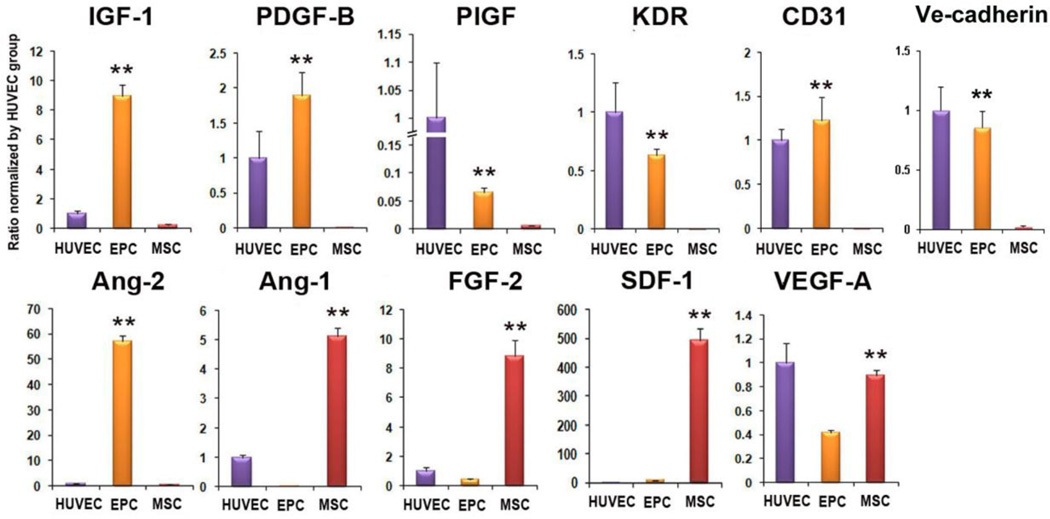
Increased expression of angiogenic and cardiac protective factors
We determined mRNA expression of angiogenic and cardiac protective factors in late EPC and MSC. qRT-PCR analyses demonstrated that expression levels of Ang-1, FGF-2, SDF1α, and VEGF-A were significantly higher in the MSC compared to the EPC (Figure 2). However, the anti-apoptotic and cardiomyogenic factor, IGF-1, and an arteriogenic factor, PDGF-B, were significantly higher in the EPC compared to MSC. In addition, levels of PlGF, KDR, CD31, VE-CADHERIN and ANG-2 were higher in EPC compared to MSC. Collectively, these findings suggest that both EPC and MSC are enriched with factors that can benefit vascularization and cardiac protection and that the two types of cells present different types of paracrine factors.
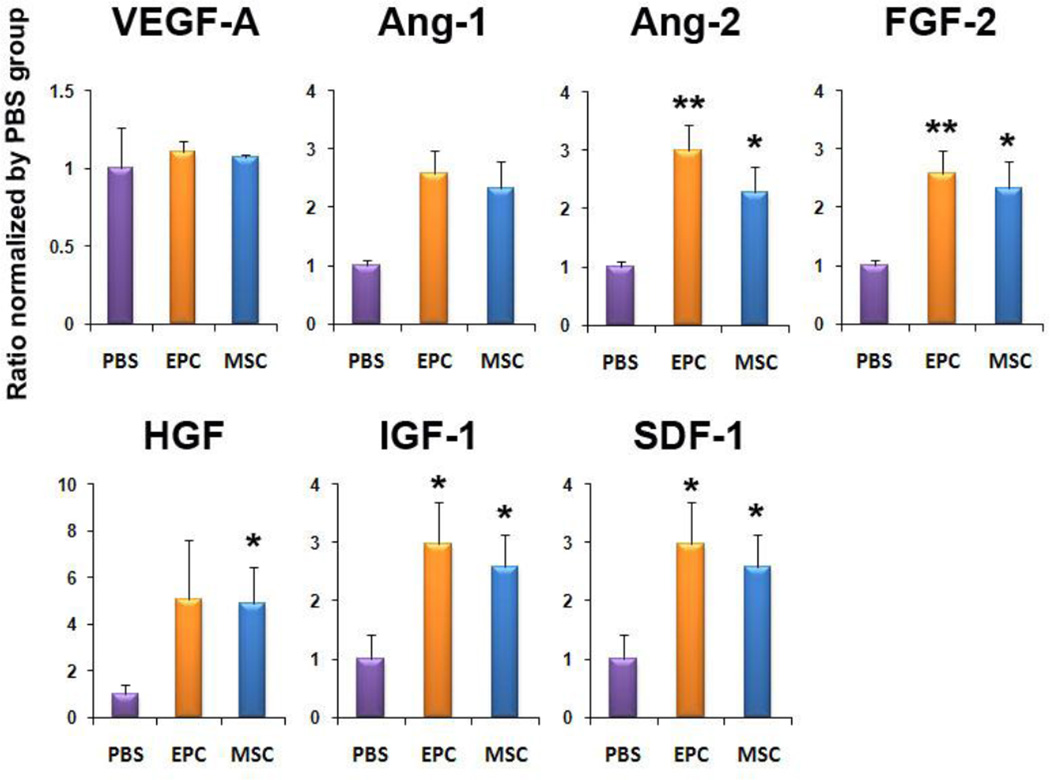
Figure 2. Increased expression of multiple humoral factors in EPC- or MSC-transplanted hearts.
qRT-PCR was performed to measure the level of gene expression in EPC and MSC. Individual values were normalized to GAPDH. (n = 3 per group). Expression of each individual gene was normalized to that of HUVEC. *P < 0.05, **P < 0.01 vs. HUVEC.
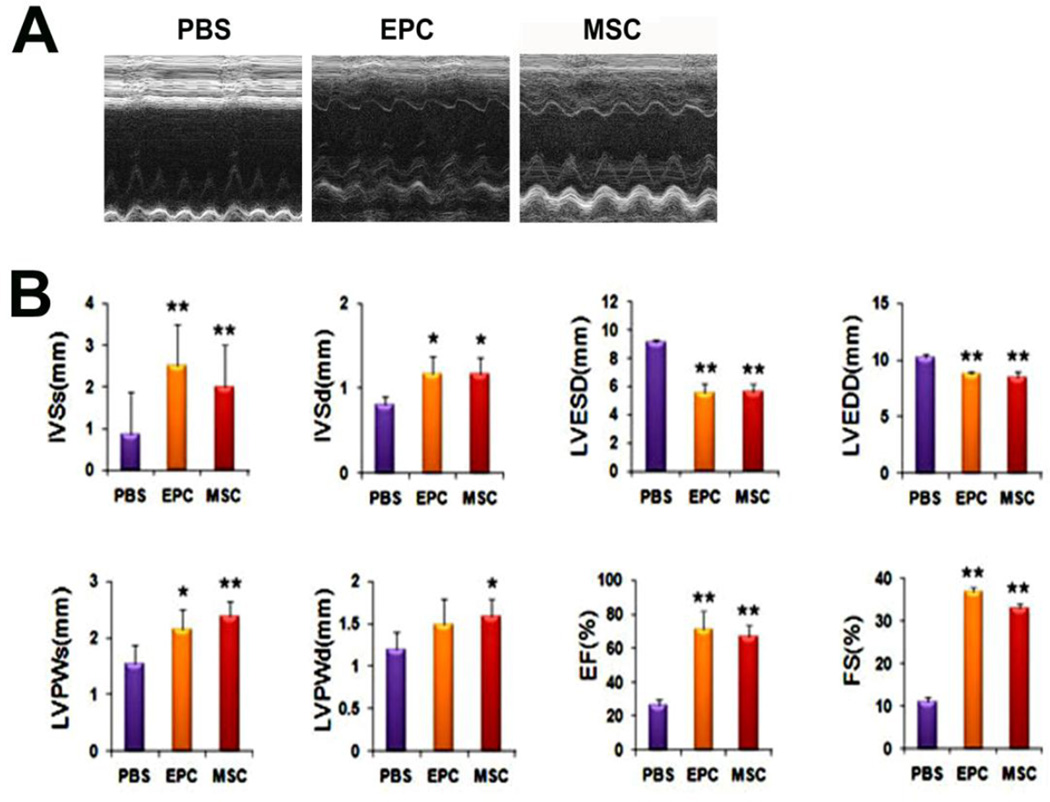
Improvement of cardiac function after EPC or MSC transplantation
We determined the therapeutic effects of direct myocardial transplantation of EPC and MSC in a rat MI model. Echocardiography demonstrated that 4 weeks after cell transplantation, the IVSs, IVSd, LVPWs, LVPWd, EF and FS were significantly greater in the EPC and MSC-transplanted groups compared to the PBS-injected group (Figure 3). The global cardiac function indices EF and FS were similar between the EPC- and MSC-transplanted groups. The LVESD and LVEDD were lower in the EPC- and MSC-groups than in the PBS group suggesting favorable cardiac remodeling (P < 0.01). These data suggest that despite minor differences, UCB-derived late EPC or MSC transplantation improves global cardiac function and induces favorable remodeling to a similar extent.
Figure 3. Enhanced cardiac function and favorable cardiac dimensions demonstrated by echocardiography.
(A) Representative M-mode echocardiograms. (B) Analysis of echocardiographic parameters. IVSs: thickness of interventricular septum at systole. IVSd: thickness of interventricular septum at diastole. LVESD: left ventricular end systolic dimension. LVEDD: left ventricular end diastolic dimension. LVPWs: thickness of left ventricular posterior wall at systole. LVPWd: thickness of left ventricular posterior wall at diastole. EF: ejection fraction. FS(%): percent fractional shortening. Each group was compared to the PBS group. (n = 5 per group). *P < 0.05, **P < 0.01 vs. PBS.
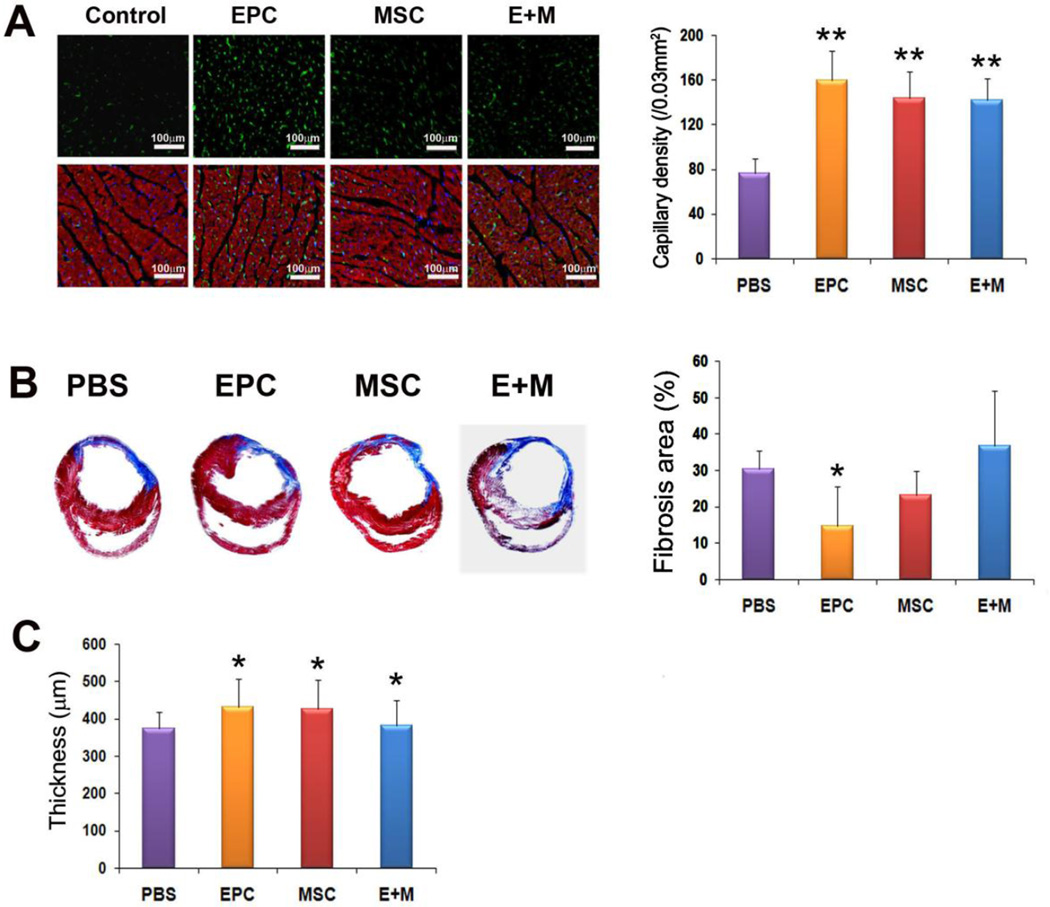
Favorable effects of EPC or MSC transplantation on cardiac fibrosis and vascularization
Four weeks after cell transplantation, rats were sacrificed and hearts were harvested, fixed and prepared for histologic examination. Masson’s trichrome staining demonstrated that circumferential fibrosis area was significantly smaller in the EPC group, but not MSC group, compared to the PBS group (Figure 4A). The infarct wall thickness was greater in the EPC- and MSC groups than in the PBS group (430.3 ± 76.9, 426.6 ± 77.9 vs. 374.6 ± 42.6) (P < 0.05) (Figure 4B). Capillary density counted after ILB4 staining was significantly higher in the EPC- and MSC groups compared to the PBS group (160.2 ± 26.3, 144.1 ± 24.1 vs. 76.7 ± 13.3) (P < 0.01) (Figure 4C).
Figure 4. Reduced fibrosis area and increased wall thickness and capillary density in the heart tissues transplanted with EPC or MSC.
(A) EPC transplantation significantly reduced percent circumferential fibrosis (n = 5 per group). (B) Wall thickness was greater in the EPC or MSC-transplanted hearts compared to PBS-injected hearts (n = 5 per group). (C) Representative examples of ILB4 staining in ischemic heart. The number of capillaries was higher in EPC- and MSC groups compared to the PBS group. (n = 5 per group). α-sarcomeric actinin (red); DAPI (blue). Bars: 100m. *P < 0.05, **P < 0.01 vs. PBS.
Cell transplantation increases paracrine factors in ischemic hearts
To determine the levels of paracrine factor expression in the ischemic hearts after cell transplantation, rats were sacrificed and heart tissues were collected from peri-infarct zones at day 7. qRT-PCR analyses demonstrated that the mRNA levels of Ang-2, FGF-2, IGF-1 and SDF-1α were significantly increased in both EPC- and MSC-transplanted groups compared to the PBS-injected group (Figure 5). These data suggest that EPC and MSC transplantation upregulates multiple paracrine factors associated with angiogenesis and cell survival. Intriguingly, the types of increased genes were similar between EPC and MSC-transplanted hearts.
Figure 5. Multiple angiogenic factors are up-regulated after EPC- or MSC transplantation.
qRT-PCR was performed to measure the level of mRNA expression from the heart tissues 7 days after cell transplantation. Various angiogenic, cardioprotective and chemoattractant cytokines were up-regulated in the EPC- or MSC transplanted hearts compared to the PBS-injected hearts. Individual values were normalized to GAPDH. Data are presented as fold difference compared to the PBS group (n = 3 per group). *P < 0.05, **P < 0.01 vs. PBS.
EPC and MSC transplantation decreased apoptosis of myocardial cells
Since biological factors associated with anti-apoptosis and cell survival such as IGF-1, FGF-2, and Ang-2 were increased after cell transplantation, we determined the effects of cell transplantation on myocardial apoptosis and proliferation. We performed TUNEL assay with tissue sections collected from the peri-infarct zones at day 7 (Supplemental Figure 1). The number of TUNEL positive nuclei was significantly lower in the EPC and MSC groups than in the control group (EPC, MSC vs. PBS, 18.3 ± 2.0, 20.3 ± 2.6 vs. 33.3 ± 4.0, P < 0.01). In addition, we also found that the number of Ki-67 positive cells was significantly higher in the EPC and MSC groups than in the control group (EPC, MSC vs. PBS, 31 ± 3.4, 34 ± 4.7 vs. 10 ± 2.6, P < 0.01).
Low transdifferentiation potential of EPC and MSC into ECs
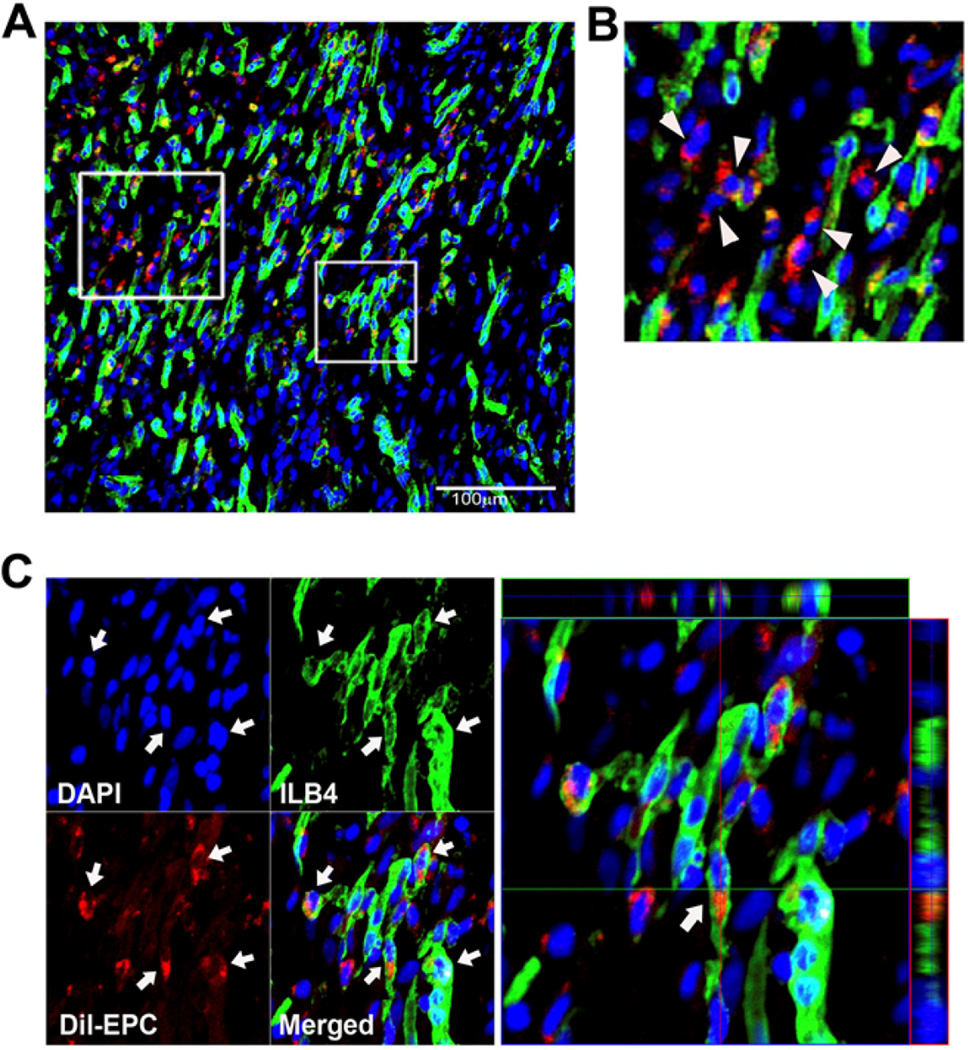
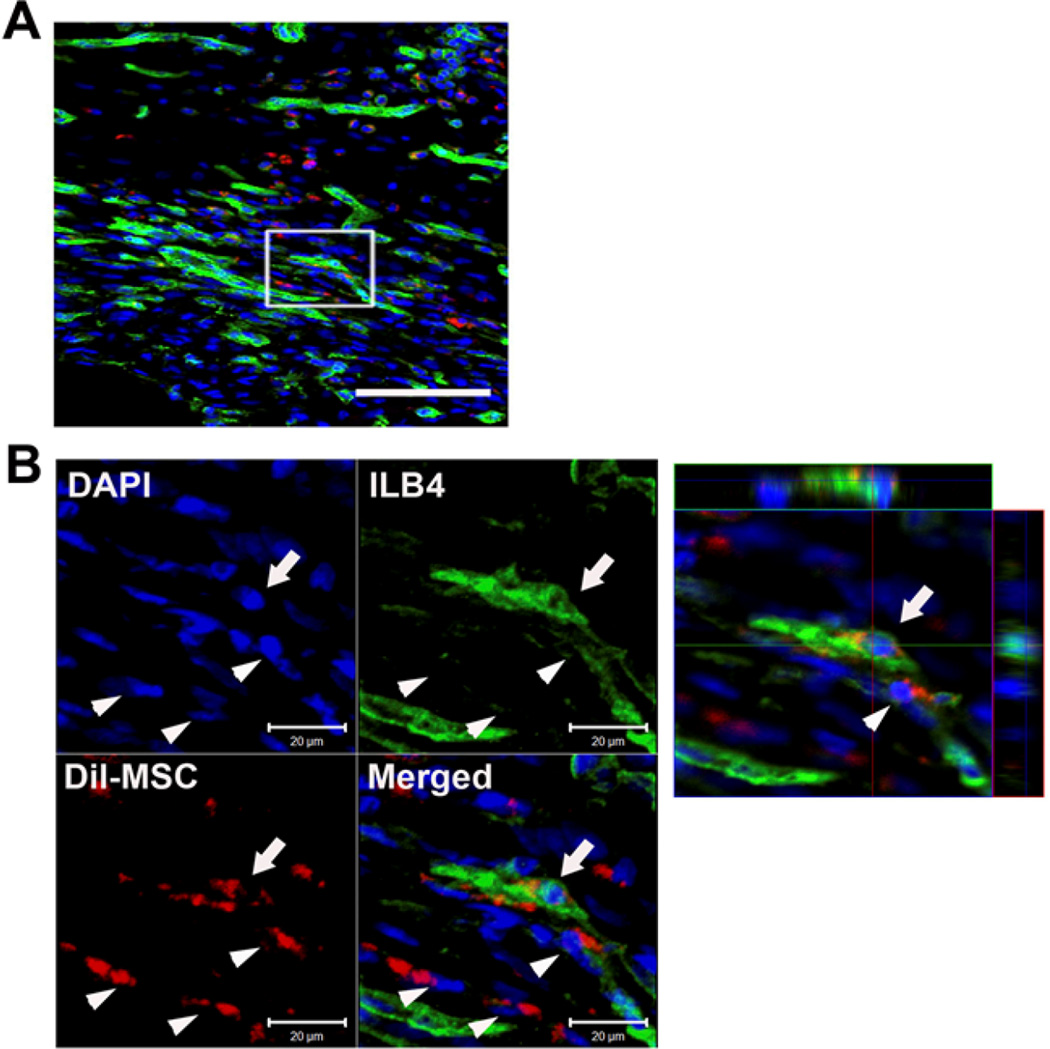
We next explored the transdifferentiation of EPC and MSC into ECs in vivo. Two weeks after myocardial transplantation of EPC or MSC which were pre-labeled with a red fluorescent dye, CM-Dil, hearts were systemically perfused with ILB4 and the heart tissues were collected. Confocal microscopic examination of peri-infarct and infarct tissues demonstrated that a large number of transplanted DiI-labeled cells were localized in the pericytic or perivascular areas in both EPC and MSC-transplanted cardiac tissues (Figure 6 and 7). Three-dimensional reconstruction followed by analyses of orthogonal images showed that a small fraction of implanted EPC was colocalized with ILB4-stained ECs (Figure 6). In MSC-transplanted heart tissues, most of the MSC were localized at the perivascular areas and only very rarely were incorporated into vascular-like structures expressing ILB4 (Figure 7). Collectively, these findings suggest that UCB-derived EPC or -MSC have low to minimal transdifferentiation effects and functions mainly through paracrine actions and vascular stabilization at the perivascular area.
Figure 6. Engraftment and colocalization of transplanted EPC with vessels in the ischemic hearts.
We directly transplanted Dil-labeled EPC into the ischemic heart. Shown are histological sections of hearts harvested at 2 weeks after cell transplantation. (A) A low magnification image. Scale bar 100 µm. (B) Most of the Injected EPCs were localized in the pericytic and perivascular areas (arrow heads) but not exactly co-localized with capillary or other vascular structures. (C) A small portion of EPCs were colocalized with the ILB4-positive capillary-like vessels (arrows). These were confirmed in the orthogonal sections in the three-dimensional reconstructed images. Green, ILB4, Red, Dil-labeled EPCs, Blue, DAPI.
Figure 7. Engraftment and colocalization of transplanted MSC with vessels in the ischemic hearts.
We directly transplanted Dil-labeled MSC into the ischemic heart of rats. Shown are histological sections of heart harvested at 2 weeks after cell transplantation. (A) Low magnification image. Scale bar 100 µm. (B) A majority of MSCs were localized in the pericytic areas (arrow heads). Rarely, we found co-localization of injected MSC with the capillary-like vessels as ECs (arrow) confirmed in the orthogonal sections in the three-dimensional reconstructed images. Green, ILB4; Red, Dil-labeled MSC; Blue, DAPI.
Discussion
The present study demonstrated, based on morphologic differences, that late outgrowth EPC and MSC can be derived from UCB via conventional EPC culture conditions. Transplantation of each of these cell types into ischemic myocardium induced effective cardiac repair with EPC being slightly superior in therapeutic potency. This is also the first study to report the therapeutic effects of both UCB-derived late EPC and MSC and to compare their effects side-by-side. Several therapeutic mechanisms were newly demonstrated. First, UCB-derived late EPC or -MSC upregulated various angiogenic, anti-apoptotic and cardioprotective factors in the injured hearts during the critical period of infarct repair. The late outgrowth EPC, conventionally known for their robust vasculogenic effects in fact were found to depend more on paracrine actions than direct vasculogenic effects in infarct repair. Second, a majority of EPC and MSC were closely localized to the vessels in the perivascular areas. Although EPC and MSC showed transdifferentiation or direct vasculogenic potential into ECs, it was low in EPC and negligible in MSC. These findings suggest that the neovascularization via UCB-EPC or –MSC occurred through their angiogenic activities and vessel-stabilizing effects.
Cell therapy with BM or peripheral blood-derived cells has shown favorable yet modest effects on cardiac repair in patients with ischemic heart disease [33]. Many studies have reported that paracrine or humoral effects are a major therapeutic mechanism of these EPC or MSC in cardiovascular regeneration [5, 13, 32, 34]. In the present study, we found that similar mechanisms underlie the therapeutic effects of cord blood-derived late outgrowth EPC and MSC.
Several prior studies have demonstrated that late outgrowth EPC derived from peripheral blood induce neovascularization mainly through direct transdifferentiation into ECs (or vasculogenesis) [10, 35, 36]. However, our confocal examination demonstrated that such transdifferentiation can occur, but to a lesser degree than can explain increased vasculature and therapeutic effects. This is arguably the first report that late outgrowth EPC have weak vasculogenic or endothelial transdifferentiation effects in the ischemic tissues. Rather, we found that UCB-derived late outgrowth EPC are enriched with factors associated with vessel formation and tissue protection such as Ang-2, IGF-1, and PDGF-B. Of interest, representative angiogenic factors such as VEGF-A, FGF-2 and Ang-1 and a chemoattractant factor, SDF-1 were more highly expressed in MSC compared to EPC. Ang-2 is conventionally known for its vascular destabilizing effects while Ang-1 is associated with vessel stabilization [37]. However, recent publications suggested that Ang-2 plays a crucial role in ischemia- and hypoxia-induced vascular remodeling and arteriogenesis [38, 39]. Increased Ang-2/Ang-1 ratio promotes blood vessel sprouting and potentiates EC’s responsiveness to VEGF [40, 41]. Another study reported that VEGF and excess Ang-2 lead to the formation of a greater number of small vessels [42]. PDGF signaling plays an important role in cardiovascular homeostasis. Deletion of PDGF-B and its receptor results in cardiac abnormalities and endothelial-specific ablation of PDGF-B leads to myocardial abnormalities due to vasculogenic defects such as pericyte loss [43, 44]. PDGF-B overexpression was shown to protect myocardium after infarction [45]. IGF-1, a well-known anti-apoptotic factor, attenuates myocardial fibrosis and interferes with necrosis during ischemia injury [46]. IGF-1 increases membrane stability of cells by Bcl-2 expression, releasing nitric oxide which prevents cardiomyocyte apoptosis [47, 48].
While there were certain differences in the types of paracrine factors between EPC and MSC in vitro, no significant differences were noted in the types of increased paracrine factors in the heart tissues after cell transplantation. Among those increased factors, SDF-1 is known for its effects on protection of myocardium by mobilizing BM cells, and IGF-1 and HGF play an important role in preventing adverse remodeling, as these factors protect cardiomyocytes from apoptosis and induce proliferation of resident cardiac stem cells for neovascularization and cardiomyogenesis [49, 50]. Recently, we demonstrated that initial up-regulation of paracrine factors is attributed to both transplanted cells and host cells and sustenance of such up-regulation after several days is almost solely due to host myocardial cells in infarcted myocardium transplanted with BM-derived early EPC [32]. The current results support this finding by showing no difference in the upregulated factors at one week regardless of the types of transplanted cells, due to the dominant contribution of host myocardium-derived humoral factors. Our prior paper in which BM-derived early EPC were similarly transplanted to mouse MI also demonstrated that SDF-1, IGF-1 and HGF are the most robustly increased factors in the first two weeks, the most critical period for infarct repair and remodeling. Together these results suggest that similar paracrine factors are involved in the injured heart following adult stem or progenitor cell transplantation for cardiac repair.
In earlier studies, transdifferentiation of BM-derived early EPC or MSC into endothelial cells or cardiomyocytes was reported as the major therapeutic mechanism; however, later studies found that such potential is minimal and the extent and durability of transdifferentiation were not sufficient to explain the therapeutic effects in cardiovascular animal models [5, 13, 28]. On the other hand, studies have demonstrated that late outgrowth EPC derived from human circulation are closer to genuine EC than early EPC, and have higher endothelial generation potential in vivo [51]. These robust vasculogenic capabilities of late EPCs were highlighted as advantages for cardiovascular cell therapy [10, 35, 36, 51]. Our current study, however, demonstrated that the fate of injected UCB-derived late EPC in ischemic hearts is similar to other BM-derived cells including early EPC [14, 52–56]. Extensive confocal microscopic examination revealed less than 1% transdifferentiation into endothelial cells in UCB-EPC and –MSC transplanted cardiac tissues. Thus, these results suggest that late outgrowth EPC do not have high endothelial transdifferentiation potential, at least in the setting of MI, and function through paracrine effects for cardiac repair similar to other adult stem or progenitor cells. Cardiomyocyte transdifferentiation was not found. As reported with BM-derived EPC or MSC, most of the engrafted UCB-EPC and -MSC were located at pericytic or perivascular areas, suggesting the vessel stabilizing effects of these cells [29, 32, 57, 58],.
Together, our data indicate that intramyocardial implantation of UCB-derived late EPC or MSC can efficiently induce myocardial repair to a similar extent, and humoral factors and perivascular localization of transplanted cells mainly mediate such therapeutic effects. These UCB-derived EPC or MSC can be attractive and promising cell sources for cardiovascular cell therapy.
Supplementary Material
Acknowledgements
This work was supported in part by NIH grants DP3DK094346, R01 HL127759, Faculty Research Assistance Program of Yonsei University College of Medicine 2015, the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIP) (No. 2015M3A9C6031514) and a Grant from the Korean Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (no. HI10C1740).
List of Abbreviations
- AMI
acute myocardial infarction
- EF
ejection fraction
- EPC
endothelial progenitor cells
- IVSd
inter ventricular septum in diastole
- IVSs
inter ventricular septum in systole
- LV
left ventricular
- LVESD
LV end-systolic dimension
- LVPWd
LV end-diastolic posterior wall
- LVPWs
LV end-systolic posterior wall
- MNC
mononuclear cells
- MSC
mesenchymal stem cells
- UCB
umbilical cord blood
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No competing financial interests exist
Author Contributions
Conceived and designed the experiments: Y-SY HOK.
Performed major experiments: S-WK HLJ.
Echocardiography : S-MK K-JY.
Analyzed the data: SK H-SK YJ S-WK.
Contributed reagents/materials/analysis tools: Y-SY HOK.
Wrote the paper: S-WK HLJ Y-SY.
References
- 1.Mayani H, Lansdorp PM. Thy-1 expression is linked to functional properties of primitive hematopoietic progenitor cells from human umbilical cord blood. Blood. 1994;83:2410–2417. [PubMed] [Google Scholar]
- 2.Ko KH, Nordon R, O'Brien TA, Symonds G, Dolnikov A. Ex vivo expansion of haematopoietic stem cells to improve engraftment in stem cell transplantation. Methods Mol Biol. 2011;761:249–260. doi: 10.1007/978-1-61779-182-6_17. [DOI] [PubMed] [Google Scholar]
- 3.Broxmeyer HE. Cord blood hematopoietic stem cell transplantation. Cambridge (MA): StemBook; 2008. [PubMed] [Google Scholar]
- 4.Lavergne M, Vanneaux V, Delmau C, Gluckman E, Rodde-Astier I, Larghero J, et al. Cord blood-circulating endothelial progenitors for treatment of vascular diseases. Cell proliferation. 2011;44(Suppl 1):44–47. doi: 10.1111/j.1365-2184.2010.00722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rehman J, Li J, Orschell CM, March KL. Peripheral blood "endothelial progenitor cells" are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 6.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 7.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 8.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 9.Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288–293. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 10.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 11.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 13.Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 14.Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JI, Uchida S, Masuda H, et al. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103:634–637. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- 15.Nagaya N, Fujii T, Iwase T, Ohgushi H, Itoh T, Uematsu M, et al. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am J Physiol Heart Circ Physiol. 2004;287:H2670–H2676. doi: 10.1152/ajpheart.01071.2003. [DOI] [PubMed] [Google Scholar]
- 16.Murohara T, Ikeda H, Duan J, Shintani S, Sasaki K, Eguchi H, et al. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J Clin Invest. 2000;105:1527–1536. doi: 10.1172/JCI8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A. 2000;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amado LC, Saliaris AP, Schuleri KH, St John M, Xie JS, Cattaneo S, et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci U S A. 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagaya N, Kangawa K, Itoh T, Iwase T, Murakami S, Miyahara Y, et al. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005;112:1128–1135. doi: 10.1161/CIRCULATIONAHA.104.500447. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Teoh SH, Chong MS, Lee ES, Mattar CN, Randhawa NK, et al. Vasculogenic and osteogenesis-enhancing potential of human umbilical cord blood endothelial colony-forming cells. Stem cells. 30:1911–1924. doi: 10.1002/stem.1164. [DOI] [PubMed] [Google Scholar]
- 21.Prasain N, Meador JL, Yoder MC. Phenotypic and functional characterization of endothelial colony forming cells derived from human umbilical cord blood. J Vis Exp. 2012 doi: 10.3791/3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beuzelin JM, Meszaros A, Reagan TE, Wilson LT, Way MO, Blouin DC, et al. Seasonal infestations of two stem borers (Lepidoptera: Crambidae) in noncrop grasses of Gulf Coast rice agroecosystems. Environmental entomology. 2011;40:1036–1050. doi: 10.1603/EN11044. [DOI] [PubMed] [Google Scholar]
- 23.O E, Lee BH, Ahn HY, Shin JC, Kim HK, Kim M, et al. Efficient nonadhesive ex vivo expansion of early endothelial progenitor cells derived from CD34+ human cord blood fraction for effective therapeutic vascularization. FASEB J. 2011;25:159–169. doi: 10.1096/fj.10-162040. [DOI] [PubMed] [Google Scholar]
- 24.Murohara T. Cord blood-derived early outgrowth endothelial progenitor cells. Microvasc Res. 2010;79:174–177. doi: 10.1016/j.mvr.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Mukai N, Akahori T, Komaki M, Li Q, Kanayasu-Toyoda T, Ishii-Watabe A, et al. A comparison of the tube forming potentials of early and late endothelial progenitor cells. Exp Cell Res. 2008;314:430–440. doi: 10.1016/j.yexcr.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 26.Reagan J, Foo T, Tracy Watson J, Jin W, Moed BR, Zhang Z. Distinct phenotypes and regenerative potentials of early endothelial progenitor cells and outgrowth endothelial progenitor cells derived from umbilical cord blood. Journal of tissue engineering and regenerative medicine. 2011 doi: 10.1002/term.354. [DOI] [PubMed] [Google Scholar]
- 27.Nishiyama N, Miyoshi S, Hida N, Uyama T, Okamoto K, Ikegami Y, et al. The significant cardiomyogenic potential of human umbilical cord blood-derived mesenchymal stem cells in vitro. Stem cells. 2007;25:2017–2024. doi: 10.1634/stemcells.2006-0662. [DOI] [PubMed] [Google Scholar]
- 28.Yoon YS, Wecker A, Heyd L, Park JS, Tkebuchava T, Kusano K, et al. Clonally expanded novel multipotent stem cells from human bone marrow regenerate myocardium after myocardial infarction. J Clin Invest. 2005;115:326–338. doi: 10.1172/JCI22326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim SW, Kim H, Cho HJ, Lee JU, Levit R, Yoon YS. Human peripheral blood-derived CD31+ cells have robust angiogenic and vasculogenic properties and are effective for treating ischemic vascular disease. J Am Coll Cardiol. 2010;56:593–607. doi: 10.1016/j.jacc.2010.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeong JO, Han JW, Kim JM, Cho HJ, Park C, Lee N, et al. Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ Res. 2011;108:1340–1347. doi: 10.1161/CIRCRESAHA.110.239848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoon YS, Uchida S, Masuo O, Cejna M, Park JS, Gwon HC, et al. Progressive attenuation of myocardial vascular endothelial growth factor expression is a seminal event in diabetic cardiomyopathy: restoration of microvascular homeostasis and recovery of cardiac function in diabetic cardiomyopathy after replenishment of local vascular endothelial growth factor. Circulation. 2005;111:2073–2085. doi: 10.1161/01.CIR.0000162472.52990.36. [DOI] [PubMed] [Google Scholar]
- 32.Cho HJ, Lee N, Lee JY, Choi YJ, Ii M, Wecker A, et al. Role of host tissues for sustained humoral effects after endothelial progenitor cell transplantation into the ischemic heart. J Exp Med. 2007;204:3257–3269. doi: 10.1084/jem.20070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeevanantham V, Butler M, Saad A, Abdel-Latif A, Zuba-Surma EK, Dawn B. Adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters: a systematic review and meta-analysis. Circulation. 126:551–568. doi: 10.1161/CIRCULATIONAHA.111.086074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 35.Yoon CH, Hur J, Park KW, Kim JH, Lee CS, Oh IY, et al. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112:1618–1627. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- 36.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 38.Mandriota SJ, Pyke C, Di Sanza C, Quinodoz P, Pittet B, Pepper MS. Hypoxia-inducible angiopoietin-2 expression is mimicked by iodonium compounds and occurs in the rat brain and skin in response to systemic hypoxia and tissue ischemia. Am J Pathol. 2000;156:2077–2089. doi: 10.1016/S0002-9440(10)65079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tressel SL, Kim H, Ni CW, Chang K, Velasquez-Castano JC, Taylor WR, et al. Angiopoietin-2 stimulates blood flow recovery after femoral artery occlusion by inducing inflammation and arteriogenesis. Arterioscler Thromb Vasc Biol. 2008;28:1989–1995. doi: 10.1161/ATVBAHA.108.175463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanahan D. Signaling vascular morphogenesis and maintenance. Science. 1997;277:48–50. doi: 10.1126/science.277.5322.48. [DOI] [PubMed] [Google Scholar]
- 41.Sandhu R, Teichert-Kuliszewska K, Nag S, Proteau G, Robb MJ, Campbell AI, et al. Reciprocal regulation of angiopoietin-1 and angiopoietin-2 following myocardial infarction in the rat. Cardiovasc Res. 2004;64:115–124. doi: 10.1016/j.cardiores.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 42.Asahara T, Chen D, Takahashi T, Fujikawa K, Kearney M, Magner M, et al. Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Circ Res. 1998;83:233–240. doi: 10.1161/01.res.83.3.233. [DOI] [PubMed] [Google Scholar]
- 43.Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- 44.Bjarnegard M, Enge M, Norlin J, Gustafsdottir S, Fredriksson S, Abramsson A, et al. Endothelium-specific ablation of PDGFB leads to pericyte loss and glomerular, cardiac and placental abnormalities. Development. 2004;131:1847–1857. doi: 10.1242/dev.01080. [DOI] [PubMed] [Google Scholar]
- 45.Hsieh PC, Davis ME, Gannon J, MacGillivray C, Lee RT. Controlled delivery of PDGF-BB for myocardial protection using injectable self-assembling peptide nanofibers. J Clin Invest. 2006;116:237–248. doi: 10.1172/JCI25878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buerke M, Murohara T, Skurk C, Nuss C, Tomaselli K, Lefer AM. Cardioprotective effect of insulin-like growth factor I in myocardial ischemia followed by reperfusion. Proc Natl Acad Sci U S A. 1995;92:8031–8035. doi: 10.1073/pnas.92.17.8031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zamzami N, Brenner C, Marzo I, Susin SA, Kroemer G. Subcellular and submitochondrial mode of action of Bcl-2-like oncoproteins. Oncogene. 1998;16:2265–2282. doi: 10.1038/sj.onc.1201989. [DOI] [PubMed] [Google Scholar]
- 48.Tsukahara H, Gordienko DV, Tonshoff B, Gelato MC, Goligorsky MS. Direct demonstration of insulin-like growth factor-I-induced nitric oxide production by endothelial cells. Kidney Int. 1994;45:598–604. doi: 10.1038/ki.1994.78. [DOI] [PubMed] [Google Scholar]
- 49.Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM, et al. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39:733–742. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 50.Urbanek K, Rota M, Cascapera S, Bearzi C, Nascimbene A, De Angelis A, et al. Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circ Res. 2005;97:663–673. doi: 10.1161/01.RES.0000183733.53101.11. [DOI] [PubMed] [Google Scholar]
- 51.Opitz CA, Rimmerman N, Zhang Y, Mead LE, Yoder MC, Ingram DA, et al. Production of the endocannabinoids anandamide and 2-arachidonoylglycerol by endothelial progenitor cells. FEBS letters. 2007;581:4927–4931. doi: 10.1016/j.febslet.2007.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 53.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A. 2001;98:10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 55.Galasso G, De Rosa R, Ciccarelli M, Sorriento D, Del Giudice C, Strisciuglio T, et al. beta2-Adrenergic receptor stimulation improves endothelial progenitor cell-mediated ischemic neoangiogenesis. Circ Res. 2013;112:1026–1034. doi: 10.1161/CIRCRESAHA.111.300152. [DOI] [PubMed] [Google Scholar]
- 56.Galasso G, Schiekofer S, Sato K, Shibata R, Handy DE, Ouchi N, et al. Impaired angiogenesis in glutathione peroxidase-1-deficient mice is associated with endothelial progenitor cell dysfunction. Circ Res. 2006;98:254–261. doi: 10.1161/01.RES.0000200740.57764.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim H, Cho HJ, Kim SW, Liu B, Choi YJ, Lee J, et al. CD31+ cells represent highly angiogenic and vasculogenic cells in bone marrow: novel role of nonendothelial CD31+ cells in neovascularization and their therapeutic effects on ischemic vascular disease. Circ Res. 2010;107:602–614. doi: 10.1161/CIRCRESAHA.110.218396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, et al. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.