Abstract
Postpartum depression is a specific type of depression that affects approximately 10-15% of mothers (Wisner et al., 2013). While many have attributed the etiology of postpartum depression to the dramatic change in hormone levels that occurs immediately postpartum, the exact causes are not well-understood. It is well-known; however, that pregnancy induces a number of dramatic changes in the peripheral immune system that foster the development of the growing fetus. It is also well-known that changes in immune function, specifically within the brain, have been linked to several neuropsychiatric disorders including depression. Thus, we sought to determine whether pregnancy induces significant neuroimmune changes postpartum and whether stress or immune activation during pregnancy induce a unique neuroimmune profile that may be associated with depressive-like behaviors postpartum. We used late-gestation sub-chronic stress and late-gestation acute immune activation to examine the postpartum expression of depressive-like behaviors, microglial activation markers, and inflammatory cytokines within the medial prefrontal cortex (mPFC) and the hippocampus (HP). The expression of many immune molecules was significantly altered in the brain postpartum, and postpartum females also showed significant anhedonia, both independently of stress. Following late-gestation immune activation, we found a unique set of changes in neuroimmune gene expression immediately postpartum. Thus, our data indicate that even in the absence of additional stressors, postpartum females exhibit significant changes in the expression of cytokines within the brain that are associated with depressive-like behavior. Additionally, different forms of antenatal stress produce varying profiles of postpartum neuroimmune gene expression and associated depressive-like behaviors.
Keywords: Pregnancy, postpartum, depression, neuroimmune, microglia, stress, inflammation, immune activation, forced swim test, LPS
1. Introduction
Postpartum depression is a specific type of major depression outlined by the National Institute of Mental Health that affects approximately 10-15% of mothers after birth (Wisner et al., 2013; National Institute of Mental Health, 2011). Approximately 30% of women will experience minor symptoms of anxiety and sadness, called “baby blues”, immediately postpartum (Faisal-Cury et al., 2008; Harris et al., 1994; Kennerley and Gath, 1989); however, these symptoms typically last only one to two weeks. The much more severe symptoms of postpartum depression including erratic mood swings, anhedonia, increased anxiety, insomnia, and social withdrawal may continue for weeks, months, or even years after giving birth (Miller, 2002; Cox et al., 1982). Pregnancy and parturition are associated with dramatic changes in hormone levels (Harris et al., 1994), which are believed to be the cause of these postpartum mood disorders. However, little research has examined whether other potential mechanisms contribute to the development or etiology of postpartum mood changes and depressive-like behaviors.
Pregnancy significantly alters peripheral immune function in order to accommodate and foster the growth of the developing fetus (Klein et al., 2010; Veenstra van Nieuwenhoven et al., 2001). As a result of these changes in peripheral immune function, the induction of classic Th1/M1 pro-inflammatory immune molecules, such as IL-1β, are decreased over the course of gestation while a unique composition of Th2/M2 immune molecules are expressed in response to immune challenges (e.g. IL-4, Arginase 1, and IL-10). The difference between the Th1/M1 and Th2/M2 phenotypes is striking and results in very distinct profiles of immune activation in response to a challenge. To date, these changes have been observed and measured in the periphery of pregnant females (Marzi et al., 1996). Very few animal studies have examined the expression of cytokines in the brain of either late-gestation or immediately postpartum females to determine if changes in central cytokine expression mimic peripheral changes, particularly in response to an immune challenge.
Thus in our first experiment, we implemented a modified protocol of stress-induced depression using a sub-chronic forced swim stressor in late-gestation females in order to determine the effect of this stressor on pro-inflammatory cytokine expression in the brain and depressive-like behaviors immediately postpartum. Our second experiment used acute immune activation via lipopolysaccharide (LPS) during late gestation to determine potential neuroimmune and behavioral changes in the early postpartum period. We predicted that physiological stress or immune activation during late gestation may increase the risk for developing postpartum depression by triggering a unique neuroinflammatory state that would be evident in the immediate postpartum period.
2. Materials and Methods
2.1 Animals
All experiments used female Sprague-Dawley rats ordered from Harlan Laboratories (Indianapolis, IN). Rats were housed in clear polypropylene cages with ad libitum access to food and water in rooms under a 12:12-hour light:dark cycle and maintained temperature and humidity. All experiments were performed in accordance with the Institutional Animal Care and Use Committee of the University of Delaware and under the Guide for the Care and Use of Laboratory Animals of the National Institute of Health.
Each experiment used both pregnant and non-pregnant females. Day of conception was determined by the presence of a sperm plug and assigned as Embryonic (E) Day 1, and day of birth (typically E23) was assigned as Postnatal (P) Day 0. All experimental manipulations in non-pregnant females were time-matched to their pregnant counterparts. Neither stress nor immune activation during late gestation produced any adverse outcomes on gestation length.
In Experiment 1, we examined the effects of a sub-chronic forced swim stressor during late gestation in pregnant females compared to time-matched non-pregnant females on the expression of cytokines, microglial activation markers, and depressive-like behavior during the immediate postpartum period. Experiments 1.1, 1.2, and 1.3 used a total of 96 female rats assigned to one of four experimental groups: Postpartum, No Stress (n=8); Postpartum, Stress (n=8); Non-pregnant, No Stress (n=8); or Non-pregnant, Stress (n=8). In this case, all female rats were mated, and rats that did not become pregnant (i.e., no sperm plug was found) were used as the Non-Pregnant control rats throughout the duration of the experiments. In Experiment 1.1, female rats were subjected to a forced swimming stressor for seven consecutive days during their last week of gestation (E16-E22) or the time matched equivalent for non-pregnant females. Rats in the No Stress group remained undisturbed during this time. Prior to the first day of testing (E15) or the time matched equivalent for No Stress and Non-pregnant groups, all females were separated to be housed individually for the remainder of the experiments. On day of birth (E23) or 24 hours after the last day of forced swimming in non-pregnant rats, the females were euthanized to examine the expression of cytokines and microglial activation markers in the brain in response to this late gestation sub-chronic stressor. Female rats in Experiment 1.2 underwent the same stress regimen; however, these rats were used to analyze immediate postpartum anhedonia on day of birth (P0) and P1. Females in Experiment 1.3 were also subjected to the forced swimming stressor, and these animals underwent behavioral testing for anhedonia at two time points: P0 and P1, to replicate our findings in Experiment 1.2, and one week after parturition on P7 and P8 in order to better understand the time course for the expression of this behavior in the early postpartum period. See Figure 1 for a timeline of these experiments.
Figure 1. Timeline of Experiments 1 and 2.
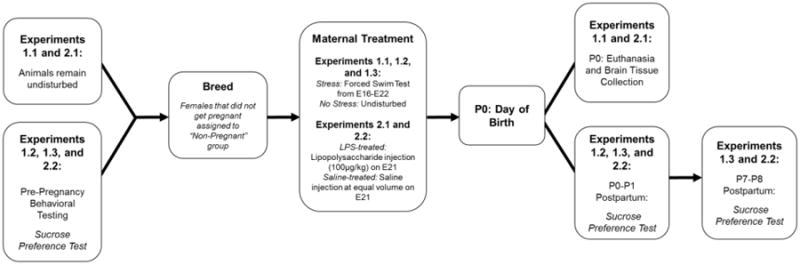
The experimental manipulations for Experiments 1.1, 1.2, 1.3, 2.1, and 2.2 are depicted. Animals in Experiments 1.1 and 2.1 did not undergo behavioral analysis and remained undisturbed during the time-matched equivalent of pre-pregnancy sucrose preference testing for females in Experiments 1.2, 1.3, and 2.2. All animals were bred, and females that did not get pregnant were assigned to the “Non-Pregnant” group. Females in Experiment 1 were subjected to forced swim stress or remained undisturbed between Embryonic (E) days 16-22. Females in Experiment 2 were treated with an i.p. injection of lipopolysaccharide (100μg/kg) or saline on E21. All females gave birth, and females in Experiments 1.1 and 2.1 were euthanized on the day of birth to collect brain tissue for analysis. Females in Experiments 1.2, 1.3, and 2.2 underwent postpartum sucrose preference testing immediately postpartum on P0-1, and animals in Experiments 1.3 and 2.2 repeated this test one week later on P7-8.
In Experiment 2, we examined the effects of acute immune activation during late gestation in pregnant females and time-matched non-pregnant females using an intraperitoneal injection of LPS on the expression of cytokines, microglial activation markers, and anhedonia during the immediate postpartum period. Experiments 2.1 and 2.2 used a total of 64 rats assigned to one of four experimental groups: Postpartum, Saline (n=8); Postpartum, LPS (n=8); Non-pregnant, Saline (n=8); or Non-pregnant, LPS (n=8). In this case, all female rats were mated, and rats that did not become pregnant (i.e., no sperm plug was found) were used as the Non-Pregnant control rats throughout the duration of the experiments. Females in Experiment 2.1 were given one injection of either LPS (100μg/kg) or sterile saline (1mL/kg) at least 24 hours prior to giving birth (approximately E22) or the time matched equivalent for non-pregnant females. Prior to the day of injection (E21, or the time-matched equivalent), all rats were separated to be housed individually for the remainder of the experiments. On the day of birth (P0) or at least 24 hours after the injection for non-pregnant animals, females were euthanized to analyze gene expression in response to late gestation immune activation. Females in Experiment 2.2 underwent the same injection procedures, and these animals were used to analyze postpartum anhedonia at two time points: P0 and P1 as in the previous experiment, and one week after parturition on P7 and P8 in order to observe a time course of the expression of this postpartum depressive-like behavior. See Figure 1 for a timeline of these experiments.
2.2 Forced Swim Test
In Experiments 1.1, 1.2, and 1.3, separate cohorts of female rats were assigned to the Stress condition and underwent the Forced Swim Test (FST) for 7 consecutive days. Rats were forced to swim in a clear cylinder (Stoelting Co., IL) filled with 20±1°C tap water with no option of rest or escape for five minutes each day for the last week of their gestation (E16-E22) or a time-matched period for non-pregnant rats. Rats were monitored by researchers to prevent accidental drowning, and during the course of this experiment, no rats in any of the treatment groups “gave up” and stopped swimming while in the apparatus. Prior to the first day of testing (E15) or the time matched equivalent for No Stress and Non-pregnant groups, all females were separated to be housed individually for the remainder of the experiments. Unstressed rats remained undisturbed during the time-matched period.
2.3 Lipopolysaccharide
Lipopolysaccharide (LPS) derived from Escherichia coli 0111:B4 was obtained from Sigma-Aldrich® (Cat. No. L2630). LPS was diluted with sterile Dulbecco's phosphate buffered saline (DPBS) to a concentration of 100μg/mL for injections and rats were injected at a volume of 1 ml/kg with a final dose of 100 μg/kg LPS.
2.4 Injections
Experiments 2.1 and 2.2 used injections of either sterile DPBS or lipopolysaccharide (LPS) as prepared above. At least 24 hours prior to giving birth (approximately E22) or the time-matched equivalent for non-pregnant rats, females were administered a one-time intraperitoneal injection of LPS at 100μg/kg of body weight or saline at 1mL/kg as a control. Prior to the day of injection (E21, or the time-matched equivalent), all rats were separated to be housed individually for the remainder of the experiments.
2.5 Sucrose Preference Testing
Experiments 1.2, 1.3, and 2.2 used identical measures of analysis to assess the depressive-like behavior of anhedonia using a sucrose preference test. All animals in these experiments underwent baseline behavioral analysis which took place prior to breeding the rats. Thus, we were not able to determine the results of our baseline data until after we bred the animals to learn which females were pregnant and subsequently assign them to stress groups at random. Sucrose preference testing took place over two consecutive days. During the test, females were individually housed in a dark room with clean testing cages without food from 3pm-6pm. The rats were allowed free access to two drinking bottles: one with regular tap water, and the other with a 1% sucrose solution. Both bottles were weighed before and after each day of testing to measure the amount of water or sucrose, in grams, the rats had consumed. The orientation of the bottles was randomized on the first day of testing and switched on the second day to control for any place preference. Data from the second test day were used to calculate the sucrose preference score with the following formula:
All rats from Experiments 1.2, 1.3, and 2.2 underwent sucrose preference testing on day of birth (P0) and P1 or the time-matched equivalent, and rats in Experiments 1.3 and 2.2 repeated this test one week after parturition on P7 and P8 or the time-matched equivalent to assess the potential time course of the expression of this behavior.
2.6 Euthanasia, Perfusion, and Tissue Collection
All euthanized rats were administered an overdose of the barbiturate Euthasol® (ANADA 200-071) via intraperitoneal injection. Sufficient anesthesia was assessed after the rat did not respond to a toe pinch. Once anesthetized, rats were perfused with 0.9% saline solution to remove peripheral blood from the brain tissue. After perfusion, half of the brain was extracted and post-fixed in 4% paraformaldehyde for immunohistochemistry, and the other half was dissected to collect the medial prefrontal cortex (mPFC) and whole hippocampus (HP) using the guide of a rat atlas. The hemisphere collected for each endpoint was randomized. Once the regions were extracted, tissue was immediately frozen on dry ice and stored at -80°C until processing.
2.7 Immunohistochemistry
After 24 hours in 4% paraformaldehyde, the half brains were transferred to a 30% sucrose/0.1% sodium azide solution to be dehydrated until slicing. Brains were flash frozen and sliced at 30 microns into 5 series using a Leica CM1950 cryostat. One series of slices were stained using anti-Iba1 ordered from Wako Chemicals (Cat No. 019-19741) and goat anti-rabbit biotinylated secondary antibody from Vector Laboratories in order to specifically visualize microglial cells. Sections were mounted onto microscope slides, and images of the mPFC and the CA1, CA3, and dentate gyrus (DG) regions of the hippocampus were taken using Stereo Investigator software. The densitometry of Iba1 staining in these brain regions was analyzed using ImageJ software, and the average integrated area densities from 6-8 sections (mPFC Bregma: from 5 to 2.5mm; CA1, CA3, and DG Bregma: from -2.5 to -4.5mm) were used to determine the average density of microglia present in these brain regions.
2.8 Real-Time PCR
Messenger RNA (mRNA) was extracted from frozen brain tissue using Isol-RNA Lysis Reagent (Cat. No. 2302700, 5 PRIME). Extracted RNA was then subjected to DNase treatment to remove any genomic DNA prior to cDNA synthesis using the QuantiTect® Reverse Transcription Kit (Cat. No. 205314, Qiagen). Relative gene expression was measured using the RealMasterMix™ Fast SYBR Kit (Cat. No. 2200830, 5 PRIME) in 10 μL reactions on a CFX96Touch™ real time PCR machine. The primer for Il-6 was a QuantiTect® Primer Assay Rn_Il6_1_SG (Cat. No. QT00182896, Qiagen) and diluted as per the Qiagen protocol for the real-time PCR reaction. All other primers were ordered through Integrated DNA Technologies and diluted to a final concentration of 0.13 μM for the real-time PCR reaction. The sequences of primers were as follows: GAPDH forward: GTTTGTGATGGGTGTGAACC, reverse: TCTTCTGAGTGGCAGTGATG (NM_017008.4); CD11b forward: CTGGGAGATGTGAATGGAG, reverse: ACTGATGCTGGCTACTGATG (NM012711.1); IL-1β forward: GAAGTCAAGACCAAAGTGG, reverse: TGAAGTCAACTATGTCCCG (NM031512.2); BDNF forward: ATCCCATGGGTTACACGAAGGAAG, reverse: AGTAAGGGCCCGAACATACGATTG (NM001270638.1); IL-4 forward: AAGGAACACCACGGAGAACG, reverse: CAGACCGCTGACACCTCTAC (NM201270.1). GAPDH was used as the reference/housekeeping gene for all samples as it was not significantly different across experimental groups. For each reaction, the quantitative threshold amplification cycle number (Cq) was determined, and the 2-ΔΔCq method was used to calculate the relative gene expression of each gene in question.
2.9 Statistical Analysis
Two-way ANOVA tests were used to assess the statistical significance of all gene expression data in this study using stress and reproductive state as factors (Experiment 2) or LPS and reproductive state (Experiment 3) as factors of analysis. Significant main effects and significant interactions of these factors were reported using p < 0.05. Significant interactions were followed up with Tukey's post hoc test (p < 0.05) to analyze individual differences. Repeated measures ANOVA tests were used to assess statistical significance of reproductive state and / or stress / or immune challenge on sucrose preference behavior within animals. Significant main effects and interactions were reported using p < 0.05. Significant interactions in the repeated measures ANOVA were followed up with two-way ANOVA tests for the individual time points of sucrose preference behavior to analyze group differences.
3. Results
3.1 Experiment 1: Effect of stress during late gestation on neuroimmune function and depressive-like behavior postpartum
Experiment 1 sought to determine the effect of antenatal stress during the last week of gestation on postpartum neuroimmune function (Experiment 1.1) and depressive-like behaviors (Experiments 1.2 and 1.3). We used a version of sub-chronic stress modified from Pan et al. (2013) that we predicted would be insufficient to induce depressive-like behavior in control, non-pregnant female rats and produce only moderate associated changes in neuroimmune function within the brain. In contrast, we predicted that pregnant females may be more sensitive to the effects of this sub-chronic stressor, such that these females may be more likely to exhibit significant changes in neuroimmune function and associated depressive-like behaviors postpartum.
3.1.1 Experiment 1.1: Impact of antenatal stress during late pregnancy on postpartum neuroinflammatory gene expression and microglia density in the medial Prefrontal Cortex and Hippocampus
First, we examined the expression of immune molecules in the mPFC immediately postpartum following stress during late gestation. We found that CD11b, a proxy of microglial activation, was significantly decreased in the mPFC of postpartum females compared to its expression in non-pregnant females (F3,29 = 6.369, p = 0.017; Figure 2A). Forced swim stress did not significantly affect the expression of CD11b. We also found a significant decrease in the expression of IL-1β in females postpartum compared to their non-pregnant counterparts (main effect of reproductive state: F3,27 = 10.664, p = 0.003; Figure 2B); as well as a significant main effect of stress on IL-1β expression such that females that received forced swimming stress had decreased IL-1β compared to non-stressed animals (F3,27 = 16.312, p < 0.001), and the effect of the stress during pregnancy was additive (Figure 2B). Next, we examined the expression of IL-6, a cytokine that has both pro- and anti-inflammatory properties. In contrast to CD11b and IL-1β expression, we found that postpartum females exhibited a significant increase in the expression of IL-6 in the mPFC compared to non-pregnant females (F3,29 = 6.236, p = 0.018; Figure 2C). Stress had no effect on the expression of IL-6. The expression of BDNF, a marker for neurogenesis and critical for learning and memory, was also significantly increased in the mPFC of females immediately postpartum compared to non-pregnant animals (main effect of reproductive state: F3,29 = 130.64, p < 0.0001; Figure 2D). Stress did not have any significant impact on the expression of BDNF in the mPFC.
Figure 2. Effects of Reproductive State and Antenatal Forced Swim Test on Relative Inflammatory Gene Expression in the Medial Prefrontal Cortex and Hippocampus Immediately Postpartum.
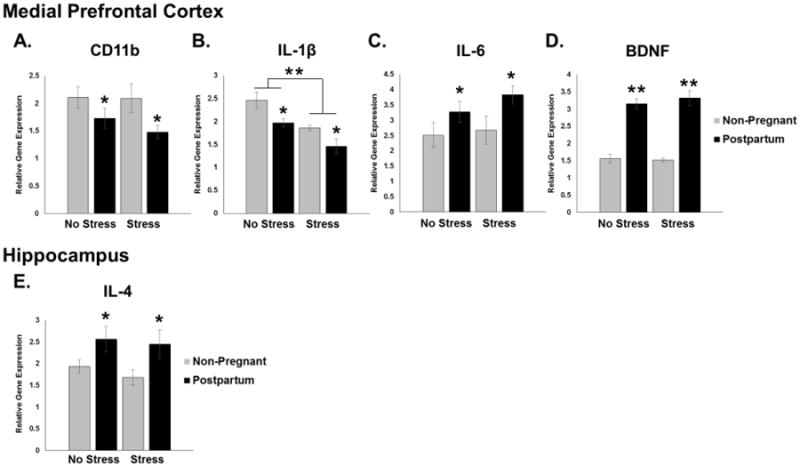
Stressed rats received daily forced swim stress from Embryonic (E) day 16 through E21 while rats in the No Stress groups remained undisturbed. Rats were sacrificed on the day of birth (P0) or 24 hours after the last day of forced swim test for non-pregnant animals. A, C, D. In the mPFC, CD11b, IL-6, and BDNF showed main effects of reproductive state. B. Il-1β analysis showed significant main effects of both reproductive state and forced swim stress. There were no effects on IL-4 expression in mPFC. E. In the HP, analysis revealed a significant main effect of reproductive state on the expression of IL-4. There were no significant changes in relative gene expression of CD11b, IL-1β, IL-6, or BDNF. *: p < 0.05; **: p < 0.001
In contrast to data obtained from the mPFC, we found very few changes in gene expression in the HP. We found no significant main effects or interactions of reproductive state and/or stress on the expression of CD11b, IL-1β, IL-6, or BDNF across these groups following either pregnancy or stress (data not shown). In addition to classical inflammatory cytokines, we examined IL-4 expression, an alternate immune gene. The expression of IL-4 was significantly increased in the HP immediately postpartum (main effect of reproductive state: F3,26 = 7.935, p = 0.009; Figure 2E). Of note, IL-4 expression was unaffected in the mPFC (data not shown). We also analyzed the density of microglia in the mPFC and CA1, CA3, and dentate gyrus (DG) of the hippocampus using an antibody for Iba1, a protein found on the membrane of microglia. There were no main effects of reproductive state or antenatal stress on the density of microglia in the mPFC or CA3 regions (Figure 3A and 3C). In the CA1 region of the HP, we found a significant main effect of reproductive state such that postpartum females had a decrease in the density of microglia compared to their non-pregnant counterparts (F3,24 = 31.572, p < 0.001; Figure 3B). Similarly, in the DG, there was a significant main effect of reproductive state indicating that postpartum females had a lower density of microglia compared to non-pregnant rats (F3,25 = 11.187, p = 0.003; Figure 3D). We also found a significant main effect of antenatal stress such that stressed animals had a higher density as compared to the undisturbed females (F3,25 = 4.530, p = 0.043; Figure 3D).
Figure 3. Effects of Reproductive State and Antenatal Forced Swim Test on Microglia Density in the Medial Prefrontal Cortex and CA1, CA3, and Dentate Gyrus Regions of the Hippocampus Immediately Postpartum.
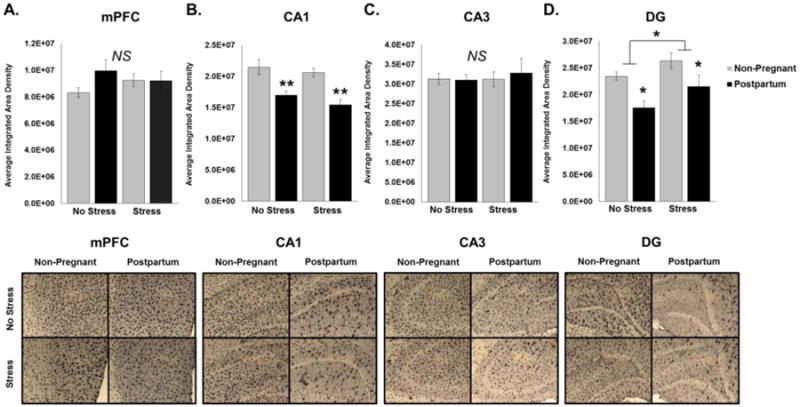
Stressed rats received daily forced swim test from Embryonic (E) day 16 through E21 while rats in the No Stress groups remained undisturbed. Rats were sacrificed on the day of birth (P0) or 24 hours after the last day of forced swim test for non-pregnant animals and the density of Iba1 stain was performed in the (A) Medial Prefrontal Cortex, (B) CA1 hippocampus, (C) CA3 hippocampus, and (D) Dentate Gyrus [DG] of the hippocampus. B. Analysis of CA1 Iba1 densitometry revealed a significant main effect of reproductive state (p < 0.001) such that only postpartum females had significantly lower integrated density of microglia compared to non-pregnant animals. D. The DG revealed both a significant main effect of reproductive state as well as a main effect of sub-chronic stress (p < 0.05). There were no significant effects on integrated density of microglia in the mPFC or CA3 regions. *: p < 0.05; #: p < 0.001
3.1.2 Experiment 1.2: Impact of antenatal stress during late pregnancy on postpartum depressive-like behaviors
In addition to postpartum changes in neuroimmune molecules, we also examined postpartum anhedonia in response to antenatal stress using the sucrose preference test. We predicted that the sub-chronic forced swim stress would not significantly affect behavior in non-pregnant animals, but that the same stressor during the last week of gestation would precipitate changes in postpartum depressive-like behavior. Using a repeated measures ANOVA test, we found a significant main effect of time point (F1,27 = 6.034, p = 0.021). Additionally, we found a trend for an interaction of time point, reproductive state, and stress (F1,27 = 3.05, p = 0.092). Following this analysis, we performed two-way ANOVA tests to determine differences across groups within the individual time points. We found no significant differences in sucrose preference between groups prior to being assigned to pregnancy or stress conditions (Figure 4). Following sub-chronic stress and parturition, analysis of sucrose preference on P1 revealed a significant interaction of reproductive state and stress (F3,28 = 5.006, p = 0.033) such that there was a decrease in sucrose preference in the postpartum females (p = 0.006) and stressed non-pregnant females (p = 0.032; Figure 4) compared to the control non-pregnant females. These data indicate that our stressor was robust enough to elicit depressive-like behavioral changes in non-pregnant females and that postpartum females also exhibited similar levels of depressive-like behavior, even in the absence of antenatal stress. Interestingly, the effects of stress and pregnancy were not additive as the anhedonia expressed by the stressed postpartum females did not significantly differ from any other groups.
Figure 4. Effects of Reproductive State and Antenatal Forced Swim Stress on Sucrose Preference Test Immediately Postpartum.
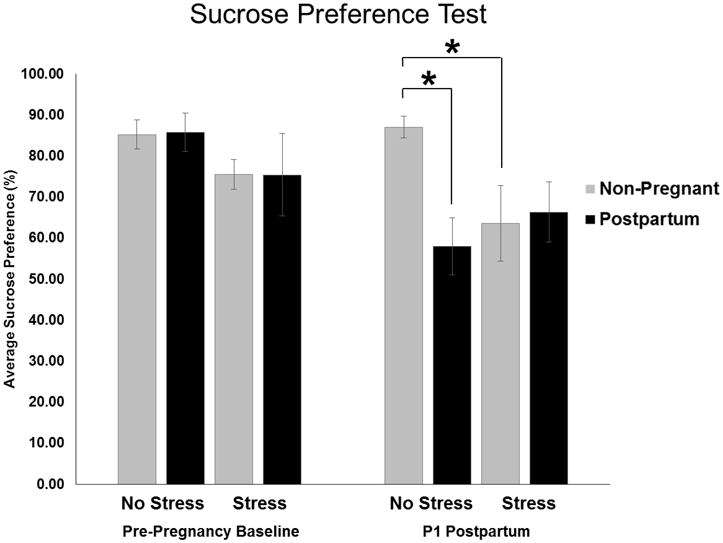
Stressed rats received daily forced swim test from Embryonic (E) day 16 through E21 while rats in the No Stress groups remained undisturbed. Pre-Pregnancy Baseline tests were completed prior to breeding the animals. Sucrose measurements were taken on Postpartum (P) day 1. Statistical analysis revealed an interaction of reproductive state and stress immediately postpartum (p < 0.05). Post hoc analysis showed that Postpartum, No Stress and Non-pregnant, Stressed groups had significantly lower sucrose preference than Non-pregnant, No Stress controls. *: p < 0.05
3.1.3 Experiment 1.3: Impact of antenatal stress during late pregnancy on long-term postpartum depressive-like behaviors
In Experiment 1.2, we found that postpartum females exhibited depressive-like anhedonia similar to that expressed by stressed females. Thus, we sought to determine whether postpartum depressive-like anhedonia would continue one week postpartum. Therefore, we repeated Experiment 1.2 with a separate cohort of females in order to replicate our previous findings and subsequently analyze depressive-like behavior one week postpartum using the sucrose preference test.
Using a repeated measures ANOVA test, we found a significant interaction of time point, reproductive state, and stress (F1,23 = 3.575, p = 0.044). Following this analysis, we performed two-way ANOVA tests within the individual time points to determine group differences. Replicating our previous findings from Experiment 1.2, we found a significant interaction of reproductive state and antenatal stress on sucrose preference immediately postpartum on P1 (F3,25 = 11.519, p = 0.002) such that the postpartum females and the stressed non-pregnant females displayed a significantly lower preference for sucrose compared to undisturbed non-pregnant females (post hoc comparisons: p = 0.008 and p = 0.006, respectively; Figure 5). Interestingly, we found no significant differences in sucrose preference one week postpartum and/or one week after sub-chronic stress, such that no group exhibited depressive-like behaviors at this later time point.
Figure 5. Effects of Reproductive State and Antenatal Forced Swim Stress on Long-Term Sucrose Preference Postpartum.
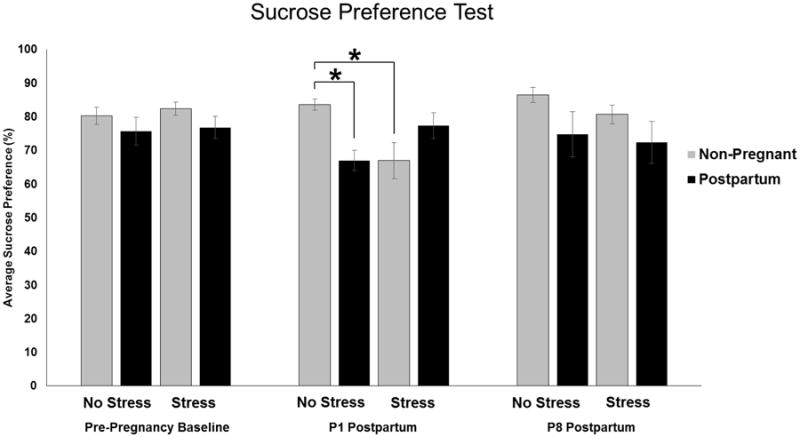
Stressed rats received daily forced swim test from Embryonic (E) day 16 through E21 while rats in the No Stress groups remained undisturbed. Pre-Pregnancy Baseline tests were completed prior to breeding the animals. Postpartum sucrose preference scores were taken on Postpartum (P) day 1 and P8. Statistical analysis revealed an interaction of reproductive state and stress immediately postpartum (P1) (p < 0.05). Post hoc analysis revealed Postpartum, No Stress and Non-pregnant, Stressed females had significantly lower sucrose preference than Non-pregnant, No Stress controls. There were no significant differences one week later. *: p < 0.05
3.2 Experiment 2: Effect of immune activation during late gestation on neuroimmune and behavioral changes postpartum
Next, we sought to determine whether immune activation during late gestation would significantly alter cytokine production in the brain with an associated increase in depressive-like behaviors postpartum. Immune activation caused by LPS administration typically increases the expression of classical pro-inflammatory cytokines and elicits depressive-like or sickness behaviors within a brief 24 hour time period (Dantzer et al., 2008; Biesmans et al., 2013). Given the significant changes in immune function produced by pregnancy, we predicted that pregnancy may prolong the antenatal immune response beyond this 24 hour period and inflammation would persist into the early postpartum period and subsequently increase the risk of associated depressive-like behaviors postpartum.
3.2.1 Experiment 2.1: Impact of acute antenatal immune activation during late pregnancy on postpartum neuroimmune function and microglial density in the medial Prefrontal Cortex and Hippocampus
In the mPFC, analysis of the microglial activation marker CD11b revealed an interaction of reproductive state and antenatal immune activation (F3,26 = 5.745, p = 0.024; Figure 6A). As expected, LPS did not increase the expression of CD11b more than 24 hours post-injection in control females; however, antenatal LPS administration significantly decreased CD11b expression in females postpartum (p = 0.022; Figure 6A). Analysis of the classical pro-inflammatory cytokine IL-1β also revealed a significant interaction of reproductive state and antenatal immune activation (F3,26 = 5.324, p = 0.029; Figure 6B). Post hoc analysis revealed a trend (p < 0.08) such that LPS administration decreased expression of IL-1β in postpartum females (p = 0.077), similar to the results of CD11b (Figure 6B). Analysis of IL-6 also revealed a significant interaction of reproductive state and antenatal LPS immune activation (F3,25 = 10.281, p = 0.004; Figure 6C). IL-6 was significantly increased within the mPFC immediately postpartum, as seen in the previous experiment, as well as 24 hours after LPS administration. However, these effects were not additive such that when LPS was administered during late gestation, IL-6 expression did not significantly differ from expression in the non-pregnant control females (Figure 6C). These data suggest that the induction of IL-6 postpartum is likely initiated via a similar mechanism to that induced by LPS immune activation, or that on-going changes in immune function postpartum modulate the induction of IL-6 produced by LPS.
Figure 6. Effects of Reproductive State and Acute Antenatal Immune Activation on Inflammatory Gene Expression in the Medial Prefrontal Cortex and Hippocampus Immediately Postpartum.
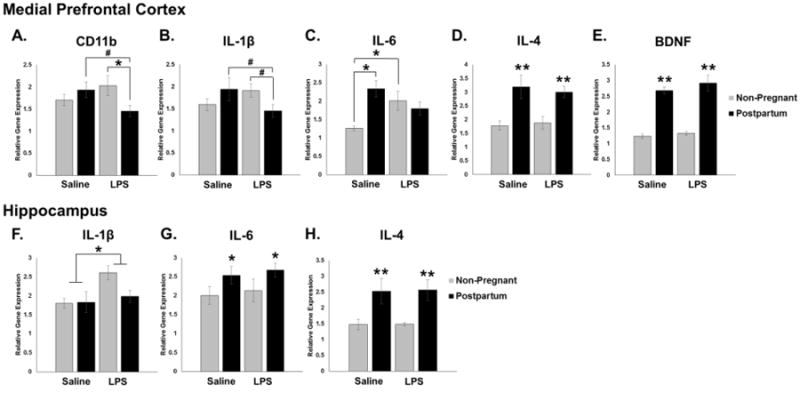
Between Embryonic (E) day 21 and 22, rats received an injection of either lipopolysaccharide (LPS) (100μg/kg) or its saline vehicle at equal volume. Animals were euthanized on day of birth (P0) or at least 24 hours after the injection for non-pregnant animals. A. In the mPFC, analysis of CD11b expression revealed an interaction of pregnancy and immune activation. Post hoc analysis indicated the Postpartum, LPS-treated group had significantly lower expression than the Non-pregnant, LPS-treated group. B. IL-1β analysis revealed a significant interaction of pregnancy and immune activation. Post hoc analysis revealed trends that the Pregnant, LPS-treated group had lower expression than the Non-pregnant, LPS-treated and Postpartum, Saline-treated groups (p < 0.08). C. Analysis of IL-6 showed a significant main effect of pregnancy and an interaction of pregnancy and immune activation such that the Postpartum, Saline-treated animals and the Non-pregnant, LPS-treated animals had significantly higher expression compared to Non-pregnant, Saline-treated controls. D, E. IL-4 and BDNF analysis revealed a significant main effect of reproductive state in that only postpartum females showed higher gene expression relative to non-pregnant rats. F. In the HP, analysis of IL-1β showed a main effect of treatment. Only the Non-pregnant, LPS-treated group showed significantly higher gene expression compared to the other three groups (p < 0.05). G, H. IL-6 and IL-4 showed a main effect of pregnancy such that postpartum rats showed higher gene expression relative to the non-pregnant groups. No significant differences were found for CD11b or BDNF in the HP (not shown). *: p < 0.05; **: p < 0.001, #: p < 0.08
In addition to classical inflammatory cytokines, we examined IL-4 expression, an alternate immune gene. Analysis revealed a significant main effect of reproductive condition such that postpartum females had higher expression of IL-4 compared to their non-pregnant counterparts (F3,27 = 23.628, p < 0.001; Figure 6D), similar to the effects found in the previous experiment. Lastly, analysis of BDNF, a crucial neurotrophic factor for neurogenesis and learning and memory, also revealed a significant main effect of reproductive condition in which postpartum females had significantly elevated expression levels of BDNF compared to non-pregnant animals (F3,27 = 107.211, p < 0.001; Figure 6E). We found no effects of LPS treatment on the expression of IL-4 or BDNF in mPFC more than 24 hours later.
In the hippocampus, there were no significant effects of reproductive state or immune activation on the microglial activation marker CD11b. Analysis of the pro-inflammatory cytokine IL-1β revealed a significant main effect of LPS treatment (F3,23 = 6.406, p = 0.019; Figure 6F). Interestingly, IL-1β remained elevated in the hippocampus more than 24 hours following LPS treatment in non-pregnant rats; however, this effect is significantly attenuated in females that were pregnant at the time of the immune activation, similar to effects seen in IL-6 in the mPFC.
Next, analysis of IL-6 expression revealed a significant main effect of reproductive state such that postpartum females had greater expression compared to non-pregnant animals (F3,25 = 4.627, p = 0.041; Figure 6G). Similarly, analysis of the alternate immune gene IL-4 revealed a significant main effect of reproductive condition in that postpartum females had higher expression compared to their non-pregnant counterparts (F3,24 = 16.499, p < 0.001; Figure 6H). There were no significant effects of LPS treatment on either IL-6 or IL-4 expression in the hippocampus. These data replicate the findings in the hippocampus from Experiment 1.1 (see Figure 2E) as well as the data obtained from the mPFC of the current study (Figure 6A-E). Lastly, there were no significant effects of reproductive state or acute antenatal LPS treatment on BDNF expression in the hippocampus at least 24 hours post-injection, similar to our findings from Experiment 1.
We also analyzed the density of microglia in the mPFC, and CA1, CA3 and DG regions of the hippocampus in these same females. Interestingly, we found no significant effects of either reproductive state or LPS treatment in the mPFC, CA1, or DG regions. In the CA3 region, however, analysis revealed a significant main effect of reproductive state such that postpartum females exhibited a significantly greater density of microglia compared to their non-pregnant counterparts (F3,26 = 28.772, p < 0.001; Figure 7C).
Figure 7. Effects of Reproductive State and Antenatal Immune Activation on Integrated Microglia Density in the Medial Prefrontal Cortex and CA1, CA3, and Dentate Gyrus Regions of the Hippocampus Immediately Postpartum.
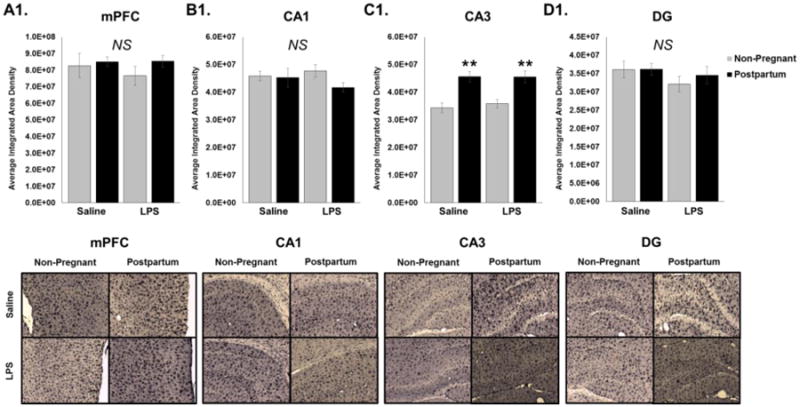
Between Embryonic (E) day 21 and 22, rats received an injection of either lipopolysaccharide (LPS) (100μg/kg) or its saline vehicle at equal volume. Animals were euthanized on day of birth (P0) or at least 24 hours after the injection for non-pregnant animals and the density of Iba1 stain was performed in the (A) Medial Prefrontal Cortex, (B) CA1 hippocampus, (C) CA3 hippocampus, and (D) Dentate Gyrus [DG] of the hippocampus. C. Analysis of CA3 revealed a significant main effect of reproductive state (p < 0.001) such that only postpartum females had significantly higher integrated density of microglia compared to non-pregnant animals. There were no significant effects on integrated density of microglia in the mPFC, CA1, or DG regions. **: p < 0.001
3.2.2 Experiment 2.2: Impact of acute immune activation during pregnancy on postpartum depressive-like behaviors
We also analyzed depressive-like behavior using sucrose preference testing immediately postpartum following the same paradigm of immune activation during late gestation. Using a repeated measures ANOVA test, we found a significant interaction of time point, reproductive state, and LPS treatment (F1,26 = 4.681, p = 0.018). Following this interaction, we performed two-way ANOVA tests to determine any group differences within the individual time points. Analysis of baseline sucrose preference (i.e., prior to breeding) did not reveal any significant differences across groups. Similar to our previous experiment, we found that postpartum females (P0-P1) exhibited significantly decreased sucrose preference compared to their non-pregnant counterparts (F3,28 = 8.181, p = 0.008; Figure 8). We found no effect of LPS administered more than 24 hours earlier on sucrose preference. Also similar to our previous findings, postpartum anhedonia did not continue one week after giving birth; however, we did find a significant main effect of the acute LPS treatment at the second time point of analysis such that females treated with LPS more than one week earlier had a higher preference for sucrose, and thus less anhedonia in comparison to saline-treated females, regardless of reproductive state (F3,30 = 5.944, p = 0.021; Figure 8). These data again highlight that postpartum females exhibit significant anhedonia immediately postpartum and also suggest that LPS immune activation may increase the expression of ingestive or reward-seeking behaviors at least one week following acute immune activation.
Figure 8. Effects of Reproductive State and Acute Antenatal Immune Activation on Immediate and Long-Term Sucrose Preference Postpartum.
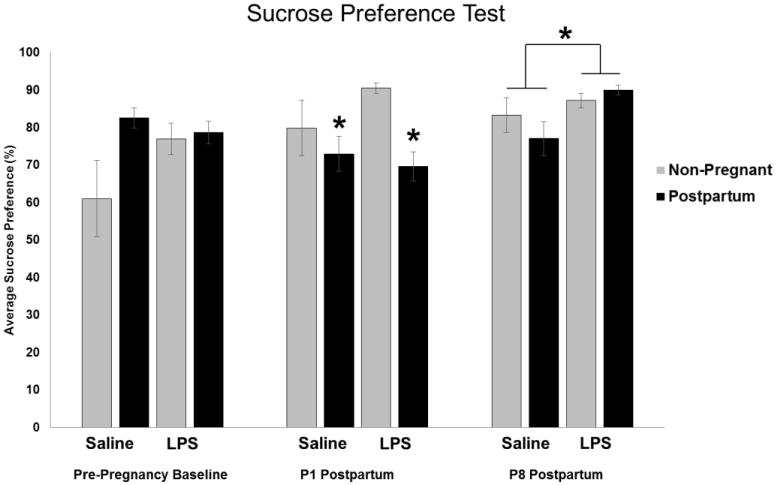
Between Embryonic (E) day 21 and 22, rats received an injection of either lipopolysaccharide (LPS) (100μg/kg) or its saline vehicle at equal volume. Pre-Pregnancy Baseline tests were completed prior to breeding the animals. Sucrose preference scores were taken on Postnatal (P) day 1, and one week later on P8. Analysis of sucrose preference revealed a significant main effect of reproductive state immediately postpartum (P1) such that females that had just given birth showed lower preference for sucrose compared to non-pregnant rats. One week later, analysis showed a main effect of treatment such that LPS-treated rats had greater sucrose preference compared to their saline counterparts. *: p < 0.05
4. Discussion
The experiments presented here sought to examine how stress or immune activation during late gestation may impact the expression of immune molecules in the brain immediately postpartum and the risk of postpartum depressive-like behaviors. These aims intended to provide us with a better understanding of how the central nervous system responds to robust physiological changes associated with pregnancy and parturition particularly in combination with stress or immune activation. We found a number of cytokines that were differentially expressed in the brain immediately postpartum, indicating that neuroimmune function is dramatically altered immediately postpartum compared to non-pregnant females. We also found significant changes in microglial density within the CA1, CA3, and DG of the hippocampus in postpartum females, indicating that microglia themselves are significantly altered immediately after giving birth. In addition, postpartum females exhibited increased anhedonia immediately postpartum that was similar to the anhedonia produced by sub-chronic stress. Finally, we found that pregnancy and/or parturition significantly attenuates the expression of cytokines in the brain following acute immune activation.
It is well known that pregnancy significantly alters peripheral immune function in the mother, a process that allows for the proper growth and development of the fetus. Given the constant communication between the peripheral and central neuroimmune systems, we hypothesized that pregnancy may also produce significant changes in immune function within the brain. Specifically, we hypothesized that challenging the immune system with either stress or the bacterial endotoxin LPS during pregnancy would induce a change in neuroimmune function in the immediate postpartum period that may simultaneously increase the risk for exhibiting postpartum depressive-like behaviors. Thus, we examined two brain regions implicated in major depressive disorder that are also particularly vulnerable to stress and immune activation: the medial prefrontal cortex and hippocampus (Pandya et al., 2012), as well as the depressive-like behavior of anhedonia in postpartum female rats.
In fact, we observed a number of immune genes that were significantly altered immediately postpartum, even in the absence of stress or overt immune activation. We found that CD11b and IL-1β were significantly decreased in the mPFC immediately postpartum compared to non-pregnant rats. These data were surprising because they suggest that the experiences of pregnancy and parturition do not create a classic pro-inflammatory state within the brain as one might predict. Instead, we see a decrease in microglia activation as marked by lower CD11b expression in postpartum females as well as a decrease in the classic proinflammatory cytokine IL-1β in the mPFC for postpartum animals. Furthermore, in the HP, postpartum females exhibited lower densities of microglia in the CA1 and DG regions, which may corroborate this neuroimmune evidence of a decreased pro-inflammatory state caused by pregnancy or parturition. However, it is particularly interesting that pregnancy had the opposite effect on the expression of two other important cytokines in the brain. Specifically, both IL-6 and IL-4 were increased immediately postpartum within the mPFC and HP. IL-6 is a cytokine that has a wide range of both pro- and anti-inflammatory properties, and the way in which IL-6 acts is determined by which molecular pathway is activated: either a “classic” or “trans-” signaling pathway. Classic signaling results in a more anti-inflammatory role, and this pathway has the ability to skew the differentiation of peripheral T cells away from the classic, pro-inflammatory Th1 subset of cells towards the alternate, more anti-inflammatory Th2 subset of cells (see Romagnani, 1999 and Scheller et al., 2011 for more comprehensive reviews, and Xing, et al., 1998). Relative gene expression data from our experiments is insufficient to determine precisely the manner in which IL-6 is acting in postpartum females; however, IL-4 is also known to be an alternate immune gene expressed under Th2 cell differentiation. Given that we find increases in IL-6 and IL-4 and associated decreases in IL-1β gene expression in the brain of postpartum females, it is likely that pregnancy or parturition increases IL-6 expression to similarly attenuate microglial cell activation away from the M1 microglial phenotype, similar to the Th1, IL-1β-driven pro-inflammatory state, and more towards the M2 microglial phenotype, similar to the Th2, IL-4-driven anti-inflammatory state (Wang et al., 2014). Thus, the findings presented here provide evidence that pregnancy and/or parturition induce a significantly altered microglial profile in the brain immediately postpartum, which can be further examined in future experiments.
This proposed function of the neuroimmune system (as M2 vs. M1 microglial phenotype) induced by pregnancy or parturition is further supported by the cytokine expression data from Experiment 2 in which we administered LPS to females during late gestation to activate the immune system and produce brief and robust pro-inflammation just before giving birth. Pregnant females that had been treated with LPS more than 24 hours before giving birth had significantly lower expression of CD11b and IL-1β than their non-pregnant counterparts as well as trends that this postpartum LPS-treated group had lower expression compared to their pregnant saline-treated counterparts. Thus, even after a robust pro-inflammatory response induced by LPS (Biesmans et al., 2013), pregnancy, parturition, and/or the immediate postpartum period are modulating the effects of this immune activation to blunt increased levels of pro-inflammatory cytokines and possibly even reverse this inflammation postpartum. One caveat to these experiments is that we were unable to replicate our findings from the first experiment indicating that CD11b and IL-1β are significantly decreased in the mPFC immediately postpartum (in the absence of LPS treatment). We hypothesize that this is likely due to either an effect of the injection producing increased cytokine expression in the postpartum brain or that differences in housing conditions between the two experiments produces differences in stress / immune activation even in untreated pregnant females. Given that the levels of IL-6 and IL-4 are robustly increased immediately postpartum, in both experiments, independent of LPS administration, it would suggest that postpartum females have altered immune function compared to non-pregnant controls which subsequently affects the way in which the central immune system responds to peripheral immune activation during pregnancy or parturition. A full time course analysis of cytokine expression following LPS-induced immune activation during pregnancy and parturition would be an important subsequent experiment to expand upon our present findings indicating significant changes in the function of microglia during late gestation and immediately postpartum.
In the context of depression, these data prove to be particularly intriguing. Numerous studies in humans have attempted to profile major depressive disorder and its associated behavioral and neurological sequelae in order to develop more accurate animal models for improved anti-depression therapies. Recently, Loftis and colleagues compared several symptoms of depression in humans such as anhedonia, fatigue, and diminished cognitive ability to their associated behaviors in rodents including decreased sucrose consumption, decreased locomotor activity, and decreased spatial memory (2010). In our current experiments, we found that postpartum females had decreased sucrose preference immediately postpartum, suggesting these new mothers were exhibiting depressive-like anhedonia behavior. We found that sub-chronic stress induced similar levels of anhedonia in non-pregnant females. Sub-chronic stress during late gestation, however, did not result in a further decrease in sucrose preference, suggesting that either the postpartum experience modulated the perception of the stressor or that the depressive-like behavior produced by these distinct life events may be initiated via similar mechanisms.
Our collective data indicate, however, that postpartum anhedonia coincides with an anti-inflammatory neuroimmune profile immediately postpartum; while previous studies have shown that stress-induced models of depression elicit an activated immune response and elevated levels of many pro-inflammatory cytokines (Walkera et al., 2013; Miller et al., 2009; Anisman and Merali, 2002). Furthermore, it is also well-documented that there is a significant decrease in the neurotrophic factor BDNF in cases of major depression, which may help to explain decreased volumes of gray matter in postmortem brains (Lee and Kim, 2010). In our current experiments we found robust increases in the expression of BDNF within the mPFC of postpartum females, likely important for the robust change in neural function necessary to induce maternal behavior (Numan, 2007) and unrelated to the depressive-like anhedonia we observed concurrently in our postpartum females. While our current experiments lack additional evidence that postpartum females are conclusively exhibiting depressive-like behaviors, the difference between our data and the literature is striking and suggest that the risk factors and etiology of postpartum anhedonia may be quite distinct from those that have been so well-examined for other major depressive disorders.
Unfortunately, pregnancy and the postpartum period have not been extensively studied in either rodents or humans, particularly in the context of postpartum depression. Postpartum depression is currently classified as a depressive disorder in which the only specific criteria necessary for diagnosis is peripartum onset of a major depressive episode (American Psychiatric Association, 2013); however, postpartum depression may in fact be much more distinct than that. Typical antidepressants prescribed for humans have proven to be an ineffective treatment of depressive-like behaviors in pregnant or postpartum female rodents (Bourke et al., 2013; Craft et al., 2010). Thus future studies should continue to examine the onset of postpartum anhedonia in rodents and the risk factors that may prolong or exacerbate this depressive-like behavior during this is unique period of dramatic physiological change. In addition, pregnancy and the postpartum period are important physiological events about which neuroscientists understand very little. Our findings indicate that important changes occur in both the neural and immune systems during pregnancy and immediately postpartum, which provides novel insight into how these life events can either transiently or permanently influence neural function in adulthood.
That said, in the current experiments, it is unclear whether these novel effects of reproductive state observed in both the brain and behavior were initiated by pregnancy itself, the process of parturition, or the novel experience of caring for and nursing a litter of pups; however, these findings outline the postpartum period as a significant time for the mother. It is possible that the postpartum period is its own period of unique stress. Combined with the stress of pregnancy, parturition and additional life stressors, the risks for developing postpartum depression may greatly increase when incorporating the stress of motherhood. Additionally, we have examined only mRNA expression within the mPFC and HP to allow for a more comprehensive examination of multiple immune molecules within the brain. At this time, we have not measured protein levels of these immune molecules to determine if our mRNA expression data correlate with subsequent changes in protein levels. Furthermore, we have not yet determined if the changes we observe in the brain correspond with immune changes that may also occur in the periphery of these postpartum females. One study examined long-term inflammatory cytokine expression three weeks postpartum following gestational restraint stress and found elevated levels of pro-inflammatory cytokines in whole-blood cultures that did not correlate with changes in protein levels of the same cytokines in the brain (O'Mahony et al., 2006). Nonetheless, our findings provide novel insight into specific changes in immune function associated with pregnancy, parturition, and/or the postpartum period. Future experiments should examine protein expression in conjunction with additional neuroimmune and behavioral endpoints before parturition, during lactation, as well as in multiparous dams in order to gain a better understanding of the individual effects of pregnancy, parturition, and new motherhood.
Our results have outlined several novel and important changes in neuroimmune gene expression within areas of the brain implicated in depression that also present with associated depressive-like anhedonia in postpartum females. While we cannot venture to say these findings lend a complete mechanism of action for postpartum depression or anhedonia, they provide an important foundation for further research on both pregnancy and the associated postpartum disorder. Additionally, an overwhelming amount of evidence has shown that unhappy, unhealthy, and stressed mothers do not adequately care for their young and in turn negatively impact the offspring such that cognitive deficits are seen during adolescence and adulthood. Thus, it is not only important that postpartum depression be understood for the sake of the mother's health, but also for the sake of the babies of suffering mothers.
Highlights.
“An investigation into the effects of antenatal stressors on the postpartum neuroimmune profile and depressive-like behaviors”
Posillico & Schwarz
Inflammatory cytokines are differentially expressed in the brain postpartum.
Pregnancy modulates the neuroimmune response to immune activation.
Pregnancy and sub-chronic stress both induce anhedonia immediately postpartum.
Acknowledgments
We would like to recognize Briana A. Basilone and Morgan L. Sherer for collecting data presented in this manuscript. This research was supported by NIH grant R21MH101663-01 to JMS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. 5th. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- Anisman H, Merali Z. Cytokines, stress, and depressive illness. Brain, Behavior, and Immunity. 2002;16(5):513–524. doi: 10.1016/s0889-1591(02)00009-0. [DOI] [PubMed] [Google Scholar]
- Biesmans S, Meert T, Bouwknecht J, Acton P, Davoodi N, De Haes P, Kuijlaars J, Langlois X, Matthews LJR, Ver Donck L, Hellings N, Nuydens R. Systemic immune activation leads to neuroinflammation and sickness behavior in mice. Mediators of Inflammation. 2013;2013:271359. doi: 10.1155/2013/271359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke CH, Capello CF, Rogers SM, Yu ML, Boss-Williams KA, Weiss JM, Stowe ZN, Owens MJ. Prenatal exposure to escitalopram and/or stress in rats: A prenatal stress model of maternal depression and its treatment. Psychopharmacology. 2013;228(2):231–241. doi: 10.1007/s00213-013-3030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509(7500):282–3. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JL, Connor Y, Kendell RE. Prospective study of the psychiatric disorders of childbirth. The British Journal of Psychiatry. 1982;140(2):111–117. doi: 10.1192/bjp.140.2.111. [DOI] [PubMed] [Google Scholar]
- Craft R, Kostick M, Rogers J, White C, Tsutsui K. Forced swim test behavior in postpartum rats. Pharmacology, Biochemistry, and Behavior. 2010;96(4):402–412. doi: 10.1016/j.pbb.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nature Reviews Neuroscience. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faisal-Cury A, Menezes PR, Tedesco J, Kahalle S, Zugaib M. Maternity "blues": Prevalence and risk factors. The Spanish Journal of Psychology. 2008;11(2):593. [PubMed] [Google Scholar]
- Harris B, Lovett L, Newcombe RG, Read GF, Walker R, Riad-Fahmy D. Maternity blues and major endocrine changes: Cardiff puerperal mood and hormone study II. Bmj. 1994;308(6934):949–953. doi: 10.1136/bmj.308.6934.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerley H, Gath D. Maternity blues. I. detection and measurement by questionnaire. The British Journal of Psychiatry. 1989;155(3):356–362. doi: 10.1192/bjp.155.3.356. [DOI] [PubMed] [Google Scholar]
- Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. The Lancet Infectious Diseases. 2010;10(5):338–349. doi: 10.1016/S1473-3099(10)70049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BH, Kim YK. The roles of BDNF in the pathophysiology of major depression and in antidepressant treatment. Psychiatry Investigation. 2010;7(4):231–235. doi: 10.4306/pi.2010.7.4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftis JM, Huckans M, Morasco BJ. Neuroimmune mechanisms of cytokine-induced depression: Current theories and novel treatment strategies. Neurobiology of Disease. 2010;37(3):519–533. doi: 10.1016/j.nbd.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzi M, Vigano A, Trabattoni D, Villa ML, Salvaggio A, Clerici E, Clerici M. Characterization of type 1 and type 2 cytokine production profile in physiologic and pathologic human pregnancy. Clinical & Experimental Immunology. 1996;106(1):127–133. doi: 10.1046/j.1365-2249.1996.d01-809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LJ. Postpartum depression. JAMA. 2002;287(6):762–765. doi: 10.1001/jama.287.6.762. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biological Psychiatry. 2009;65(9):732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Mental Health. National Institutes of Health. 2011. Depression. [Google Scholar]
- Numan M. Motivational systems and the neural circuitry of maternal behavior in the rat. Developmental Psychobiology. 2007;49(1):12–21. doi: 10.1002/dev.20198. [DOI] [PubMed] [Google Scholar]
- O'Mahony SM, Myint AM, van den Hove D, Desbonnet L, Steinbusch H, Leonard BE. Gestational stress leads to depressive-like behavioural and immunological changes in the rat. Neuroimmunomodulation. 2006;13(2):82–88. doi: 10.1159/000096090. [DOI] [PubMed] [Google Scholar]
- Pan Y, Lin W, Wang W, Qi X, Wang D, Tang M. The effects of central pro-and anti-inflammatory immune challenges on depressive-like behavior induced by chronic forced swim stress in rats. Behavioural Brain Research. 2013;247:232–240. doi: 10.1016/j.bbr.2013.03.031. [DOI] [PubMed] [Google Scholar]
- Pandya M, Altinay M, Malone DA, Jr, Anand A. Where in the brain is depression? Current Psychiatry Reports. 2012;14(6):634–642. doi: 10.1007/s11920-012-0322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnani S. Th1/Th2 cells. Inflammatory Bowel Diseases. 1999;5(4):285–294. doi: 10.1097/00054725-199911000-00009. [DOI] [PubMed] [Google Scholar]
- Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochimica Et Biophysica Acta (BBA) - Molecular Cell Research. 2011;1813(5):878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- Veenstra van Nieuwenhoven AL, Bouman A, Moes H, Heineman M, de Leij LFMH, Santema J, Faas MM. Cytokine production in natural killer cells and lymphocytes in pregnant women compared with women in the follicular phase of the ovarian cycle. Fertility and Sterility. 2001;77(5):1032–1037. doi: 10.1016/s0015-0282(02)02976-x. [DOI] [PubMed] [Google Scholar]
- Walkera FR, Nilsson M, Jones K. Acute and chronic stress-induced disturbances of microglial plasticity, phenotype and function. Current Drug Targets. 2013;14:1262–1276. doi: 10.2174/13894501113149990208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Frontiers in Immunology. 2014;5:614. doi: 10.3389/fimmu.2014.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisner KL, Sit DY, McShea MC, Rizzo DM, Zoretich RA, Hughes CL, Eng HF, Luther JF, Wisniewski SR, Costantino ML, Confer AL, Moses-Kolko EL, Famy CS, Hanusa BH. Onset timing, thoughts of self-harm, and diagnoses in postpartum women with screen-positive depression findings. JAMA Psychiatry. 2013;70(5):490–498. doi: 10.1001/jamapsychiatry.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, Achong MK. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. The Journal of Clinical Investigation. 1998;101(2):311–320. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]


