Abstract
Background
We have previously demonstrated that Tubastatin A, a selective inhibitor of histone deacetylase (HDAC) 6, improves survival, and increases circulating monocyte count and bacterial clearance in a lethal model of cecal ligation and puncture (CLP) in mice. The aim of the present study was to characterize the effects of inhibition of HDAC6 on the bone marrow cell population.
Methods
C57BL/6J mice were subjected to CLP, and 1 h later given an intraperitoneal injection of either Tubastatin A (70 mg/kg) dissolved in dimethyl sulfoxide (DMSO), or DMSO alone (n=9/group). Sham-operated animals were treated in an identical fashion, without CLP. Forty-eight hours later, bone marrow cells were flushed out from the femurs and tibias. Erythrocytes were lysed, and a single-cell suspension was made for analysis. Cells were washed, blocked with anti-mouse CD16/32, stained with anti-mouse B220 PE-Cy7, CD3 APC-eFluor® 780, CD11b FITC, Gr-1 PerCP-Cy5.5 and F4/80 Antigen APC, and subjected to flow cytometry. Data was acquired on an LSRII Flow Cytometer (BD Biosciences) and analyzed with FlowJo (Flowjo, LLC).
Results
In comparison to the sham group, CLP animals showed decreased percentage of innate immune cells (CD11b+; 62.1±3.1 vs. 32.9±4.9%, p=0.0025) and macrophages (CD11b+ F4/80+; 44.6±3.4 vs. 19.8±2.6%, p=0.0002), and increased percentage of T lymphocytes (CD3+; 1.1±0.2 vs. 3.3±0.4%, p=0.0082) in the bone marrow 48 h after CLP. Treatment with Tubastatin A restored the innate immune cells (32.9±4.9 vs. 54.0±4.1%, p=0.0112) and macrophages (19.8±2.6 vs. 47.1±4.6%, p=0.0001), and increased the percentage of neutrophils (CD11b+ Gr-1+; 28.4±3.9 vs. 48.0±4.0%, p=0.0075). The percentages of B (B220+) and T lymphocytes were not significantly altered by Tubastatin A, compared to the vehicle-treated CLP animals.
Conclusions
Selective inhibition of HDAC6 in this lethal septic model restored the innate immune cell and macrophage populations, and increased the neutrophil composition in the bone marrow. These results may explain the previously reported beneficial effects of Tubastatin A treatment in a septic model.
Levels of Evidence
not applicable (animal study).
Keywords: HDAC6, bone marrow, innate immune cells, mice, sepsis
INTRODUCTION
Severe sepsis and septic shock are the leading cause of death in critically ill patients in the United States. Multiple clinical trials have failed to identify an effective treatment for severe sepsis and septic shock. 1, 2 The innate immune system is the first line of defense against pathogenic invasion. Bone marrow is an important immune organ that plays an essential role in maintaining a robust and healthy immune system. Treatments that can enhance bone marrow function are therefore of interest.
Histone acetylation is an essential epigenetic mechanism that determines the amplitude of immune signaling, by controlling the chromatin structure, accessibility of transcription factors to the DNA, and the subsequent gene transcription. The acetylation status of chromatin is balanced by the activities of histone acetyltransferases (HATs) and histone deacetylases (HDACs), and hypoacetylation can suppress gene transcription. HDAC6 is a unique HDAC localized in the cytoplasm, where it associates with non-histone substrates. 3 Inhibition of HDAC6 has been shown to ameliorate central nervous system injury, and HDAC6 has become an emerging target for development of anti-cancer drugs. 4, 5 Tubastatin A is a selective HDAC6 inhibitor with simple synthesis and superior target selectivity. 6
We have previously demonstrated that Tubastatin A treatment improves survival, increases circulating monocyte count and bacterial clearance, and attenuates bone marrow atrophy in a mouse model of lethal cecal ligation and puncture (CLP). 7-9 The aim of the present study was to characterize the effects of inhibition of HDAC6 on the bone marrow cell population. Our hypothesis was that treatment with Tubastatin A in a CLP model would enhance the bone marrow cellular profile to make the animals better equipped to fight an infection.
METHODS
Sepsis Model: Cecal Ligation and Puncture (CLP)
Male C57BL/6J mice (18-26 gm) were purchased from The Jackson Laboratory (The Jackson Laboratory, Bar Harbor, ME), and were housed for 3 days before manipulations. A well-established CLP model 10,11 was utilized to induce fecal peritonitis. Briefly, the peritoneal cavity was opened under inhaled isoflurane anesthesia. Cecum was eviscerated, ligated below the ileocecal valve using a 5-0 suture, and punctured through and through (2 holes) with a 20-gauge needle. The punctured cecum was squeezed to expel a small amount of fecal material and returned to the peritoneal cavity. The abdominal incision was closed in two layers with 4-0 silk suture, and animals were resuscitated by subcutaneous injection of 1 mL of saline. Sham-operated animals were handled in the same manner, except that the cecum was neither ligated nor punctured. This protocol was approved by the Institutional Animal Care and Use Committee. All of the surgical procedures were performed under anesthesia, and all possible efforts were made to minimize suffering and stress.
Administration of HDAC Inhibitor and Experimental Design
Animals were randomly assigned to the following three groups (n = 9/group): (a) Sham-operated animals (SHAM); (b) vehicle treated animals after CLP (CLP+DMSO), and (c) Tubastatin A treated animals after CLP (CLP+Tub.A). Animals were given intra-peritoneal Tubastatin A dissolved in DMSO (70 mg/kg) or vehicle DMSO 1 h after the CLP. Sham-operated animals were subjected to laparotomy and intestinal manipulation, but the cecum was not ligated or punctured. Forty-eight hours later, animals were killed and long bones (2 femurs and 2 tibias) were harvested.
Flow Cytometry of Bone Marrow Cells
We have previously shown that a double puncture (20-gauge needle) CLP with ligation results in 100% mortality, and is associated with significant bone marrow atrophy. 8 Therefore, we used the same model in the present study to investigate the effects of Tubastatin A, and harvested the bone marrow 48 hours post-surgery based upon previous experience to coincide with a time point when the animals were septic. Bone marrow cells were flushed out from the bone cavity with cold phosphate-buffered saline (Corning, Manassas, VA), and single-cell suspension was obtained by passing through 70 μm cell strainers. Erythrocyte lysis was performed using RBC Lysis Buffer (Sigma Aldrich, St. Louis, MO). Bone marrow cells were then washed, blocked with anti-mouse CD16/32 (eBiosicence, San Diego, CA) for 10 min on ice, and stained with anti-mouse B220 PE-Cy7, CD3 APC-eFluor® 780, CD11b FITC, Gr-1 PerCP-Cy5.5, and F4/80 Antigen APC (eBiosicence, San Diego, CA) for 30 min on ice. Cells were washed before flow cytometry analysis. Data were acquired on an LSRII (BD Biosciences, San Jose, California) and analyzed with FlowJo (Flowjo, LLC, Ashland, OR). 12
Statistical Analysis
Data are presented as group mean ± SEM. Differences between 3 or more groups were assessed using one way analysis of variance (ANOVA) followed by Bonferroni post hoc testing for multiple comparisons. Student’s t-test was used to compare the differences between two groups. Analyses were performed with GraphPad Prism. P values of 0.05 or less were considered significant.
RESULTS
Tubastatin A restored the percentage of innate immune cells and macrophages in the bone marrow
In comparison to the sham group, vehicle-treated CLP animals showed decreased percentage of innate immune cells (CD11b+; 62.1±3.1 vs. 32.9±4.9%, p=0.0025; Figure 1) and macrophages (CD11b+ F4/80+; 44.6±3.4 vs. 19.8±2.6%, p=0.0002; Figure 2) in the bone marrow 48 h after CLP. Treatment with Tubastatin A restored the innate immune cells (32.9±4.9 vs. 54.0±4.1%, p=0.0112; Figure 1) and macrophages (19.8±2.6 vs. 47.1±4.6%, p=0.0001; Figure 2).
Figure 1. Tubastatin A treatment restored the percentage of innate immune cells in the bone marrow.
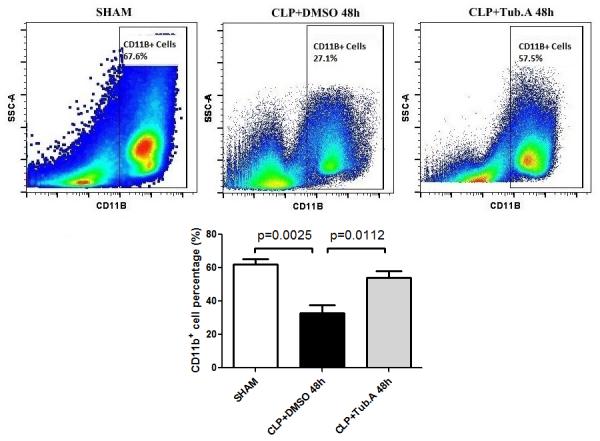
Representative plots of innate immune cells are shown on the right side of panel (CD11b+). The percentages of innate immune cells were quantified and compared among groups (means ± SEM, n = 9/group). CLP: cecal ligation and puncture; Tub.A: Tubastatin A; SSC: side scatter.
Figure 2. Tubastatin A treatment restored the percentage of macrophages in the bone marrow.
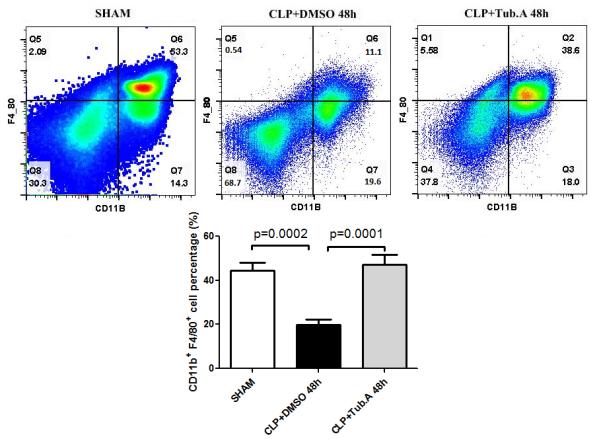
Representative plots of macrophages are shown in the right upper quadrant of panel (CD11b+ F4/80+). The percentages of macrophages was quantified and compared among groups. CLP: cecal ligation and puncture; Tub.A: Tubastatin A.
Tubastatin A increased the percentage of neutrophils in the bone marrow
The percentage of neutrophils was not significantly different between the sham-operated and vehicle-treated CLP animals. Treatment with Tubastatin A increased the percentage of neutrophils (CD11b+ Gr-1+; 28.4±3.9 vs. 48.0±4.0%, p=0.0075; Figure 3).
Figure 3. Tubastatin A increased the percentage of neutrophils in the bone marrow.
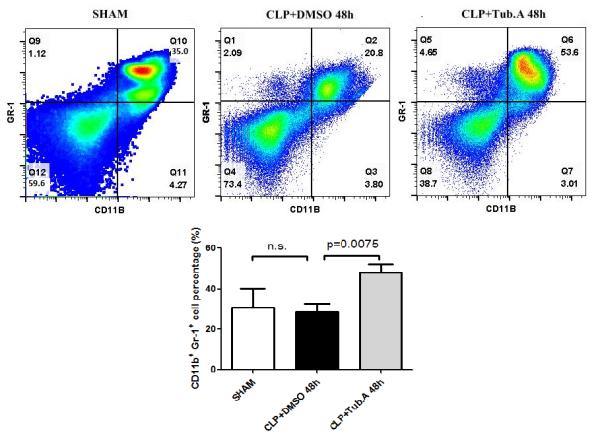
Representative plots of innate immune cells are shown in the right upper quadrant of panel (CD11b+ Gr-1+). The percentages of neutrophils were quantified and compared among groups. CLP: cecal ligation and puncture; Tub.A: Tubastatin A.
Tubastatin A did not significantly alter the percentages of B and T lymphocytes
In comparison to the sham group, vehicle-treated CLP animals showed increased percentage of T lymphocytes (CD3+; 1.1±0.2 vs. 3.3±0.4%, p=0.0082) in the bone marrow 48 h after CLP. The percentages of B (B220+) and T lymphocytes were not significantly altered by Tubastatin A, compared to vehicle-treated CLP animals (Figures 4 and 5).
Figure 4. Tubastatin A treatment did not alter the percentages of B lymphocytes.
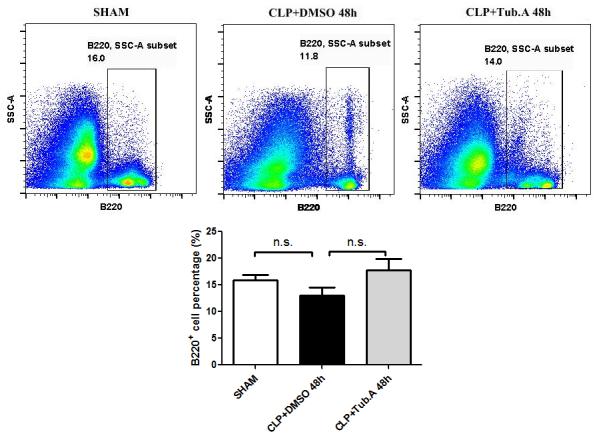
Representative plots B lymphocytes are shown on the right side of panel (B220+). The percentages of B lymphocytes were quantified and compared among groups. CLP: cecal ligation and puncture; Tub.A: Tubastatin A; SSC: side scatter.
Figure 5. Tubastatin A treatment did not alter the percentages of T lymphocytes.
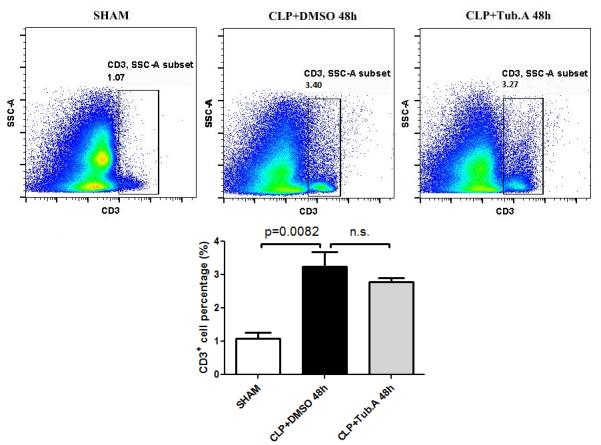
Representative plots T lymphocytes are shown on the right side of panel (CD3+). The percentages of T lymphocytes were quantified and compared among groups. CLP: cecal ligation and puncture; Tub.A: Tubastatin A; SSC: side scatter.
DISCUSSION
We investigated the effects of an HDAC6 inhibitor, Tubastatin A, on the composition of different bone marrow cell types in a severe CLP model. We discovered that treatment with Tubastatin A restored the innate immune cell and macrophage populations, and increased the neutrophil composition in the bone marrow.
In humans, at least eighteen HDACs have been reported that are sub-divided into three classical (Zn++ dependent) classes (I, II, and IV), as well as class III that contains NAD+ dependent sirtuins.13, 14 HDAC6 (group IIb) are mostly cytoplasmic in location with tissue-specific expression, and are known to be associated with non-histone substrates, such as heat shock protein 90 (HSP90), α-tubulin and cortactin. HDAC6 has an ubiquitin binding domain and two catalytic domains.3 Overexpression of HDAC6 deacetylates tubulin and increases cell motility 15, whereas specific inhibition of HDAC6 activity or its down regulation by siRNA increases the acetylation of α-tubulin and HSP90. This reduces cellular motility and induces the degradation of HSP90 client proteins, including Bcr-Abl, Raf-1, Akt, HER2/Neu, interleukin-1 receptor associated kinase 1, and hypoxia-inducible factor-1α.16-18 HDAC6 has become a target for drug development to treat cancer, including leukemia, multiple myeloma, and breast cancer, 5, 19-22 and cardiac HDAC6 catalytic activity has been shown to increase in response to chronic hypertension. 23 Meanwhile, inhibition of HDAC6 promotes survival and regeneration of neurons. 4 Our laboratory has demonstrated that treatment with Tubastatin A (an HDAC6 specific inhibitor) improves survival, increases circulating monocyte count and bacterial clearance, and attenuates bone marrow atrophy in a lethal model of CLP in mice. 7-9 However, whether this was a direct effect or exerted indirectly through alterations in the bone marrow composition was unknown.
Macrophages and neutrophils are critical effector cells contributing to the altered innate immune response against infection. They are efficient pathogen scavengers and the predominant source of inflammatory cytokines, making them critical elements of a robust immune response. 24 The dysfunction of macrophages and neutrophils during late stages of sepsis results in a relatively immunosuppressed state. 25, 26 In this study, we have discovered that treatment with Tubastatin A restores the innate immune cell and macrophage populations, and increases the neutrophil composition in the bone marrow, following CLP-induced sepsis. Tubastatin A appears to replenish the circulating monocyte and neutrophil pool from bone marrow, which may potentially enhance the host’s ability to phagocytize foreign pathogens and eliminate bacteria from the circulation. These findings may explain the significantly better outcomes that we have previously noted with Tubastatin A treatment in the lethal CLP-sepsis model. 7 The present study is a logical extension of our previous research. Based upon the previous survival data, we selected the time point of 48 hours after the CLP for tissue harvest, as by this time almost all of the untreated animals were moribund, and the bone marrow demonstrated significant atrophy.
Bone marrow is the critical organ in the production and maturation of lymphocytes and phagocytes. We have recently reported that treatment with HDAC6 inhibitor improves survival, alters the composition of circulating blood cells, increases circulating monocyte count, and enhances bacterial clearance and splenocyte phagocytosis in a lethal septic model. 7 We also discovered that HDAC6 inhibition attenuated the stress response, and prevented bone marrow atrophy and splenic apoptosis 48 hours post-CLP 9. It also altered the composition of the circulating blood cells with a decrease in granulocytes, increase in lymphocytes and monocytes, and an increase in the red cell mass. 8 The current experiment provides yet another piece in this complex jigsaw puzzle by characterizing the effects of the HDAC6 inhibitor administration on the bone marrow cell population. We still do not know whether the beneficial effects of Tub A are due to a direct impact on the bone marrow cells, or an indirect effect due to the overall attenuation of inflammation and better survival. However, by taking the findings of these studies together, a plausible explanation can be put together, where inhibition of HDAC6 in sepsis can7-9: (1) prevent bone marrow atrophy; (2) restore bone marrow innate immune cell and macrophage cell populations (the current finding), and augment the neutrophil composition in the bone marrow; and (3) enhance circulating monocyte count and bacterial clearance (Figure 6).
Figure 6. Histone deacetylase 6 inhibition in septic shock and potential mechanisms.
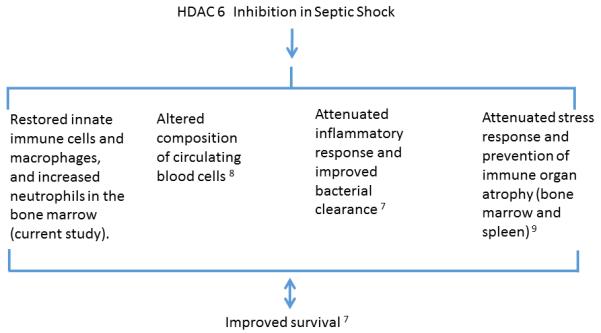
Findings from several studies are summarized to highlight the possible mechanisms that may be involved in improving the survival. The double arrow is to acknowledge that it is unclear whether these pathways are specifically responsible for the improved survival (direct effect), or if the enhanced survival results in the observed findings (indirect effect). Attenuation of sepsis-induced bone marrow atrophy by Tubastatin A can (1) prevent bone marrow from damage; 9 (2) restore innate immune cell and macrophage populations, and increase neutrophil composition in the bone marrow (the current finding); (3) enhance circulating monocyte count and bacterial clearance 7, and alter composition of circulating blood cells. 8
The precise relationship between the HDAC6 and bone marrow differentiation currently remains elusive. The inhibition of HDAC6 may increase the percentages of macrophages and neutrophils through epigenetic modulation. Theoretically, HDAC6 inhibitors may activate the gene promoters of bone marrow mesenchymal stem cells, and facilitate their differentiation into macrophages and neutrophils. HDAC6 expression is elevated in patients with primary myelofibrosis. 27 This finding is consistent with our results, as inhibition of HDAC6 may stimulate the proliferation of certain bone marrow progenitors. Meanwhile, histone acetylation has been reported to be beneficial for the monocytes. Acetylated histone H4 levels in the circulating monocytes were significantly elevated in complication-free diabetic patients, but not in patients with complications, suggesting that protein acetylation in the monocytes may be a protective mechanism. 28
This study has certain limitations that must be acknowledged. We have described broad changes in the bone marrow cellular composition after Tubastatin A treatment in the CLP-sepsis model. However, further molecular mechanisms need to be investigated to discover how exactly histone modifications control bone marrow stem cell differentiation in sepsis. We did not investigate the long-term consequences of the treatment, and functional assays were not performed in this study. Although our understanding is improving, clearly additional research is still required to better understand the mechanism by which Tubastatin A affects bone marrow during septic shock, and how to best exploit its therapeutic potential.
In summary, we have demonstrated that inhibition of HDAC6, in a CLP-induced septic model, restores the innate immune cell and macrophage populations, and increases the neutrophil composition in the bone marrow. These results may explain the previously reported beneficial effects of Tubastatin A treatment in this model.
Acknowledgements
This work was funded by a grant from NIH RO1 GM084127 to HBA. Data was presented at 74th Annual Meeting of the American Association for the Surgery of Trauma (AAST) and Clinical Congress of Acute Care Surgery in Las Vegas, NV (September, 2015).
ABBREVIATIONS AND ACRONYMS
- ANOVA
one-way analysis of variance
- CLP
cecal ligation and puncture
- DMSO
dimethyl sulfoxide
- HATs
histone acetyltransferases
- HDAC
histone deacetylase
- HSP90
heat shock protein 90
- SSC
side scatter
Footnotes
AUTHOR CONTRIBUTION
T.Z., Y.L. and H.B.A. designed this study, for which H.B.A. secured funding. T.Z. performed the experiments, collected and analyzed data. B.L. and X.C. provided experimental help. B.P. and P.G. provided additional support, including review of the manuscript. T.Z. and Y.L. wrote the manuscript, which was critically reviewed and revised by Y.L. and H.B.A. All of the authors read and approved the final manuscript.
CONFLICT-OF-INTEREST DISCLOSURE: No.
REFERENCE
- 1.Wenzel RP, Edmond MB. Septic shock--evaluating another failed treatment. N Engl J Med. 2012 May 31;366(22):2122–4. doi: 10.1056/NEJMe1203412. [DOI] [PubMed] [Google Scholar]
- 2.Ranieri VM, Thompson BT, Barie PS, Dhainaut JF, Douglas IS, Finfer S, Gardlund B, Marshall JC, Rhodes A, Artigas A, et al. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012 May 31;366(22):2055–64. doi: 10.1056/NEJMoa1202290. [DOI] [PubMed] [Google Scholar]
- 3.Zou H, Wu Y, Navre M, Sang BC. Characterization of the two catalytic domains in histone deacetylase 6. Biochemical and biophysical research communications. 2006 Mar 3;341(1):45–50. doi: 10.1016/j.bbrc.2005.12.144. [DOI] [PubMed] [Google Scholar]
- 4.Rivieccio MA, Brochier C, Willis DE, Walker BA, D'Annibale MA, McLaughlin K, Siddiq A, Kozikowski AP, Jaffrey SR, Twiss JL, et al. HDAC6 is a target for protection and regeneration following injury in the nervous system. Proc Natl Acad Sci U S A. 2009 Nov 17;106(46):19599–604. doi: 10.1073/pnas.0907935106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aldana-Masangkay GI, Sakamoto KM. The role of HDAC6 in cancer. J Biomed Biotechnol. 2011;2011:875824. doi: 10.1155/2011/875824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butler KV, Kalin J, Brochier C, Vistoli G, Langley B, Kozikowski AP. Rational design and simple chemistry yield a superior, neuroprotective HDAC6 inhibitor, tubastatin A. Journal of the American Chemical Society. 2010 Aug 11;132(31):10842–6. doi: 10.1021/ja102758v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Zhao T, Liu B, Halaweish I, Mazitschek R, Duan X, Alam HB. Inhibition of histone deacetylase 6 improves long-term survival in a lethal septic model. J Trauma Acute Care Surg. 2015 Feb;78(2):378–85. doi: 10.1097/TA.0000000000000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao T, Li Y, Liu B, Halaweish I, Mazitschek R, Alam HB. Selective inhibition of histone deacetylase 6 alters the composition of circulating blood cells in a lethal septic model. J Surg Res. 2014 Aug;190(2):647–54. doi: 10.1016/j.jss.2014.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao T, Li Y, Bronson RT, Liu B, Velmahos GC, Alam HB. Selective histone deacetylase-6 inhibition attenuates stress responses and prevents immune organ atrophy in a lethal septic model. Surgery. 2014 Aug;156(2):235–42. doi: 10.1016/j.surg.2014.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao T, Li Y, Liu B, Liu Z, Chong W, Duan X, Deperalta DK, Velmahos GC, Alam HB. Novel pharmacologic treatment attenuates septic shock and improves long-term survival. Surgery. 2013 Aug;154(2):206–13. doi: 10.1016/j.surg.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao T, Li Y, Liu B, Bronson RT, Halaweish I, Alam HB. Histone deacetylase III as a potential therapeutic target for the treatment of lethal sepsis. J Trauma Acute Care Surg. 2014 Dec;77(6):913–9. doi: 10.1097/TA.0000000000000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rauch PJ, Chudnovskiy A, Robbins CS, Weber GF, Etzrodt M, Hilgendorf I, Tiglao E, Figueiredo JL, Iwamoto Y, Theurl I, et al. Innate response activator B cells protect against microbial sepsis. Science. 2012 Feb 3;335(6068):597–601. doi: 10.1126/science.1215173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim H, Bae S. Histone deacetylase inhibitors: molecular mechanisms of action and clinical trials as anti-cancer drugs. Am J Transl Res. 2011;3:166–179. [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Alam HB. Modulation of acetylation: creating a pro-survival and anti-inflammatory phenotype in lethal hemorrhagic and septic shock. J Biomed Biotechnol. 2011;2011:523481. doi: 10.1155/2011/523481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP. HDAC6 is a microtubule-associated deacetylase. Nature. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S.] 2002 May 23;417(6887):455–8. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- 16.Bali P, Pranpat M, Bradner J, Balasis M, Fiskus W, Guo F, Rocha K, Kumaraswamy S, Boyapalle S, Atadja P, et al. Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors. The Journal of biological chemistry. 2005 Jul 22;280(29):26729–34. doi: 10.1074/jbc.C500186200. [DOI] [PubMed] [Google Scholar]
- 17.Kovacs JJ, Murphy PJ, Gaillard S, Zhao X, Wu JT, Nicchitta CV, Yoshida M, Toft DO, Pratt WB, Yao TP. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Molecular cell. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.] 2005 May 27;18(5):601–7. doi: 10.1016/j.molcel.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 18.Triantafilou M, Triantafilou K. Heat-shock protein 70 and heat-shock protein 90 associate with Toll-like receptor 4 in response to bacterial lipopolysaccharide. Biochem Soc Trans. 2004 Aug;32(Pt 4):636–9. doi: 10.1042/BST0320636. [DOI] [PubMed] [Google Scholar]
- 19.Eklund EA, Platanias LC. Inhibition of histone deacetylase 6 as a therapeutic strategy for acute lymphocytic leukemia. Leuk Lymphoma. 2011 Aug;52(8):1421–2. doi: 10.3109/10428194.2011.577259. [DOI] [PubMed] [Google Scholar]
- 20.Hackanson B, Rimmele L, Benkisser M, Abdelkarim M, Fliegauf M, Jung M, Lubbert M. HDAC6 as a target for antileukemic drugs in acute myeloid leukemia. Leuk Res 2012. 2012;36(8):1055–62. doi: 10.1016/j.leukres.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 21.Santo L, Hideshima T, Kung AL, Tseng JC, Tamang D, Yang M, Jarpe M, van Duzer JH, Mazitschek R, Ogier WC, et al. Preclinical activity, pharmacodynamic, and pharmacokinetic properties of a selective HDAC6 inhibitor, ACY-1215, in combination with bortezomib in multiple myeloma. Blood. 2012 Mar 15;119(11):2579–89. doi: 10.1182/blood-2011-10-387365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schulz R, Marchenko ND, Holembowski L, Fingerle-Rowson G, Pesic M, Zender L, Dobbelstein M, Moll UM. Inhibiting the HSP90 chaperone destabilizes macrophage migration inhibitory factor and thereby inhibits breast tumor progression. J Exp Med. 2012 Feb 13;209(2):275–89. doi: 10.1084/jem.20111117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lemon DD, Horn TR, Cavasin MA, Jeong MY, Haubold KW, Long CS, Irwin DC, McCune SA, Chung E, Leinwand LA, et al. Cardiac HDAC6 catalytic activity is induced in response to chronic hypertension. J Mol Cell Cardiol. 2011 Jul;51(1):41–50. doi: 10.1016/j.yjmcc.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Underhill DM, Ozinsky A. Phagocytosis of microbes: complexity in action. Annual review of immunology. [Research Support, U.S. Gov't, P.H.S. Review] 2002;20:825–52. doi: 10.1146/annurev.immunol.20.103001.114744. [DOI] [PubMed] [Google Scholar]
- 25.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003 Jan 9;348(2):138–50. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 26.Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, Bricker TL, Jarman SD, 2nd, Kreisel D, Krupnick AS, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011 Dec 21;306(23):2594–605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang JC, Chen C, Dumlao T, Naik S, Chang T, Xiao YY, Sominsky I, Burton J. Enhanced histone deacetylase enzyme activity in primary myelofibrosis. Leuk Lymphoma. 2008 Dec;49(12):2321–7. doi: 10.1080/10428190802527699. [DOI] [PubMed] [Google Scholar]
- 28.Chen SS, Jenkins AJ, Majewski H. Elevated plasma prostaglandins and acetylated histone in monocytes in Type 1 diabetes patients. Diabet Med. 2009 Feb;26(2):182–6. doi: 10.1111/j.1464-5491.2008.02658.x. [DOI] [PubMed] [Google Scholar]


