Abstract
Objective
To explore the relationship between gender, native artery diameters and outcomes of stent revascularization in the Claudication: Exercise versus Endoluminal Revascularization (CLEVER) trial.
Methods
A comparative analysis was performed of the impact of gender, age, weight, height, body mass index, and body surface area, on revascularization outcomes at baseline and 6 months in 55 arterial segments of aorta, common iliac and external iliac (EIA) arteries.
Results
Women demonstrated smaller diameter of the EIA. However, the clinical outcomes of revascularization were not negatively affected by the gender-based differences.
Conclusion
Gender-based differences are unlikely to significantly impact outcome of stent revascularization.
Keywords: Peripheral arterial disease, gender differences, Aortoiliac stent
1. INTRODUCTION
Many previous reports suggest that gender correlates with the outcome of several peripheral artery revascularization procedures such as percutaneous endovascular, surgical bypass and endarterectomy procedures.1-6 In particular, localization of disease to the external iliac artery has been reported by some to be a significant predictor of poor outcomes in women after iliac angioplasty and stent placement.2,5,6 In patients with coronary artery disease undergoing revascularization procedures, the potential explanation for poorer outcome in women has been the smaller diameter of coronary artery in women.7-13 However the data describing impact of gender based on peripheral arterial interventions lacks a strong evidence14 and is also quite limited.2,6,15-17
We utilized the database from the “Claudication: Exercise versus Endoluminal Revascularization” (CLEVER) study, which provided an opportunity to carefully measure native artery diameters and outcomes of revascularization by gender with the intent to assess if the diameter of iliac arteries were smaller in women and/or female gender and was this associated with a worse outcome after endovascular treatment. This analysis used only those patients who were randomly assigned to have undergone aortoiliac artery revascularization i.e.; the Stent Revascularization (ST) group in CLEVER trial cohort and these arteries were measured using quantitative vessel analysis. While the study sample is small; very well characterized subjects from the prospective, randomized trial permitted a careful analysis to be performed.
2. MATERIALS AND METHODS
2.1. STUDY POPULATION
The enrollment criteria, protocol design, subject, demographics and baseline characteristics of the CLEVER trial have been previously published. 18-20 As noted above, this study represents an analysis of the data derived only from the stent revascularization (ST) group. This group of patients, although enrolled based on noninvasive vascular testing, had all undergone catheter angiograms prior to endovascular treatment, which was performed according to the CLEVER study treatment protocol. For each participant, pre- and post- intervention images were obtained and sent to the Vascular Disease Research Center at Rhode Island Hospital for analysis, which was done using the QVA module of the Medis QAngio XA 7.1 software package (Leiden, the Netherlands). Previous studies have comparably utilized this system for various peripheral vascular anatomies and have confirmed its validity for vessel measurements.21,22
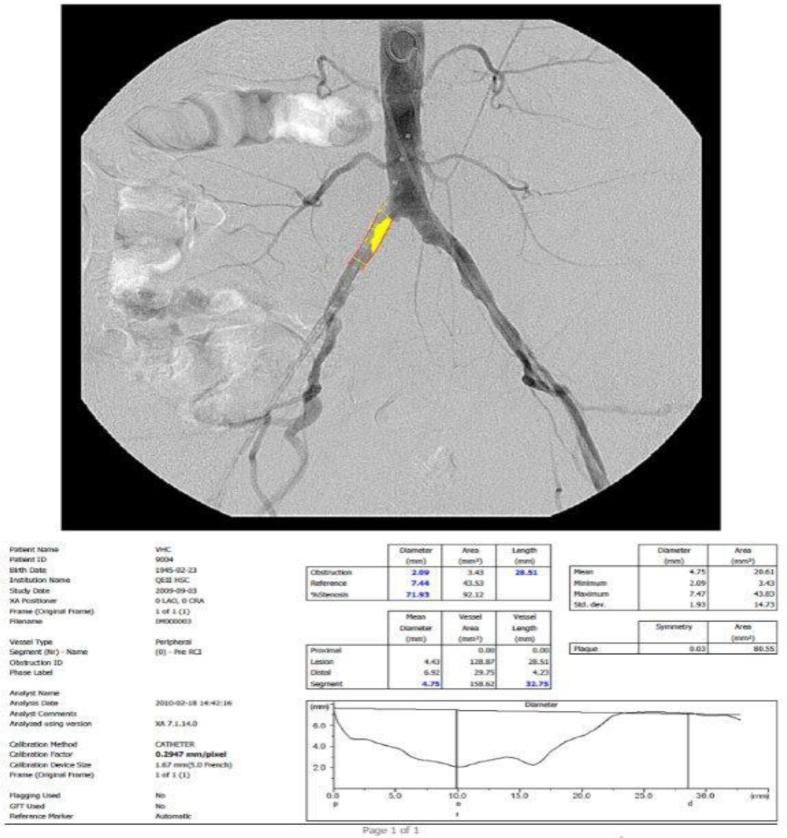
Arteries were grouped and analyzed by segment (e.g., common and external iliac artery). To analyze the arteriograms, the reader calibrated the system using the known diameter of the catheter (figure 1). Next, reference diameters were measured from regions proximal to the target lesions. The reference areas were plaque-free. Lastly, the diameter and length of stenosis were measured prompting the Medis QVA system to automatically calculate the percent stenosis, plaque symmetry, and vessel area. If a calibration was not possible due to catheter diameter being too small or if an image was received from a site that was not formatted correctly, calipers were visually placed on the normal vessel maximal diameter. If the vessel segment was occluded or stenotic throughout its length, the contralateral vessel of the same segment was used as a proxy. These measurements served as variables in our statistical analysis. These methods were consistent for all subjects and for all images pre- and post- stenting. In two cases, subjects 105-159 and 105-154, an additional bifurcation analysis was performed due to lesions that involved the aortoiliac bifurcation.
Figure 1.
A pre-stenting pelvic angiogram showing the catheter calibration method. The segment used for calibration is displayed in red lines. Information listed on the lower left refers to the analysis parameters (particular to each subject). The tables display quantitative values for diameters of the measured obstruction, reference, and subsequent percent stenosis (blue). Mean diameter is displayed (in blue) as well as the segment profile from proximal to distal (line graph) image.
Revascularization outcomes were defined pre hoc and included variables such as the ankle-brachial index (ABI), and change in both claudication onset time (COT) and peak walking time (PWT) between baseline and 6 months. At baseline, all subjects walked ≥ 2 minutes and <11 minutes using the Gardner-Skinner graded treadmill protocol. The COT was defined as the time at which subjects first reported symptoms of claudication. The PWT was defined as the length of time a subject was able to walk before claudication forced him/her to rest. The resting ABI was measured pre-exercise with subjects prone for 5 minutes. The post-exercise ABI was measured immediately after exercise, and then at 2, 4, 6, 8, and 10 minutes post-exercise.
Subjects were followed up to 6 months post-stenting. The follow up included a brief physical exam, assessment of any adverse events, ABI/thigh BI, biochemistry samples, treadmill test, 7 day electronic step monitoring, health cost data collection, quality of life questionnaires, independent exercise assessment, concurrent medication updates, and cilostazol dispensation. The protocol did not include any specific imaging studies to capture restenosis of the stents.
2.2. STATISTICAL ANALYSIS
The artery segments used in the analyses were the aorta, common iliac artery (CIA) and external iliac artery (EIA). An independent sample t-test was used to assess the difference in artery diameter with respect to the gender. Descriptive statistics were calculated for artery segment diameters, age in years, gender, weight, height, body mass index (BMI) and body surface area (BSA). An independent sample t-test was conducted to compare the reference vessels diameter, age, weight, BMI, BSA and height between males and females. These baseline variables were correlated with artery diameter and outcomes of revascularization. Statistical analyses were done using SPSS (Statistical Package for Social Sciences; version 16.0, Illinois, USA).
Correlations were conducted using the Spearman correlation to investigate the relationship among variables. Linear regression analysis was performed taking into account sex as an independent variable and was regressed against the reference vessel diameters of aorta, CIA and EIA. Then the independent variables (age, gender, weight, height, BMI and BSA) were put (in a step-wise fashion) into a multiple linear regression and were analyzed against the dependent variable (which was arterial reference diameter). Revascularization outcome variables such as ankle-brachial index (ABI), claudication onset time (COT), and peak walking time (PWT) at baseline and 6-months were analyzed in a multiple linear regression against the reference vessel diameters and other baseline variables. Various models were created to assess the variance in arterial diameters based on the independent variables. P-values <0.05 are considered statistically significant. As these analyses are exploratory in nature, no adjustments for multiple comparisons were done.
3. RESULTS
All stents were successfully placed in the ST group. This study included a total of 39 subjects (mean age 63.9 ± 10.6 (s.d.) years; 13 females) from the CLEVER stent revascularization (ST) cohort who had angiograms that were available for analysis. The numbers of arterial segments treated were as follows: 1 aorta, 19 right common iliac arteries, 8 right external iliac arteries, 20 left common iliac arteries, and 7 left external arteries. The mean lesion length was 3.9±3.4 cm and mean stenosis pre-procedure was 83±19%. For each subject, the diseased arterial segment(s) was (were) measured and analyzed to define the percent stenosis per the CLEVER protocol. While each subject had five arterial segments – the aorta, the left and right CIAs, and the left and right EIAs only 55 segments in 39 subjects were actually diseased and targeted for analysis. Reference diameters were obtained from areas proximal to the lesions within the same vascular segment in all but 9 target segments which required contralateral measurement of the same segment to serve as a proxy reference. Automatic calibration using the Medis QVA system was achieved for 30 segments while manual calibration was required for 21 segments. The remaining four segments belong to the three subjects and required visual stenosis estimation. Descriptive statistics are shown in table 1. The reference vessel diameters for the aorta, common iliac and external iliac arteries (expressed as mean ± SD in mm) were 17.42 ± 3.94, 9.79 ± 2.40 and 6.96 ± 2.11, respectively
Table 1.
Descriptive variables with comparison between men and women.
| Variable | All patients (N= 39) | Men (N= 26) | Women (N= 13) | p-value |
|---|---|---|---|---|
| Age, mean±s.d. (N), years | 63.9±10.6 (39) | 65.1±11.3 (26) | 61.4±8.8 (13) | 0.307 |
| Height, mean±s.d. (N), inches | 67.3±4.9 (39) | 69.5±3.1 (26) | 62.8 ±0.7 (13) | <0.001 |
| Weight, mean±s.d. (N), lbs. | 176.7±38.9 (39) | 184.1±40.9 (26) | 161.9±30.8 (13) | 0.094 |
| BSA, mean±s.d. (N), m2. | 1.91±0.23 (39) | 1.99±0.22 (26) | 1.75±0.17 (13) | 0.002 |
| BMI, mean±s.d. (n). | 28.14±6.24 (39) | 27.52±5.39 (26) | 29.38±7.75 (13) | 0.387 |
| Reference vessel size of aorta, mean±s.d. (N), mm | 17.42±3.94 (39) | 17.49±3.67 (26) | 17.25±4.65 (13) | 0.861 |
| Reference vessel size of CIA, mean±s.d. (N), mm | 9.79±2.40 (39) | 26 (10.20±2.36) | 13 (8.97±2.37) | 0.136 |
| Reference vessel size of EIA,, mean±s.d. (N), mm | 6.96±2.11 (22) | 7.50±2.14 (15) | 5.81±1.61 (7) | 0.080 |
| ABI at baseline, mean±s.d. (N) | 0.62±0.17 (39) | 0.63±0.17 (26) | 0.59±0.17 (13) | 0.410 |
| ABI at 6 months, mean±s.d. (N) | 1.00±0.29 (39) | 1.03±0.32 (26) | 0.95±0.25 (13) | 0.446 |
| COT at baseline, mean±s.d. (N), min | 1.5±1.0 (39) | 1.6±0.9 (26) | 1.4±1.1 (13) | 0.630 |
| COT at 6 months, mean±s.d. (N), min | 7.0±6.7 (39) | 7.5±7.8 (26) | 6.1±3.5 (13) | 0.564 |
| PWT at baseline, mean±s.d. (N), min | 4.8±1.9 (39) | 5±2.1 (26) | 4.4±1.5 (13) | 0.367 |
| PWT at 6 months, mean±s.d. (N), min | 9.8±5.1 (39) | 10±5.6 (26) | 9.3±4.1 (13) | 0.697 |
BSA= Body surface area, BMI= Body Mass Index, CIA= Common iliac artery, EIA= External iliac artery, ABI= Ankle-Brachial index, COT= Claudication onset time on the graded treadmill test, PWT= Peak walking time on the graded treadmill test. The total number of subjects was 39. The numbers less than 39 indicate the missing data for the arterial segments not intervened
There was trend toward larger EIA diameters in males compared with females (mean 7.50 ± 2.14 vs 5.81 ± 1.61 mm (p= 0.080). The aorta and CIA diameters were not different between males and females. The revascularization outcomes (ABI, COT and PWT changes from baseline to 6 months) between males and females are shown in table 1.
Spearman correlations were done to assess bivariate correlations between these variables (Table 2). There was statistically significant positive correlation between gender and EIA diameter (r = 0.392, p= 0.035). A marginally significant correlation was observed between gender and CIA diameter, r = 0.266, p= 0.051. There was no other variable that was statistically correlating with reference vessel diameters although age showed marginal significant positive correlation with CIA diameter, r = 0.265, p= 0.052. Simple linear regression was done to assess the influence of gender on reference vessel diameters of aorta, CIA and EIA (not shown in table). The result of the regression indicated that gender explained each 0.1% variance in aorta diameter (β = 0.029, t = 0.176, p = 0.861), 5.9% variance in CIA diameter (β = 0.243, t = 1.524, p= 0.136) and 14.6% variance in EIA (β = 0.381, t = 1.846, p = 0.080). Multiple linear regression was done using the baseline variables of age, weight, height, BMI, gender and BSA to test the ability of these variables to predict the diameters of the aorta, CIA and EIA. None of these models indicated more statistical significance than the univariate models.
Table 2.
Spearman correlation with significance values among the study variables.
| Study variables correlations [p-values] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | Weight | Height | BMI | BSA | Gender | Aorta@ | CIA@ | EIA@ | ||
| Study Variables correlations [p-values] | Age | - | 0.269 [0.049]** | 0.021 [0.450] | 0.276 [0.045]** | 0.188 [0.126] | 0.184 [0.131] | 0.032 [0.425] | 0.265 [0.052] | 0.053 [0.408] |
| Weight | 0.269 [0.049]** | - | 0.406 [0.005]** | 0.603 [<0.001]** | 0.861 [<0.001]** | 0.273 [0.046]** | −0.161 [0.166] | 0.150 [0.181] | 0.082 [0.359] | |
| Height | 0.021 [0.450] | 0.406 [0.005]** | - | −0.251 [0.062] | 0.757 [<0.001]** | 0.655 [<0.001]** | 0.007 [0.484] | 0.119 [0.236] | 0.233 [0.149] | |
| BMI | 0.276 [0.045]** | 0.603 [<0.001]** | −0.251 [0.062] | - | 0.253 [<0.001]** | −0.102 [0.269] | −0.178 [0.143] | 0.058 [0.363] | −0.086 [0.351] | |
| BSA | 0.188 [0.126] | 0.861 [<0.001]** | 0.757 [<0.001]** | 0.253 [<0.001]** | - | 0.500 [0.001]** | −0.164 [0.163] | 0.116 [0.241] | 0.120 [0.297] | |
| Gender | 0.184 [0.131] | 0.273 [0.046]** | 0.655 [<0.001]** | −0.102 [0.269] | 0.500 [0.001]** | - | −0.015 [0.463] | 0.266 [0.051] | 0.392 [0.035]** | |
| Aorta@ | 0.032 [0.425] | −0.161 [0.166] | 0.007 [0.484] | −0.178 [0.143] | −0.164 [0.163] | −0.015 [0.463] | - | 0.515 [<0.001]** | 0.466 [0.017]** | |
| CIA@ | 0.265 [0.052] | 0.150 [0.181] | 0.119 [0.236] | 0.058 [0.363] | 0.116 [0.241] | 0.266 [0.051] | 0.515 [<0.001]** | - | 0.871 [<0.001]** | |
| EIA@ | 0.053 [0.408] | 0.082 [0.359] | 0.233 [0.149] | −0.086 [0.351] | 0.120 [0.297] | 0.392 [0.035]** | 0.466 [0.017]** | 0.871 [<0.001]** | - | |
BSA= Body surface area, BMI= Body Mass Index, CIA= Common iliac artery, EIA= External iliac artery.
Reference vessel sizes in mm
Significant 1-tailed p-values (p<0.05)
– indicates the perfect correlation, [ ] indicates p-values for the respective correlation.
There was a significant strong positive correlation between the CIA diameter with ABI at 6 months (r = 0.440, p = 0.003). COT at baseline and age (r = 0.276, p = 0.045) and COT at baseline and BMI (r = 0.388, p = 0.007) were positively correlated. PWT at 6 months had negative correlation with age (r = −0.407, p = 0.006), weight (r = −0.409, p = 0.005) and BSA (r = −0.375, p = 0.010). Similarly, negative correlations were observed for the COT at 6 month with age (r = −0.346, p = 0.017), height (r = −0.394, p = 0.007) and BSA (r = −0.352, p = 0.015). For ABI (at baseline and 6 month), COT (at baseline and 6 month) and PWT (at baseline and 6 month), we found that in multiple linear regression CIA diameters explained 47.4% of the variance in ABI change from baseline to 6 months (β = 0.474, p = 0.035). The other models failed to explain any variance.
4. DISCUSSION
Our analysis demonstrates the presence of sex-based differences in baseline iliac artery diameters, with a clear sex-based diameter difference in the external iliac arteries of female and male patients with atherosclerotic lower extremity PAD but no outcome difference. These data, measured with precision in a limited study cohort, may seem to be marginal, and in this sample was not associated with some differences in post-endovascular outcomes. The external iliac artery diameters were smaller by an average of 1.69 mm in females compared to males.
The effects of various factors on arterial diameter, have been well established in many vascular territories, such as the carotid23-25, coronary26,27 and brachial arteries28, but have not been well characterized in the abdominal and lower extremity vascular beds.29-34 In the carotid arteries, the major cardiovascular risk factors (smoking, hypertension, and cholesterol levels) are inversely related to the diameter of the internal carotid artery25 and male sex is associated with a larger diameter.24 In both carotid and coronary arteries, this plaque burden is directly correlated with larger luminal diameters.25-27
In our study, regression analysis indicated that sex explained 0.1% of the variance in aorta diameter and 5.9% of the variance in common iliac diameter, although these trends were not statistically significant in this small cohort, and thus these findings should be considered hypothesis generating, and worthy of evaluation in future investigations. Previous studies have reported an inconsistent correlation between sex and aortic diameters.30,32-34
These studies have generally used ultrasound measurements in a healthy population and may not be relevant once severe atherosclerotic disease is manifest. The sex based differences in aortic diameter in prior studies has been reported to be between 0.23 cm and 0.35 cm. These are diameter differences that could well influence outcomes in larger endovascular treatment cohorts.
Multiple linear regression analysis of other variables did not show a significant influence of other variables (age, height, weight, BMI and BSA) on the baseline artery diameters. However, the highest variance (19%) was noted between the BMI and BSA measurements and aortic diameter. This finding is similar to previously reported publications that have documented statistically significant, but small effects for all of these variables on the diameter of vessels.30-34 The CIA and EIA did not show significant variance with any of the variables in our study.
The impact of these arterial diameter distinctions has not been well-described in past studies. Some prior studies have described lower patency rates after revascularization of the EIA particularly in women.35-39 Smaller vessel diameter has been suggested as a possible reason for decreased patency rates in female patients, but this relationship is not certain.35-37,40-43 In addition to smaller iliac artery diameters in women, social factors exist that may also contribute to differential outcomes. For example, operator preference for smaller devices to accommodate smaller caliber vessels is also considered to be one of the contributing factors.2 The study by Timaran et al also found EIA disease to be an independent predictor for decreased primary patency in women after angioplasty and stenting, but not in men.2 In our study, the average diameter of the EIA in women was smaller compared to men. However, the discrepant artery diameter did not negatively affect the outcome (ABI, COT and PWT) of aortoiliac stenting among females in our cohort. This is a significant deviation from conclusions of the previous studies and highlights a gap in scientific evidence, strongly advocating further studies on influence of gender on outcome of endovascular revascularization in iliac arteries.
5. LIMITATIONS
The major limitation of our study is the small sample size. However, we note that a majority of PAD descriptive and treatment studies include such small samples, and there is a relative absence of larger study cohorts now available for these anatomic descriptive surveys. For these data, the endovascular treatment cohort was obtained from the CLEVER trial, a very well-characterized study cohort, with all measurements occurring within carefully controlled investigative conditions. As such, the quantitative measurements used consistent methods in a core laboratory.
6. CONCLUSIONS
Our data demonstrates a small gender-based difference in iliac artery diameters at baseline in a cohort of individuals with atherosclerotic lower extremity PAD. These distinctions were modest and did not affect the ability of the endovascular intervention to achieve beneficial clinical outcome measures (ABI, COT and PWT) at six months. The conclusion of this study is significantly different from previous studies. Thus there is considerable uncertainty regarding how gender might influence baseline PAD anatomy and outcomes, and it is hoped that these results highlight this evidence gap. Due to very small sample size in our study, there remains a need to confirm the impact of gender on PAD outcomes in larger and more diverse populations in a prospective manner.
Acknowledgments
Funding: The study was funded by National Heart, Lung, and Blood Institute of the National Institutes of Health and co-funded by the National Institute of Health for Research on Women's Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Venkataramu N. Krishnamurthy, 2215 Fuller Road, Mail code #114, Ann Arbor VA Health System, Ann Arbor, Michigan, USA..
Muhammad Naeem, Department of Diagnostic Imaging, Division of Vascular and Interventional Radiology, Rhode Island Hospital, Warren Alpert Medical School of Brown University, Providence, Rhode Island, USA. mnaeem@Lifespan.org/ Muhammad_naeem@brown.edu.
Timothy P. Murphy, Department of Diagnostic Imaging, Division of Vascular and Interventional Radiology, Rhode Island Hospital, Warren Alpert Medical School of Brown University, Providence, Rhode Island, USA. TMurphy@Lifespan.org.
Joselyn Cerezo, Department of Diagnostic Imaging, Division of Vascular and Interventional Radiology, Rhode Island; Hospital, Warren Alpert Medical School of Brown University, Providence, Rhode Island, USA. jvcerezo1@gmail.com.
Paul Gaither Jordan, Department of Diagnostic Imaging, Division of Vascular and Interventional Radiology, Rhode Island Hospital, Warren Alpert Medical School of Brown University, Providence, Rhode Island, USA. pjordan@Lifespan.org.
Suzanne Goldberg, Nutrition branch, Division of Cardiovascular diseases, National Heart, Lung and Blood Institute, National Institute of Health, Bethesda, Maryland, USA. Suzanneg2@gmail.com.
Abby G. Ershow, Division of Cardiovascular Sciences, National Heart, Lung, and Blood Institute, 6701 Rockledge Drive, Room 8160, MSC 7956, Bethesda MD 20892-7956 ErshowA@nhlbi.nih.gov.
Alan T. Hirsch, Cardiovascular Division and Lillehei Heart Institute, 420 Delaware Street SE, MMC 508, University of Minnesota Medical School, Minneapolis, Minnesota, USA. hirsc005@umn.edu.
Niki Oldenburg, Cardiovascular Division and Lillehei Heart Institute, 420 Delaware Street SE, MMC 508, University of Minnesota Medical School, Minneapolis, Minnesota, USA. olden019@umn.edu.
Donald E. Cutlip, Clinical Investigations, 930 Commonwealth Avenue, Harvard Clinical Research Institute, Boston, Massachusetts, USA. don.cutlip@hcri.harvard.edu.
REFERENCES
- 1.Vouyouka AG, Kent KC. Arterial vascular disease in women. J Vasc Surg. 2007;46:1295–302. doi: 10.1016/j.jvs.2007.07.057. [DOI] [PubMed] [Google Scholar]
- 2.Timaran CH, Stevens SL, Freeman MB, Goldman MH. External iliac and common iliac artery angioplasty and stenting in men and women. J Vasc Surg. 2001;34:440–6. doi: 10.1067/mva.2001.117148. [DOI] [PubMed] [Google Scholar]
- 3.Colapinto RF, Stronell RD, Johnston WK. Transluminal angioplasty of complete iliac obstructions. AJR Am J Roentgenol. 1986;146:859–62. doi: 10.2214/ajr.146.4.859. [DOI] [PubMed] [Google Scholar]
- 4.Colapinto RF, Harries-Jones EP, Johnston KW. Percutaneous transluminal recanalization of complete iliac artery occlusions. Arch Surg. 1981;116:277–81. doi: 10.1001/archsurg.1981.01380150011003. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen LL, Hevelone N, Rogers SO, et al. Disparity in outcomes of surgical revascularization for limb salvage: race and gender are synergistic determinants of vein graft failure and limb loss. Circulation. 2009;119(1):123–30. doi: 10.1161/CIRCULATIONAHA.108.810341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Enzler MA, Ruoss M, Seifert B, Berger M. The influence of gender on the outcome of arterial procedures in the lower extremity. Eur J Vasc Endovasc Surg. 1996;11(4):446–52. doi: 10.1016/s1078-5884(96)80180-8. [DOI] [PubMed] [Google Scholar]
- 7.Yang F, Minutello RM, Bhagan S, et al. The impact of gender on vessel size in patients with angiographically normal coronary arteries. J Interv Cardiol. 2006;19(4):340–4. doi: 10.1111/j.1540-8183.2006.00157.x. [DOI] [PubMed] [Google Scholar]
- 8.Argulian E, Patel AD, Abramson JL, et al. Gender differences in short-term cardiovascular outcomes after percutaneous coronary interventions. Am J Cardiol. 2006;98(1):48–53. doi: 10.1016/j.amjcard.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 9.Arnold AM, Mick MJ, Piedmonte MR, et al. Gender differences for coronary angioplasty. Am J Cardiol. 1994;74(1):18–21. doi: 10.1016/0002-9149(94)90484-7. [DOI] [PubMed] [Google Scholar]
- 10.Bell MR, Grill DE, Garratt KN, et al. Long-term outcome of women compared with men after successful coronary angioplasty. Circulation. 1995;91(12):2876–81. doi: 10.1161/01.cir.91.12.2876. [DOI] [PubMed] [Google Scholar]
- 11.O'Connor GT, Morton JR, Diehl MJ, et al. Differences between men and women in hospital mortality associated with coronary artery bypass graft surgery. The Northern New England Cardiovascular Disease Study Group. Circulation. 1993;88(5 Pt 1):2104–10. doi: 10.1161/01.cir.88.5.2104. [DOI] [PubMed] [Google Scholar]
- 12.Peterson ED, Lansky AJ, Kramer J, et al. Effect of gender on the outcomes of contemporary percutaneous coronary intervention. Am J Cardiol. 2001;88(4):359–64. doi: 10.1016/s0002-9149(01)01679-4. [DOI] [PubMed] [Google Scholar]
- 13.Dodge JT, Jr., Brown BG, Bolson EL, et al. Lumen diameter of normal human coronary arteries. Influence of age, sex, anatomic variation, and left ventricular hypertrophy or dilation. Circulation. 1992;86(1):232–46. doi: 10.1161/01.cir.86.1.232. [DOI] [PubMed] [Google Scholar]
- 14.Hirsch AT, Allison MA, Gomes AS, et al. A Call to Action: Women and Peripheral Artery Disease: A Scientific Statement From the American Heart Association. Circulation. 2012;125(11):1449–72. doi: 10.1161/CIR.0b013e31824c39ba. [DOI] [PubMed] [Google Scholar]
- 15.Sandgren T, Sonesson B, Ahlgren R, et al. The diameter of the common femoral artery in healthy human: influence of sex, age, and body size. J Vasc Surg. 1999;29(3):503–10. doi: 10.1016/s0741-5214(99)70279-x. [DOI] [PubMed] [Google Scholar]
- 16.AhChong AK, Chiu KM, Wong M, et al. The influence of gender difference on the outcomes of infrainguinal bypass for critical limb ischaemia in Chinese patients. Eur J Vasc Endovasc Surg. 2002;23(2):134–9. doi: 10.1053/ejvs.2001.1564. [DOI] [PubMed] [Google Scholar]
- 17.Dinter DJ, Neff KW, Visciani G, et al. Peripheral bolus-chase MR angiography: analysis of risk factors for nondiagnostic image quality of the calf vessels--a combined retrospective and prospective study. AJR Am J Roentgenol. 2009;193(1):234–40. doi: 10.2214/AJR.08.1814. [DOI] [PubMed] [Google Scholar]
- 18.Murphy TP, Hirsch AT, Ricotta JJ, et al. The Claudication: Exercise Vs. Endoluminal Revascularization (CLEVER) study: Rationale and methods. J Vasc Surg. 2008;47(6):1356–63. doi: 10.1016/j.jvs.2007.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy TP, Hirsch AT, Cutlip DE. Claudication: Exercise vs Endoluminal Revascularization (CLEVER) study update. J Vasc Surg. 2009;50(4):942–5. doi: 10.1016/j.jvs.2009.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy TP, Cutlip DE, Regensteiner JG, et al. Supervised Exercise Versus Primary Stenting for Claudication Resulting From Aortoiliac Peripheral Artery Disease: Six-Month Outcomes From the Claudication: Exercise Versus Endoluminal Revascularization (CLEVER) Study. Circulation. 2012;125(1):130–9. doi: 10.1161/CIRCULATIONAHA.111.075770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schnyder G, Sawhney N, Whisenant B, et al. Common femoral artery anatomy is influenced by demographics and comorbidity: implications for cardiac and peripheral invasive studies. Catheter Cardiovasc Interv. 2001;53(3):289–95. doi: 10.1002/ccd.1169. [DOI] [PubMed] [Google Scholar]
- 22.van Assen HC, Vasbinder GBC, Stoel BC, et al. Quantitative Assessment of the Morphology of Renal Arteries from X-ray Images: Quantitative Vascular Analysis. Invest Radiol. 2004;39(6):365–73. doi: 10.1097/01.rli.0000126178.51618.13. [DOI] [PubMed] [Google Scholar]
- 23.Patel AS, Mackey RH, Wildman RP, et al. Cardiovascular risk factors associated with enlarged diameter of the abdominal aortic and iliac arteries in healthy women. Atherosclerosis. 2005;178(2):311–7. doi: 10.1016/j.atherosclerosis.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 24.Bonithon-Kopp C, Touboul PJ, Berr C, et al. Factors of carotid arterial enlargement in a population aged 59 to 71 years: the EVA study. Stroke. 1996;27(4):654–60. doi: 10.1161/01.str.27.4.654. [DOI] [PubMed] [Google Scholar]
- 25.Crouse JR, Goldbourt U, Evans G, et al. Risk factors and segment-specific carotid arterial enlargement in the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1996;27(1):69–75. doi: 10.1161/01.str.27.1.69. [DOI] [PubMed] [Google Scholar]
- 26.Zarins CK, Weisenberg E, Kolettis G, et al. Differential enlargement of artery segments in response to enlarging atherosclerotic plaques. J Vasc Surg. 1988;7(3):386–94. [PubMed] [Google Scholar]
- 27.Glagov S, Weisenberg E, Zarins CK, et al. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316(22):1371–5. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 28.Urbina EM, Brinton TJ, Elkasabany A, Berenson GS. Brachial artery distensibility and relation to cardiovascular risk factors in healthy young adults (The Bogalusa Heart Study). Am J Cardiol. 2002;89(8):946–51. doi: 10.1016/s0002-9149(02)02244-0. [DOI] [PubMed] [Google Scholar]
- 29.Ahlgren AR, Hansen F, Sonesson B, et al. Stiffness and diameter of the common carotid artery and abdominal aorta in women. Ultrasound Med Biol. 1997;23(7):983–8. doi: 10.1016/s0301-5629(97)00082-3. [DOI] [PubMed] [Google Scholar]
- 30.Lederle FA, Johnson GR, Wilson SE, et al. Relationship of age, gender, race, and body size to infrarenal aortic diameter. J Vasc Surg. 1997;26(4):595–601. doi: 10.1016/s0741-5214(97)70057-0. [DOI] [PubMed] [Google Scholar]
- 31.Liddington MI, Heather BP. The relationship between aortic diameter and body habitus. Eur J Vasc Surg. 1992;6(1):89–92. doi: 10.1016/s0950-821x(05)80101-x. [DOI] [PubMed] [Google Scholar]
- 32.Pearce WH, Slaughter MS, Lemaire S, et al. Aortic Diameter as a Function of Age, Gender, and Body-Surface Area. Surgery. 1993;114(4):691–7. [PubMed] [Google Scholar]
- 33.Pedersen OM, Aslaksen A, Vikmo H. Ultrasound Measurement of the Luminal Diameter of the Abdominal-Aorta and Iliac Arteries in Patients without Vascular-Disease. J Vasc Surg. 1993;17(3):596–601. doi: 10.1067/mva.1993.39525. [DOI] [PubMed] [Google Scholar]
- 34.Sonesson B, Lanne T, Hansen F, et al. Infrarenal Aortic Diameter in the Healthy Person. Eur J Vasc Surg. 1994;8(1):89–95. doi: 10.1016/s0950-821x(05)80127-6. [DOI] [PubMed] [Google Scholar]
- 35.Hood DB, Hodgson KJ. Percutaneous transluminal angioplasty and stenting for iliac artery occlusive disease. Surg Clin North Am. 1999;79(3):575–96. doi: 10.1016/s0039-6109(05)70025-6. [DOI] [PubMed] [Google Scholar]
- 36.Johnston KW. Iliac arteries: reanalysis of results of balloon angioplasty. Radiology. 1993;186(1):207–12. doi: 10.1148/radiology.186.1.8416566. [DOI] [PubMed] [Google Scholar]
- 37.Strecker EP, Boos IB, Hagen B. Flexible tantalum stents for the treatment of iliac artery lesions: long-term patency, complications, and risk factors. Radiology. 1996;199(3):641–7. doi: 10.1148/radiology.199.3.8637980. [DOI] [PubMed] [Google Scholar]
- 38.Powell RJ, Fillinger M, Bettmann M. The durability of endovascular treatment of multisegment iliac occlusive disease. J Vasc Surg. 2000;31(6):1178–84. doi: 10.1067/mva.2000.104569. [DOI] [PubMed] [Google Scholar]
- 39.Powell RJ, Fillinger M, Walsh DB, et al. Predicting outcome of angioplasty and selective stenting of multisegment iliac artery occlusive disease. J Vasc Surg. 2000;32(3):564–9. doi: 10.1067/mva.2000.107760. [DOI] [PubMed] [Google Scholar]
- 40.Ballard JL, Bergan JJ, Singh P, et al. Aortoiliac stent deployment versus surgical reconstruction: analysis of outcome and cost. J Vasc Surg. 1998;28(1):94–101. doi: 10.1016/s0741-5214(98)70204-6. [DOI] [PubMed] [Google Scholar]
- 41.Sapoval MR, Chatellier G, Long AL, et al. Self-expandable stents for the treatment of iliac artery obstructive lesions: long-term success and prognostic factors. AJR Am J Roentgenol. 1996;166(5):1173–9. doi: 10.2214/ajr.166.5.8615265. [DOI] [PubMed] [Google Scholar]
- 42.Bosch JL, Hunink MG. Meta-analysis of the results of percutaneous transluminal angioplasty and stent placement for aortoiliac occlusive disease. Radiology. 1997;204(1):87–96. doi: 10.1148/radiology.204.1.9205227. [DOI] [PubMed] [Google Scholar]
- 43.Horejs D, Gilbert PM, Burstein S, et al. Normal aortoiliac diameters by CT. J Comput Assist Tomogr. 1988;12(4):602–3. doi: 10.1097/00004728-198807000-00011. [DOI] [PubMed] [Google Scholar]